Abstract
YAP is the major downstream effector of the Hippo pathway, which controls cell growth, tissue homeostasis, and organ size. Aberrant YAP activation, resulting from dysregulation of the Hippo pathway, is frequently observed in human cancers. YAP is a transcription co‐activator, and the key mechanism of YAP regulation is its nuclear and cytoplasmic translocation. The Hippo pathway component, LATS, inhibits YAP by phosphorylating YAP at Ser127, leading to 14‐3‐3 binding and cytoplasmic retention of YAP. Here, we report that osmotic stress stimulates transient YAP nuclear localization and increases YAP activity even when YAP Ser127 is phosphorylated. Osmotic stress acts via the NLK kinase to induce YAP Ser128 phosphorylation. Phosphorylation of YAP at Ser128 interferes with its ability to bind to 14‐3‐3, resulting in YAP nuclear accumulation and induction of downstream target gene expression. This osmotic stress‐induced YAP activation enhances cellular stress adaptation. Our findings reveal a critical role for NLK‐mediated Ser128 phosphorylation in YAP regulation and a crosstalk between osmotic stress and the Hippo pathway.
Keywords: 14‐3‐3, Hippo, NLK, osmotic stress, YAP
Subject Categories: Post-translational Modifications, Proteolysis & Proteomics; Signal Transduction
Introduction
The Hippo pathway is a highly conserved signaling cascade that plays an important role in organ growth and tissue homeostasis through regulation of cell proliferation, death, and differentiation 1, 2, 3. Dysregulation of the Hippo pathway is an important mechanism in tumorigenesis and other diseases 4, 5, 6. For instance, high YAP activities have been reported in many human cancers. However, genetic mutations of Hippo pathway components are relatively rare in cancer 7. Therefore, identification of upstream regulators has become an extensively studied subject in the field.
The mammalian Hippo pathway core kinase cascade consists of Mammalian Ste20‐like kinases 1/2 (MST1/2), MAP kinase kinase kinase kinase (MAP4Ks), large tumor suppressor 1/2 (LATS1/2) and the downstream effectors Yes‐associated protein (YAP) and transcriptional co‐activator with PDZ‐binding motif (TAZ) 7, 8, 9. MST1/2 or MAP4Ks activate LATS1/2 by phosphorylating the hydrophobic motif residues in LATS1/2 8, 9, 10, 11, 12, 13, 14. Activated LATS1/2 then phosphorylate YAP and TAZ, leading to 14‐3‐3 binding and cytoplasmic retention 15. Because YAP and TAZ do not contain DNA‐binding domains, they bind to transcription factors, primarily the TEA domain family members 1‐4 (TEAD1‐4), to induce transcription of downstream target genes, including CTGF, c‐Myc, and BIRC5, which have been shown to promote cell proliferation and inhibit cell death 16, 17, 18. Cytoplasmic YAP and YAZ are physically prevented from interaction with TEADs, and are therefore inactive. Extensive studies have established that the LATS‐dependent YAP phosphorylation of Ser127 and resulting cytoplasmic localization is one of the most important mechanisms in physiological regulation of YAP activity. Phosphorylation of YAP Ser127 results in YAP‐14‐3‐3 binding and therefore cytoplasmic retention. This phosphorylation‐dependent cytoplasmic localization is highly conserved in Drosophila (Yki Ser 168) and mice (Yap S112) 17, 19. Furthermore, phosphorylation on Ser397 (Ser381 in mice) by LATS leads to phosphodegron‐mediated YAP degradation 20.
The physiological effects of the Hippo pathway are mainly exerted through YAP and TAZ. Many upstream signals of YAP and TAZ have been identified 21. Most notably, cell contact inhibition, mechanotransduction, cellular energy status, and mitogens in serum can potently regulate YAP activity 15, 22, 23, 24, 25, 26, 27. For example, serum deprivation induces YAP Ser127 phosphorylation through activation of LATS, resulting in increased YAP binding with 14‐3‐3 and cytoplasmic retention 27. Most upstream signals regulate YAP activity by influencing YAP Ser127 phosphorylation. Inhibition of Ser127 phosphorylation, by mutating YAP serine 127 to alanine (S127A), was shown to abolish 14‐3‐3 binding and increase nuclear localization 15. Consistently, in vivo study also supports the notion that S127 (S112 in mouse YAP) phosphorylation is critical for YAP cytoplasmic localization as a more prominent nuclear YAP is found in the liver and the colon of YAP S112A knock‐in mice 19. Therefore, YAP Ser127 phosphorylation has been widely used as an indicator of YAP inactivation.
In this study, we discovered that osmotic stress regulates YAP activity. Surprisingly, mild osmotic stress induces YAP nuclear localization and target gene expression despite the high level of Ser127 phosphorylation. We further show that the osmotic stress‐induced YAP nuclear translocation is mediated through a mitogen‐activated protein (MAP) kinase family member nemo‐like kinase (NLK), which phosphorylates YAP at the Ser128 residue. Ser128 phosphorylation disrupts YAP binding with 14‐3‐3 even when Ser127 is phosphorylated, leading to YAP nuclear translocation. This report identifies osmotic stress and NLK as new upstream regulators of YAP, and reveals a mechanism that can override canonical YAP regulation by Hippo pathway‐induced Ser127 phosphorylation. Our study also uncovers a functional interplay between osmotic stress response and the Hippo pathway.
Results
Osmotic stress activates LATS and induces YAP phosphorylation
A wide range of extracellular and intracellular signals, including stress signals, have been shown to regulate YAP and TAZ 28. For example, energy stress activates AMPK to inhibit YAP via both LATS‐dependent and LATS‐independent mechanisms 24, 25, 26, and oxidative stress inhibits YAP activity by activating the Hippo pathway 29. Here, we investigated whether osmotic stress was able to affect YAP phosphorylation, and whether the Hippo pathway was involved. Our previous studies have shown that YAP phosphorylation is strongly regulated by serum 27. As expected, YAP was dephosphorylated in the presence of serum whereas it was highly phosphorylated in the absence of serum, as determined by mobility shift on phos‐tag gel (Fig 1A). Treatment of HEK293A cells with 0.2 M sorbitol induced rapid and robust YAP phosphorylation in the presence of serum. In the absence of serum, YAP was already highly phosphorylated and sorbitol treatment had no obvious effect on YAP phosphorylation. The effect of YAP phosphorylation by sorbitol was dose‐dependent. We found that sorbitol concentrations lower than 100 mM sorbitol had little effect on YAP phosphorylation (Fig EV1A). Only when sorbitol concentrations reached 200 mM or higher was YAP phosphorylation induced, in addition to LATS phosphorylation. YAP Ser127 is an important site phosphorylated by LATS, and its phosphorylation inhibits YAP activity by inducing 14‐3‐3‐mediated cytoplasmic retention. Western blot analysis showed that osmotic stress induced YAP Ser127 phosphorylation (Fig 1A). TAZ is a YAP homolog similarly regulated by LATS. As expected, sorbitol induced a mobility shift of TAZ, suggesting an increased phosphorylation (Fig 1A). TAZ is known to be strongly destabilized upon phosphorylation by LATS 30. Consistently, TAZ protein levels were decreased upon prolonged sorbitol treatment. In addition to HEK293A cells, osmotic stress induced YAP phosphorylation in MCF10A cells (Fig EV1B), indicating that osmotic stress induces YAP/TAZ phosphorylation in a cell type‐independent manner.
Figure 1. Osmotic stress induces YAP phosphorylation and LATS activation but does not inhibit YAP.
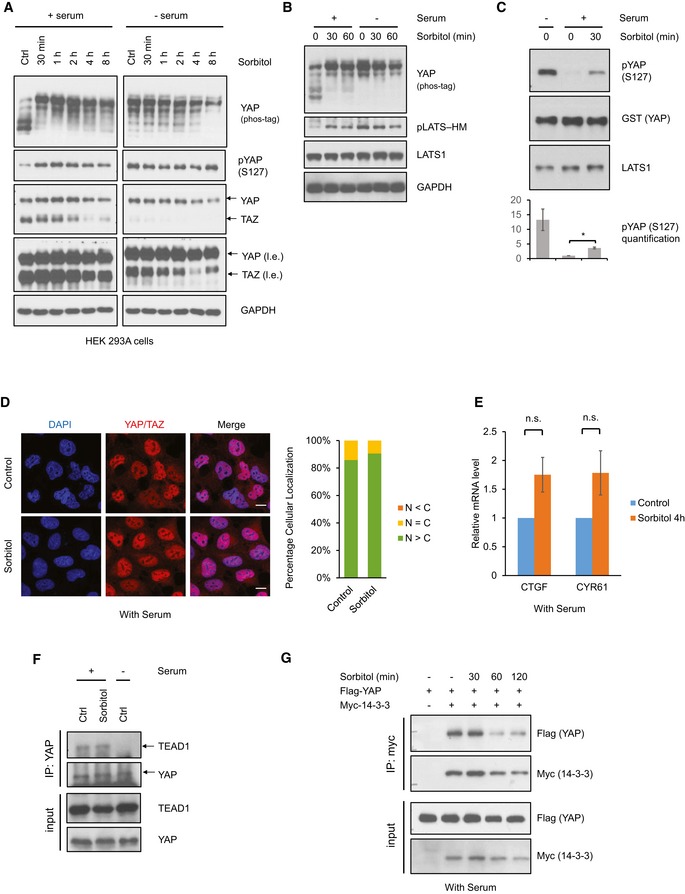
- Sorbitol stimulates YAP phosphorylation. HEK293A cells were cultured in the presence or absence of serum, and treated with 0.2 M sorbitol for the indicated time points. Phos‐tag gel was used to assess total YAP phosphorylation based on mobility shift. Note that YAP and TAZ are recognized by the same antibody. l.e. denotes long exposure of YAP and TAZ.
- Osmotic stress induces LATS phosphorylation. HEK293A cells were treated with 0.2 M sorbitol for 30 or 60 min in the presence or absence of serum. LATS phosphorylation at the hydrophobic motif (HM) was detected with the phosphospecific pLATS antibody.
- Osmotic stress increases LATS kinase activity. HEK293A cells were treated with 0.2 M sorbitol for 30 min. Lats1 was immunoprecipitated, and an in vitro kinase assay was performed using recombinant GST‐YAP as a substrate. Phosphorylation of YAP was determined by immunoblotting with phospho‐YAP (S127) antibody. Data are presented as mean ± SEM. *P < 0.05 (two‐tailed Student's t‐test, n = 3).
