Abstract Abstract
Pulmonary hypertension (PH) and right ventricular function are the focus of cardiovascular effects of bronchopulmonary dysplasia (BPD). We assessed cardiac indexes reflecting systemic afterload and pulmonary venous back pressure as pathophysiologic factors. Cardiac parameters were measured by conventional echocardiography in 20 preterm infants with severe BPD and compared with those of 10 preterm infants with no BPD and 20 healthy term infants. In infants with severe BPD, PH was noted in 5 (25%) by tricuspid regurgitation Doppler jet ≥2.8 m/s and in 15 (75%) by time to peak velocity/right ventricular ejection time <0.34. Among systemic cardiac indexes, significant impairment of diastolic measures was noted in the BPD group compared with infants with no BPD and term infants. The significance persisted after adjusting for gestational age and birth weight. These included transmitral E/A ratio (1.07 ± 0.07 vs. 0.91 ± 0.04 vs. 0.89 ± 0.09; P < 0.0001), isovolumic relaxation time (68.8 ± 3.9 vs. 58.5 ± 7.8 vs. 54.2 ± 5.7 ms ; P < 0.0001), mitral valve stroke volume (4.7 ± 0.7 vs. 5.6 ± 0.6 vs. 5.9 ± 0.1; P = 0.002), and myocardial performance index (0.33 ± 0.05 vs. 0.28 ± 0.01 vs. 0.27 ± 0.05; P = 0.03). Left ventricular output was significantly lower in the BPD cohort (183 ± 45 vs. 189 ± 9 vs. 191 ± 32 mL/kg/min; P = 0.03). Altered systemic (left-sided) cardiac function was noted in infants with BPD, which may lead to pulmonary venous congestion contributing to a continued need for respiratory support.
Keywords: infants, chronic lung disease, cardiac function, pulmonary hypertension
Bronchopulmonary dysplasia (BPD) is the most common and most significant respiratory complication of preterm birth, with an incidence of about 60% in infants ≤25 weeks gestational age (GA).1 It is associated with prolonged hospitalization, the need for home oxygen therapy, and hospital readmissions during infancy and beyond. A proportion of these infants develop pulmonary hypertension (PH), which may increase the risk of mortality.2 Among infants with severe BPD, the reported incidence of PH is between 15% and 58%.2-4 Although cardiac catheterization is the gold standard to accurately measure pulmonary pressures, the assessment is generally made noninvasively with echocardiography (ECHO) using various markers, such as tricuspid regurgitation jet velocity (TRJV), pulmonary artery Doppler (time to peak velocity/right ventricular ejection time; TPV/RVETc), and flattened or bowed interventricular septum (IVS).
Pulmonary hypertension complicating BPD is generally the end result of a variety of pathophysiologic processes. The assessment and therapy are almost exclusively focused on decreasing pulmonary vascular resistance (PVR) and increasing pulmonary blood flow (PBF).2,5,6 The contribution of the left-sided (systemic) circulation toward BPD pathophysiology is poorly understood. A small number of investigators have previously noted impaired left ventricular (LV) function or systemic hypertension in infants with BPD.7-11Mourani et al.8 first reported on the contribution of LV hemodynamics on BPD pathophysiology. The identification and treatment of LV diastolic dysfunction were accompanied by a significant clinical improvement.8 It was postulated that the elevated left atrial (LA) pressure may lead to pulmonary venous hypertension (and subsequently may contribute to the development of pulmonary arterial hypertension). PH is a well-recognized complication of BPD. It is classified separately in the Panama Classification of Paediatrics Hypertensive Vascular Diseases and, among pathophysiology, includes diastolic dysfunction.12 The European Society of Cardiology Guidelines categorized patients with PH into groups based on the underlying disease process.13 This classification includes chronic lung diseases and, separately, patients with LV systolic/diastolic dysfunction. In adults, PH is very common among patients with left-sided cardiac disease (>60% of patients with LV systolic dysfunction and >80% of patients with LV diastolic dysfunction).14,15 Left-sided structural heart disease or other nonstructural conditions (systemic hypertension, coarctation of the aorta) have all been reported in association with PH.16 Systemic hypertension and aortic wall thickness have been noted in infants with BPD and may contribute to elevated systemic afterload.10-12,17
We hypothesize that systemic cardiac dysfunction (especially diastolic dysfunction) may be present in infants with BPD. This may contribute to ongoing respiratory morbidity by way of increasing the back pressure pulmonary venous effects. The objective of this study was to ascertain the left-sided cardiac function in infants with severe BPD using conventional ECHO.
