Abstract
Introduction
Defunctioning loop ileostomy (LI) and loop colostomy (LC) are used widely to protect/treat anastomotic leakage after colorectal surgery. However, it is not known which surgical approach has a lower prevalence of surgical complications after low anterior resection for rectal carcinoma (LARRC).
Methods
We conducted a literature search of PubMed, MEDLINE, Ovid, Embase and Cochrane databases to identify studies published between 1966 and 2013 focusing on elective surgical complications related to defunctioning LI and LC undertaken to protect a distal rectal anastomosis after LARRC.
Results
Five studies (two randomized controlled trials, one prospective non-randomized trial, and two retrospective trials) satisfied the inclusion criteria. Outcomes of 1,025 patients (652 LI and 373 LC) were analyzed. After the construction of a LI or LC, there was a significantly lower prevalence of sepsis (p=0.04), prolapse (p=0.03), and parastomal hernia (p=0.02) in LI patients than in LC patients. Also, the prevalence of overall complications was significantly lower in those who received LIs compared with those who received LCs (p<0.0001). After closure of defunctioning loops, there were significantly fewer wound infections (p=0.006) and incisional hernias (p=0.007) in LI patients than in LC patients, but there was no significant difference between the two groups in terms of overall complications.
Conclusions
The results of this meta-analysis show that a defunctioning LI may be superior to LC with respect to a lower prevalence of surgical complications after LARRC.
Keywords: Rectal surgery, loop ileostomy, loop colostomy, complications
One of the most severe complications after low rectal resection is anastomotic leakage. Trying to prevent this complication by undertaking proximal faeces diversion, with or without a defunctioning stoma, is controversial. Four meta-analyses have reported that a defunctioning stoma does not prevent anastomotic leak, but limits sequelae. However, a lower prevalence of surgical re-intervention may reduce the disastrous clinical consequences of leakage if a stoma is constructed to protect anastomotic leakage after rectal resection.1–4
Consensus on the best surgical procedure for constructing a defunctioning stoma is lacking. This scenario persists despite the fact that four meta-analyses of the clinical course of patients undergoing ileostomy compared with colostomy after colorectal cancer anastomoses have been reported: comparison of the outcomes between loop ileostomy (LI) and loop colostomy (LC) failed to establish the superiority of either method.5–8
Several of the meta-analyses reported previously had limitations: they included surgery for different diseases (Crohn’s disease, diverticulosis) and operation times for emergency surgery and elective surgery. Here, we focused on complications related to the construction and closure of stomas during elective low anterior resection for rectal carcinoma (LARRC) to exclude the confounding factors associated with other diseases.
We conducted a meta-analysis of all randomized controlled trials (RCTs) and observational studies that compared the complications related to defunctioning LI with those of LC after LARRC. The results of our survey will help surgeons in making decisions with regard to these two procedures.
Methods
A literature search of PubMed, MEDLINE, Ovid, Embase and Cochrane databases was undertaken to identify studies published between 1966 and 2013. Medical subject headings (MeSH) and free text words in combination were used in the search for RCTs and observational studies.
The following MeSH search headings were used: ‘rectal neoplasms’, ‘defunctioning stoma’, ‘colostomy’, and ‘ileostomy’; the ‘related articles’ function was used to broaden the search. All abstracts, studies, and citations were reviewed. Reference lists of all relevant studies (including reviews) were screened. References of identified articles were searched manually. Authors and device companies were contacted for additional published and unpublished studies. The latest date of the literature search was 31 March 2013.
There were two inclusion criteria for this study: (i) RCTs and controlled clinical trials that evaluated defunctioning LI compared with LC for patients who had undergone an elective procedure for rectal cancer: (ii) reports citing ‘loop ileostomy’ vs ‘loop colostomy’.
Studies were excluded from the analysis if: (i) LI or LC was excluded; (ii) relevant data could not be extracted or calculated from the publication.
Two reviewers (H.G. and D.N.) extracted the following relevant data for each study independently: first author; year of publication; characteristics of the study population; study design; inclusion/exclusion criteria; matching criteria; number of patients who underwent procedures using the two methods; number of males: number of females. Discrepancies in the outcome of extraction were resolved by re-examination of relevant studies until consensus was achieved.
Selected complications associated with stoma construction were skin irritation, dehydration, sepsis, prolapse, parastomal herniation, enterocutaneous fistula, retraction, stenosis, necrosis, and haemorrhage. Selected complications associated with stoma closure were wound infection, incisional hernia, obstruction, anastomotic leakage at stoma closure site, and mortality.