- Osmotic stress does not lead to YAP cytoplasmic localization. HEK293A cells were treated with 0.2 M sorbitol for 1 h in the presence of serum. YAP subcellular localization was determined by immunofluorescence staining (red). DAPI (blue) was used to stain for DNA. Scale bars: 20 μm. Quantification of more nuclear (N > C) or more cytosolic (N < C) YAP signal was determined with randomly chosen fields, each with approximately 100 cells.
- Osmotic stress does not reduce YAP target gene expression. HEK293A cells were treated with 0.2 M sorbitol for 4 h. mRNA levels of CTGF and CYR61 were measured by quantitative RT–PCR and normalized to GAPDH control. Data are presented as mean ± SEM. n.s. means P > 0.05 (two‐tailed Student's t‐test, n = 3).
- Osmotic stress does not disrupt the interaction between YAP and TEAD1. HEK293A cells were treated with 0.2 M sorbitol for 1 h. Endogenous YAP was immunoprecipitated with YAP antibody, and TEAD1 and YAP were detected by Western blot. Cells were serum starved for 1 h as indicated (‐ serum).
- Osmotic stress does not increase the interaction between YAP and 14‐3‐3. HEK293A cells were transiently transfected with Flag‐YAP and Myc‐14‐3‐3, then treated with 0.2 M sorbitol for the indicated time points. Myc‐14‐3‐3 was immunoprecipitated, and the co‐precipitated Flag‐YAP was detected.
Source data are available online for this figure.
Figure EV1. Osmotic stress induces YAP phosphorylation but not cytoplasmic retention.
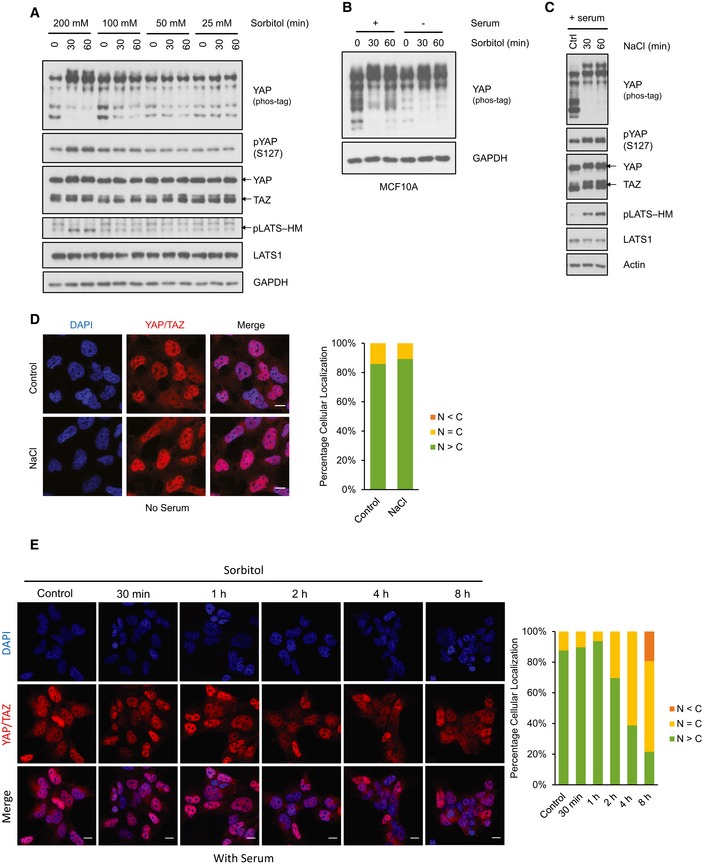
- Osmotic stress‐induced YAP phosphorylation is dose‐dependent. HEK293A cells were treated with different doses of sorbitol for 30 and 60 min. YAP phosphorylation is determined by mobility shift on phos‐tag gel and a S127 phosphospecific antibody.
- Sorbitol stimulates YAP phosphorylation in MCF10A cells. MCF10A cells were cultured in the presence or absence of serum, and were treated with 0.2 M sorbitol for the indicated time points. A phos‐tag gel was used to assess total YAP phosphorylation.
- NaCl stimulates YAP and LATS phosphorylation. HEK293A cells were cultured in the presence of serum and were treated with 0.1 M NaCl for the indicated time points. A phos‐tag gel was used to assess YAP phosphorylation.
- NaCl does not induce YAP cytoplasmic localization. HEK293A cells were treated with 0.1 M NaCl for 1 h in the presence of serum. YAP/TAZ subcellular localization was determined by immunofluorescence staining with an antibody that recognizes both YAP and TAZ (red). DAPI (blue) was used to stain for DNA (cell nuclei). Scale bars: 20 μm. Quantification of YAP/TAZ more nuclear (N > C) or more cytosolic (N < C) is determined with randomly chosen fields, each with approximately 100 cells.
- Sustained osmotic stress induces YAP cytoplasmic localization in the presence of serum. HEK293A cells were treated with 0.2 M sorbitol from 30 min to 8 h in the presence of serum. YAP/TAZ subcellular localization was determined by immunofluorescence staining. Scale bars: 20 μm.
Source data are available online for this figure.
To investigate whether the Hippo pathway is involved, we checked for LATS kinase activity in response to osmotic stress. We found that osmotic stress increased LATS1/2 phosphorylation in their hydrophobic motif (HM, Thr1079 for LATS1 and Thr1041 for LATS2) (Fig 1B), which is known to promote LATS kinase activity 12, 13, 14, 31, 32. LATS activation was further confirmed by LATS in vitro kinase assay using immunoprecipitated LATS1 from cells treated with or without sorbitol. Purified recombinant YAP was used as a substrate for LATS kinase assay. The result showed a significant increase of LATS1 activity by 0.2 M sorbitol treatment as measured by YAP Ser127 phosphorylation (Fig 1C). As expected, serum starvation strongly activated LATS1. In addition to sorbitol, NaCl was also used to test the effects of osmotic stress on YAP. Similar to sorbitol treatment, 0.1 M NaCl induced YAP phosphorylation at Ser127 and LATS phosphorylation at HM, indicating that the Hippo pathway activation by osmotic stress is not restricted to organic osmolyte‐induced hyperosmolarity (Fig EV1C). In summary, these observations demonstrate that osmotic stress activates the Hippo pathway component LATS and increases YAP phosphorylation.
YAP/TAZ are transcription co‐activators and function by binding to transcription factors in the nucleus, such as TEAD, to induce gene expression. In order to determine YAP activity, we first checked its subcellular localization under sorbitol treatment. Immunofluorescence staining showed that YAP and TAZ remained in the nucleus 1 h after sorbitol treatment in the presence of serum although YAP Ser127 was highly phosphorylated (Fig 1D). Similar results were observed when cells were treated with NaCl (Fig EV1D). Notably, some YAP proteins eventually accumulated in the cytoplasm after prolonged osmotic stress (Fig EV1E). In our immunofluorescence staining experiments, an antibody recognizing both YAP and TAZ was used. Our later result showed that TAZ behaved similarly to YAP in terms of localization, whereas in this study, we mainly focused on YAP. The above results are surprising and perplexing because it has been well established that YAP Ser127 phosphorylation promotes 14‐3‐3 binding and cytoplasmic localization. Here, the coupling between YAP Ser127 phosphorylation and cytoplasmic localization is obviously disrupted under osmotic stress condition; YAP Ser127 phosphorylation and nuclear localization are simultaneously induced. These observations are not consistent with the current dogma of YAP regulation.
To further test whether YAP is inhibited by osmotic stress, we measured expression of YAP target gene CTGF and CYR61. We found that expression of these two genes was not decreased 4 h after sorbitol treatment (Fig 1E). We then checked whether this sorbitol‐induced YAP phosphorylation could disrupt its binding with TEAD. Co‐immunoprecipitation (co‐IP) results of endogenous proteins showed that YAP–TEAD interaction was not disrupted by 1 h of sorbitol treatment (Fig 1F). As a positive control, serum starvation reduced YAP–TEAD interaction. Furthermore, co‐IP experiments showed that YAP‐14‐3‐3 binding was not increased upon sorbitol treatment (Fig 1G). These data suggest that YAP is neither associated with 14‐3‐3 nor inhibited upon osmotic stress, despite activation of the Hippo pathway and high levels of YAP phosphorylation.
Osmotic stress induces YAP nuclear localization and activation
Phosphorylation of YAP Ser127 is widely used as a marker of YAP inactivation. However, YAP Ser127 phosphorylation induced by osmotic stress does not appear to inhibit YAP, as its nuclear localization is not affected by sorbitol. We then tested whether osmotic stress might activate YAP. To this end, we determined YAP localization upon sorbitol treatment in HEK293A cells under serum‐free condition in which YAP is cytoplasmic. Immunofluorescence staining, using either an antibody recognizing both YAP and TAZ or a YAP‐specific antibody, showed YAP was translocated into the nucleus upon sorbitol or NaCl treatment (Figs 2A and EV2A). We also wanted to know whether TAZ was similarly regulated by osmotic stress. Since we did not have TAZ antibody suitable for immunofluorescence staining, we used the YAP/TAZ antibody in YAP KO cells; YAP/TAZ signals detected in YAP KO cells should reflect only endogenous TAZ localization. We found that sorbitol also induced TAZ nuclear translocation (Figs 2B and EV2B). Western blot analysis showed that YAP was completely deleted in YAP KO cells (Fig EV2C). The nuclear translocation of YAP by osmotic stress occurred at a short time point (peaking at 1 h upon osmotic stress), whereas at later time points YAP started to move to the cytoplasm (Fig 2C). Therefore, osmotic stress induces a transient YAP nuclear localization. In addition to subcellular localization of YAP, we queried whether osmotic stress activates YAP‐mediated gene transcription in the absence of serum. We measured the YAP downstream target gene CTGF and CYR61 expression and found a significant increase 4 h after sorbitol treatment (Fig 2D). This effect was abolished in YAP/TAZ knockout cells, suggesting that sorbitol‐induced expression of CTGF and CYR61 was YAP/TAZ‐dependent. Consistent with YAP nuclear localization and activation, we found that sorbitol treatment significantly reduced the interaction between YAP and 14‐3‐3 (Fig 2E). Collectively, our data show that osmotic stress induces a transient activation of YAP in the absence of serum, while osmotic stress had a minor effect on YAP in the presence of serum. This osmotic stress‐induced YAP regulation is uncoupled with YAP Ser127 phosphorylation.
Figure 2. Osmotic stress induces YAP nuclear translocation and activation.