Methods
This cross-sectional study with detailed echocardiographic assessments was conducted at a busy perinatal center with a capacity of 58 beds (28 with ventilation support). Approximately 160 infants with very low birth weight (<1,500 g) are admitted annually; half of these infants have extremely low birth weight (<1,000 g). The enrollment was consecutive and took place over a 1-year period. There were no cases of refusal of consent. Twenty preterm infants (≤28 completed weeks GA and birth weight <1,000 g) were evaluated at 36 weeks corrected GA if they fulfilled the diagnostic criteria for BPD (group 1). For the infants born at <32 weeks GA, the Australia and New Zealand Neonatal Network (ANZNN) defines BPD as lung disease with ongoing requirement for supplemental oxygen therapy or ventilation support (high-flow oxygen, continuous positive airway pressure, or mechanical ventilation) at 36 weeks postmenstrual age. All the infants belonged to the severe BPD category (need for ≥30% oxygen and/or positive pressure at 36 weeks postmenstrual age).18 None of the infants were intubated and given mechanical ventilation at the time of the study. They were compared with preterm infants with no BPD (group 2) and healthy, asymptomatic term infants in the postnatal ward between 2 and 5 days of life (group 3). Infants with congenital malformations, chromosomal abnormalities, or born to mothers with diabetes were excluded. In this busy perinatal center, preterm infants not needing respiratory support are transferred to step-down units unless residing locally, which limits access to the non-BPD population. The study was approved by the institutional research ethics board, and informed parental consent was obtained. Baseline neonatal characteristics, including GA and weight, mode of delivery, and common neonatal morbidities were recorded. Noninvasive blood pressure measurements were obtained using an appropriate-sized cuff on the right arm with the infant in a quiet state and positioned supine in a cot at the level of the sphygmomanometer (model M3046A; Philips, Boeblingen, Germany). The average values of two readings were recorded.
Echocardiographic assessments
Detailed ECHO was performed by a single operator (A.S.) using the Vivid 7 Advantage cardiovascular ultrasound system (GE Medical Systems, Milwaukee, WI) with 7.5- and 10-MHz high-frequency probes. Table 1 describes the various parameters and their roles in the assessment of various aspects of cardiac function. The TPV/RVETc ratio is inversely related to pulmonary artery pressure.19 The IVS septal position was graded as <50% of the systemic pressure (round septum at end systole), ≥50% but <100% of the systemic pressure (end-systolic flattening), and ≥100% of the systemic pressure (end-systole bowing into the left ventricle).20 The aortic cross-sectional area was measured from the left atrial/aortic ratio images following a continuous sweep from the left ventricle to the aortic root at a position where the valve cusps were well visualized.21 The E wave deceleration time (EDT) was measured from the maximum E point to the baseline. Left ventricular diastolic dysfunction was characterized by increased E/A ratio, prolongation of EDT and isovolumic relaxation time (IVRT), and impaired pulmonary venous and mitral flow. End-systolic wall stress (ESWS) was assessed as a measure of afterload.22 All pulse wave Doppler measurements were calculated from the average of three consecutive cardiac cycles. Doppler analysis of all four pulmonary veins was performed to rule out pulmonary vein stenosis. The infants were not sedated; the unit prefers swaddling, dummy, and sucrose instead.
Table 1.
Summary of echocardiographic measures performed in the study population
| Component of function | Technique | View | Cursor position | Comments | Reference |
|---|---|---|---|---|---|
| Pulmonary hemodynamic characteristic: | |||||
| TR jet velocity | CWD | Apical 4 chamber | Aligned with the jet | Angle dependant, ≥2.8 m/s (∼36 mmHg) | Lau et al.32 |
| TPV/RVETc | PWD | Parasternal short axis (PA view) | Aligned with the flow, sample just beyond pulmonary valve | Angle and cursor position dependant (normal, >0.34) | Evans and Archer33 |
| Interventricular septum configuration | 2D | Short axis | View at mitral valve or papillary muscle level | Bowed into left ventricle or flat with high PA pressure | King et al.20 |
| Systemic systolic function: | |||||
| Fractional shortening (FS) | M-mode | Parasternal short axis | Just distal to mitral valve leaflet tips at end diastole | (LVEDD-LVESD)/LVEDD | Mertens et al.34 |
| Left ventricular output | PWD | Apical 5 chamber | Aligned with the flow, sample just beyond aortic valve | Angle and cursor position dependant | Silverman and Shiller35 |
| mVCFc, circ/s | 2D/M-mode | Combination of above two | Combination of above two | FS/LVET | Colan et al.36 |
| Tei index | PWD | Apical 4 and 5 chamber | Transmitral and apical 5-chamber | (IVCT + IVRT)/LVET | Eidem et al.37 |
| Diastolic function: | |||||
| E/A ratio, EDT, MVSV | PWD | Apical 4 chamber | Aligned with the flow, sample at tips of mitral leaflets | E wave: early passive filling; A wave: late active filling | Schmitz et al.38 |
| IVRT | PWD/CWD | Apical 5 chamber | PWD: sample volume placed within LVOT (in proximity to the anterior mitral leaflet to record both inflow and outflow signals); CWD: Doppler beam at an intermediate position (between inflow and outflow) to record both velocities | From closure of the aortic valve to the opening of the mitral valve | Quiñones et al.39 |
| Pulmonary venous flow | PWD | Apical 4 chamber | Aligned with the flow, sample into the vein | Biphasic wave | Ito et al.40 |
CWD: continuous wave Doppler; EDT: E wave deceleration time; IVCT: isovolumic contraction time; IVRT: isovolumic relaxation time; LVEDD: left ventricular end-diastolic dimension; LVESD: left ventricular end-systolic dimension; LVET: left ventricular ejection time; mVCFc: mean velocity of circumferential fiber shortening; MVSV: mitral valve stroke volume; PA: pulmonary artery; PWD: pulse wave Doppler; TPV/RVETc: time to peak velocity/right ventricular ejection time; TR: tricuspid regurgitation.