The meta-analysis was carried out in accordance with the recommendations set by the Quality Reporting of Meta-analyses, the PRISMA statement (www.prisma-statement.org).9 The quality of included studies was assessed using the Newcastle–Ottawa score for one prospective non-RCT and two retrospective trials, and modified to match the needs of this study.10 Studies with a rating of five stars (from a maximum of 11 stars) were considered to be of high quality. The Revised Jadad Scale (low quality, 1–3 points; high quality, 4–7 points) was used for evaluation of two RCTs.11 Begg test and Egger test were used for assessment of publication bias.12,13
Statistical analyses
Statistical analyses of dichotomous outcomes were carried out, and odds ratios (ORs) used as the summary statistic; both were reported with 95% confidence intervals (CIs). p<0.05 was considered significant.
Clinical heterogeneity was tested using the I2 value. I2>50% was indicative of clinical heterogeneity. If heterogeneity was found, random-effects analyses were carried out. Otherwise, the results of fixed-effects analyses are presented.14 ORs for the outcomes of interest were combined using the Mantel–Haenszel method and a ‘random effect’ meta-analytical method. In a random-effect model, variations between studies are assumed. Consequently, the calculated OR is a more conservative value.15,16 Publication bias was tested using Stata v11.0. Funnel plots were used to investigate publication bias. The χ2 test was employed for assessment of heterogeneity. Analyses were conducted using Statistical Review Manager v5.0 (Cochrane Collaboration, Software Update, Oxford, UK).
Results
The initial literature search identified 129 studies from PubMed, MEDLINE, Ovid, Embase and Cochrane databases. An additional nine studies were identified through ‘grey literature’ sources. Four studies were removed because they were duplicates. Hence, 134 studies were screened. However, 111 articles were excluded because their titles described inappropriate topics, such as discussion of ileostomy or colostomy only for colorectal anastomosis, or the clinical outcome of patients treated with or without derivative enterostomy after colorectal surgery. The remaining 23 studies were evaluated.
Thirteen studies were excluded because they did not include the complications when comparing LI with LC after LARRC.17–29 A further four studies were excluded because relevant data could not be extracted or calculated from them; these studies had included surgery for different diseases (eg neoplasms, Crohn’s disease, diverticulitis) and operation times for defunctioning stomas (eg emergency surgery, elective surgery).30–33 One article was merely an abstract and reported outcomes on defunctioning stomas after LARRC. This article was found in the literature but its full-text evaluations were not available, so it was excluded.34
Thus, five comparative studies were included in the analyses.35–39 Two were RCTs,35,36 one was a prospective non-randomized study,37 and two were retrospective reviews (Fig 1).38,39
Figure 1.
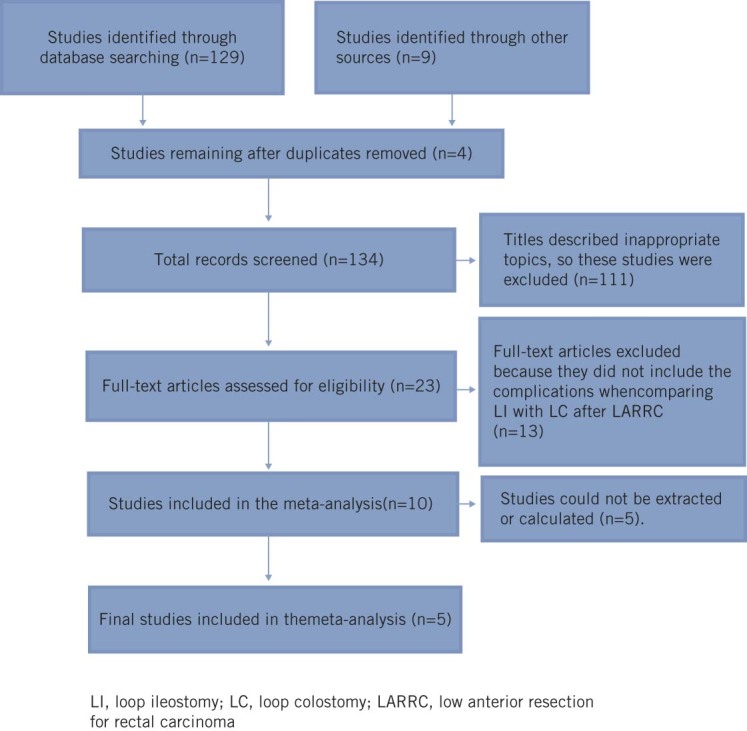
Flow diagram showing study selection
In total, selected studies reported on 1,025 patients: 652 LI (63.61%) and 373 LC (36.39%). The two RCTs35,36 involved 150 patients: 76 (50.67%) underwent LI and 74 underwent LC (49.33%). The other three non-randomized studies37–39 involved 875 patients, of whom 576 (65.83%) underwent LI and 299 (34.17%) underwent LC. The characteristics of patients and the study, as well as the outcomes of interest reported by each of these studies, are summarized in Table 1.