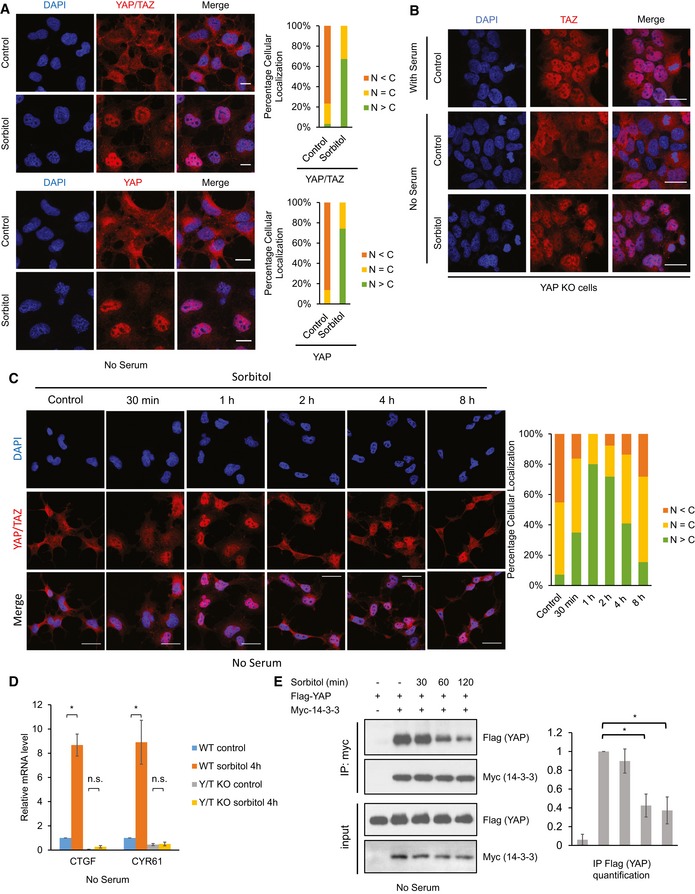
- Osmotic stress induces YAP nuclear translocation. HEK293A cells were serum starved for 1 h followed by 0.2 M sorbitol treatment for 1 h. YAP/TAZ (red, upper panels) or YAP (red, lower panels) were stained with two specific antibodies. DAPI (blue) was used to visualize cell nuclei. Scale bars: 20 μm. Quantification of more nuclear (N > C) or more cytosolic (N < C) YAP signal is determined with randomly chosen fields.
- Osmotic stress induces TAZ nuclear translocation. YAP knockout (KO) HEK293A cells were serum starved for 1 h followed by 0.2 M sorbitol treatment for 1 h. TAZ localization was determined by immunofluorescence staining with the YAP/TAZ antibody (red). Scale bars: 20 μm.
- Osmotic stress induces a transient YAP nuclear translocation. HEK293A cells were serum starved for 1 h followed by 0.2 M sorbitol treatment for the indicated time points. Scale bars: 20 μm.
- Osmotic stress induces YAP target gene expression. Wild‐type (WT) or YAP/TAZ double knockout (Y/T KO) HEK293A cells were serum starved for 1 h and treated with 0.2 M sorbitol for 4 h. mRNA levels of CTGF and CYR61 were measured by quantitative RT–PCR and normalized to GAPDH control. Data are presented as mean ± SEM. *P < 0.05 (two‐tailed Student's t‐test, n = 3).
- Osmotic stress decreases YAP and 14‐3‐3 interaction. HEK293A cells were transiently transfected with Flag‐YAP and Myc‐14‐3‐3, and were serum starved for 16 h. Cells were then treated with 0.2 M sorbitol for the indicated time points. Myc‐14‐3‐3 was immunoprecipitated, and the associated Flag‐YAP was detected by Western blot. Data are presented as mean ± SEM. *P < 0.05 (two‐tailed Student's t‐test, n = 3).
Source data are available online for this figure.
Figure EV2. Osmotic stress induces YAP and TAZ nuclear translocation in serum‐free conditions.
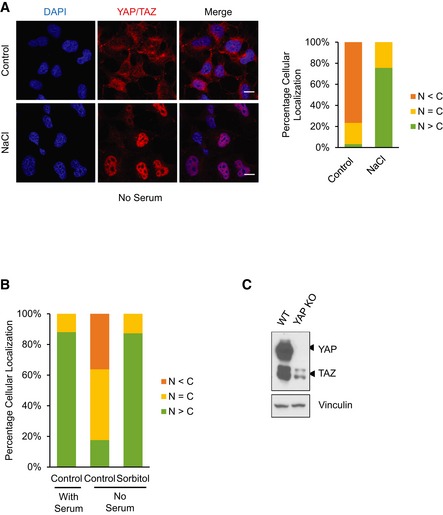
- NaCl induces YAP nuclear translocation under serum‐starved conditions. HEK293A cells were serum starved for 1 h followed by 0.1 M NaCl treatment for 1 h. YAP/TAZ subcellular localization was determined by immunofluorescence staining (red). Scale bars: 20 μm.
- Quantification of Fig 2B. YAP knockout (KO) HEK293A cells were serum starved for 1 h followed by 0.2 M sorbitol treatment for 1 h. Quantification of more nuclear (N > C) or more cytosolic (N < C) YAP signal was determined with randomly chosen fields.
- YAP expression is completely abolished in YAP knockout (KO) cells. Western blot shows that YAP is absent and TAZ is still present in the YAP KO HEK293A cells. Vinculin serves as a loading control.
NLK mediates osmotic regulation of YAP
To understand the mechanism of YAP regulation by osmotic stress, we searched for signaling molecules that may be responsible for YAP nuclear translocation induced by osmotic stress. p38 and JNK are members of the MAP kinase family, and they are activated by various cellular stresses, including osmotic stress 33, 34, 35. To test whether p38 and JNK are involved in osmotic stress‐induced YAP activation, we pretreated cells with the p38 inhibitor SB203580 (2 μM) or JNK inhibitor SP600125 (20 μM) followed by sorbitol treatment, and YAP localization was determined. In both cases, inhibition of p38 or JNK did not abolish osmotic stress‐induced YAP nuclear translocation (Fig 3A). Furthermore, a combined inhibition of both p38 and JNK did not block the osmotic stress‐induced YAP nuclear localization (Fig EV3A). The efficiency of these inhibitors was confirmed by checking the phosphorylation level of downstream substrates MK‐2 at Thr334 for p38, and c‐Jun at Ser63 for JNK (Fig 3A). Collectively, the above observations show that p38 and JNK are not required for YAP nuclear translocation in response to osmotic stress, indicating that other signaling molecules are involved in regulation of YAP upon osmotic stress.
Figure 3. NLK mediates osmotic stress signal to induce YAP nuclear localization.
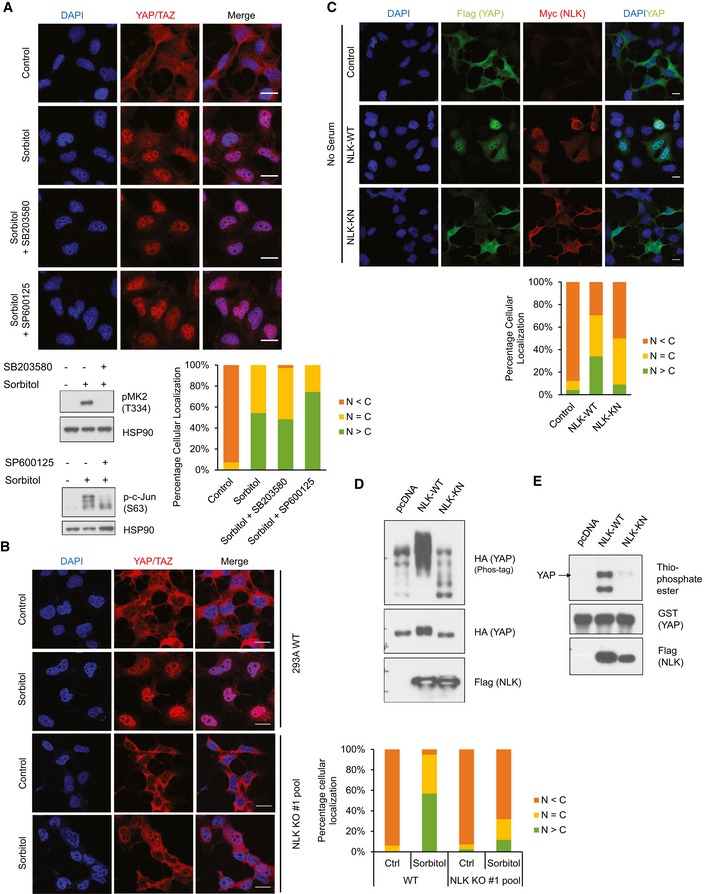
- Inhibition of p38 or JNK does not block osmotic stress‐induced YAP nuclear localization. HEK293A cells were pretreated with 2 μM p38 inhibitor (SB203580) or 20 μM JNK inhibitor (SP600125) before treatment followed by 0.2 M sorbitol for 1 h in the absence of serum. Quantification of YAP/TAZ subcellular staining is shown. Scale bars: 20 μm. Cell lysates from identically treated samples were examined for phosphorylation of p38 substrate MK‐2 and JNK substrate c‐Jun.
- NLK knockout blocks osmotic stress‐induced YAP nuclear localization. HEK293A cells were transiently transfected with CRISPR/Cas9 to knock out NLK. Wild‐type (WT) cells and the NLK KO cell pool were treated with 0.2 M sorbitol for 1 h in the absence of serum. YAP/TAZ subcellular localization was determined by immunofluorescence staining. Scale bars: 20 μm.
- NLK induces YAP nuclear translocation. HEK293A cells were co‐transfected with Flag‐YAP together with vector control, Myc‐NLK‐WT (wild‐type NLK), or NLK‐KN (kinase‐negative mutant). Cells were serum starved for 6 h, and YAP localization and NLK expression were determined by Flag (green) and Myc (red) antibodies, respectively. Scale bars: 20 μm.
- NLK induces YAP phosphorylation. HA‐YAP was co‐transfected with NLK‐WT or NLK‐KN in HEK293A cells. The phos‐tag gel showed NLK‐WT but not NLK‐KN caused a significant mobility shift of YAP.
- NLK phosphorylates YAP in vitro. NLK‐WT and NLK‐KN were immunoprecipitated from HEK293A cells, and an in vitro kinase assay was performed using recombinant GST‐YAP as a substrate in the presence of ATP‐γ‐S. Total phosphorylation of YAP was detected by immunoblotting with thiophosphate ester antibody, which identifies the alkylated thiophosphorylation on YAP.
Source data are available online for this figure.
Figure EV3. NLK mediates osmotic stress‐induced YAP nuclear translocation.