Statistical analyses
A convenient sample of 50 subjects was chosen due to the exploratory nature of this study. We assessed the effect of group (BPD, no BPD, and control) on cardiac parameters via general linear regression models with group as a categorical variable. To assess the independent effect of group on outcomes, analyses were further adjusted by including GA and birth weight as covariates in the regression models separately as well as together. Post hoc comparisons were performed using Bonferroni adjustment for multiple comparisons. Comparisons of proportions were made using Fisher exact test. Correlation between mean velocity of circumferential fiber shortening (mVCFc) and ESWS was assessed by Pearson product-moment correlation coefficient. Continuous variables were summarized using means and standard deviations (SDs). Categorical variables were reported as counts and proportions. All calculated P values were two tailed. P < 0.05 indicated statistical significance. Analyses were performed with SAS software, version 9.4 (SAS Institute, Cary, NC).
Results
Twenty infants were enrolled as BPD subjects (group 1). Table 2 depicts salient demographic characteristics of the whole population. Nine (45%) of the infants in the BPD group were treated for a patent ductus arteriosus in the first 4 weeks of life; none needed surgical duct ligation. Fifteen (75%) were discharged from the hospital with home oxygen support. All except 2 infants were receiving oral diuretics (a combination of chlorothiazide and spironolactone), which is a fairly standard practice in the unit. The average time between receipt of an oral diuretic and the echocardiographic assessment was 6 hours. Of the 20 infants with BPD, 8 had capillary gas tests performed on the day of the echocardiogram. All had compensated mean (±SD) pH (7.34 ± 0.03) and acceptable CO2 levels (52 ± 4 mmHg). In this cohort, only 1 infant received systemic steroids 1 week before the echocardiographic assessment. The unit practice is to administer systemic steroids only to facilitate extubation.
Table 2.
Demographic features of the study population
| Variable | Group 1 (N = 20) |
Group 2 (N = 10) |
Group 3 (N = 20) |
P |
|---|---|---|---|---|
| GA at birth, w | 26.2 ± 1.7 | 26.2 ± 0.6 | 38.6 ± 1.1 | <0.001a |
| Birth weight, g | 772.7 ± 271 | 704.8 ± 53 | 3,402 ± 472 | <0.001a |
| GA at assessment, w | 36.5 ± 0.1 | 36.5 ± 0.1 | 38.6 ± 1.1 | <0.001a |
| Weight at assessment, g | 1,898 ± 409 | 1,943.5 ± 206 | 3,403 ± 472 | <0.001a |
| Male sex, no. (%) | 11 (55) | 4 (40) | 11 (55) | 0.81 |
| Prolonged rupture of membranes (>18 h), no. (%) | 2 (10) | 1 (10) | 2 (10) | 1.00 |
| Antenatal steroids, no. (%) | 19 (95) | 10 (100) | 0 (0) | <0.001a |
| Mode of delivery, no. (%) | ||||
| Vaginal | 7 (35) | 4 (40) | 9 (45) | 0.93 |
| Cesarean | 13 (65) | 6 (60) | 11 (55) | |
| Surfactant replacement therapy, no. (%) | 19 (95) | 10 (100) | 0 (0) | <0.001a |
| Pulmonary hemorrhage, no. (%) | 1 (5) | 1 (10) | 0 (0) | 0.67 |
| Medical treatment for PDA, no. (%) | 9 (45) | 4 (40) | 0 (0) | 0.001a |
| Necrotizing enterocolitis, no. (%) | 1 (5) | 1 (10) | 0 (0) | 0.67 |
| Culture proven sepsis anytime during stay, no. (%) | 7 (35) | 3 (30) | 0 (0) | 0.008a |
| Discharge with home oxygen, no. (%) | 15 (75) | 0 (0) | 0 (0) | <0.001b |
| Systolic blood pressure, mmHg | 80 ± 6 | 70 ± 2 | 66 ± 5 | <0.001b |
| Diastolic blood pressure, mmHg | 44 ± 5 | 41 ± 2 | 40 ± 4 | 0.02a |
| Mean blood pressure, mmHg | 57 ± 5 | 49 ± 3 | 48 ± 4 | <0.001b |
Except where otherwise noted, data are mean value ± standard deviation. BW: birth weight; GA: gestational age; group 1: preterm infants with bronchopulmonary dysplasia (BPD); group 2: preterm infants with no BPD; group 3: term healthy infants; PDA: patent ductus arteriosus.
Group 1 versus group 3 is significant (P < 0.05).
Group 1 versus group 2 and group 1 versus group 3 are significant (P < 0.05).