Table 1.
Study characteristics and outcomes of interest reported by each of the included studies
| Reference | Design | I | C | FemalesN (%) | Matching | Stoma indication | Study quality | Complications of stoma construction | Complications of stoma closure | |
|---|---|---|---|---|---|---|---|---|---|---|
| Edwards et al.[35] | RCT | 34 | 36 | 21 (30.0) | 1, 2, 4, 5, 7 | n/c | 4 | a3 a4, a5, a7 | b1, b2, b4, | |
| Law et al.[36] | RCT | 42 | 38 | 31 (38.8) | 1, 2, 4, 5,6, 7, 8 | c | 4 | a1, a3, a4, a5, a9, a11 | b1, b3 | |
| Gastinger et al.[37] | PNR | 407 | 229 | n/c | 1, 2, 4, 5 | n/c | **** | b1, b3, b5 | ||
| Rullier et al.[38] | RET | 107 | 60 | 46 (27.5) | 1, 2, 3, 4,5, 7 | a, b | ****** | a1, a2, a3, a4, a5, a6, a7, a8, a9, a10 | b1, b2, b3, b4, b5 | |
| Mala et al.[39] | RET | 62 | 10 | 32 (44) | 1, 2, 4, 6, | c | ***** | a1, a5, a11 | b1, b2, b3, b4, b5 |
I, ileostomy; C, colostomy; PNR, prospective non-randomized; RET, retrospective; RCT, randomized controlled trial; n/c, no comment. 1, age; 2, gender, 3, body mass index; 4, diagnosis of rectal cancer, 5, tumor level; 6, anastomosis level; 7, tumor stage; 8, anastomosis method; a, poor bowel preparation; b, technical anastomotic problem; c, anastomosis <6cm.
Complications of stoma construction: dehydration (a1); necrosis (a2); prolapse (a3); retraction (a4); parastomal hernia (a5); stenosis (a6); sepsis (a7); haemorrhage (a8); skin irritation (a9); obstruction (a10); anastomotic leak or fistula (a11).
