Abstract
Accurate wound repair is a crucial step to protect organisms from environmental damage, for example infection and toxin exposure. In this issue of The EMBO Journal, Miyoshi et al (2017) have elucidated a new mechanism underpinning this process within the intestine where mesenchymal prostaglandin E2 produced following damage drives intestinal regeneration.
Subject Categories: Development & Differentiation, Molecular Biology of Disease, Stem Cells
The intestine requires an efficiently orchestrated repair process given the contents of the intestinal lumen and its primary function as an absorptive organ. Unless damage is repaired quickly, barrier function would be compromised which would rapidly lead to infection, sepsis and ultimately death. After injury, the highly replicative nature of the intestine leads to rapid wound closure, to minimize the exposure to luminal antigens and microorganisms (Peterson & Artis, 2014). However, given repair/wound healing often activates many pathways deregulated in cancer, for example WNT and TGFβ, uncontrolled repair and loss of homoeostasis could lead to carcinogenesis (Ashton et al, 2010). To guarantee proper wound repair, a process termed epithelial restitution occurs in the intestine to rapidly reseal superficial injuries (Lacy, 1988). The healing process is most likely to be performed by a cell population that has transient repair features, wound‐associated epithelial (WAE) cells, and the induction of non‐canonical Wnt signalling is required (Stappenbeck & Miyoshi, 2009). WAE cells are controlled by an orchestra of growth factors and chemokines. However, both upstream activators of WAE cells and downstream effector pathways need to be elucidated.
Figure 1. Mechanistic model describing the role of PGE2–EP4–PKA–ß‐catenin signalling in intestinal wound repair.
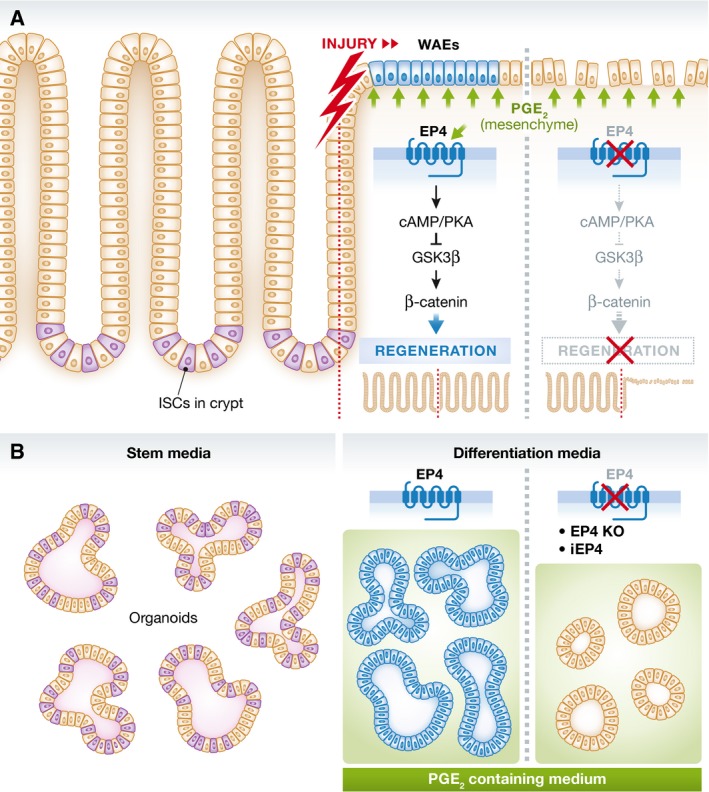
(A) During intestinal injury, PGE2 levels increase locally. This triggers a non‐canonical Wnt signalling cascade resulting in regeneration and wound repair. Pharmacological inhibition or genetic deletion of EP4 impairs this signalling and the tissue remains refractory for regeneration and wound healing. (B) In vitro organoid propagation using stem cell medium enables the culture of stem cell rich organoids. Growth factor‐depleted but PGE2‐containing medium leads to WAE cell differentiation if cells are EP4 proficient; those cells resemble their in vivo analogue. EP4 deficiency or pharmacological inhibition of EP4 (iEP4) leads to enterocyte differentiation and a reduced regenerative capacity.
