Abstract
Statement of Problem:
One of the most challenging tasks for forensic science is to identify the unknown human skeletal remains of deceased individuals. Study of sex by distinguishing the various morphological characteristics of bones is utmost important in forensic anthropology and for medico-legal assessment.
Purpose:
The purpose of this article is to review the literature, to find if there is sufficient evidence to establish the use of mandible in sex identification.
Materials and Methods:
An electronic search was performed to identify suitable literature, using database of MEDLINE, PubMed, and EBSCOhost. Published articles in between January 2000 and April 2015 were searched. The main focus of search was on the various parameters of mandible studied in last 15 years for sex dimorphism. The focus was on the articles published on radiographic studies as well as on morphometric studies of dry mandible in which skeletal parameters were studied. The screening of titles and abstracts were done, suitable literature that fulfilled the inclusion criteria was selected for a full-text reading.
Results:
The initial literature search resulted in 89 articles, out of which only 36 articles fulfilled the inclusion criteria and were included in this systematic review.
Conclusion:
Out of 16 radiographic studies, 14 showed statistically significant results that the adult mandible could be used with increased sensitivity and objectivity to identify both sex and population affinity compared to other standard analytical techniques, whereas two studies showed insignificant results. Out of 20 morphometric studies of dry mandible 15 studies showed a positive correlation between sex dimorphism and mandibular parameters and five studies did not show any positive correlations between the two.
Key words: Anthropology, dry mandible, flexure, osteologic, radiographic, sex dimorphism
Introduction
The aim of osteology is to establish the attributes for an individual from their skeletal remains. In anthropological, archaeological, and forensic studies along with the ethnicity and stature, age determination, the identification of sex from human skeletal remains is an important element.[1] The pelvis is the most reliable source for sex dimorphism among human bones,[2] but when a complete pelvis is absent in such cases, other bones such as mandible can be an important aid in identification. Since mandible retains its shape better than other bones and as it is most durable facial bone, it is appropriate for study. Many researchers claim a sexing accuracy of 80% from the cranium alone, 90% from the skull and mandible, and 98% from the pelvis.[3] This review of literature is an attempt to summarize the morphometric parameters of dry mandible and radiographic parameters of mandibular bone, which can be used for sex dimorphism and to find out whether mandible can be used successfully as a tool for sex dimorphism?
Search strategy
A broad search of the literature in MEDLINE, PubMed, and EBSCOhost database was performed for articles published between January 2000 and April 2015. A focus was made on peer-reviewed journals. The key words searched were anthropology, dry mandible, flexure, sex dimorphism, radiographic, osteologic, morphometric. The search strategy included the combination of the following terms: “Mandible and sex dimorphism; radiograph in mandible sex dimorphism; osteologic studies, dry mandible and sex dimorphism; ramus in sex differentiation; mental foramen in sex differentiation, morphometric study of dry mandible.” Manual searches of the references of all full-text articles and relevant review articles selected from the electronic search were also performed.
Selection criteria
To determine the studies to be included in this systematic review, the following inclusion criterias were decided. Articles related to mandible in sex dimorphism were only included. Only original articles were included. Both abstract and full text articles were included. Review articles and case reports were excluded. On articles with both maxilla and mandible parameters in sex dimorphism, only mandibular parameters were included. Studies that did not meet any of the inclusion criteria were excluded from the review. The literature search initially resulted in 89 articles out of which only 36 articles fulfilled the inclusion criteria and were included in this systematic review. A systematic review of available articles from the MEDLINE and PubMed on morphometric studies of dry mandible and radiologic studies was done. A synopsis of various radiographic studies on mandible in sex dimorphism was presented in Table 1.[4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19] And synopsis of skeletal parameters of morphometric studies on dry mandible in sex dimorphism was presented in Table 2.[1,2,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37]
Table 1.