The GA and birth weight among preterm infants with and without BPD was comparable. Systolic, diastolic, and mean blood pressure were higher in the infants with BPD. Among infants with BPD, 5 (25%) had TRJV ≥2.8 m/s, and 2 each had flattened or bowed IVS.2 On pulmonary artery Doppler examination, TPV/RVETc <0.34 was noted in 15 cases (75%). Table 3 compares systemic cardiac parameters among the study groups. Diastolic function parameters (E/A ratio, IVRT, Tei index, EDT, and pulmonary vein flow) were significantly altered, suggesting impaired cardiac relaxation (diastolic dysfunction) in group 1. The significance persisted after adjustment for GA and birth weight. Among systolic parameters, fractional shortening was comparable, although load independent marker (mVCFc) was significantly impaired. Figure 1 depicts the significant negative correlation between mVCFc and ESWS in the infants with severe BPD (r = −0.74, P = 0.0002). A higher ESWS (surrogate for afterload) correlated with a lower mVCFc (contractility). A small patent foramen ovale was noted in 7 infants with BPD, and all had a peak velocity of >0.5 m/s (restrictive). Among those with closed foramen ovale, 5 of 13 had bowing into the right atrium. Shunt was either closed or restrictive. A small patent foramen ovale was noted in 8 control subjects, with bidirectional low-velocity shunt in 2 of them.
Table 3.
Comparison of echocardiographic parameters in the study population
| P | |||||||
|---|---|---|---|---|---|---|---|
| Variable | BPD group (N = 20) |
Preterm no BPD group (N = 10) |
Term infants (N = 20) |
Unadjusted overall group effect | GA adjusted group effect | BW adjusted group effect | GA and BW adjusted group effect |
| Heart rate, beats/min | 146 ± 10 | 152 ± 11 | 145 ± 8 | 0.24 | 0.2 | 0.13 | 0.44 |
| Diastolic parameter: | |||||||
| EDT, ms | 75.6 ± 7.9 | 63.6 ± 8.2 | 60.5 ± 7 | <0.0001 | 0.001 | 0.001 | 0.001a |
| ESWS, g/cm2 | 72 ± 10.8 | 44.2 ± 6.5 | 41.8 ± 6.4 | <0.0001 | <0.0001 | <0.0001 | <0.0001b |
| E/A | 1.07 ± 0.07 | 0.91 ± 0.04 | 0.89 ± 0.09 | <0.0001 | <0.0001 | <0.0001 | <0.0001b |
| IVRT, ms | 68.8 ± 3.9 | 58.5 ± 7.8 | 54.2 ± 5.7 | <0.0001 | <0.0001 | <0.0001 | <0.0001a |
| MVSV, mL/kg | 4.7 ± 0.7 | 5.6 ± 0.6 | 5.9 ± 0.1 | <0.0001 | 0.01 | <0.0001 | 0.002b |
| PV VTI, cm | 5.4 ± 1.8 | 7.8 ± 0.9 | 8.2 ± 1.3 | <0.0001 | <0.0001 | <0.0001 | <0.0001a |
| Tei index | 0.33 ± 0.05 | 0.28 ± 0.01 | 0.27 ± 0.05 | 0.001 | 0.03 | 0.004 | 0.03a |
| Systolic parameter: | |||||||
| mVCFc | 1.7 ± 0.4 | 2.7 ± 0.2 | 3 ± 0.6 | <0.0001 | <0.0001 | <0.0001 | <0.0001a |
| LVSV, mL/kg | 1.25 ± 0.32 | 1.29 ± 0.09 | 1.32 ± 0.21 | 0.72 | 0.03 | 0.48 | 0.046 |
| LVO, mL/kg/min | 183 ± 45 | 189 ± 9 | 191 ± 32 | 0.75 | 0.054 | 0.21 | 0.03 |
| FS, % | 33.1 ± 3.3 | 33.3 ± 2.8 | 33.5 ± 0.5 | 0.96 | 0.95 | 0.93 | 0.92 |
| LVPW, mm | 1.98 ± 0.13 | 4.1 ± .19 | 5.8 ± 0.13 | <0.0001 | <0.0001 | <0.0001 | <0.0001a |
Data are presented as mean ± standard deviation. BPD: bronchopulmonary dysplasia; EDT: E wave deceleration time; ESWS: end-systolic wall stress; FS: fractional shortening; IVRT: end-systolic wall stress; LVO: left ventricular output; LVPW: left ventricular posterior wall; LVSV: left ventricular stroke volume; mVCFc: mean velocity of circumferential fiber shortening; MVSV: mitral valve stroke volume; PV VTI: pulmonary vein velocity time integral.
BPD group versus No BPD group is significant (P < 0.05).
BPD group versus No BPD group and BPD group versus Term controls are significant (P < 0.05).
Figure 1.