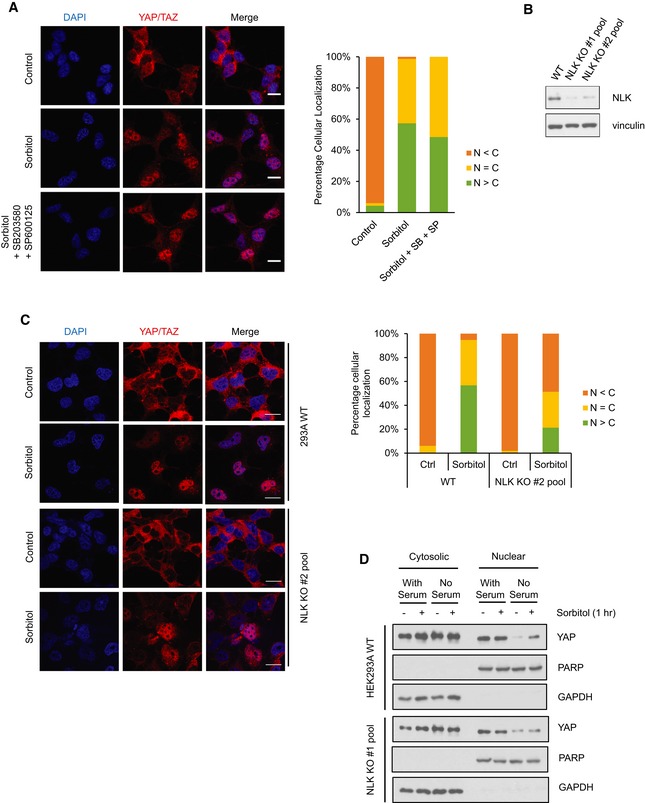
- Inhibition of both p38 and JNK does not block osmotic stress‐induced YAP nuclear translocation. HEK293A cells were pretreated with 2 μM p38 inhibitor (SB203580) and 20 μM JNK inhibitor (SP600125) before treatment followed by 0.2 M sorbitol for 1 h in the absence of serum. Endogenous YAP/TAZ subcellular localization was determined by immunofluorescence staining (red). Scale bars: 20 μm.
- Transient NLK CRISPR/Cas9 transfection reduces NLK expression levels. HEK293A cells were transiently transfected with CRISPR/Cas9 and guide RNAs to knock out NLK. Two NLK guide RNAs were used. NLK protein levels were measured by Western blot with vinculin as a loading control.
- NLK knockout blocks osmotic stress‐induced YAP/TAZ nuclear localization. HEK293A cells were transiently transfected with CRISPR/Cas9 to knock out NLK with gRNA #2. Wild‐type (WT) cells and the NLK KO cell pool were treated with 0.2 M sorbitol for 1 h in the absence of serum. YAP/TAZ subcellular localization was determined by immunofluorescence staining (red). Scale bars: 20 μm.
- Sorbitol‐induced YAP nuclear accumulation requires NLK. Both WT and NLK KO HEK293A cells were cultured in the presence or absence and with or without sorbitol as indicated. Cytosolic and nuclear fractions were collected by differential fractionation. PARP and GAPDH were used as nuclear and cytosolic markers, respectively.
Source data are available online for this figure.
Recently, we have discovered that nemo‐like kinase (NLK), an atypical MAP kinase, can be activated by osmotic stress and plays a role in cellular response to osmotic stress 36. In order to test the role of NLK, we used CRISPR/Cas9 system to knock out NLK by transfecting Cas9 and a guide RNA targeting NLK into HEK293A cells. Two NLK guide RNA sequences were used, and both resulted in an efficient deletion of NLK in transiently transfected cells (Fig EV3B). YAP nuclear translocation by osmotic stress was blocked in the majority of these KO cell pools, which presumably had no NLK (Fig 3B). Similar results were observed in cells with two independent NLK guide sequences (Figs 3B and EV3C). Quantification of the staining results showed a dramatic decrease of nuclear YAP in the NLK KO cell pool after sorbitol treatment (Figs 3B and EV3C). We do not have a high‐quality NLK antibody suitable for immunofluorescence staining to verify NLK KO in individual cells. However, Western blotting with a NLK antibody did confirm a strong reduction in NLK protein in the pooled cells transfected with the NLK CRISPR/Cas9 guide sequences (Fig EV3B). Subcellular fractionation was performed to confirm the localization of YAP. Consistent with the immunofluorescence data, osmotic stress increased nuclear YAP in WT cells under serum starvation, and this effect was diminished in NLK KO cells (Fig EV3D). These results suggest that NLK serves as a mediator between osmotic stress and YAP. In addition, overexpression of wild‐type NLK (NLK‐WT) but not the kinase‐negative mutant (NLK‐KN) induced nuclear localization of YAP in the absence of serum (Fig 3C), supporting the notion that NLK induces YAP nuclear localization. To further investigate the role of NLK in YAP regulation, NLK‐WT and NLK‐KN were overexpressed in HEK293A cells. We observed that only wild‐type NLK induced YAP mobility shift on a phos‐tag gel, indicating that NLK could promote YAP phosphorylation (Fig 3D). This notion was further confirmed by in vitro kinase assays, which showed that immunoprecipitated NLK‐WT, but not NLK‐KN, was able to phosphorylate purified GST‐YAP (Fig 3E).
Osmotic stress disrupts YAP and 14‐3‐3 binding by inducing Ser128 phosphorylation
YAP phosphorylation at Ser127 site increases its binding with 14‐3‐3 and results in cytoplasmic retention 15, 20. Here, we observed that although osmotic stress increased YAP S127 phosphorylation (Fig 1A), YAP‐14‐3‐3 binding was actually decreased (Fig 2E). There is an apparent uncoupling between YAP S127 phosphorylation and its interaction with 14‐3‐3 under osmotic stress. Furthermore, this osmotic stress‐induced uncoupling between YAP S127 phosphorylation and cytoplasmic localization requires NLK. In order to understand how NLK regulates YAP localization under osmotic stress, it is critical to identify the NLK‐induced YAP phosphorylation site that may be responsible for the disruption of 14‐3‐3 binding. As a MAP kinase family member, NLK is a proline‐directed kinase that phosphorylates serines/threonines that are followed by a proline. We searched YAP amino acid sequence and identified ten putative NLK phosphorylation sites. Notably, the NLK consensus site Ser128 in YAP is adjacent to the Ser127 site and is within the 14‐3‐3 binding pocket. Previous phosphoproteomic studies also showed that YAP Ser128 is a phosphorylation site 25, 37, 38. Thus, we hypothesized that NLK phosphorylates YAP Ser128 to disrupt YAP‐14‐3‐3 binding, leading to the uncoupling event between S127 phosphorylation and cytoplasmic localization.
To test the above hypothesis, we obtained YAP Ser128 phosphospecific antibody, which is described in the accompanying paper by Moon et al 41. To confirm the specificity of the pYAP Ser128 antibody, we mutated this serine to alanine (S128A) to prevent phosphorylation. Flag‐YAP wild‐type (WT) and Flag‐YAP S128A were expressed in HEK293A cells. YAP proteins were then immunoprecipitated, and samples were subjected to Western blot analysis. The result showed that this antibody recognizes Ser128 phosphorylated YAP, as there was no signal detected in the YAP S128A mutant (Fig EV4A). To determine whether YAP Ser128 could be phosphorylated by NLK, NLK and YAP were ectopically co‐expressed in HEK293A cells. Results showed that NLK expression indeed induced Ser128 phosphorylation of WT YAP, and this phosphorylation was abolished in YAP S128A mutant (Fig 4A). In addition, immunoprecipitated NLK, but not the kinase‐inactive mutant NLK‐KN, phosphorylated YAP Ser128 in vitro (Fig 4B). The low level of signal detected in the pcDNA control or NLK‐KN by the YAP S128 phosphoantibody might be due to a weak recognition of the unphosphorylated YAP protein by the antibody. Next, we tested whether sorbitol could induce YAP Ser128 phosphorylation. We found that sorbitol increased Ser128 phosphorylation of the endogenous YAP in both HEK293A cells and MCF10A cells (Figs 4C and EV4B). Consistently, sorbitol also induced Ser128 phosphorylation of the transfected YAP (right panel, Fig 4C). Furthermore, this sorbitol‐induced YAP Ser128 phosphorylation was diminished in the NLK knockout cell pool (Fig 4D). Collectively, these results suggest that osmotic stress induces YAP Ser128 phosphorylation and this phosphorylation is mediated by NLK.
Figure EV4. Osmotic stress induces YAP Ser128 phosphorylation to determine its subcellular localization.
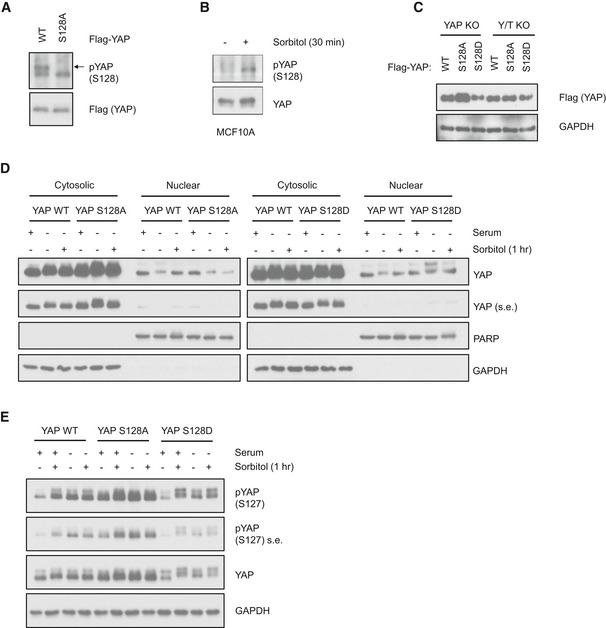
- pYAP S128 antibody is specific to YAP S128 site. Flag‐YAP wild‐type (WT) and S128A mutant constructs were transfected into HEK293A cells. Cell lysates were collected for Western blot analysis by indicated antibodies.
- Osmotic stress induces endogenous YAP Ser128 phosphorylation in MCF10A cells. MCF10A cells were treated with sorbitol and endogenous YAP was immunoprecipitated. YAP Ser128 phosphorylation and protein levels were determined by Western blot.
- Expressions of Flag‐YAP WT, S128A, and S128D mutant stable cell lines are at similar levels. Stable cell lines were generated with retroviral infection of YAP WT and mutant constructs into YAP KO or YAP/TAZ dKO HEK293A cells. Cell lysates were collected for Western blot analysis. YAP expression level was detected by Flag antibody, with GAPDH as a loading control.
- Subcellular fractionation of YAP WT‐, S128D‐, and S128A‐reconstituted cells. YAP KO HEK293A cells were stably reconstituted with YAP WT, S128D, or S128A. Cytosolic and nuclear fractions were collected by differential fractionation. PARP and GAPDH were used as nuclear and cytosolic markers, respectively. s.e. denotes short exposure of the YAP Western blot.
