Abstract
Aims:
This study was conducted to assess the sex determination potential from mesiodistal (MD) and buccolingual (BL) dimensions of permanent maxillary first molar.
Subjects and Methods:
The study was conducted in the Department of Oral Medicine and Radiology, Al-Badar Rural Dental College and Hospital, Gulbarga, Karnataka, on 600 subjects (300 male and 300 female), aged 17–25 years. The subjects were selected based on the inclusion and exclusion criteria set forth for the study. After obtaining informed consent, the intraoral measurements of MD and BL dimensions on casts of the first maxillary molars were taken using digital vernier caliper with resolution of 0.01 mm.
Statistical Analysis Used:
The data obtained were subjected to statistical analysis using paired and unpaired t-test to compare MD and BL dimensions between males and females. P ≤0.05 was considered statistically significant.
Results:
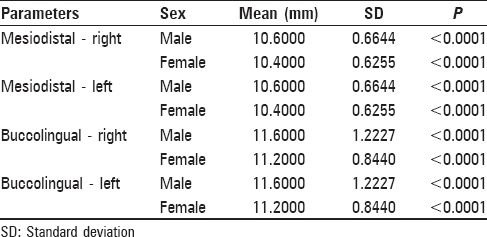
The mean MD width of the first maxillary molar was 10.60 ± 0.6644 mm (right) and 10.60 ± 0.6644 mm (left) in males and 10.40 ± 0.6255 mm (right) and 10.40 ± 0.6255 mm (left) in females. The mean BL width of the first maxillary molar was 11.60 ± 1.2227 mm (right) and 11.60 ± 1.2227 mm (left) in males and 11.20 ± 0.8440 mm (right) and 11.20 ± 0.8440 mm (left) in females. The differences between males and females in MD and BL dimensions measured were statistically significant (P < 0.05). Right and left MD dimensions exhibited sexual dimorphism of 1.92% and right and left BL dimensions exhibited sexual dimorphism of 3.57%.
Conclusions:
The MD and BL dimensions of the maxillary first molars may be used as an aid in sex discrimination.
Key words: Forensic odontology, gender, maxillary first molar, sex determination, sexual dimorphism
Introduction
Human beings are born with an identity.[1] The identification of a dead body may be required in cases of sudden and unexpected death, explosions, fires, road/railway or aircraft accidents, mutilated or hidden decomposed bodies, or foul.[2] Gender determination of skeletal remains is a part of the archaeological and many medico-legal examinations.[3] An important initial step in the identification of the dismembered remains of mass disaster victims is the separation of sexes.[4] Complete skeletons with or without soft tissue present fewer problems. Those bodies, which are less complete and consisting of parts of a skeleton only, present more problems in the identification and in many instances, may not be identified at all.[5] Various methods are used to establish the identity of unknown remains. The only method with totally accurate result is the DNA technique, but in many cases and for several reasons, it cannot be used.[2,6] This study was conducted to assess the sex determination potential from mesiodistal (MD) and buccolingual (BL) dimensions of permanent maxillary first molar.
Subjects and Methods
This study was conducted in the Department of Oral Medicine and Radiology, Al-Badar Rural Dental College and Hospital, Gulbarga, Karnataka, on 600 subjects (300 male and 300 female), aged 17–25 years. Sample size was estimated before the start of the study and based on statistical analysis, number of subjects (n) needed were 299 (so, approximately, 300 male and 300 females were included in the study). The subjects were selected based on the inclusion and exclusion criteria set forth for the study.
The subjects for the study were selected based on simple random sampling technique. The subjects were told about the aim and purpose of the present study and only those who gave their voluntary consent were participated (in accordance with the International Ethical Guidelines for Biomedical Research involving Human Subjects). Accordingly, the Research and Ethics Committee of the Al-Badar Rural Dental College and Hospital, Rajiv Gandhi University of Health Sciences, approved the procedure employed in the study. Written consent was obtained from each subject and the consent was obtained from parents/guardian of all participants under the age of 18 who were involved in the study. This research was in full accordance with the World Association Declaration of Helsinki.