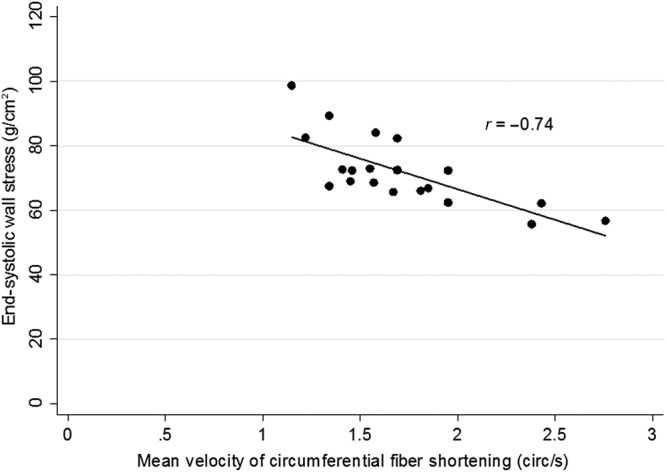
Relationship between end-systolic wall stress and cardiac contractility in infants with bronchopulmonary dysplasia. circ: circulations.
Discussion
Along with precapillary PH and parenchymal disease, LV diastolic dysfunction may contribute to BPD pathophysiology. These alterations may contribute to pulmonary venous congestion and pulmonary edema, leading to reduced lung compliance. We noted impaired relaxation (diastolic dysfunction) and contractility (mVCFc and myocardial performance index [MPI]) with preserved conventional parameter (fractional shortening). The diastolic dysfunction in our cohort was characterized by abnormalities in transmitral measurements. These included E/A ratio, lower mitral stroke volume and EDT, and elevated MPI and IVRT. These may suggest elevated LA pressure and were accompanied by reduced pulmonary venous flow. These ECHO features may support the postulation of postcapillary pathology as a contributor to the overall BPD pathophysiology.
Evidence from adult literature
Postcapillary pathology, whether organic (pulmonary venous occlusive disease, mitral valve disease, or aortic valve disease) or functional (LV systolic or diastolic dysfunction), may contribute to the transudation of fluid across the intrapulmonary capillary beds and may represent an “edemagenic” factor in BPD affecting compliance, prolonging the need for respiratory support,14,15,23 and guides therapeutic options. The standard vasodilator testing (increasing PBF) runs the risk of development of acute pulmonary edema and sudden respiratory compromise in this group. Of note, patients with PH secondary to mitral valve disease have been excluded from the clinical trials evaluating the current vasodilator therapies.16 The pathogenesis of BPD is multifactorial. This consists of precapillary pulmonary hypertension and parenchymal abnormalities (compounded by oxygen toxicity and baro/volutrauma). We propose that postcapillary pathology may have an important bearing where elevated systemic afterload and LV diastolic dysfunction (with accompanied elevated LA pressure) may lead to pulmonary venous congestion and edema, providing the postcapillary component. In a recent study involving infants with BPD, Khemani et al.2 highlighted the alteration in catheter-measured pulmonary capillary wedge pressure after nitric therapy at 80 ppm or 100% oxygen. In 7 (58%) of 12, a net increase in wedge pressure was noted after nitric therapy, an overall increase from 10.8 ± 3.1 to 11.6 ± 4.1 mmHg. In 6 infants, a net increase was noted with 100% oxygen (overall from 10.8 ± 3.1 to 11.5 ± 3.9 mmHg). Although this increase was not significant enough to cause clinical deterioration, the expected improvement with pulmonary vasodilatation did not materialize. This could be multifactorial; pulmonary capacitance, left heart compliance, or both could be contributory to a variable extent.
The signs and symptoms of postcapillary pathology often mimic those of primary lung disease of BPD. Clinical cues could be persistent late pulmonary edema despite aggressive diuretic use or worsening pulmonary edema in response to PH treatment with vasodilators. LV dysfunction in the infants with BPD has suggested a role of decreased compliance and increased filling pressure in the pathogenesis of increased interstitial fluid.24,25 Melnick et al.24 studied patients with BPD who were 2–7 months of age and noted ECHO evidence of elevated LA pressure, suggesting diminished compliance. Mourani et al.8reported infants with severe BPD in whom LV diastolic dysfunction contributed to clinical abnormalities, including PH and recurrent pulmonary edema.8 These hemodynamic alterations seem to be physiologically plausible contributors to disease pathology. We noted alterations in multiple ECHO parameters, which supports the relevance of systemic diastolic dysfunction in BPD pathophysiology.
PH can develop with preserved ejection fraction, underlining the importance of assessing and monitoring diastolic function. In adult patients with PH, heart failure with preserved LV ejection fraction is noted to be the most common cause, the common link being an impaired diastolic filling.26 Usefulness of MPI in infants with BPD and preschool survivors of BPD has been noted.7,27 In our cohort, the conventional measure of contractility (fractional shortening) was comparable between the 3 groups, in contrast to load-independent measure (mVCFc), which was reduced in the BPD subjects. The mVCFc also correlated with elevated ESWS (surrogate measure of LV afterload). Yates et al.7 noted that, in patients with severe BPD, abnormal LV MPI accompanied normal fractional shortening. It is possible that measures such as mVCFc may be more sensitive, allowing for an earlier detection, and the relaxation properties of the heart may provide early clues toward this pathology.