- Osmotic stress induces YAP Ser127 phosphorylation despite Ser128 phosphorylation status. YAP KO HEK293A cells were stably reconstituted with YAP WT, S128D, or S128A mutants and were treated with sorbitol in the presence or absence of serum. YAP Ser127 phosphorylation and protein levels were determined by Western blot. s.e. denotes short exposure.
Source data are available online for this figure.
Figure 4. Osmotic stress induces YAP Ser128 phosphorylation and inhibits its 14‐3‐3 binding.
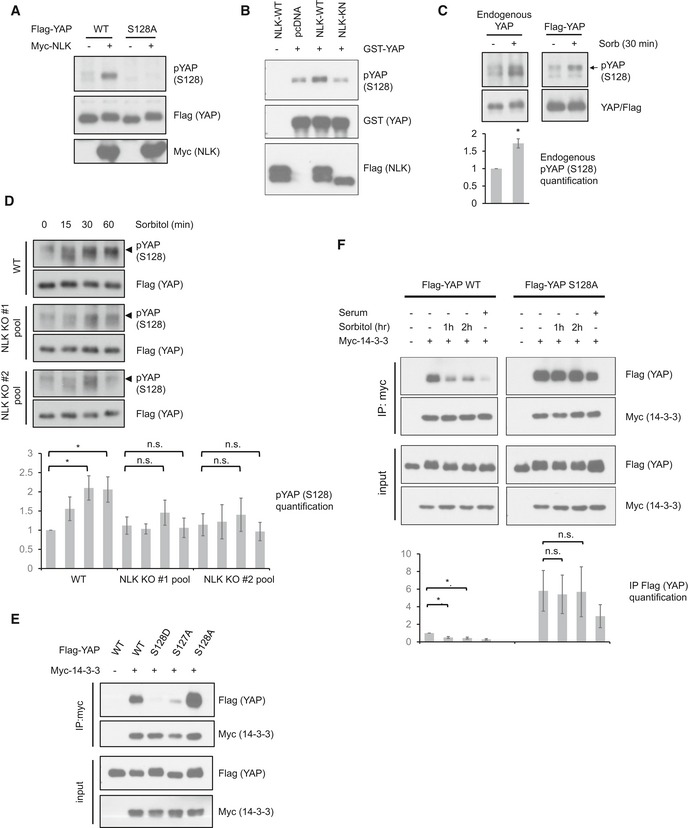
- NLK induces YAP Ser128 phosphorylation. Flag‐YAP WT or S128A mutant was co‐transfected with or without Myc‐NLK. Phosphorylation was determined by Western blot using YAP S128 phosphospecific antibody.
- NLK phosphorylates YAP Ser128 in vitro. NLK‐WT or NLK‐KN was transfected into HEK293A cells and immunoprecipitated. An in vitro NLK kinase assay was performed using recombinant GST‐YAP as a substrate. Phosphorylation of YAP Ser128 was determined by immunoblotting with phospho‐YAP (S128) antibody.
- Osmotic stress induces YAP phosphorylation at Ser128. In the left panel, HEK293A cells were treated with sorbitol and endogenous YAP was immunoprecipitated. In the right panel, Flag‐YAP was transfected into HEK293A cells, and Flag‐YAP was immunoprecipitated. YAP Ser128 phosphorylation was detected by a phosphospecific antibody. Data are presented as mean ± SEM. *P < 0.05 (two‐tailed Student's t‐test, n = 3).
- NLK deficiency reduces YAP S128 phosphorylation. Two CRISPR/Cas9 gRNA plasmids targeting different sites of NLK were transfected into HEK293A. WT cells and two pools of NLK CRISPR/Cas9‐transfected cells were treated with 0.2 M sorbitol for the indicated time points. Data are presented as mean ± SEM. *P < 0.05 (two‐tailed Student's t‐test, n = 4).
- The S128D phosphomimetic mutant abolishes YAP interaction with 14‐3‐3. Flag‐YAP WT and mutant constructs were co‐transfected with Myc‐14‐3‐3 into HEK293A cells. Cells were serum starved for 16 h. 14‐3‐3 was immunoprecipitated with Myc antibody, and the associated YAP was detected with Flag antibody.
- YAP Ser128 phosphorylation is required for disruption of YAP‐14‐3‐3 interaction by osmotic stress. Flag‐YAP WT or Flag‐YAP S128A constructs were co‐transfected with Myc‐14‐3‐3 into HEK293A cells. Cells were serum starved for 16 h, and treated with 0.2 M sorbitol for the indicated time points or refreshed with serum containing medium for 1 h. Refreshing medium served as a control to disrupt YAP and 14‐3‐3 binding. 14‐3‐3 was immunoprecipitated with Myc antibody, and the associated YAP was detected with Flag antibody. Data are presented as mean ± SEM. *P < 0.05 (two‐tailed Student's t‐test, n = 3).
Source data are available online for this figure.
To examine the effect of Ser128 phosphorylation on YAP and 14‐3‐3 binding, YAP Ser128 was mutated to nonphosphorylatable alanine (S128A) or phosphomimetic aspartate (S128D). Myc‐tagged 14‐3‐3 was co‐transfected with WT or mutant YAP. Co‐immunoprecipitation showed that the phosphomimetic YAP S128D mutant abolished its interaction with 14‐3‐3 (Fig 4E), supporting that Ser128 phosphorylation interferes with YAP and 14‐3‐3 interaction. In contrast, YAP S128A showed stronger interaction with 14‐3‐3, further supporting the notion that phosphorylation on YAP Ser128 disrupts YAP‐14‐3‐3 interaction (Fig 4E). As expected, YAP S127A showed a weak 14‐3‐3 interaction because its phosphorylation is required for this interaction 15. We next tested the effect of sorbitol on the interaction between 14‐3‐3 and wild‐type YAP or YAP S128A mutant. Osmotic stress reduced the interaction between 14‐3‐3 and wild‐type YAP, but not the S128A mutant (Fig 4F). These results indicate a model in which sorbitol induces YAP S128 phosphorylation to disrupt YAP and 14‐3‐3 association.
YAP Ser128 phosphorylation is required for sorbitol‐induced YAP nuclear localization
We then tested whether YAP Ser128 mutation would affect YAP subcellular localization by NLK and osmotic stress. By co‐transfection, NLK induced nuclear accumulation of WT YAP, but failed to do so in the YAP S128A mutant (Fig 5A), suggesting that phosphorylation on the Ser128 site is required for NLK to induce YAP nuclear translocation. Furthermore, YAP WT and mutant stable cell lines were generated. WT, S128A mutant, and S128D mutant YAP were stably expressed in the YAP KO HEK293A cells (Fig EV4C). The stably expressing Flag‐tagged YAP WT behaved similarly as endogenous YAP. For instance, serum starvation induced YAP cytoplasmic localization, and sorbitol treatment induced its nuclear translocation in the absence of serum (Fig 5B). YAP S128D mutant was constitutively nuclear even in the absence of serum, consistent with the result that the YAP S128D mutant is defective in 14‐3‐3 binding. Of note, sorbitol treatment had a minor effect on the subcellular localization of YAP S128D mutant compared with WT YAP. To test whether YAP Ser128 phosphorylation is required for its osmotic stress‐induced nuclear translocation, localization of YAP S128A was examined. We observed that YAP S128A mutant retained a normal serum response; that is, serum starvation induced its cytoplasmic localization (Fig 5B). Actually, upon serum starvation, YAP S128A mutant showed an even more cytoplasmic localization than the wild‐type YAP. Importantly, YAP nuclear localization by sorbitol treatment was strongly diminished in YAP S128A mutant cells. Subcellular fractionation experiments showed a consistent result with the immunofluorescence observations (Fig EV4D). Notably, sorbitol induces YAP Ser127 phosphorylation in both YAP S128A and YAP S128D mutants in the presence of serum, while in the absence of serum, Ser127 phosphorylation remained high (Fig EV4E). These results show that YAP Ser127 phosphorylation does not correlate with its localization in the presence of osmotic stress, which is consistent with our model that S128 phosphorylation (mimicked by S128D mutation) overrides the S127 regulation on YAP. Collectively, we show that Ser128 plays a critical role in YAP subcellular localization and its phosphorylation is required for sorbitol to induce YAP nuclear localization, consistent with the notion that NLK‐induced YAP nuclear translocation requires phosphorylation of YAP on Ser128 (Fig 5A). In summary, when S128 is dephosphorylated, as mimicked by the S128A mutant, sorbitol could not induce YAP nuclear localization. When YAP S128 is phosphorylated, as mimicked by the S128D mutant, it is constitutively nuclear and serum starvation could not efficiently induce YAP cytoplasmic localization. Our data suggest a critical role of S128 in YAP regulation and that osmotic stress induces YAP nuclear localization by phosphorylating Ser128 and disrupting 14‐3‐3 binding.
Figure 5. YAP Ser128 phosphorylation affects YAP subcellular localization.
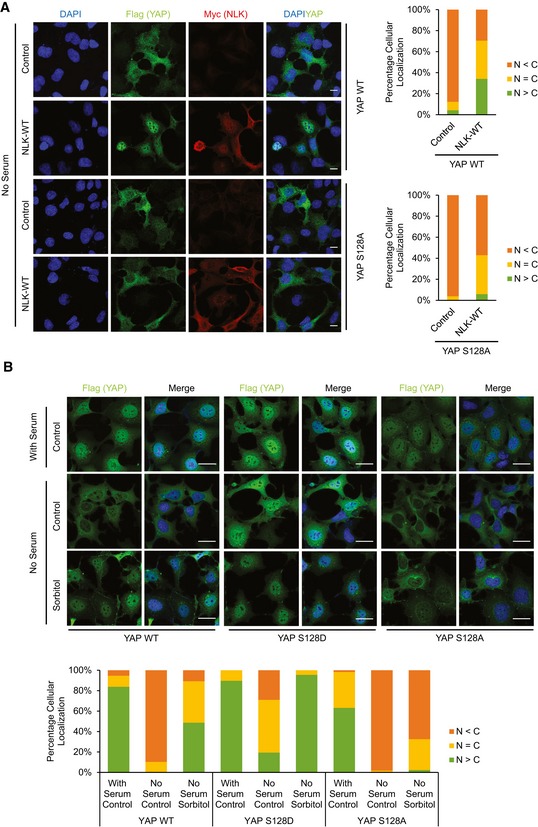
- NLK does not induce nuclear translocation of the S128A mutant YAP. HEK293A cells were co‐transfected with Myc‐NLK and Flag‐YAP WT or S128A mutant. Cells were serum starved for 6 h, and YAP localization and NLK expression were determined by Flag (green) and Myc (red) antibodies, respectively. Scale bars: 20 μm.