Inclusion criteria
The subjects having a complete set of fully erupted, morphologically well-formed, noncarious, periodontally healthy, nonattrited, intact, and satisfactorily aligned maxillary and mandibular teeth with Angle's Class I Malocclusion, no history of orthodontic treatment, and no evidence of crown restorations or cleft palate were included in the study. Very tall and very short persons suffering from any endocrine disturbances were excluded from the study, although individuals who are well built or short built but in normal range are included in the study.
Exclusion criteria
The subjects with anodontia, partially edentulous, malformed/hypoplastic teeth and positional variations in any of the segments and the individuals who wear bridges, crowns, and other appliances, or had any anomalies that could influence the measurements and developmental disturbances, metabolic disorders, history of prolonged illness and medically compromised states, and subjects with endocrine disturbances, for example, gigantism and acromegaly were excluded from the study.
Measurements were taken intraorally with a digital vernier caliper (Mitutoyo, Japan) with resolution of 0.01 mm, with the subject sitting in the dental chair, followed by full arch maxillary and mandibular impressions were taken by irreversible hydrocolloid impression material (Hydrogumsoft; Zhermack clinical, Germany) and poured immediately by Type III Gypsum product, dental stone (Stone plaster; Neelkanth Healthcare Pvt. Ltd., India).
The following parameters were measured on casts:
MD width of the crown of the maxillary first permanent molar: this is measured as the maximum contour of the tooth (in mm) between the contact points with the second premolar and second molar teeth[3] and BL width of the crown of the maxillary first permanent molar: this is measured as the maximum contour of the tooth (in mm) between facial and lingual surface of the crown parallel to the long axis of tooth.[3]
All measurements were made by a single observer who was blinded to the sex of the person's cast being measured.
Thirty subjects who were not a part of this study were randomly sampled to assess the degree of error of the measurements in this study, and the measurements of MD and BL crown dimensions of the maxillary first permanent molar were taken twice at an interval of 10 days. Intra-observer error was calculated. The mean error as calculated was 0.08 mm for MD width and 0.30 mm for BL width. Pearson correlation between respective first and second measurements was highly significant at the 0.01 level (P = 0.000); Pearson r is 0.930 for MD width and 0.980 for BL width. The findings indicate that the errors were minimal and were not significant, showing good method reproducibility.
The readings obtained were subjected for analysis to derive conclusions. Sexual dimorphism in the right and left maxillary first molars were calculated using the following formula.[9]
Sexual dimorphism = ([xm/xy] – 1) ×100
xm = Mean value for males; xy = Mean value for females
Statistical analysis
The data obtained were quantified and analyzed statistically using SPSS (Statistical Package for the Social Sciences, BM Corp. Released 2011. IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp.) to determine the significance of differences between the sexes. Normality assumption tested by Kolmogorov–Smirnov Z-test was done and it showed that the distribution scores of all parameters in male and female groups are satisfying or normally distributed. Unpaired t-test was used to test the odontometric features of the permanent maxillary first molar for statistically significant sexual dimorphisms in males and females. Paired t-test was used to determine the differences in the mean values of the parameters between the left and the right sides measured intraorally and on study casts. P < 0.05 was considered statistical significant.[10] Power of the study was also estimated.
Results
The following parameters were determined intraorally and on the study cast in males and females:
MD diameter of the left and right maxillary first molars
-
BL diameter of the left and right maxillary first molars.