Mechanism of development of LV diastolic dysfunction
A high incidence of systemic hypertension has been noted in the infants with BPD.9-11 The metabolic function of the lung circulation is also impaired (lack of pulmonary clearance of circulating noradrenaline across the lung).28 Aortic stiffness has recently emerged as a possible pathophysiologic factor that may additionally contribute to elevated afterload.17 It is postulated that systemic hypertension (and aortic stiffness) over a period of time may generate enough LV afterload to cause left-sided hemodynamic alterations for the back pressure cascade to increase pulmonary venous pressure leading to pulmonary edema. This has been noted in adults with chronic obstructive pulmonary disease, where arterial stiffness is a strong predictor of cardiovascular events and is independently associated with the severity of the disease.29,30 Systemic hypertension, aortic wall stiffness and compliance, and preterm myocardial maturation issues may provide a multifactorial effect in our cohort of preterm infants.
Physiology-based therapeutic approach: potential for after-load reduction
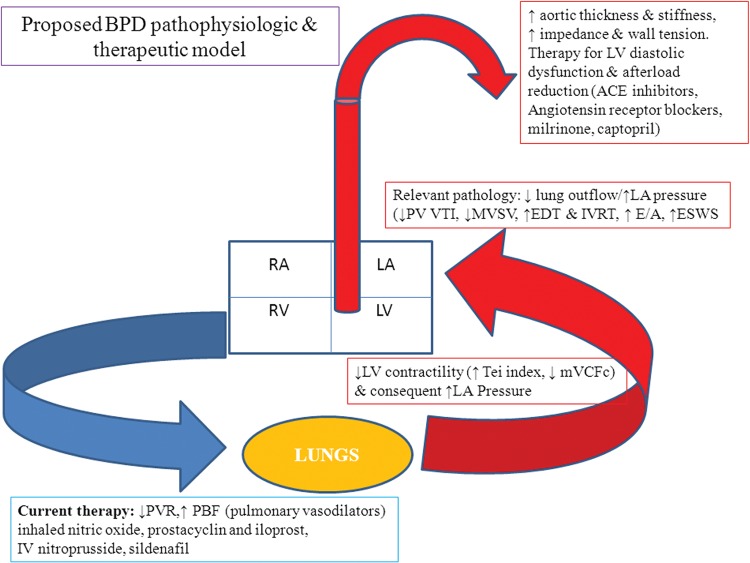
Postcapillary PH component is relevant in BPD pathophysiology to guide monitoring and therapy. Figure 2 proposes a pathophysiologic model outlining various variables. Milrinone (inotrope, lusitrope, and systemic vasodilator used in the management of LV dysfunction) has shown promise by indirectly decreasing PVR and augmenting RV function.31 In adults, the cornerstone of therapy for patients with PH and accompanying LV dysfunction includes systemic (left-side-acting) beta-blockers, angiotensin-converting enzyme inhibitors, and aldosterone antagonists. These reduce LV afterload and improve diastolic properties, leading to lower LA pressure with a corresponding reduction in pulmonary back pressure.
Figure 2.
Proposed bronchopulmonary dysplasia (BPD) pathophysiologic and therapeutic model. ACE: angiotensin-converting enzyme; EDT: E wave deceleration time; ESWS: end-systolic wall stress; IVRT: isovolumic relaxation time; LA: left atrium; LV: left ventricle; mVCFc: mean velocity of circumferential fiber shortening; MVSV: mitral valve stroke volume; PBF: pulmonary blood flow; PV: pulmonary vein; PVR: pulmonary vascular resistance; RA: right atrium; RV: right ventricle; VTI: velocity time integral.
Neonatal and pediatric experience with these strategies is limited. Mourani et al.8 described two infants born at 28 and 24 weeks GA, respectively, where the strategy of systemic afterload reduction was successful. The PH and ECHO features of LV diastolic dysfunction had not improved with diuretics and inhaled nitric oxide (pulmonary vasodilator). Milrinone, followed by ongoing captopril led to clinical improvement and subsequent extubation; echocardiogram findings normalized, and no further hospital admissions were required. In the second case, the addition of captopril improved pulmonary edema, decreasing the need for oxygen, diuretics, and steroids, with no further hospitalizations.
The overall small number of infants (especially in the group of preterm infants without BPD) is acknowledged. As a unit policy in our perinatal referral center, preterm infants, once no longer receiving respiratory support, are transferred to level II units (unless in local catchment). Analysis was adjusted for GA and birth weight separately and GA and birth weight together to address this. The infants were not followed up with serial ECHO to assess the evolution of the disease process. Our institution does not have the facilities for invasive catheterization, and bedside ECHO was used as an easily accessible, noninvasive, and commonly used tool.
BPD is considered to be predominantly an airway and parenchymal disease with contribution from PH. We propose that close cardiac function monitoring be included in the assessments, because it may assist with the clinical management and improve outcomes. Relevant therapeutic options need prospective analysis.