Summary of various radiographic studies on mandible in sex dimorphism
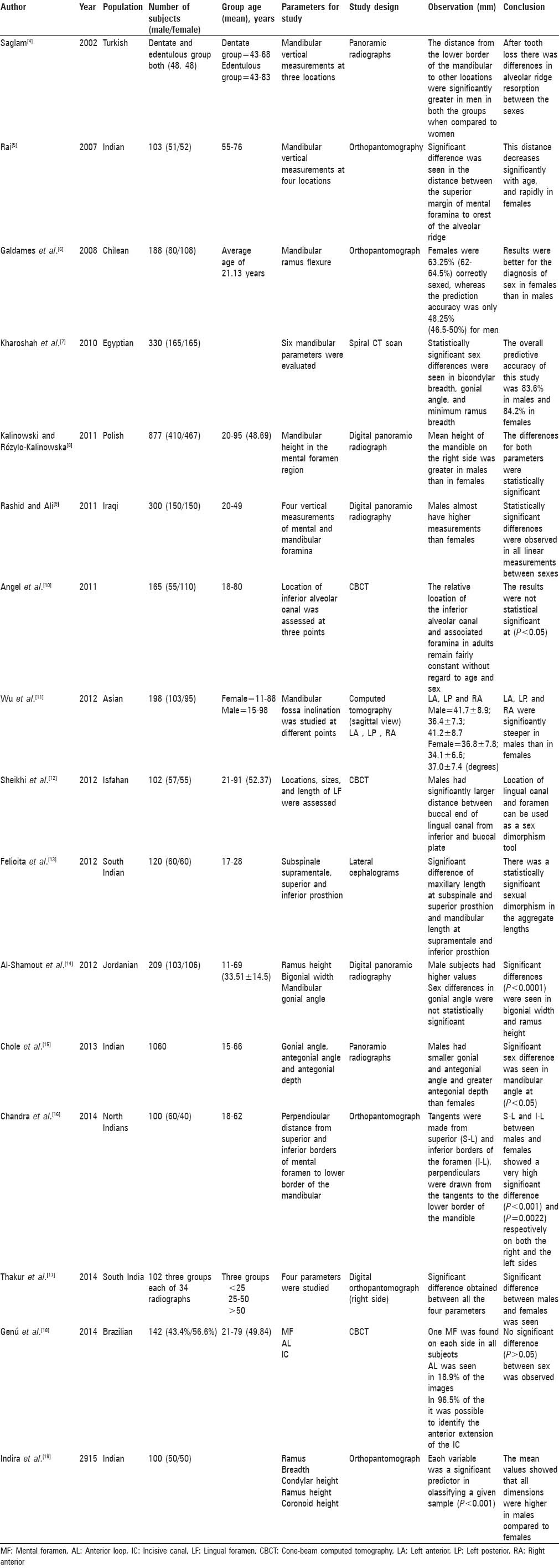
Table 2.
Synopsis of skeletal parameters of morphometric studies of dry mandible in sex dimorphism

Results
Most of the studies reviewed showed statistically significant sex differentiations. Among the most prominent parameters showing sex dimorphism was the ramus of the mandible showing demarcating measurements in ramus breadth and ramus height. Other major parameters which showed statistically significant sex dimorphism were the bicondylar and bigonial width, position of the mental foramen, mandibular length, and chin height. Mandibular ramus flexure was one parameter which has been studied extensively but showed inconsistent results. Very few studies were available on factors such as condylar length and breadth, gonial angle, symphyseal height, and fossa inclinations, but those available showed higher values for males.
Discussion
This article reviewed the various parameters of mandible studied in last 15 years for sex dimorphism. Most of the studies done showed statistically significant sex demarcations. Among the most prominent parameters showing significant sex dimorphism was the ramus of the mandible which showed differences in minimum ramus breath and height. In the study by Ongkana and Sudwan[1] on Thai population minimum ramus breadth for male and females was 32.8 mm and 31.4 mm, respectively. In another study by Pokhrel and Bhatnagar[33] in Indian population, the ramus breadth values were 36.59 ± 6.01 and 28.71 ± 2.72 for male and female, respectively. Kharoshah et al.[7] also studied that the predictive accuracy for sex difference was 83.6% in males and 84.2% in females in Egyptian population using minimum ramus height as a factor. Using ramus height as study parameter in Jordanian population Al-Shamout et al.[14] concluded that the difference was statistically significant with right side values being 54.02 mm in males and 49.77 mm in females and the left side values for male, i.e., 52.62 mm in relation to females i.e. 48.44 mm. In another study using ramus height on Indian population, Thakur et al.[32] also concluded that ramus height can be successfully used as a tool for sex dimorphism with higher values for males. The right side values for male and female were 53 mm and 45.8 mm, respectively, and the left side values were 59.4 mm for male and 36.5 mm for female, respectively. The measured values for both the parameters were higher for male irrespective of the populations studied by many researchers.