In an elegant study, Miyoshi et al (2017) used a series of models to address this important question: mouse, organoid and human cell lines. Transcriptomic profiling revealed that postmitotic properties of WAE cells can be generated by growth factor depletion of intestinal organoids. This resembles an atypical differentiation state of WAE cells that has been previously described during the process of epithelial reconstitution (Lacy, 1988; Stappenbeck & Miyoshi, 2009). Importantly, this differentiation state was prostaglandin E2 (PGE2) dependent. Using a genetically engineered model for Ptger4 (encoding for the prostaglandin E receptor 4, EP4) deletion, the authors show that PGE2 triggered WAE cell differentiation is transmitted by EP4 in vitro and in vivo. In an array of elegant in vitro assays, the authors could show that the PGE2–EP4 axis irreversibly leads to WAE cell differentiation, while inhibition of the PGE2–EP4 axis leads to enterocyte differentiation. Mechanistically, the authors describe that EP4 signalling acts via cAMP–PKA–GSK3β cascade that controls β‐catenin stabilization (Wnt signalling activity) and eventually differentiation to WAE cells. Notably, the authors demonstrated a concentration‐dependent differentiation into WAE cells, which may explain the spatial–temporal appearance of WAE cells during healing. To note, mesenchymal cells as source for PGE2 have been described by the authors previously (Manieri et al, 2015).
Cancer has been suggested to be a “wound that cannot heal” so it is notable that many of the pathways described here have been suggested to be important in colorectal carcinogenesis. Stromal COX2 has been shown to be important for growth of adenomas in Apc +/− mice, and COX2 produces Prostaglandin H2 of which Prostaglandin E2 is a downstream metabolite. Loss of EP2 and EP4 was shown to suppress tumorigenesis in Apc +/− mice (Sonoshita et al, 2001, 2002) and, recently, a PGE2–EP4 axis was suggested to control colon cancer stem cell expansion and metastasis (Wang et al, 2015).
Taken together, this study using a sophisticated in vitro tool to study wound repair has generated important new findings into this process. This in vitro system may also be able to define many more novel regulators of wound repair. Importantly, trials have already been performed using EP4 agonist in inflammatory bowel disease (Nakase et al, 2010). It would be interesting to assess whether these patients had more WAE cells and whether they showed increased wound repair. Conversely, further work examining the inhibition of the Prostaglandin E2–EP4 axis in both the initiation and progression of colorectal cancer should provide key insights into the hijacking of repair processes by cancer cells.
See also: H Miyoshi et al (January 2017)
References
- Ashton GH, Morton JP, Myant K, Phesse TJ, Ridgway RA, Marsh V, Wilkins JA, Athineos D, Muncan V, Kemp R, Neufeld K, Clevers H, Brunton V, Winton DJ, Wang X, Sears RC, Clarke AR, Frame MC, Sansom OJ (2010) Focal adhesion kinase is required for intestinal regeneration and tumorigenesis downstream of Wnt/c‐Myc signaling. Dev Cell 19: 259–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacy ER (1988) Epithelial restitution in the gastrointestinal tract. J Clin Gastroenterol 10(Suppl. 1): S72–S77 [DOI] [PubMed] [Google Scholar]
- Manieri NA, Mack MR, Himmelrich MD, Worthley DL, Hanson EM, Eckmann L, Wang TC, Stappenbeck TS (2015) Mucosally transplanted mesenchymal stem cells stimulate intestinal healing by promoting angiogenesis. J Clin Invest 125: 3606–3618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi H, VanDussen KL, Malvin NP, Ryu SH, Wang Y, Sonnek NM, Lai CW, Stappenbeck TS (2017) Prostaglandin E2 promotes intestinal repair through an adaptive cellular response of the epithelium. EMBO J 36: 5–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakase H, Fujiyama Y, Oshitani N, Oga T, Nonomura K, Matsuoka T, Esaki Y, Murayama T, Teramukai S, Chiba T, Narumiya S (2010) Effect of EP4 agonist (ONO‐4819CD) for patients with mild to moderate ulcerative colitis refractory to 5‐aminosalicylates: a randomized phase II, placebo‐controlled trial. Inflamm Bowel Dis 16: 731–733 [DOI] [PubMed] [Google Scholar]
- Peterson LW, Artis D (2014) Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol 14: 141–153 [DOI] [PubMed] [Google Scholar]
- Sonoshita M, Takaku K, Sasaki N, Sugimoto Y, Ushikubi F, Narumiya S, Oshima M, Taketo MM (2001) Acceleration of intestinal polyposis through prostaglandin receptor EP2 in Apc(Delta 716) knockout mice. Nat Med 7: 1048–1051 [DOI] [PubMed] [Google Scholar]
- Sonoshita M, Takaku K, Oshima M, Sugihara K, Taketo MM (2002) Cyclooxygenase‐2 expression in fibroblasts and endothelial cells of intestinal polyps. Cancer Res 62: 6846–6849 [PubMed] [Google Scholar]
- Stappenbeck TS, Miyoshi H (2009) The role of stromal stem cells in tissue regeneration and wound repair. Science 324: 1666–1669 [DOI] [PubMed] [Google Scholar]
- Wang D, Fu L, Sun H, Guo L, DuBois RN (2015) Prostaglandin E2 promotes colorectal cancer stem cell expansion and metastasis in mice. Gastroenterology 149: 1884–1895 [DOI] [PMC free article] [PubMed] [Google Scholar]