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Chow SSW. 2014. Report of the Australian and New Zealand Neonatal Network (ANZNN) 2012. Sydney: ANZNN.
- 2.Khemani E, McElhinney DB, Rhein L, Andraade O, Lacro RV, Thomas KC, Mullen MP. Pulmonary artery hypertension in formerly premature infants with bronchopulmonary dysplasia: clinical features and outcomes in the surfactant era. Pediatrics 2007;120:1260–1269. [DOI] [PubMed]
- 3.Bhat R, Salas AA, Foster C, Carlo WA, Ambalavanan N. Prospective analysis of pulmonary hypertension in extremely low birth weight infants. Pediatrics 2012;129:e682–e629. [DOI] [PMC free article] [PubMed]
- 4.An HS, Bae EJ, Kim GB, Kwon BS, Beak JS, Kim EK, Kim HS, Choi JH, Noh CI, Yun YS. Pulmonary hypertension in preterm infants with bronchopulmonary dysplasia. Korean Circ J 2010;40:131–136. [DOI] [PMC free article] [PubMed]
- 5.Berkelhamer SK, Mestan KK, Steinhorn RH. Pulmonary hypertension in bronchopulmonary dysplasia. Semin Perinatol 2013;37:124–131. [DOI] [PMC free article] [PubMed]
- 6.Mourani PM, Sontag MK, Ivy DD, Abman SH. Effects of long-term sildenafil treatment for pulmonary hypertension in infants with chronic lung disease. J Pediatr 2009;154(3):379–384. [DOI] [PMC free article] [PubMed]
- 7.Yates AR, Welty SE, Gest AL, Cua CL. Changes in patients with bronchopulmonary dysplasia. J Pediatr 2008;152:766–770. [DOI] [PubMed]
- 8.Mourani PM, Ivy DD, Rosenberg AA, Fagan TE, Abman SH. Left ventricular diastolic dysfunction in bronchopulmonary dysplasia. J Pediatr 2008;152:291–293. [DOI] [PMC free article] [PubMed]
- 9.Anderson AH, Warady BA, Daily DK, Johnson JA, Thomas MK. Systemic hypertension in infants with severe bronchopulmonary dysplasia: associated clinical factors. Am J Perinatol 1993;10:190–193. [DOI] [PubMed]
- 10.Alagappan A, Malloy MH. Systemic hypertension in very low birth weight infants with bronchopulmonary dysplasia: incidence and risk factors. Am J Perinatol 1998;15:3–8. [DOI] [PubMed]
- 11.Abman SH, Warady BA, Lum GM, Koops BL. Systemic hypertension in infants with bronchopulmonary dysplasia. J Pediatr 1984;104:928–931. [DOI] [PubMed]
- 12.Cerro MJ, Abman S, Diaz G, Freudenthal AH, Freudenthal F, Harikrishnan S, Haworth SG, et al. A consensus approach to the classification of pediatric pulmonary hypertensive vascular disease: report from the PVRI Pediatric Taskforce, Panama 2011. Pulm Circ 2011;1:286–298. [DOI] [PMC free article] [PubMed]
- 13.Gali N, Hoeper MM, Humbert M. Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J 2009;30:2493–2537. [DOI] [PubMed]
- 14.Lam CSP, Roger VL, Rodeheffer RJ, Borlaug BA, Enders FT, Redfield MM. Pulmonary hypertension in heart failure with preserved ejection fraction: a community based study. J Am Coll Cardiol 2009;52:1119–1126. [DOI] [PMC free article] [PubMed]
- 15.Walls MC, Cimino N, Bolling SF, Bach DS. Persistent pulmonary hypertension after mitral valve surgery: does surgical procedure affect outcome? J Heart Valve Dis 2008;17:1–9. [PubMed]
- 16.Kiefer TL, Bashore TM. Pulmonary hypertension related to left-sided cardiac pathology. Pulm Med 2011;2011:381787. [DOI] [PMC free article] [PubMed]
- 17.Sehgal A, Malikiwi A, Paul E, Tan K, Menahem S. Systemic arterial stiffness in infants with bronchopulmonary dysplasia: potential cause of systemic hypertension. J Perinatol 2016;36(7):564–569. doi: 10.1038/jp.2016.10. [DOI] [PubMed]
- 18.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med 2001;163:1723–1729. [DOI] [PubMed]
- 19.Ziino AJA, Ivanovska J, Belcastro R, Kantores C, Xu EZ, Lau M, McNamara PJ, Tanswell AK, Jankov RP. Effects of rho-kinase inhibition on pulmonary hypertension, lung growth, and structure in neonatal rats chronically exposed to hypoxia. Pediatr Res 2010;67:177–182. [DOI] [PubMed]
- 20.King ME, Braun H, Goldblatt A, Liberthson R, Weyman AE. Interventricular septal configuration as a predictor of right ventricular systolic hypertension in children: a cross-sectional echocardiographic study. Circulation 1983;68:68–75. [DOI] [PubMed]
- 21.Sahn DJ, Vaucher Y, Williams DE, Allen HD, Goldberg SJ, Friedman WF. Echocardiographic detection of large left-to-right shunts and cardiomyopathies in infants and children. Am J Cardiol 1976;38:73–79. [DOI] [PubMed]