Complications of stoma closure: obstruction (b1); wound infection (b2); anastomotic leak or fistula (b3); incisional hernia (b4); death (b5).
Outcome measures for stoma construction
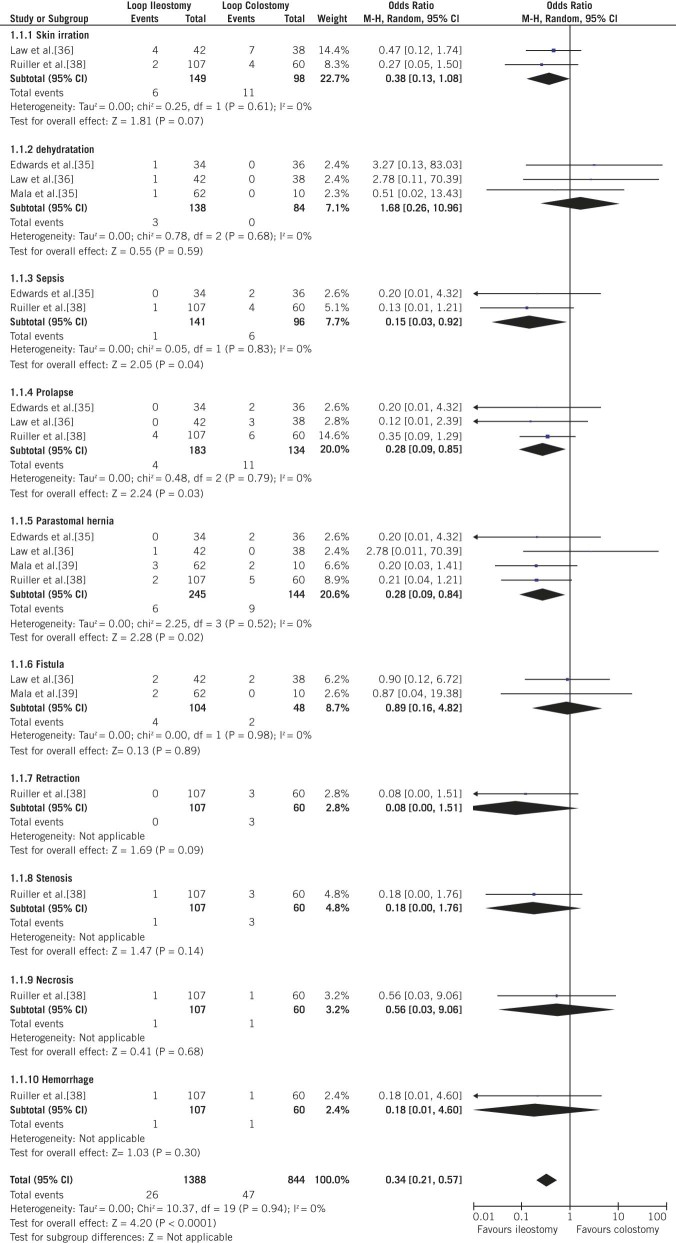
There was a lower prevalence of prolapse in LI patients (2.19%; 4/183) compared with LC patients (8.21%; 11/134 patients) (OR: 0.28; 95% CI: 0.09–0.85; p=0.03; Fig 2). LI patients also had a lower prevalence of sepsis (0.71%; 1/141) compared with that in LC patients (6.25%; 6/96) (OR: 0.15; 95% CI: 0.03–0.92; p=0.04; Fig 2). A trend towards a lower prevalence of parastomal hernias was observed in LI patients (2.45%; 6/245) compared with that in LC patients (6.25%; 9/144) (OR: 0.28; 95% CI: 0.09–0.84; p=0.02; Fig. 2).
Figure 2.
Forest plots illustrating overall meta-analysis of complications of stomal construction, complications of stomal construction, and total complications, as well as summary plots of sepsis, prolapse, and parastomal hernia after stoma construction
Outcome measures for stoma closure
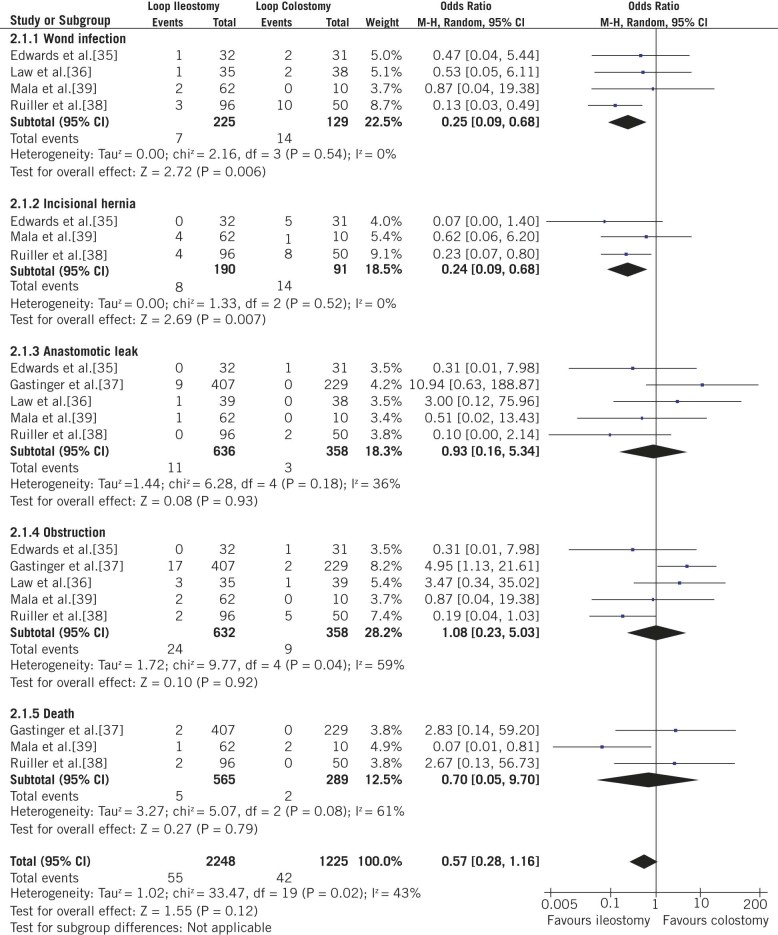
A lower prevalence of incisional hernia was reported in LI patients (4.21%; 8/190) compared with that in LC patients (15.38%; 14/91) (OR: 0.24; 95% CI: 0.09–0.68; p=0.007; Fig 3). Similarly, LI patients had a lower prevalence of wound infection (3.11%; 7/225) compared with that in LC patients (10.85%; (14/129) (OR: 0.25; 95% CI: 0.09–0.68; p=0.006; Fig 3).