- YAP Ser128 phosphorylation is required for osmotic stress‐induced YAP nuclear localization. HEK293A YAP KO cells reconstituted with Flag‐YAP WT, YAP S128D, or YAP S128A mutant were treated with 0.2 M sorbitol for 1 h. YAP subcellular localization was determined by Flag immunofluorescence staining (green). Scale bars: 20 μm.
YAP Ser128 phosphorylation is important for cell survival in hyperosmotic environment
Osmotic stress causes a wide spectrum of signaling events leading to alteration of gene expression in order for cells to adapt to the hyperosmotic environment. Cells may undergo apoptosis if adaptive mechanisms fail to balance the biochemical homeostasis, such as osmolyte concentrations 39. YAP activation has been implicated in inducing gene expression for cell survival. To investigate the function of YAP activation in cellular osmotic adaptation, we generated cells with re‐expression of WT YAP, YAP S128A, and YAP S128D in the YAP/TAZ dKO HEK293A cells (Fig EV4C). Cell growth was determined in the presence or absence of osmotic stress. Under normal osmotic conditions, these three cell pools showed similar growth rates (Fig 6A). However, in the presence of osmotic stress, YAP S128D cells were able to resume growth whereas the YAP WT or the S128A mutant expressing cells were more sensitive to osmotic stress (Fig 6A). Next, we performed cell cycle distribution by FACS analysis and observed that WT YAP and YAP S128A mutant cells showed more severe cell cycle arrest and displayed much high levels of cell death as indicated by the sub‐G1 phase (Figs 6B and EV5). Annexin V staining indicated that the YAP S128D expressing cells were more resistant to apoptosis induced by hyperosmotic stress (Fig 6C). The above data indicate that the transient YAP activation may be advantageous for cell adaptation and survival under osmotic stress.
Figure 6. YAP Ser128 phosphorylation protects cells from hyperosmotic stress.
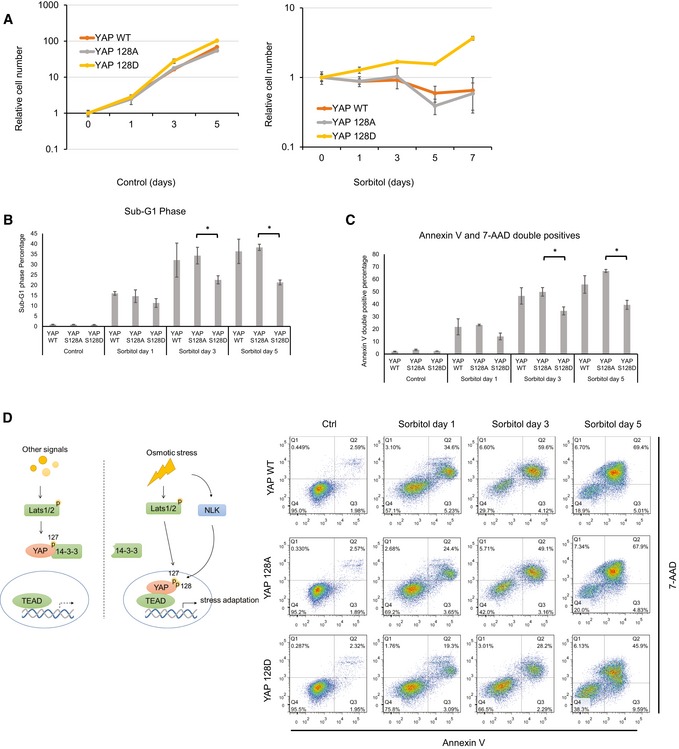
- YAP S128D‐reconstituted cells show growth advantage under hyperosmotic conditions. YAP/TAZ dKO HEK293A cells with stable expression of YAP WT, S128A, or S128D were cultured in the absence (left panel) or presence of 0.2 M sorbitol (right panel) for the indicated amount of time. Cell numbers were counted and normalized to day 0. Data are presented as mean ± SEM, n = 3.
- YAP S128D‐reconstituted cells have lower cell death in a hyperosmotic environment. Cell cycle analyses of YAP/TAZ dKO HEK293A cells with stable expression of YAP WT, S128A, or S128D after 0.2 M sorbitol treatment were determined using flow cytometry. Propidium iodine (PI) was used for DNA staining (Fig EV5). Quantification of sub‐G1 phase cells is shown. Data are presented as mean ± SEM. *P < 0.05 (two‐tailed Student's t‐test, n = 3).
- YAP S128D‐reconstituted cells show reduced apoptosis under hyperosmotic conditions. Annexin V analyses of YAP/TAZ dKO HEK293A cells with stable expression of YAP WT, S128A, or S128D after 0.2 M sorbitol treatment were determined using flow cytometry. PE‐Annexin V and 7‐AAD stained for phospholipid phosphatidylserine (PS) and DNA, respectively. Data are presented as mean ± SEM. *P < 0.05 (two‐tailed Student's t‐test, n = 3).
- A proposed model for osmotic stress regulation of YAP via NLK kinase. See Discussion for details.
Figure EV5. YAP Ser128 phosphorylation prevents cell death under osmotic stress.
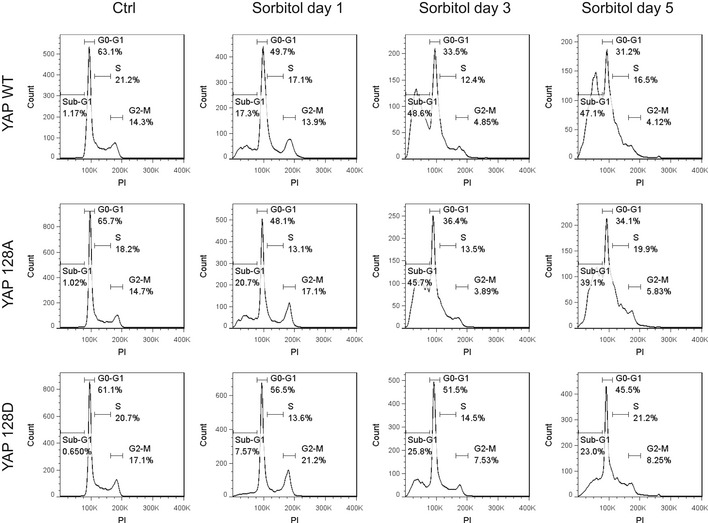
Cell cycle analysis of YAP‐reconstituted cells in hyperosmotic environment. Cell cycle analyses of YAP/TAZ dKO HEK293A cells with stable expression of YAP WT, S128A, or S128D after 0.2 M sorbitol treatment were determined using flow cytometry. Propidium iodine (PI) was used for DNA staining. Quantification of sub‐G1 phase is shown in Fig 6B.
Discussion
The Hippo pathway is an exciting young field with great importance in both normal physiological regulations and pathological conditions such as tumorigenesis. YAP and TAZ are the major downstream effectors of the Hippo pathway, and their increased expression and activity are frequently associated with human cancers 6. Elucidating upstream regulators of the Hippo pathway remains an important research direction. Many studies have revealed that the Hippo–YAP pathway can integrate various upstream extracellular and intracellular signals. This report adds a new dimension to YAP/TAZ regulation. We show that NLK mediates osmotic signals to activate YAP, revealing an intricate interplay between osmotic stress via phosphorylation of YAP Ser128 and the Hippo pathway via phosphorylation of YAP Ser127.
As a transcription co‐activator, YAP nuclear localization determines its activity. The current dogma of Hippo pathway regulation is that LATS inhibits YAP by phosphorylating Ser127, leading to increased YAP binding with 14‐3‐3 and cytoplasmic retention 15. Our results show that osmotic stress induces transient YAP nuclear localization and activation despite activation of LATS and YAP Ser127 phosphorylation. These observations demonstrate a LATS‐independent mechanism utilized by osmotic stress to override the inhibitory effect of LATS on YAP. We show that YAP nuclear translocation induced by sorbitol treatment is mediated, at least in part, by NLK. Mechanistically, NLK phosphorylates YAP on Ser128, which is located in the 14‐3‐3 binding region of YAP. We propose that YAP Ser128 phosphorylation is not compatible with 14‐3‐3 binding, therefore, inhibiting YAP association with 14‐3‐3 and inducing its accumulation in nucleus (Fig 6D). Consistent with this model, osmotic stress cannot influence YAP subcellular localization when YAP Ser128 is mutated to alanine. Furthermore, the YAP S128D mutant appears to mimic the effect of phosphorylation as this mutant shows constitutively nuclear localization even under serum starvation, which normally induces Ser127 phosphorylation and cytoplasmic localization of YAP. Yorkie (Yki) is the Drosophila homolog of YAP. Consistent with the mammalian YAP protein, the phosphorylation of Yki Ser169, which is the analogous residue of YAP Ser128, has also been shown to promote Yki activity in vivo 40. In the accompanying paper by Moon et al, these authors suggest a model that YAP S128 phosphorylation inhibits S127 phosphorylation 41. However, we would like to propose that YAP S128 phosphorylation mainly interferes 14‐3‐3 binding to promote its nuclear accumulation. Nevertheless, our data could not exclude the possibility that S128 phosphorylation may interfere with S127 phosphorylation in YAP.
In addition to the regulation of YAP by NLK, it is possible that YAP is regulated by osmotic stress‐induced cytoskeleton remodeling. One immediate osmotic stress response in mammalian cells is the rapid reorganization of the actin cytoskeleton. The pattern of actin remodeling varies between cell types, but typically, an increase in F‐actin level is observed 42, 43, 44. Various studies have shown that YAP activity is associated with actin remodeling in response to different mechanical cues. For example, F‐actin is known to be important for YAP regulation by cell shape and cell attachment/detachment 23, 45, 46. It will be interesting to explore the relationship between actin regulation and YAP activity under osmotic stress, and whether it is mediated by NLK.
It has also been reported that Ser127 may not be the only site regulating YAP localization although the mechanistic insights for this observation have not been provided 45, 47. For example, YAP S127A protein is not restricted to the nucleus in the intestine of transgenic mice 47. It is possible that YAP Ser128 phosphorylation may regulate YAP localization under such conditions. It is worth noting that YAP Ser128 phosphorylation has been observed in many phosphoproteomic studies 25, 37, 38. We speculate that YAP Ser128 may be a common regulatory phosphorylation site for proline‐directed kinases, such as CDK family and MAP kinase family. Notably, YAP Ser128 has been implicated as a phosphorylation target site of cyclin‐dependent kinase 1 (CDK1) 37. Future study is needed to determine the role of CDK1 in regulation of YAP activity and its involvement in Ser128 phosphorylation. Phosphorylation of Ser128 in YAP would suppress the effect of the LATS‐dependent Ser127 phosphorylation and therefore, provides a mechanism for the integration of numerous signaling pathways with Hippo.