- The comparison of the mean values of BL and MD parameters showed highly statistically significant differences between males and females, with P < 0.001, measured both intraorally and on the study casts
- The mean values of the parameters were same on the right and left sides whether measured intraorally or on the study casts
- Percentage of sexual dimorphism in permanent maxillary first molars, which was 3.57% for the right and left BL dimensions of maxillary first molars as compared to 1.92% for the right and left MD dimensions of the same teeth, was measured intra-orally and on casts and
- Among the intraoral and cast group, the right and left maxillary first molars were found to exhibit the greatest sexual dimorphism (3.57%) in BL dimension whereas it is the least dimorphic (1.92%) in MD dimensions
- When BL and MD measurements were compared, the BL dimensions were found to exhibit greater sexual dimorphism than MD dimensions of the permanent maxillary first molars.[10]
- Power of the study was estimated and it was 85%. Standard deviation in the 1st Group was S1 = 0.6508 and standard deviation in the 2nd Group was S2 = 0.6111.
Mean difference between the 1st and 2nd samples was 0.1561; effect size was 0.247404707187574; and alpha error (%) was 5.
Discussion
Osteometry is considered the preferred technique because it is more effective in determining sex.[7] On an individual basis, however, gender differences are always distinctive, but taken collectively can give a good indication in majority of the cases.[4] The determination of sex is among the important aspect of forensic anthropology. These characteristics display population-specific variation and therefore, need further attention for major populations of the world.[8]
Many authors have done the measurements of crown in teeth between males and females and found certain variations. Although the morphology of the structure is similar to male and female, there is no need that the size of the structure should remain the same, as the size of the structure is determined by various factors such as exercise, nutrition, and metabolic activities. Measurements of tooth dimensions are quick, less time-consuming, noninvasive, and can be easily performed compared to DNA technique.
A permanent human dentition has a complement of 32 teeth; at least a few teeth may be recovered.[10] Hence, they are routinely used in comparative identification of human remains. The fact that most teeth complete development before skeletal maturation makes the dentition a valuable sex indicator, particularly in young individuals.[1,10]
Teeth form an excellent material in living and nonliving populations for anthropological, genetic, odontogenic, and forensic investigations. Measurements of tooth dimensions are quick, noninvasive, and can be easily performed. Dimensions of teeth are used to establish the sex of a victim in major disasters/accidents, medico-legal cases, and natural disasters. Sex can be determined well in mature individuals if the human skeletal remains are intact.[2]
In the present study, the comparison of mean values of parameters measured between males and females showed highly statistically significant differences with P < 0.001, and these results are in agreement with the studies done by Sonika et al.,[10] Perzigian,[11] Ghose and Baghdady,[12] Stroud et al.,[9] Hattab et al.,[13] Rai et al.,[14] and Ghodosi et al.,[15] in which the authors have observed that males had larger teeth than females in all the dimensions. Differences in dimensions of the teeth are due to greater dentine thickness in males as compared to females, as the Y-chromosome increases the mitotic potential of the tooth germ and induces dentinogenesis; whereas the X-chromosome induces amelogenesis.[10,16,17]
The present study showed bilateral symmetry of the maxillary permanent first molar as no significant difference was found in the dimensions of the maxillary right and left permanent first molar in both males and females, indicating almost symmetric dimensions [Tables 1–3]. This finding is in agreement with most of the studies done by Preeti et al.,[18] Rai et al.,[19] and Garn et al.[20] that showed no tendency for the MD as well as BL crown dimensions on one side to be consistently larger than on the other side.
Table 1.
Comparison of mean values of parameters in males and females measured intraorally by using unpaired t-test (right and left maxillary molars)
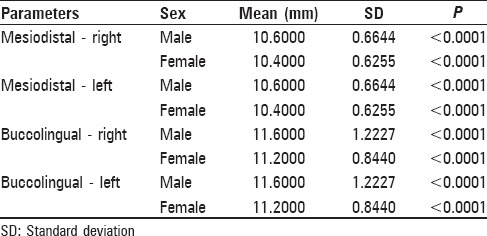
Table 3.
Percentage of sexual dimorphism in permanent maxillary first molars

Table 2.