Figure 3.
Forest plots illustrating overall meta-analysis of complications of stoma closure, complications of stoma closure, and total complications, as well as summary plots of wound infection and incisional hernias after stoma closure
Overall complications
Overall, 26 and 47 events related to stoma construction were observed in LI and LC. Cumulative analyses of outcome measures demonstrated a significant difference between LI and LC (OR: 0.34; 95% CI: 0.21–0.57; p<0.0001; Fig 2).
Overall, 55 and 42 events related to stoma closure were observed in LI and LC patients. Cumulative analyses of outcome measures did not reveal a significant difference between LI and LC (OR: 0.57; 95% CI: 0.28–1.16; p=0.12; Fig 3).
Heterogeneity analyses
There were no heterogeneity differences between the stoma-construction group (χ2=10.37, df=19, p=0.94, I2=0%; Fig 2) and the stoma-closure group (χ2=33.47, df=19, p=0.02, I2=43%; Fig 3).
Publication bias
There was no significant difference in publication bias as determined by the Begg rank correlation test (p=0.806; Fig 4) and the Egger linear regression method (p=0.762; Fig 4).
Figure 4A.
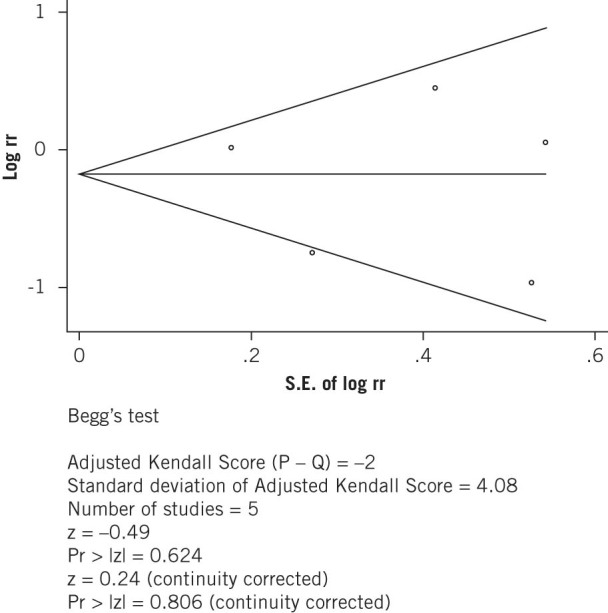
Begg�s funnel plot with pseudo 95% confidence limits
Figure 4B.
Funnel plot of studies comparing ileostomy and colostomy for publication bias
Discussion
Our meta-analysis used the method of pooled data for RCTs and observational studies for 1,025 patients who underwent LARRC. Outcome measures analyzed for construction of a defunctioning stoma showed a lower prevalence of sepsis, prolapse, and parastomal hernia in LI recipients than in LC recipients. Outcome measures analyzed for stoma closure revealed advantages for LI in terms of postoperative wound infection and incisional hernia. Complications described in the present study and in other studies5–8are very different in terms of the severity of the consequences.
Our data may have differed from the results of other scholars due to four main reasons. Firstly, the endpoint might have been judged differently. For instance, we did not use the endpoints used by Guenaga et al.:6 general outcome measures; stoma construction; stoma closure; stoma function. Secondly, some complications were not detected, which lowered the reliability of the studies spesis.35 However, it is statistically significant in our study, the present study was a similar result in another meta-analysis.8 Thirdly, we focused on elective LARRC and stoma construction and stoma closure-related complications. Those studies5–5 included surgery for different diseases (eg primary cancer, Crohn’s disease, diverticulosis) and operation times for defunctioning stomas (eg emergency surgery, elective surgery). The reason for this result is not completely clear but several relevant differences between emergency and elective procedures (eg pathology, bowel distention, bowel preparation, mesenteric edema) may have played a part. In addition, different health statuses for patients and surgeon experience may have had roles.8 Finally, follow-up after stoma closure varied among the included studies: 1–6 months,38 1 year,37 and 5 years.35,36,39 For instance, two of the studies35,38 compared LC and LI with different durations of follow-up; a longer follow-up might explain the difference between the two patient groups with regard to the risk for stoma-site incisional hernias.
