Abstract
As zebrafish become an increasingly popular model organism in biomedical research, lines of genetically modified fish are a valuable resource. A centralized European archive of fish lines would thus help to improve both research and animal welfare.

Subject Categories: Methods & Resources; S&S: Ethics; S&S: Politics, Policy & Law
The use of animals in scientific research in Europe is governed by the DIRECTIVE 2010/63/EU of 22 September 2010. This framework is built on the 3R principle with the aim to reduce, refine and replace animal experiments to the indispensable minimum and to conduct them humanely 1. Careful experimental planning and statistical evaluation can reduce the number of animals necessary for an experiment. Refinement can, for example, include appropriate analgesia and anaesthesia, the improvement of assay procedures and non‐invasive methods. The third and most rigorous strategic line of the 3R principle is the replacement of animals altogether by in vitro methods including cell, organ and embryo culture or by in silico simulation.
Since its adoption in 2010, DIRECTIVE 2010/63/EU has been implemented by national legal guidelines such as the Animal Protection Act (TierSchG—Tierschutzgesetz) in Germany. The administrative burden for scientists has increased along with the new guidelines, prompting researchers to avoid this additional workload by employing of alternative research models. Big hopes rest on artificial stem cell‐derived in vitro organ systems. However, significant technological advances are still required to faithfully reproduce organ function in vitro. In addition, many studies involving interactions at a system level or complex behavioural outcomes require the intact animal model.
The zebrafish embryo is increasingly used as an alternative model system. Many aspects of biology, including disease processes, can be studied during embryonic stages of the zebrafish prior to feeding. Zebrafish embryos can be grown easily in vitro in embryo cultures. They hatch from their transparent egg shells after about 48 h of development at standard cultivation temperature (28°C). The hatched embryos, called “eleutheroembryos”, depend entirely on their yolk supply for nutrition until at about 120 h of development when they start to hunt for food. From this time on, they are referred to as free‐feeding larvae. However, only after complete consumption of the yolk, at up to 240 h of development, they become strictly dependent on external food 2. According to the EU Directive, embryo and eleutheroembryo cultures are regarded as equivalent to in vitro cell culture experiments. By contrast, experiments with the free‐feeding larvae older than 120 h of development are classified as animal experiments and require permission. The rationale is that active hunting reflects a perception of environmental stimuli that go beyond simple reflexes. This is taken as an indication of a mature nervous system that controls behaviour.
Zebrafish embryo research not only offers an alternative to animal experiments in mammals, but has its own advantages with unique contributions to research. More than 10,000 mutants in protein coding genes 3 and numerous transgenic lines allow the modelling of many disease processes in transparent embryos at a resolution unmatched by other vertebrate models. The UK Home Office recently reported that zebrafish was the second most frequently used animal system in biomedical research 4, and the steady increase in publications using zebrafish further underlines its growing popularity and impact (Fig 1).
Figure 1. Annual number of PubMed‐listed articles using zebrafish.
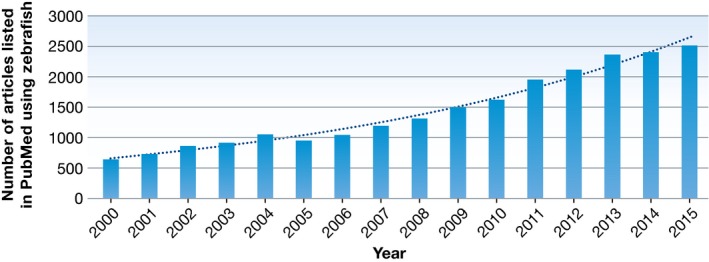
Research publications using zebrafish as animal model have been steadily increasing over the years. PubMed‐filed abstracts of articles were searched for the presence of the term “zebrafish” or “Danio rerio”.
Moreover, no specific permission is needed to breed existing genetic strains if carriers do not show signs of suffering. Article 3 of the EU Directive states, however, that the generation of genetically altered animals combined with raising them beyond the free‐feeding stage requires authorization. This generates a significant load of paperwork for scientists, for institutions and for regulatory authorities. Furthermore, after the fish have been generated, they must undergo a severity assessment to determine whether they suffer as a result of the genetic modification 5. Importantly, when the results of this assessment for a given genetically altered line have been published or are available through a repository, the assessment does not have to be carried out again when the line is transferred to a different institution. Thus, reuse of existing lines has enormous practical benefits.