Though ramus flexure has been widely studied it showed inconsistent results mainly due to improper grading system.[6,20,21,23,27] Coqueugniot et al.[20] and Saini et al.[27] concluded that ramus flexure can be successfully used to determine sex with an high average accuracy of up to 82%, while Hill[21] conclude that the results were not consistent as 79.1% accuracy was seen in first evaluation and only 64.7% of the scores were duplicated in the second session. Kemkes-Grottenthaler et al.[23] concluded an overall accuracy of 59%.
Other parameter showing consistently significant higher values for male was the bicondylar width. In the study by Ongkana and Sudwan[1] on Thai population bicondylar width for male was 123.8 mm and for females was found to be 116.1 mm. Marinescu et al.[29] also concluded similar results in Romanian population with males showing higher values, i.e., 120 mm and 113.1 mm for females. Similarly, Vinay et al.[30] in his study on Indian population showed similar results with male values of 129 mm and 96.9 mm for females, and Kranioti et al.[2] showed male values of 118.72 mm and 113.34 mm in females for Greek population, respectively. These results were further supported by the study of Kumar and Lokanadham[31] on Indian population who concluded the bicondylar diameters to be in range of 91–126 mm.
Bigonial breadth can also be considered as a statistically significant factor for sex dimorphism. In the study conducted by Vinay et al.[30] on Indian Population, the measured values were 103.5 mm for males and 78 mm for females, which were supported by the findings of Kranioti et al.[2] who found the male measurements to be 101.169 mm and female measurements to be 93.974 mm in Greek population.
A large number of studies were conducted on the position of the mental foramen. Rai[5] studied the Indian population and concluded that a statistically significant sex difference existed between the superior margins of mental foramina to crest of the alveolar ridge. These finding were further supported by the study conducted by Rashid and Ali[9] were the distance was 17.4 ± 0.119 mm for males and 16.01 ± 0.121 mm for females in Iraqi population. Thakur et al.[17] also studied the same parameter and found the distance to be statistically more in males and concluded that the distance between the superior margins of mental foramina to crest of the alveolar ridge can be used as a tool for sex dimorphism with high accuracy. The distance between the inferior margins of the mental foramen to the inferior border of the mandible also showed a high accuracy for sex dimorphism.[8,9,16,17] Chandra et al.[16] found the lower height to be 12.67 mm in males and 11.46 mm in females on the right side and 12.58 mm in males and 11.25 mm in females on the left side in Indian. Rashid and Ali[9] found these measurements to be 10.06 ± 0.101 mm in males and 9.24 ± 0.095 mm in female which coincided with other similar studies.
Mandibular length and chin height were also evaluated, and measurements were higher for males in all studies.[1,30] Ongkana and Sudwan[1] found the mandibular length to be 8.94 mm in males and 8.53 mm in females of Thai origin. Similar values were reported by Vinay et al.[30] in Indian population who found the male measurements to be 8.81 mm and female measurements to be 6.22 mm for mandibular length. Chin height values were 29.78 for males and 26.12 for females in the study conducts by Kranioti et al.[2] and 32.1 mm and 29.4 mm in the study conducted by Marinescu et al.[29] in Romanian population for males and females respectively.