The present study exhibited identical results as those for two meta-analyses that focused on the prevalence of overall complications.7,8 LI was associated with a lower prevalence of surgical complications in the construction of stomas (but not in their closure) compared with LC. However, the cause of the difference between stoma construction and stoma closure in terms of overall complications is not clear. The present study revealed that the difference between LI and LC in terms of stoma construction and stoma closure may be due to anatomy and the tissue/physiology characteristics of the ileum and colon (eg bacterial counts, peristalsis, electrolytes). Lower prevalence of wound sepsis after ileostomy closure may be because anaerobic bacterial counts from ileostomy effluent have been found to be 105-times lower compared with anaerobic bacterial counts from normal faeces, whereas the bacterial flora of colostomy effluent is very similar to that of faeces.40,41 Recently, Murray et al. analyzed the prevalence of incisional hernias related with surgical-site infections in colorectal surgery.42 Veljkovic et al. reported that patients with a surgical-site infection were 1.9-times more likely to have an incisional hernia than those without an infection.43 The above-mentioned analyses revealed that the difference in bacterial counts in the ileum and colon lead to sepsis, wound infection, and hernia. Faeces in the colon is a semisolid material and the movement of such a mass is faster than the peristaltic wave in the ileum.44 We suggest that this phenomenon makes prolapse of the colon more likely than in the ileum after stoma construction.
The articles included in our meta-analysis had limitations. The low number and quality of the included studies weakened the results of our meta-analysis. Our study included RCTs and observational studies. The major drawback of this approach is that including studies other than RCTs means data pooling, which weakens the meta-analysis. Conversely, Bayesian approaches to meta-analysis can be useful if few data from RCTs are available.45,46 They can also be used to account for the uncertainty introduced by estimation of between-study variance in the random-effects model, which can lead to reliable estimates and predictions of treatment effects.47 In addition, using just two RCTs, analyses of a single surgical complication as a subgroup becomes very difficult. However, we did not find evidence of heterogeneity using pooled data. In contrast, a previous meta-analysis5 demonstrated pronounced heterogeneity between LI and LC for the included studies. Random-effect models are preferable if meta-analytical methods are used in surgical research because, for a given surgical procedure, each centre has its own criteria for patient selection, and patients may have different risk profiles.7 Thus, caution is needed when drawing conclusions from the reports. However, it appears that LI is associated with fewer surgical complications than LC, though large RCTs are needed to clarify this issue.
Conclusions
The results of this meta-analysis suggest that LI may be superior to LC with regard to the prevalence of surgical complications associated with construction of a defunctioning stoma in the management of low rectal anastomoses. Compared with LC, LI had a lower prevalence of sepsis, prolapse, and parastomal hernias. In the closure of stomas, wound infection and incisional hernias occurred less frequently with LI than with LC. In terms of overall outcomes, LI was superior to LC for the construction of stomas, but not for their closure.