A major function of YAP is to promote cell survival and proliferation. YAP activation by osmotic stress might serve as an immediate stress response for cells to adapt to the stressful environment. Consistently, cells expressing the more active YAP S128D mutant show increased cell viability and proliferation under the osmotic stress. The phosphorylation of Ser127 has been widely recognized as the indicator of YAP cellular localization. Here, we report that YAP Ser128 phosphorylation can override the inhibitory regulation of the canonical Hippo pathway and Ser127 phosphorylation, leading to YAP nuclear localization and activation. Our study not only identifies osmotic stress and NLK as novel regulators of YAP, but suggests that the current dogma of YAP regulation needs to be modified. YAP Ser127 phosphorylation does not necessarily equal to YAP cytoplasmic localization and inhibition. Additional layers of regulation, such as Ser128 phosphorylation, can influence the outcome of Ser127 phosphorylation on YAP activity.
Materials and Methods
Cell culture and transfection
HEK293A cells were cultured in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum (FBS) (Gibco) and 100 units/ml penicillin and streptomycin (Invitrogen). Cells were incubated in a humidified incubator with 5% CO2. MCF10A cells were cultured in DMEM/F12 supplemented with 5% horse serum, 20 ng/ml EGF, 0.5 μg/ml hydrocortisone, 10 μg/ml insulin, 100 ng/ml cholera toxin, and 100 units/ml penicillin and streptomycin. Cells were harvested 24 h post‐transfection for protein analysis. PolyJet In Vitro DNA Transfection Reagent (SignaGen Lab) was used for transfection.
Antibodies and chemicals
Anti‐YAP/TAZ, GAPDH, and Myc‐HRP antibodies were obtained from Santa Cruz Biotechnology. Anti‐phospho‐YAP (S127), LATS1, phospho‐Lats1/2 (Thr 1079/1041), phospho‐MK2 (T334), phospho‐c‐Jun (S63), and HA‐HRP antibodies for Western blot, and Myc and Flag (DYKDDDDK) tag antibodies for IF were obtained from Cell Signaling. Anti‐YAP antibody for IP was obtained from Bethyl Laboratory. Anti‐TEAD1 (TEF‐1) and HSP90 antibodies were obtained from BD Biosciences. Anti‐GST, Flag‐HRP, and Flag (M2) for IP were obtained from Sigma‐Aldrich. Anti‐thioesterphosphate antibody was obtained from Abcam. Anti‐Myc (9E10) antibody for IP was a homemade reagent. Anti‐phospho‐YAP (Ser128) was a kind gift from Professor Eek‐hoon Jho. All pYAP S128 blotting were done with immunoprecipitated YAP. Alexa Fluor 488 and 546‐conjugated secondary antibody for IF were obtained from Invitrogen. Chemicals SB203580 (p38 inhibitor) and SP600125 (JNK inhibitor) were from Tocris.
Immunofluorescence staining
HEK293A cells were plated on fibronectin‐coated coverslips. After sorbitol or NaCl treatment, cells were fixed with 4% paraformaldehyde for 15 min and permeabilized with 0.1% Triton X‐100 for 5 min. After blocking in 3% BSA in PBS for 30 min, cells were incubated with primary antibodies diluted in 3% BSA overnight at 4°C. After three washes with PBS, cells were incubated with Alexa Fluor secondary antibodies (Invitrogen, 1:1,000 dilution) for 1 h in the dark at room temperature. Coverslips were mounted with ProLong Gold antifade mountant with DAPI (Life Technologies). Slides were detected using Olympus FV1000 confocal microscopy. The final images were obtained and analyzed by using confocal microscopy with FLUOVIEW viewer software.
Co‐immunoprecipitation
Cells were lysed with mild lysis buffer (20 mM Tris–HCl pH 7.5, 100 mM NaCl, 50 mM NaF, 2 mM EDTA, 1% NP‐40 substitute) supplemented with protease inhibitor (Roche), phosphatase inhibitor (Thermo Scientific), and 1 mM PMSF for 10 min on ice and centrifuged at 12,000 g for 15 min at 4°C. The supernatants were incubated with the appropriate antibodies overnight at 4°C, and protein A/G magnetic beads (Thermo Scientific) were added for 1 h. Proteins were washed with lysis buffer three times and were eluted with SDS–PAGE sample buffer. Samples were followed by Western blot analysis.
In vitro kinase assay
For the LATS1 kinase assay, HEK293A cells were treated with sorbitol and were lysed with mild lysis buffer. For NLK kinase assay, cells were transfected with Flag‐NLK‐WT or Flag‐NLK‐KN constructs. Twenty‐four hours after transfection, cells were lysed with mild lysis buffer. Proteins were immunoprecipitated using LATS1 antibody or FLAG antibody. The immunoprecipitates were washed three times with lysis buffer, followed by a single wash with TBS. Immunoprecipitated kinases were subjected to kinase assay in the kinase buffer (NEBuffer for Protein Kinases) in the presence of 500 μM cold ATP or ATP‐γ‐S and 1 μg GST‐YAP. The reaction mixtures were incubated for 30 min at 30°C. For ATP‐γ‐S reaction, p‐Nitrobenzyl mesylate (PNBM) was added after the kinase reaction for 1 h to alkylate the thiophosphorylation site on the substrates. The reactions were terminated with SDS sample buffer, and subjected to SDS–PAGE. Phosphorylation of YAP was determined by phospho‐YAP Ser127 or Ser128, and thiophosphate ester antibodies were used to detect total substrate phosphorylation.
RNA isolation and real‐time PCR
Total RNAs were extracted using a RNeasy kit (Qiagen). cDNAs were synthesized by reverse transcription using iScript reverse transcriptase (Bio‐Rad). cDNAs were then used for quantitative real‐time PCR with gene‐specific primers and KAPA SYBR FAST qPCR master mix (Kapa Biosystems) using the 7300 Real‐time PCR system (Applied biosystems). The relative abundance of mRNAs was calculated by normalizing to GAPDH mRNA.
CRISPR
CRISPR genomic editing technology was used for the deletion of NLK. The guide RNA sequences were cloned into the px459 plasmid (Addgene 48319, a gift from Dr. Feng Zhang.). The constructed plasmids were transfected into HEK293A. 24 h after transfection, the transfected cells were enriched by 1 μg/ml puromycin selection for 2–3 days and then were used for experiments. Two guide RNA sequences were used, #1: 5′‐AAAATGATGGCGGCTTACAA‐3′ and 5′‐TTGTAAGCCGCCATCATTTT‐3′, #2: 5′‐ACACCATCTTCATCCGGGGT‐3′ and 5′‐ACCCCGGATGAAGATGGTGT‐3′. Knockout efficiency of the cell pool was assayed with Western blot.
Stable cell lines
To generate YAP mutant expressing stable cells, retrovirus infection was performed by transfecting 293T cells with Retroviral gene (pQCXIH‐Flag‐YAP‐WT/S128A/S128D), pCGP (pCMV‐Gag‐Pol), and pCMV‐VSVG (envelop) constructs. Retroviral supernatant was collected at 12, 24 and 48 h, and filtered through 0.45‐μm syringe filter. Filtered viral supernatant was used to infect HEK293A YAP KO or YAP/TAZ dKO cells with 10 μg/ml polybrene (Sigma‐Aldrich). Infected cells were selected with 200 μg/ml hygromycin (Invitrogen).
Cell cycle analysis
After HEK293A cells were treated with sorbitol for indicated time points, cells were collected and fixed with cold 70% ethanol in PBS, incubated with 50 μg/ml propidium iodide (PI) and 0.5 μg/ml RNase A at 37°C for 30 min, and processed with the BD FACSCanto Flow Cytometer (BD Biosciences). The results were analyzed with FlowJo 7.6 software.
Annexin V staining
HEK293A cells were collected by trypsinization after sorbitol treatment for indicated time points. The PE Annexin V Apoptosis Detection Kit (BD Biosciences) was used as per manufacturer protocol. Samples were then processed by the FASCanto Flow Cytometer (BD Biosciences), and the results were analyzed with FlowJo 7.6 software.
Subcellular fractionation
Cells were lysed in hypotonic buffer A (10 mM HEPES pH 7.6, 1.5 mM MgCl2, 10 mM KCl, 1 mM DTT, 1× protease inhibitor cocktail (Roche), 1× phosphatase inhibitor cocktail (Thermo Fisher Scientific), 2 mM PMSF). Nuclei were pelleted at 800 g for 5 min and washed with hypotonic buffer A twice, then incubated in buffer B (20 mM HEPES pH 7.6, 5% glycerol, 420 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 1× protease inhibitor cocktail, 1× phosphatase inhibitor cocktail, 2 mM PMSF) with vigorous rotation for 30 min. Samples were then spun down at 18,000 g for 5 min, and supernatant was collected for nuclear proteins. Samples were handled at 4°C.
Statistical analysis
Each experiment was repeated at least three times. Data are presented as mean ± SEM. All statistical tests were performed using a Student's t‐test (unpaired, two‐tailed), *P < 0.05. No statistical method was used to predetermine sample size.
Author contributions
AWH carried out most of the experiments and wrote the manuscript; ZM contributed to intellectual inputs; H‐XY contributed to the initial observation; SWP provided some important cell lines; SM, WK, and E‐hJ provided the YAP S128 phosphoantibody; and K‐LG supervised the project and wrote the manuscript.
Conflict of interest
K‐LG is a cofounder of Vivace Therapeutics.
Supporting information
Expanded View Figures PDF
Source Data for Expanded View
Review Process File
Source Data for Figure 1
Source Data for Figure 2E
Source Data for Figure 3
Source Data for Figure 4
Acknowledgements
We thank all the members in the Guan lab for intellectual inputs and providing reagents, Kimberly Lin for critical reading of the manuscript, and the Sanford Consortium Stem Cell Core facility for technical support. A.W.H. and S.W.P. are supported in part by the T32 GM007752 training grant; E.H.J. is supported by NRF grants funded by the MSIP, Republic of Korea (2013R1A1A2064012). K.L.G. is supported by grants from National Institute of Health (CA196878, GM51586, DEO15964, P30CA023100).
