Abstract
The Hippo growth control pathway coordinates cell proliferation, death, differentiation and stemness through regulatory phosphorylation of the YAP proto‐oncoprotein, a major nuclear effector of Hippo signalling. In particular, YAP phosphorylation on Ser127 can promote inhibitory 14‐3‐3 interactions and cytoplasmic sequestration. Two studies in this issue of EMBO Reports show that Ser128 phosphorylation of YAP by the Nemo‐like kinase (NLK) disrupts 14‐3‐3 interactions, thereby promoting nuclear accumulation of active YAP 1, 2. Notably, Ser127‐phosphorylated YAP can be nuclear and co‐transcriptionally active upon osmotic stress, thus, challenging the dogma of YAP regulation by Ser127 phosphorylation.
Subject Categories: Post-translational Modifications, Proteolysis & Proteomics; Signal Transduction
Organ size control in multicellular organisms is essential. During development tissues must form to the correct size, and the dimensions of adult organs must be maintained. The Hippo pathway is a pivotal regulator of tissue growth and homoeostasis by signalling through three main levels: (i) a central core cassette composed of protein kinases and scaffold adaptors, (ii) downstream regulators of diverse transcriptional programmes and (iii) upstream regulators responding to microenvironmental cues 3, 4. In Drosophila, the Hippo core cassette comprises the Hippo and Warts kinases in complex with scaffold adaptors, which inhibit the transcriptional co‐activator Yorkie through Warts‐mediated phosphorylation 3, 5. In mammals, the core consists of the MST1/2 and LATS1/2 kinases bound to adaptors, regulating the transcriptional co‐activators YAP/TAZ 3, 5, 6.
Upon stimulation, MST1/2 activate LATS1/2 by phosphorylation, which triggers LATS1/2‐dependent YAP phosphorylation at Ser127, Ser397 and other residues, resulting in cytoplasmic retention (due to the interaction of the protein 14‐3‐3 with phosphorylated Ser127) and/or degradation (primed by Ser397 phosphorylation) 5. In the off‐state, Hippo signalling does not trigger YAP phosphorylation, causing nuclear accumulation of YAP, where it drives growth‐promoting transcription. Studies on Ser127 phosphorylation of human YAP (and the corresponding Ser112 and Ser168 residues in mice and flies, respectively) showing its nuclear exclusion led to the dogma that Ser127‐phosphorylated YAP is cytoplasmic and hence transcriptionally inactive 5. However, accumulating evidence indicates that the Hippo cascade is only one of several tumour suppressor pathways that can regulate YAP 6. Mice carrying a homozygous S112A substitution in YAP (corresponding to human S127A) are surprisingly normal, although YAP(S112A) displays defective cytoplasmic retention 7. S112‐phosphorylated YAP is also nuclear in murine cells 8. Thus, additional mechanisms must exist that control the subcellular localisation of YAP.
In this issue of EMBO Reports, the Jho and Guan laboratories uncover a novel regulatory mechanism of YAP 1, 2. Both studies found that YAP is phosphorylated at Ser128 by the Nemo‐like kinase (NLK), which disrupts 14‐3‐3 binding, hence promoting nuclear accumulation of active YAP (Fig 1). Fly Nemo and mammalian NLK are atypical MAP kinase‐related kinases regulating diverse signalling pathways 9. NLK expression is deregulated in numerous human cancers, most likely as NLK plays context dependent roles through its functions in cell proliferation, migration, invasion and apoptosis 10. However, a connection between NLK and Hippo signalling was so far unknown. Therefore, Moon et al 2 analysed phospho‐mobility shifts of Hippo components upon NLK overexpression. They found that NLK phosphorylates YAP at Ser128 in vitro and in vivo. Considering that Ser128 is adjacent to the key regulatory site S127, the effects on Ser127 phosphorylation were studied, revealing that NLK overexpression diminished Ser127 phosphorylation, while NLK knockout increased Ser127 phosphorylation. Noteworthy, Ser397 phosphorylation followed the same pattern, suggesting that NLK‐mediated phosphorylation of YAP is also negatively affecting other LATS1/2‐dependent inhibitory phosphorylation sites, and possibly also sites targeted by other kinases such as NDR1/2 11.