References
- 1.Hüser N, Michalski CW, Erkan M, et al. Systematic review and meta-analysis of the role of defunctioning stoma in low rectal cancer surgery. Ann Surg 2008; : 52–60. [DOI] [PubMed] [Google Scholar]
- 2.Chen J, Wang DR, Yu HF, et al. Defunctioning stoma in low anterior resection for rectal cancer: a meta-analysis of five recent studies. Hepatogastroenterology 2012; : 1,828–1,831. [DOI] [PubMed] [Google Scholar]
- 3.Montedori A, Cirocchi R, Farinella E, et al. Covering ileo- or colostomy in anterior resection for rectal carcinoma (review). Cochrane Database Syst Rev 2010; 12: CD006878. [DOI] [PubMed] [Google Scholar]
- 4.Tan WS, Tang CL, Shi L, et al. Meta-analysis of defunctioning stomas in low anterior resection for rectal cancer. Br J Surg 2009; : 462–472. [DOI] [PubMed] [Google Scholar]
- 5.Lertsithichai P, Rattanapichart P. Temporary ileostomy versus temporary colostomy: a meta-analysis of complications. Asian J Surg 2004; : 202–210. [DOI] [PubMed] [Google Scholar]
- 6.Güenaga KF, Lustosa SA, Saad SS, et al. Ileostomy or colostomy for temporary decompression of colorectal anastomosis. Cochrane Database Syst Rev 2007; 24: CD004647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tilney HS, Sains PS, Lovegrove RE, et al. Comparison of outcomes following ileostomy versus colostomy for defunctioning colorectal anastomoses. World J Surg 2007; : ,1,143—1,152. [DOI] [PubMed] [Google Scholar]
- 8.Rondelli F, Reboldi P, Rullin A, et al. Loop ileostomy versus loop colostomy for fecal diversion after colorectal or coloanal anastomosis: a meta-analysis. Int J Colorectal Dis 2009; : 479–488. [DOI] [PubMed] [Google Scholar]
- 9.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; ; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stang A. Critical evaluation of the Newcastle–Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010; : 603–605. [DOI] [PubMed] [Google Scholar]
- 11.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996; : 1–12. [DOI] [PubMed] [Google Scholar]
- 12.Idris N.A comparison of methods to detect publication bias for meta-analysis of continuous data. J Appl Sci 2012; ,1,413–1,417. [Google Scholar]
- 13.Peters JL, Sutton AJ, Jones DR, et al. Comparison of two methods to detect publication bias in meta-analysis. JAMA 2006; : 676–680. [DOI] [PubMed] [Google Scholar]
- 14.Friedman HP, Goldberg JD. Meta-analysis: an introduction and point of view. Hepatology 1996; : 917–928. [DOI] [PubMed] [Google Scholar]
- 15.DerSimonian R, Laird N.Meta-analysis in clinical trials. Control Clin Trials 1986; : 177–188. [DOI] [PubMed] [Google Scholar]
- 16.Egger M, Davey Smith G, Schneider M, et al.Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; : 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alexander-Williams J, Loop ileostomy and colostomy for faecal diversion. Ann R Coll Surg Engl 1974; : 141–148. [PMC free article] [PubMed] [Google Scholar]
- 18.Fasth S, Hultén L, Palselius I. Loop ileostomy-an attractive alternative to a temporary transverse colostomy. Acta Chir Scand 1980; : 203–207. [PubMed] [Google Scholar]
- 19.Nordstrӧm G, Hultén L. Loop ileostomy as an alternative to transverse loop colostomy. J Enterostomal Ther 1983; : 92–94. [DOI] [PubMed] [Google Scholar]
- 20.Williams NS, Nasmyth DG, Jones D, et al. Defunctioning stomas: a prospective controlled trial comparing loop ileostomy with loop transverse colostomy. Br J Surg 1986; : 566–570. [DOI] [PubMed] [Google Scholar]
- 21.Khoury GA, Lewis MC, Meleagros L, et al. Colostomy or ileostomy after colorectal anastomosis?: a randomized trial. Ann R Coll Surg Engl 1987; : 5–7. [PMC free article] [PubMed] [Google Scholar]
- 22.Rutegård J, Dahlgren S. Transverse colostomy or loop ileostomy as diverting stoma in colorectal surgery. Acta Chir Scand 1987; : 229–232. [PubMed] [Google Scholar]
- 23.Gooszen AW, Geelkerken RH, Hermans J, et al. Temporary decompression after colorectal surgery: randomized comparison of loop ileostomy and loop colostomy. Br J Surg 1998; : 76–79. [DOI] [PubMed] [Google Scholar]
- 24.Edwards DP, Chisholm EM, Donaldson DR. Closure of transverse loop colostomy and loop ileostomy. Ann R Coll Surg Engl 1998; : 33–35. [PMC free article] [PubMed] [Google Scholar]