Conclusion
The present review revealed that there was a statistically significant sex dimorphism in mandible. 87.5% of radiographic studies showed statistically significant results that the adult mandible could be used to identify both sex and population affinity compared to other standard analytical techniques. Out of twenty morphometric studies of dry mandible 75% of studies showed a positive correlation between sex dimorphism and mandibular parameters. The review further concludes that it is better to use more number of variables than single parameters for higher accuracy in identification of the mandible in sex dimorphism. Growing mandible cannot be used as a very accurate method in sex differentiation as its studies showed a lower rate of sex differentiation than adult bone. Hence, due to the differences in ethnic patterns parameters and methodology must be validated and standardized for the different groups of population.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Ongkana N, Sudwan P. Gender difference in Thai mandibles using metric analysis. Chiang Mai Med J. 2009;48:43–8. [Google Scholar]
- 2.Kranioti EF, García-Donas JG, Langstaff H. Sex estimation of the Greek mandible with the aid of discriminant function analysis and posterior probabilities. Rom J Legal Med. 2014;22:101–4. [Google Scholar]
- 3.Cox M, Mays S. Human Osteology in Archaeology and Forensic Science. London, UK: Greenwich Medical Media, Ltd.; 2000. p. 548. [Google Scholar]
- 4.Saglam AA. The vertical heights of maxillary and mandibular bones in panoramic radiographs of dentate and edentulous subjects. Quintessence Int. 2002;33:433–8. [PubMed] [Google Scholar]
- 5.Rai B. Possible identification marker in orthopantomograms: Edentulous. Middle East J Sci Res. 2007;2:82–3. [Google Scholar]
- 6.Galdames IC, Valenzuela JS, Quezada NA, Contreras CE, Rivas ZA, López MC. Ortopantomographic blind test of mandibular ramus flexure as a morphological indicator of sex in Chilean young adults. Int J Morphol. 2008;26:89–92. [Google Scholar]
- 7.Kharoshah MA, Almadani O, Ghaleb SS, Zaki MK, Fattah YA. Sexual dimorphism of the mandible in a modern Egyptian population. J Forensic Leg Med. 2010;17:213–5. doi: 10.1016/j.jflm.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 8.Kalinowski P, Rózylo-Kalinowska I. Panoramic radiomorphometric parameters in Polish patients. Folia Morphol (Warsz) 2011;70:168–74. [PubMed] [Google Scholar]
- 9.Rashid SA, Ali J. Sex determination using linear measurements related to the mental and mandibular foramina vertical positions on digital panoramic images. J Baghdad Coll Dent. 2011;23:59–64. [Google Scholar]
- 10.Angel JS, Mincer HH, Chaudhry J, Scarbecz M. Cone-beam computed tomography for analyzing variations in inferior alveolar canal location in adults in relation to age and sex. J Forensic Sci. 2011;56:216–9. doi: 10.1111/j.1556-4029.2010.01508.x. [DOI] [PubMed] [Google Scholar]
- 11.Wu CK, Hsu JT, Shen YW, Chen JH, Shen WC, Fuh LJ. Assessments of inclinations of the mandibular fossa by computed tomography in an Asian population. Clin Oral Investig. 2012;16:443–50. doi: 10.1007/s00784-011-0518-y. [DOI] [PubMed] [Google Scholar]
- 12.Sheikhi M, Mosavat F, Ahmadi A. Assessing the anatomical variations of lingual foramen and its bony canals with CBCT taken from 102 patients in Isfahan. Dent Res J (Isfahan) 2012;9(Suppl 1):S45–51. [PMC free article] [PubMed] [Google Scholar]
- 13.Felicita AS, Chandrasekar S, Shanthasundari KK. Determination of craniofacial relation among the subethnic Indian population: a modified approach – (Sagittal relation) Indian J Dent Res. 2012;23:305–12. doi: 10.4103/0970-9290.102210. [DOI] [PubMed] [Google Scholar]
- 14.Al-Shamout R, Ammoush M, Alrbata R, Al-Habahbah A. Age and gender differences in gonial angle, ramus height and bigonial width in dentate subjects. Pak Oral Dent J. 2012;32:81–7. [Google Scholar]
- 15.Chole RH, Patil RN, Balsaraf Chole S, Gondivkar S, Gadbail AR, Yuwanati MB. Association of mandible anatomy with age, gender, and dental status: A radiographic study. ISRN Radiol. 2013;2013:453763. doi: 10.5402/2013/453763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chandra A, Singh A, Badni M, Jaiswal R, Agnihotri A. Determination of sex by radiographic analysis of mental foramen in North Indian population. J Forensic Dent Sci. 2014;5:52–5. doi: 10.4103/0975-1475.114556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thakur M, Reddy KV, Sivaranjini Y, Khaja S. Gender determination by mental foramen and height of the body of the mandible in dentulous patients a radiographic study. J Indian Acad Forensic Med. 2014;36:13–8. [Google Scholar]