Comparison of mean values of different parameters in males and females measured on study casts using unpaired t-test (right and left maxillary molars)
The right and left maxillary first molars were found to exhibit the greatest sexual dimorphism (3.57%) in terms of BL dimension among the intraoral group. The results of the present study were in agreement with the study done by Rai et al.[14] and Sonika et al.[10] The right and left maxillary first molars were found to exhibit the greatest sexual dimorphism (3.57%) in terms of BL dimension whereas the least dimorphic value was that for the right maxillary first molar (1.92%) in terms of MD dimensions among the study cast group.[10]
Comparing the MD and BL measurements, the BL dimensions in the present study were found to exhibit greater sexual dimorphism than MD dimensions of permanent maxillary first molars. The results of this study are in agreement with the study done by Garn et al.[20]
Iscan and Kedici[21] stated that an advantage of BL dimension is that it is more reliably measured than others, while this is true for posterior teeth. The major disadvantage of MD measurements is that they are more difficult to obtain than BL measurements considering the proximal contact that exists between teeth.
In contrast, Potter[22,23] has observed that MD variables contributed more to stepwise discriminant analysis, i.e., 10 out of 12 variables that entered the analysis in his study were MD dimensions. The reason why MD dimensions have better sex discriminatory ability could be that these variables are related to the maxillary and mandibular arch dimensions considering the observations that anteroposterior jaw measurements are statistically larger in males and that arch size influences teeth size, one may infer that jaws in males result in correspondingly larger MD dimensions. However, the difference in size between male and female teeth has been explained as part of genetic expression of the male being larger than female.
The explanations proposed for tooth size dimorphism between males and females as quoted by Hattab et al.[13] and reviewed by Kieser includes sex variation in odontogenic timing and enamel thickness. Males have larger bodies than females and effects of sex chromosomes in promoting tooth growth and other hormonal influences. However, according to Garn et al. as quoted by Hattab et al.,[13] intra-individual variations in crown size and similarities between isomers and antimeres might be well derived from specific intrauterine events during odontogenesis and less from genetic effects.
Conclusions
The power of the study was 85% which reflects the strength of the present study. The BL dimensions in the present study were found to exhibit greater sexual dimorphism than MD dimensions of permanent maxillary first molars. Thus, this study indicates that maxillary first molar show significant sexual dimorphism and can be used as an adjunct along with other accepted procedures for sex determination.
The present study measured only linear dimensions because of simplicity, reliability, inexpensibilty, and in setup where latest technology utilizing DNA analysis methods is not available and gender estimation has to be managed based on jaw fragments.
Further investigations are desired with larger samples and in populations of varied ethnic origin in the direction of improving accuracy of using linear dimensions of teeth as a method of sex identification.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Lund H, Mörnstad H. Gender determination by odontometrics in a Swedish population. J Forensic Odontostomatol. 1999;17:30–4. [PubMed] [Google Scholar]
- 2.Reddy VM, Saxena S, Bansal P. Mandibular canine index as a sex determinant: A study on the population of western Uttar Pradesh. J Oral Maxillofac Pathol. 2008;12:56–9. [Google Scholar]