Figure 1. The regulation of YAP by NLK‐mediated Ser128 phosphorylation.
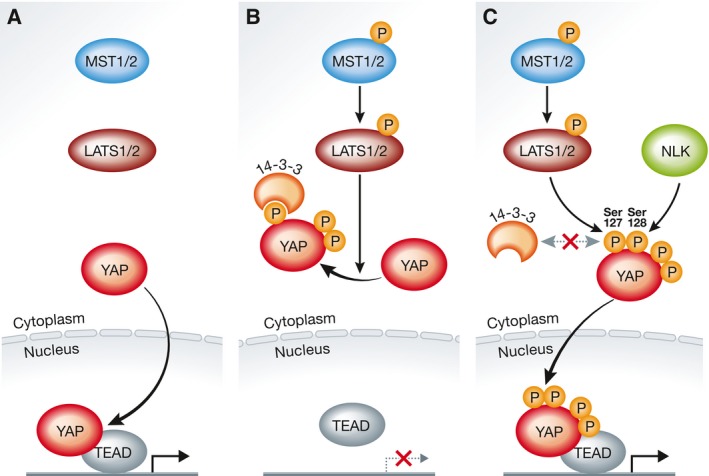
(A) In the off‐state, the MST1/2‐LATS1/2 kinase cascade does not phosphorylate YAP, allowing the nuclear accumulation of YAP, which in complex with TEADs and other transcription factors can drive pro‐growth and pro‐survival transcriptional programmes. (B) Upon stimulation, LATS1/2 phosphorylate YAP at Ser127 (and other serine residues), resulting in 14‐3‐3 binding to Ser127‐phosphorylated YAP and cytoplasmic retention of YAP. (C) Activated LATS1/2 may phosphorylate YAP on Ser127 without promoting 14‐3‐3 binding and cytoplasmic sequestration in case YAP is phosphorylated at Ser128 by NLK, which can override YAP inhibition and consequently support nuclear accumulation of Ser127‐phosphorylated and co‐transcriptionally active YAP.
Intriguingly, Ser128 phosphorylation inversely correlates with Ser127 phosphorylation 2, suggesting that these phosphorylation events are mutually exclusive, although Hong et al 1 do not entirely support this conclusion, since YAP(S128D) is phosphorylated on Ser127. Nevertheless, both studies demonstrate that NLK phosphorylation of YAP at Ser128 results in nuclear accumulation of transcriptionally active YAP by disrupting complex formation between YAP and 14‐3‐3 (Fig 1).
In addition to human cells, Moon et al studied the role of Nemo (Drosophila NLK) in Yorkie‐dependent (fly YAP) gene expression, revealing that Yorkie functions epistatically to Nemo in fly wing imaginal discs 2, further supporting the notion that NLK promotes YAP activity. In this regard, the Irvine laboratory already reported that Ser168 phosphorylation of Yorkie is reduced upon introducing phospho‐mimetic mutations at Ser169 and Ser172 12, suggesting that Ser169/Ser172 phosphorylation of Yorkie (corresponding to Ser128 and Ser131 of human YAP) promotes Yorkie activity. Considering the reports in this issue 1, 2, it is likely that Nemo functions as the kinase phosphorylating Yorkie on Ser169.
Hong et al 1 draw similar conclusions, namely that NLK phosphorylation of YAP on Ser128 disrupts 14‐3‐3 interaction and consequently promotes nuclear localisation of active YAP. However, they came from a different angle, studying the relationship between osmotic stress and the Hippo pathway. General hyperosmolarity (caused by sorbitol or NaCl) was sufficient to trigger elevated Ser127 phosphorylation. Surprisingly, Ser127‐phosphorylated YAP remained nuclear and bound to TEAD transcription factors without increased 14‐3‐3 interactions. In other words, Ser127 phosphorylation, subcellular localisation and 14‐3‐3 binding of YAP are temporarily uncoupled upon osmotic stress. Thus, the current dogma of YAP regulation by Ser127 phosphorylation needs revision (Fig 1).