- 18.Genú PR, Vasconcellos RJ, de Oliveira BP, de Vasconcelos BC, da Cruz Delgado NC. Analysis of anatomical landmarks of the mandibular interforaminal region using CBCT in a Brazilian population. Braz J Oral Sci. 2014;13:303–7. [Google Scholar]
- 19.Indira AP, Markande A, David MP. Mandibular ramus: An indicator for sex determination – A digital radiographic study. J Forensic Dent Sci. 2015;4:58–62. doi: 10.4103/0975-1475.109885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coqueugniot H, Tillier A, Bruzek J. Mandibular ramus posterior flexure: A sex indicator in homo sapiens fossil hominids? Int J Osteoarchaeol. 2000;10:426–31. [Google Scholar]
- 21.Hill CA. Technical note: Evaluating mandibular ramus flexure as a morphological indicator of sex. Am J Phys Anthropol. 2000;111:573–7. doi: 10.1002/(SICI)1096-8644(200004)111:4<573::AID-AJPA11>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 22.Loth SR, Henneberg M. Sexually dimorphic mandibular morphology in the first few years of life. Am J Phys Anthropol. 2001;115:179–86. doi: 10.1002/ajpa.1067. [DOI] [PubMed] [Google Scholar]
- 23.Kemkes-Grottenthaler A, Löbig F, Stock F. Mandibular ramus flexure and gonial eversion as morphologic indicators of sex. Homo. 2002;53:97–111. doi: 10.1078/0018-442x-00039. [DOI] [PubMed] [Google Scholar]
- 24.Hu KS, Koh KS, Han SH, Shin KJ, Kim HJ. Sex determination using nonmetric characteristics of the mandible in Koreans. J Forensic Sci. 2006;51:1376–82. doi: 10.1111/j.1556-4029.2006.00270.x. [DOI] [PubMed] [Google Scholar]
- 25.Franklin D, Oxnard CE, O'Higgins P, Dadour I. Sexual dimorphism in the subadult mandible: Quantification using geometric morphometrics. J Forensic Sci. 2007;52:6–10. doi: 10.1111/j.1556-4029.2006.00311.x. [DOI] [PubMed] [Google Scholar]
- 26.Galdames IC, Matamala DA, Smith RL. Sex determination in mandibles in the first year of life by a quantitative approach. Int J Morphol. 2009;27:113–6. [Google Scholar]
- 27.Saini V, Srivastava R, Shamal SN, Singh TB, Pandey AK, Tripathi SK. Sex determination using mandibular ramus flexure: A preliminary study on Indian population. J Forensic Leg Med. 2011;18:208–12. doi: 10.1016/j.jflm.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 28.Carvalho SP, Brito LM, Paiva LA, Bicudo LA, Crosato EM, Oliveira RN. Validation of a physical anthropology methodology using mandibles for gender estimation in a Brazilian population. J Appl Oral Sci. 2013;21:358–62. doi: 10.1590/1679-775720130022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marinescu M, Panaitescu V, Rosu M. Sex determination in Romanian mandible using discriminant function analysis: Comparative results of a time – Efficient method. Rom J Legal Med. 2013;21:305–8. [Google Scholar]
- 30.Vinay G, Gowri SR, Anbalagan J. Sex determination of human mandible using metrical parameters. J Clin Diag Res. 2013;7:2671–3. doi: 10.7860/JCDR/2013/7621.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar MP, Lokanadham S. Sex determination morphometric parameters of human mandible. Int J Res Med Sci. 2013;1:93–6. [Google Scholar]
- 32.Thakur KC, Choudhary AK, Jain SK, Kumar LL. Racial architecture of human mandible – An anthropological study. J Evol Med Dent Sci. 2013;2:4177–88. [Google Scholar]
- 33.Pokhrel R, Bhatnagar R. Sexing of mandible using ramus and condyle in Indian population: A discriminant function analysis. Eur J Anat. 2013;17:39–42. [Google Scholar]
- 34.Raj JD, Sindhu R. Sexual dimorphism in mandibular ramus of South Indian population. Antrocom Online J Anthropol. 2013;9:1973–2880. [Google Scholar]
- 35.Akhlaghi M, Khalighi Z, Vasigh S, Yousefinejad V. Sex determination using mandibular anthropometric parameters in subadult Iranian samples. J Forensic Leg Med. 2014;22:150–3. doi: 10.1016/j.jflm.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 36.Lin C, Jiao B, Liu S, Guan F, Chung NE, Han SH, et al. Sex determination from the mandibular ramus flexure of Koreans by discrimination function analysis using three-dimensional mandible models. Forensic Sci Int. 2014;236:191.e1–6. doi: 10.1016/j.forsciint.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 37.Pillai TJ, Devi TS, Devi CK. Studies on human mandibles. IOSR J Dent Med Sci. 2014;13:8–15. [Google Scholar]