A critical issue is therefore the availability of zebrafish lines for future experiments. When strains are generated for a specific project, one cannot safely assume that they will remain available forever. Cryopreservation of sperm for long‐term storage is not practiced by most laboratories, since it incurs costs and requires specialized skills. Loss of transgenic and mutant lines may not appear to be a major concern, because, in principle, they can be regenerated on demand by following the experimental procedures used for their construction. However, besides requiring a substantial amount of time and resources, recreating genetically altered lines may create problems owing to different insertion sites or off‐target effects 6. Each recreation of a line requires new experimental animals contrasting with the aim of avoiding unnecessary animal experimentation. Furthermore, it creates an additional administrative burden.
The solution is to deposit genetically altered lines at a resource centre for safekeeping and distribution to the research community. Cryopreservation is carried out routinely and cheaply at such a centre given the high throughput of animal lines. Centralized storage, with an appropriate backup, provides greater safety against the loss of biological material. Moreover, storing fish as cryopreserved sperm is cheaper than maintaining lines for archiving and possible reuse at a later stage.
The large‐scale mutagenesis screens in Europe led to the creation of thousands of new zebrafish lines 7, 8. No European repository was available to store these. Transatlantic shipping of live fish to and from the American Zebrafish International Resource Centre (ZIRC) is difficult and expensive. European researchers therefore established the European Zebrafish Resource Centre (EZRC) at the Karlsruhe Institute of Technology in 2012. It exchanges lines with ZIRC, offers long‐term storage as frozen sperm and maintains living fish of frequently requested lines 9.
The EZRC was established with funds from the Klaus Tschira Foundation, a 7th Framework EU project (ZF‐HEALTH) and the Helmholtz Association. However, despite its vital role for European science, there has been no long‐term funding available from other European and national funding agencies. The roadmap of the European Strategy Forum on Research Infrastructures (ESFRI) provided funding specifically for archiving of mouse models, but not for other organisms.
A central European archive of zebrafish lines would fulfil the spirit of animal protection laws by reducing animal experimentation. It would make more efficient use of research funds by preserving resources that are costly and time‐consuming to generate and maintain. Perhaps most importantly, it would implement the agreement of publishers and public funders that all research materials should be freely available for the research community. This ensures reproducibility of results and allows for re‐evaluation of findings by others as a fundamental motor of progress in science. In conclusion, it would be wise to invest in unique infrastructures such as the EZRC since they can make crucial contributions to both animal welfare and science in Europe.
Conflict of interest
R.G. and U.S. are employed by the EZRC.
References
- 1. Russell WMS, Burch RL (1959) The principles of humane experimental technique. London, UK: Methuen; [Google Scholar]
- 2. Strähle U, Scholz S, Geisler R et al (2012) Reprod Toxicol 33: 128–132 [DOI] [PubMed] [Google Scholar]
- 3. Howe K, Clark MD, Torroja CF et al (2013) Nature 496: 498–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Home Office (2016) Annual statistics of scientific procedures on living animals Great Britain 2015. London, UK: Dandy Booksellers; [Google Scholar]
- 5. Bert B, Chmielewska J, Bergmann S et al (2016) EMBO J 35: 1151–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Han AP (2015) New sequencing methods reveal off‐target effects of CRISPR/Cas9. https://www.genomeweb.com/sequencing-technology/new-sequencing-methods-reveal-target-effects-crisprcas9 (Accessed 27 September 2016)
- 7. Haffter P, Granato M, Brand M et al (1996) Development 123: 1–36 [DOI] [PubMed] [Google Scholar]
- 8. Kettleborough RN, Busch‐Nentwich EM, Harvey SA et al (2013) Nature 496: 494–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Geisler R, Borel N, Ferg M et al (2016) Zebrafish 13(Suppl. 1): S19–S23 [DOI] [PMC free article] [PubMed] [Google Scholar]