- 3.Franklin CA. Modi's Textbook of Medical Jurisprudence and Toxicology. 21st ed. Bombay, India: N.M. Tripathi Pvt. Ltd.; 1988. [Google Scholar]
- 4.Fleming WC. Localisation of pyorrheal involvement. Dent Cosmos. 1926;68:538–41. [Google Scholar]
- 5.Miller SC, Seider BB. Relative alveoclastic experiences of various teeth. J Dent Res. 1942;21:365–71. [Google Scholar]
- 6.Rai B, Narula SC, Madan M, Dhattarwal SK. Evidence of tooth in sex. Medico Legal Update. 2004;4:119–26. [Google Scholar]
- 7.Krogh HW. Permanent tooth mortality: A clinical study of causes of loss. J Am Dent Assoc. 1958;57:670–5. doi: 10.14219/jada.archive.1958.0256. [DOI] [PubMed] [Google Scholar]
- 8.McDonald RE, Avery DR. Eruption of teeth: Local, systemic and congenital factors that influences the process. St. Louis: C.V. Mosby; 2004. Dentistry for child and adolescent; pp. 175–202. [Google Scholar]
- 9.Stroud JL, Buschang PH, Goaz PW. Sexual dimorphism in mesiodistal dentin and enamel thickness. Dentomaxillofac Radiol. 1994;23:169–71. doi: 10.1259/dmfr.23.3.7835519. [DOI] [PubMed] [Google Scholar]
- 10.Sonika V, Harshaminder K, Madhushankari GS, Sri Kennath JA. Sexual dimorphism in the permanent maxillary first molar: A study of the Haryana population (India) J Forensic Odontostomatol. 2011;29:37–43. [PMC free article] [PubMed] [Google Scholar]
- 11.Perzigian AJ. The dentition of the Indian Knoll skeletal population: Odontometrics and cusp number. Am J Phys Anthropol. 1976;44:113–21. doi: 10.1002/ajpa.1330440116. [DOI] [PubMed] [Google Scholar]
- 12.Ghose LJ, Baghdady VS. Analysis of the Iraqi dentition: Mesiodistal crown diameters of permanent teeth. J Dent Res. 1979;58:1047–54. doi: 10.1177/00220345790580030301. [DOI] [PubMed] [Google Scholar]
- 13.Hattab FN, al-Khateeb S, Sultan I. Mesiodistal crown diameters of permanent teeth in Jordanians. Arch Oral Biol. 1996;41:641–5. doi: 10.1016/s0003-9969(96)00066-0. [DOI] [PubMed] [Google Scholar]
- 14.Rai B, Dhattarwal SK, Anand SC. Sex determination from tooth. Medico Legal Update. 2008;8:3–5. [Google Scholar]
- 15.Ghodosi A, Mosharraf R, Nia FF. Sexual variation in bucco-lingual dimensions in Iranian dentition. Int J Dent Anthropol. 2008;12:1–7. [Google Scholar]
- 16.Vodanovic M, Demo Z, Njemirovskij V, Keros J, Brkic H. Odontometrics: A useful method for sex determination in an archaeological skeletal population? J Archaeol Sci. 2007;34:905–13. [Google Scholar]
- 17.Garn SM, Lewis AB, Kerewsky RS. Buccolingual size asymmetry and its developmental meaning. Angle Orthod. 1967;37:186–93. doi: 10.1043/0003-3219(1967)037<0186:BSAAID>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 18.Preeti N, Balaji Rao B, Anniger RG. A study of tooth size, symmetry and sexual dimorphism. JFMT. 1999;16:10–3. [Google Scholar]
- 19.Rai B, Jain R, Duhan J, Dutta S, Dhattarwal S. Importance of maxillary first molar for sex determination. Internet J Dent Sci. 2007;4:2. [Google Scholar]
- 20.Garn SM, Lewis AB, Kerewsky RS. Sexual dimorphism in the buccolingual tooth diameter. J Dent Res. 1966;45:1819. doi: 10.1177/00220345660450064301. [DOI] [PubMed] [Google Scholar]
- 21.Iscan MY, Kedici PS. Sexual variation in bucco-lingual dimensions in Turkish dentition. Forensic Sci Int. 2003;137:160–4. doi: 10.1016/s0379-0738(03)00349-9. [DOI] [PubMed] [Google Scholar]
- 22.Potter RH. Univariate versus multivariate differences in tooth size according to sex. J Dent Res. 1972;51:716–22. doi: 10.1177/00220345720510030501. [DOI] [PubMed] [Google Scholar]
- 23.Eboh DEO. A dimorphic study of maxillary first molar crown dimensions of Urhobos in Abraka, South-Southern Nigeria. J Morphol Sci. 2012;29:96–100. [Google Scholar]