EMBO Reports (2017) 18: 72–86
See also: S Moon et al (January 2017) and A Hergovich (January 2017)
References
- 1. Piccolo S, Dupont S, Cordenonsi M (2014) The biology of YAP/TAZ: Hippo signaling and beyond. Physiol Rev 94: 1287–1312 [DOI] [PubMed] [Google Scholar]
- 2. Varelas X (2014) The Hippo pathway effectors TAZ and YAP in development, homeostasis and disease. Development 141: 1614–1626 [DOI] [PubMed] [Google Scholar]
- 3. Barry ER, Camargo FD (2013) The Hippo superhighway: signaling crossroads converging on the Hippo/Yap pathway in stem cells and development. Curr Opin Cell Biol 25: 247–253 [DOI] [PubMed] [Google Scholar]
- 4. Harvey KF, Zhang X, Thomas DM (2013) The Hippo pathway and human cancer. Nat Rev Cancer 13: 246–257 [DOI] [PubMed] [Google Scholar]
- 5. Moroishi T, Hansen CG, Guan KL (2015) The emerging roles of YAP and TAZ in cancer. Nat Rev Cancer 15: 73–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Plouffe SW, Hong AW, Guan KL (2015) Disease implications of the Hippo/YAP pathway. Trends Mol Med 21: 212–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pan D (2010) The hippo signaling pathway in development and cancer. Dev Cell 19: 491–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Meng Z, Moroishi T, Mottier‐Pavie V, Plouffe SW, Hansen CG, Hong AW, Park HW, Mo JS, Lu W, Lu S et al (2015) MAP4K family kinases act in parallel to MST1/2 to activate LATS1/2 in the Hippo pathway. Nat Commun 6: 8357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zheng Y, Wang W, Liu B, Deng H, Uster E, Pan D (2015) Identification of happyhour/MAP4K as alternative Hpo/Mst‐like kinases in the Hippo kinase cascade. Dev Cell 34: 642–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ni L, Zheng Y, Hara M, Pan D, Luo X (2015) Structural basis for Mob1‐dependent activation of the core Mst‐Lats kinase cascade in Hippo signaling. Genes Dev 29: 1416–1431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Callus BA, Verhagen AM, Vaux DL (2006) Association of mammalian sterile twenty kinases, Mst1 and Mst2, with hSalvador via C‐terminal coiled‐coil domains, leads to its stabilization and phosphorylation. FEBS J 273: 4264–4276 [DOI] [PubMed] [Google Scholar]
- 12. Chan EH, Nousiainen M, Chalamalasetty RB, Schafer A, Nigg EA, Sillje HH (2005) The Ste20‐like kinase Mst2 activates the human large tumor suppressor kinase Lats1. Oncogene 24: 2076–2086 [DOI] [PubMed] [Google Scholar]
- 13. Praskova M, Xia F, Avruch J (2008) MOBKL1A/MOBKL1B phosphorylation by MST1 and MST2 inhibits cell proliferation. Curr Biol 18: 311–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hoa L, Kulaberoglu Y, Gundogdu R, Cook D, Mavis M, Gomez M, Gomez V, Hergovich A (2016) The characterisation of LATS2 kinase regulation in Hippo‐YAP signalling. Cell Signal 28: 488–497 [DOI] [PubMed] [Google Scholar]
- 15. Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim J, Xie J, Ikenoue T, Yu J, Li L et al (2007) Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev 21: 2747–2761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhao B, Ye X, Yu J, Li L, Li W, Li S, Yu J, Lin JD, Wang CY, Chinnaiyan AM et al (2008) TEAD mediates YAP‐dependent gene induction and growth control. Genes Dev 22: 1962–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dong J, Feldmann G, Huang J, Wu S, Zhang N, Comerford SA, Gayyed MF, Anders RA, Maitra A, Pan D (2007) Elucidation of a universal size‐control mechanism in Drosophila and mammals. Cell 130: 1120–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vassilev A, Kaneko KJ, Shu H, Zhao Y, DePamphilis ML (2001) TEAD/TEF transcription factors utilize the activation domain of YAP65, a Src/Yes‐associated protein localized in the cytoplasm. Genes Dev 15: 1229–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen Q, Zhang N, Xie R, Wang W, Cai J, Choi KS, David KK, Huang B, Yabuta N, Nojima H et al (2015) Homeostatic control of Hippo signaling activity revealed by an endogenous activating mutation in YAP. Genes Dev 29: 1285–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhao B, Li L, Tumaneng K, Wang CY, Guan KL (2010) A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(beta‐TRCP). Genes Dev 24: 72–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Meng Z, Moroishi T, Guan KL (2016) Mechanisms of Hippo pathway regulation. Genes Dev 30: 1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim NG, Koh E, Chen X, Gumbiner BM (2011) E‐cadherin mediates contact inhibition of proliferation through Hippo signaling‐pathway components. Proc Natl Acad Sci USA 108: 11930–11935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S et al (2011) Role of YAP/TAZ in mechanotransduction. Nature 474: 179–183 [DOI] [PubMed] [Google Scholar]
- 24. DeRan M, Yang J, Shen CH, Peters EC, Fitamant J, Chan P, Hsieh M, Zhu S, Asara JM, Zheng B et al (2014) Energy stress regulates hippo‐YAP signaling involving AMPK‐mediated regulation of angiomotin‐like 1 protein. Cell Rep 9: 495–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang W, Xiao ZD, Li X, Aziz KE, Gan B, Johnson RL, Chen J (2015) AMPK modulates Hippo pathway activity to regulate energy homeostasis. Nat Cell Biol 17: 490–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mo JS, Meng Z, Kim YC, Park HW, Hansen CG, Kim S, Lim DS, Guan KL (2015) Cellular energy stress induces AMPK‐mediated regulation of YAP and the Hippo pathway. Nat Cell Biol 17: 500–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yu FX, Zhao B, Panupinthu N, Jewell JL, Lian I, Wang LH, Zhao J, Yuan H, Tumaneng K, Li H et al (2012) Regulation of the Hippo‐YAP pathway by G‐protein‐coupled receptor signaling. Cell 150: 780–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hansen CG, Moroishi T, Guan KL (2015) YAP and TAZ: a nexus for Hippo signaling and beyond. Trends Cell Biol 25: 499–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shao D, Zhai P, Del Re DP, Sciarretta S, Yabuta N, Nojima H, Lim DS, Pan D, Sadoshima J (2014) A functional interaction between Hippo‐YAP signalling and FoxO1 mediates the oxidative stress response. Nat Commun 5: 3315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liu CY, Zha ZY, Zhou X, Zhang H, Huang W, Zhao D, Li T, Chan SW, Lim CJ, Hong W et al (2010) The hippo tumor pathway promotes TAZ degradation by phosphorylating a phosphodegron and recruiting the SCF{beta}‐TrCP E3 ligase. J Biol Chem 285: 37159–37169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hergovich A, Schmitz D, Hemmings BA (2006) The human tumour suppressor LATS1 is activated by human MOB1 at the membrane. Biochem Biophys Res Commun 345: 50–58 [DOI] [PubMed] [Google Scholar]
- 32. Yin F, Yu J, Zheng Y, Chen Q, Zhang N, Pan D (2013) Spatial organization of Hippo signaling at the plasma membrane mediated by the tumor suppressor Merlin/NF2. Cell 154: 1342–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Han J, Lee JD, Bibbs L, Ulevitch RJ (1994) A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science 265: 808–811 [DOI] [PubMed] [Google Scholar]
- 34. Berl T, Siriwardana G, Ao L, Butterfield LM, Heasley LE (1997) Multiple mitogen‐activated protein kinases are regulated by hyperosmolality in mouse IMCD cells. Am J Physiol 272: F305–F311 [DOI] [PubMed] [Google Scholar]
- 35. Kyriakis JM, Avruch J (2001) Mammalian mitogen‐activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev 81: 807–869 [DOI] [PubMed] [Google Scholar]
- 36. Yuan HX, Wang Z, Yu FX, Li F, Russell RC, Jewell JL, Guan KL (2015) NLK phosphorylates Raptor to mediate stress‐induced mTORC1 inhibition. Genes Dev 29: 2362–2376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhao Y, Khanal P, Savage P, She YM, Cyr TD, Yang X (2014) YAP‐induced resistance of cancer cells to antitubulin drugs is modulated by a Hippo‐independent pathway. Cancer Res 74: 4493–4503 [DOI] [PubMed] [Google Scholar]
- 38. Lee KK, Yonehara S (2012) Identification of mechanism that couples multisite phosphorylation of Yes‐associated protein (YAP) with transcriptional coactivation and regulation of apoptosis. J Biol Chem 287: 9568–9578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Burg MB, Ferraris JD, Dmitrieva NI (2007) Cellular response to hyperosmotic stresses. Physiol Rev 87: 1441–1474 [DOI] [PubMed] [Google Scholar]
- 40. Oh H, Irvine KD (2009) In vivo analysis of Yorkie phosphorylation sites. Oncogene 28: 1916–1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Moon S, Kim W, Kim S, Kim Y, Song Y, Blouson O, Kim J, Lee T, Cha B, Kim M et al (2017) Phosphorylation by NLK inhibits YAP‐14‐3‐3‐interactions and induces its nuclear localization. EMBO Rep 18: 61–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yamamoto M, Chen MZ, Wang YJ, Sun HQ, Wei Y, Martinez M, Yin HL (2006) Hypertonic stress increases phosphatidylinositol 4,5‐bisphosphate levels by activating PIP5KIbeta. J Biol Chem 281: 32630–32638 [DOI] [PubMed] [Google Scholar]
- 43. Di Ciano C, Nie Z, Szaszi K, Lewis A, Uruno T, Zhan X, Rotstein OD, Mak A, Kapus A (2002) Osmotic stress‐induced remodeling of the cortical cytoskeleton. Am J Physiol Cell Physiol 283: C850–C865 [DOI] [PubMed] [Google Scholar]
- 44. Bustamante M, Roger F, Bochaton‐Piallat ML, Gabbiani G, Martin PY, Feraille E (2003) Regulatory volume increase is associated with p38 kinase‐dependent actin cytoskeleton remodeling in rat kidney MTAL. Am J Physiol Renal Physiol 285: F336–F347 [DOI] [PubMed] [Google Scholar]
- 45. Wada K, Itoga K, Okano T, Yonemura S, Sasaki H (2011) Hippo pathway regulation by cell morphology and stress fibers. Development 138: 3907–3914 [DOI] [PubMed] [Google Scholar]
- 46. Zhao B, Li L, Wang L, Wang CY, Yu J, Guan KL (2012) Cell detachment activates the Hippo pathway via cytoskeleton reorganization to induce anoikis. Genes Dev 26: 54–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Barry ER, Morikawa T, Butler BL, Shrestha K, de la Rosa R, Yan KS, Fuchs CS, Magness ST, Smits R, Ogino S et al (2013) Restriction of intestinal stem cell expansion and the regenerative response by YAP. Nature 493: 106–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Expanded View Figures PDF
Source Data for Expanded View
Review Process File
Source Data for Figure 1
Source Data for Figure 2E
Source Data for Figure 3
Source Data for Figure 4