- 25.Sakai Y, Nelson H, Larson D, et al. Temporary transverse colostomy vs loop ileostomy in diversion. Arch Surg 2001;: 338–342. [DOI] [PubMed] [Google Scholar]
- 26.Caricato M, Ausania F, Ripetti V, et al. Retrospective analysis of long-term defunctioning stoma complications after colorectal surgery. Colorectal Dis 2007; : 559–561. [DOI] [PubMed] [Google Scholar]
- 27.Harish K. The loop stoma bridge – a new technique. J Gastrointest Surg 2008; : 958–961 [DOI] [PubMed] [Google Scholar]
- 28.Daluvoy S, Gonzalez F, Vaziri K, et al. Factors associated with ostomy reversal. Surg Endosc 2008; 22: ,2,168–2,170. [DOI] [PubMed] [Google Scholar]
- 29.Klink CD, Lioupis K, Binnebosel M, et al. Diversion stoma after colorectal surgery: loop colostomy or ileostomy? Int J Colorectal Dis 2011; : 431–436. [DOI] [PubMed] [Google Scholar]
- 30.Chen F, Stuart M. The morbidity of defunctioning stomata. Aust NZ J Surg 1996; : 218–221. [DOI] [PubMed] [Google Scholar]
- 31.Bailey CM, Wheeler JM, Birks M, et al. The incidence and causes of permanent stoma after anterior resection. Colorectal Dis 2003; : 331–334. [DOI] [PubMed] [Google Scholar]
- 32.Baloyiannis I, Christodoulidis G, Symeonidis D, et al. Loop stomas with a subcutaneously placed bridge device. Tech Coloproctol 2010; : S75–S76. [DOI] [PubMed] [Google Scholar]
- 33.Lindgren R, Hallböök O, Rutegård J, et al. What is the risk for a permanent stoma after low anterior resection of the rectum for cancer? A six-year follow-up of a multicenter trial. Dis Colon Rectum 2011; : 41–47. [DOI] [PubMed] [Google Scholar]
- 34.Tocchi A, Mazzoni G, Miccini M, et al. Use of ileostomy and colostomy as temporal derivation in colorectal surgery. G Chir 2002; : 48–52. [PubMed] [Google Scholar]
- 35.Edwards DP, Leppington-Clarke A, Sexton R, et al. Stoma-related complications are more frequent after transverse colostomy than loop ileostomy: a prospective randomized clinical trial. Br J Surg 2001; : 360–363. [DOI] [PubMed] [Google Scholar]
- 36.Law WL, Chu KW, Choi HK. Randomized clinical trial comparing loop ileostomy and loop transverse colostomy for faecal diversion following total mesorectal excision. Br J Surg 2002; : 704–708. [DOI] [PubMed] [Google Scholar]
- 37.Gastinger I, Marusch F, Steinert R, et al. Protective defunctioning stoma in low anterior resection for rectal carcinoma. Br J Surg 2005; ,1,137–1,142. [DOI] [PubMed] [Google Scholar]
- 38.Rullier E, Le Toux N, Laurent C, et al. Loop ileostomy versus loop colostomy for defunctioning low anastomoses during rectal cancer surgery. World J Surg 2001; : 274–278. [DOI] [PubMed] [Google Scholar]
- 39.Mala T, Nesbakken A. Morbidity related to the use of a protective stoma in anterior resection for rectal cancer. Colorectal Dis 2008; : 785–788. [DOI] [PubMed] [Google Scholar]
- 40.Gorbach SL, Tabaqchali S. Bacteria, bile and the small bowel. Gut 1969; : 963–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hill GL. Ileostomy: Surgery, Physiology and Management. New York: Grune & Stratton, 1976. [Google Scholar]
- 42.Murray BW, Cipher DJ, Pham T, et al. The impact of surgical site infection on the development of incisional hernia and small bowel obstruction in colorectal surgery. Am J Surg 2011; : 558–560. [DOI] [PubMed] [Google Scholar]
- 43.Veljkovic R, Protic M, Gluhovic A, et al. Prospective clinical trial of factors predicting the early development of incisional hernia after midline laparotomy. J Am Coll Surg 2010; : 210–219. [DOI] [PubMed] [Google Scholar]
- 44.Guyton AC, Hall JE. Textbook of Medical Physiology, 10th edition. Beijing: Health Sciences Asia, Elsevier; 2002. [Google Scholar]
- 45.Price D, Jefferson T, Demicheli V. Methodological issues arising from systematic reviews of the evidence of safety of vaccines. Vaccine 2004; ,2,080–2,084. [DOI] [PubMed] [Google Scholar]
- 46.Liford RJ, Thornton JG, Braunholtz D. Clinical trials and rare diseases: a way out of a conundrum. BMJ 1995; : 1,621–1,625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dumouchel W. Meta-analysis for dose–response models. Stat Med 1995; : 679–685. [DOI] [PubMed] [Google Scholar]