Significantly, Hong et al 1 found that osmotic stress can induce a transient activation of nuclear YAP despite increased Ser127 phosphorylation and that NLK mediates these osmotic stress signals and activates YAP. In full agreement with Moon et al 2, they discovered that NLK phosphorylation of Ser128 is central for the regulation of YAP localisation. YAP(S128D), carrying a permanent phospho‐mimetic substitution at Ser128, showed constitutive nuclear localisation even upon increased Ser127 phosphorylation. This indicates that Ser128 phosphorylation (as mimicked by S128D) can override YAP regulation through Ser127 phosphorylation.
Collectively, these studies demonstrate that NLK is required for YAP nuclear localisation in human cells and flies 1, 2. Ser128 phosphorylation of YAP is not compatible with inhibitory 14‐3‐3 binding, even when YAP is phosphorylated at Ser127, which normally promotes 14‐3‐3 binding (Fig 1). This point is crucial, since Ser127 phosphorylation has been widely used as an indirect readout of YAP activity. Consequently, more stringent criteria, such as complementary localisation studies combined with YAP target gene quantifications, are needed to accurately assess YAP activity.
Based on these studies, it is tempting to propose the development of NLK inhibitors to pharmacologically dampen YAP‐mediated pro‐growth and pro‐survival signalling in cancer. Other proline‐directed kinases that may phosphorylate YAP on Ser128 should also be considered for inhibitor development. However, it is essential to understand these kinases and to determine whether Ser89 phosphorylation of TAZ by LATS1/2 5 is also overridden by proline‐directed kinases through Ser90 phosphorylation (the TAZ residue corresponding to Ser128), taking into account that Hong et al 1 found that osmotic stress can transiently induce TAZ nuclear translocation. Moreover, researchers will need to consider possible detrimental effects on healthy tissues in response to NLK inhibition given the various important biological roles of the protein 9, 10.
Acknowledgements
We are very grateful to Joanna Lisztwan and all members of the Hergovich laboratory for their critical review of the commentary. The Hergovich laboratory has been supported by the Wellcome Trust (090090/Z/09/Z), BBSRC (BB/I021248/1), Worldwide Cancer Research (AICR; 11‐0634), UCL Cancer Research UK Centre funding and the National Institute for Health Research University College London Hospitals Biomedical Research Centre.
References
- 1. Hong AW, Meng Z, Yuan H‐X et al (2017) EMBO Rep 18: 72–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Moon S, Kim W, Kim S et al (2017) EMBO Rep 18: 61–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sun S, Irvine KD (2016) Trends Cell Biol 26: 694–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yu FX, Zhao B, Guan KL (2015) Cell 163: 811–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Meng Z, Moroishi T, Guan KL (2016) Genes Dev 30: 1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zanconato F, Cordenonsi M, Piccolo S (2016) Cancer Cell 29: 783–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen Q, Zhang N, Xie R et al (2015) Genes Dev 29: 1285–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wada K, Itoga K, Okano T et al (2011) Development 138: 3907–3914 [DOI] [PubMed] [Google Scholar]
- 9. Ishitani T, Ishitani S (2013) Cell Signal 25: 190–197 [DOI] [PubMed] [Google Scholar]
- 10. Huang Y, Yang Y, He Y et al (2015) Tumour Biol 36: 9147–9152 [DOI] [PubMed] [Google Scholar]
- 11. Hergovich A (2016) Genes 7: 21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Oh H, Irvine KD (2009) Oncogene 28: 1916–1927 [DOI] [PMC free article] [PubMed] [Google Scholar]