- 22.Rowland DG, Gutgesell HP. Non-invasive assessment of myocardial contractility, preload, and afterload in healthy newborn infants. Am J Cardiol 1995;75:818–821. [DOI] [PubMed]
- 23.Brown ER, Stark A, Sosenko I, Lawson EE, Avery ME. Bronchopulmonary dysplasia: possible relationship to pulmonary edema. J Pediatr 1978;92:982. [DOI] [PubMed]
- 24.Melnick G, Pickoff AS, Ferrer PL, Peyser J, Bancalari E, Gelband H. Normal pulmonary vascular resistance and left ventricular hypertrophy in young infants with bronchopulmonary dysplasia: an echocardiographic and pathologic study. Pediatrics 1980;66:589–596. [PubMed]
- 25.Abman SH, Burchell MF, Schaffer MS, Rosenberg AA. Late sudden unexpected deaths in hospitalized infants with bronchopulmonary dysplasia. Am J Dis Child 1989;143:815–819. [DOI] [PubMed]
- 26.Shapiro BP, McGoon MD, Redfield MM. Unexplained pulmonary hypertension in elderly patients. Chest 2007;131:94–100. [DOI] [PubMed]
- 27.Fitzgerald D, Evans N, Van Asperen P, Henderson-Smart D. Subclinical persisting pulmonary hypertension in chronic neonatal lung disease. Arch Dis Child Fetal Neonatal Ed 1994;70:F118–F122. [DOI] [PMC free article] [PubMed]
- 28.Abman SH. Pulmonary hypertension in chronic lung disease of infancy: pathogenesis, pathophysiology and treatment. In: Bland RD, Coalson JJ, eds. Chronic lung disease of infancy. New York: Marcel Dekker, 2000:619–668.
- 29.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol 2010;55:1318–1327. [DOI] [PubMed]
- 30.Vivodtzev I, Tamisier R, Baguet JP, Borel JC, Levy P, Pépin JL. Arterial stiffness in COPD. Chest 2014;145:861–875. [DOI] [PubMed]
- 31.James AT, Corcoran JD, McNamara PJ, Franklin O, El-Khuffash AF. The effect of milrinone on right and left ventricular function when used as a rescue therapy for term infants with pulmonary hypertension. Cardiol Young 2015;20:1–10. [DOI] [PubMed]
- 32.Lau EMT, Manes A, Celermajer DS, Galie` N. Early detection of pulmonary vascular disease in pulmonary arterial hypertension: time to move forward. Eur Heart J 2011;32:2489–2498. [DOI] [PubMed]
- 33.Evans NJ, Archer LN. Doppler assessment of pulmonary artery pressure and extra pulmonary shunting in the acute phase of hyaline membrane disease. Arch Dis Child 1999;66:6–11. [DOI] [PMC free article] [PubMed]
- 34.Mertens L, Seri I, Marek J, Arlettaz R, Barker P, McNamara PJ, Moon-Grady AJ, et al. Targeted neonatal echocardiography in the neonatal intensive care unit: practice guidelines and recommendations for training. Writing Group of the American Society of Echocardiography (ASE) in collaboration with the European Association of Echocardiography (EAE) and the Association for European Pediatric Cardiologists (AEPC). J Am Soc Echocardiogr 2011;24:1057–1078. [DOI] [PubMed]
- 35.Silverman NH, Schiller NB. Cross-sectional echocardiographic assessment of cardiac chamber size and ejection fraction in children. Ultrasound Med Biol 1984;10:757–769. [DOI] [PubMed]
- 36.Colan SD, Parness IA, Spevak PJ, Sanders SP. Developmental modulation of myocardial mechanics: age-and growth-related alterations in afterload and contractility. J Am Coll Cardiol 1992;19:619–629. [DOI] [PubMed]
- 37.Eidem BW, Tei C, O’Leary PW, Cetta F, Seward JB. Non-geometric quantitative assessment of right and left ventricular function: myocardial performance index in normal children and patients with Ebstein anomaly. J Am Soc Echocardiogr 1998;11:849–856. [DOI] [PubMed]
- 38.Schmitz L, Stiller B, Pees C, Koch H, Xanthopoulos A, Lange P. Doppler-derived parameters of diastolic left ventricular function in preterm infants with a birth weight, 1500 g: reference values and differences to term infants. Early Hum Dev 2004;76:101–114. [DOI] [PubMed]
- 39.Quiñones MA, Otto CM, Stoddard M, Waggoner A, Zoghbi WA. Recommendations for quantification of Doppler echocardiography: a report from Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography. J Am Soc Echocardiogr 2002;15:167–184. [DOI] [PubMed]
- 40.Ito T, Harada K, Takada G. Changes in pulmonary venous flow patterns in patients with ventricular septal defect. Pediatr Cardiol 2002;23:491–495. [DOI] [PubMed]