Abstract
Urothelial cell carcinoma (UCC) of the bladder is one of the most common malignancies, causing considerable morbidity and mortality worldwide. It is unique among the epithelial carcinomas as two distinct pathways to tumourigenesis appear to exist: low grade, recurring papillary tumours usually contain oncogenic mutations in FGFR3 or HRAS whereas high grade, muscle invasive tumours with metastatic potential generally have defects in the pathways controlled by the tumour suppressors p53 and retinoblastoma. Over the last two decades, a number of transgenic mouse models of UCC, containing deletions or mutations of key tumour suppressor genes or oncogenes, have helped us understand the mechanisms behind tumour development. In this summary, I present my work investigating the role of the WNT signalling cascade in UCC.
Keywords: Urothelial cell carcinoma, Bladder cancer, Mouse models, Transgenics, WNT
The incidence of urothelial cell carcinoma (UCC) continues to rise, with significant implications for our healthcare systems. In the UK, it is the seventh most common cancer, accounting for 3% of all new cases, with an estimated 10,399 new cases diagnosed in the UK in 2011.1
UCCs are predominantly transitional cell carcinomas (TCCs), arising from the transitional epithelium that lines the bladder. Of these, 70–80% of cases are low grade (LG), papillary, non-invasive tumours that tend to recur in over 30% of patients despite local excision.2 There is controversy regarding whether these papillary tumours progress to invasive disease, with progression occurring in approximately 5% of cases.3 However, whether this is a true progression or development of de novo muscle invasive tumours remains unclear. Most morbidity and subsequent mortality secondary to UCC is due to the high grade (HG), non-papillary, muscle invasive form of the disease (20–30% of new TCC cases).4 These invasive tumours penetrate through the muscularis of the bladder and 50% of patients relapse with tumours that metastasise to distant sites despite treatment.5 The five-year survival rate for metastatic bladder cancer is approximately 7%.6
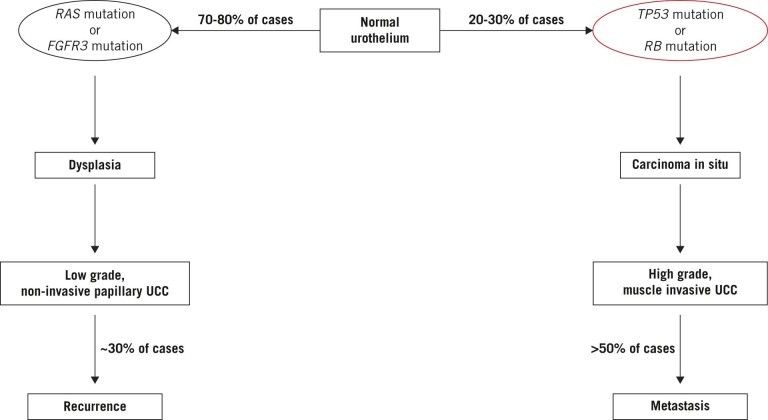
Clinical and pathological studies have found that these two forms of UCC arise through at least two separate mechanisms (Fig 1).7,8 Recently, genomic profiling and expression studies have identified two separate signatures for different molecular subtypes of UCC that stratify into LG or HG disease and can independently predict the likelihood of development of metastasis and disease specific survival.9–11
Figure 1 .
Important genetic defects that characterise the diverse pathways underlying urothelial cell carcinoma (UCC): Low grade, non-invasive papillary tumours are associated with mutations in either RAS pathway components or FGFR3, which are thought to be mutually exclusive. High grade, muscle invasive tumours are associated with loss of p53 or retinoblastoma protein function.
Multiple transgenic murine models that attempt to recapitulate the different subtypes of UCC have been developed to better understand the disease process (Table 1).
Table 1.
At a glance reference of published genetically engineered mouse models of UCC57
[Matt to advise on whether permission is needed to reproduce.]
| Mutation | Promoter | Phenotype | References |
|
Hras+ (low copy) Hras+ (high copy) |
UPII UPII |
Low grade, non-invasive (long latency) Low grade, non-invasive (short latency) |
Zhang, 200144 Zhang, 200144 |
|
Egfr+ Egfr+Hras+ |
UPII UPII |
Hyperplasia Hyperplasia |
Cheng, 200245 Cheng, 200245 |
|
Fgfr3+ Fgfr3+KrasG12D Fgfr3+β-cateninexon3/+ Fgfr3+Ptenfl/fl |
UPII UPII UPII UPII |
Hyperplasia Hyperplasia Hyperplasia Hyperplasia |
Ahmad, 201146 Ahmad, 201146 Ahmad, 201146 Foth, 201447 |
| Ncstnfl/fl | R26rtTA; tetO- | Invasive UCC | Rampias, 201448 |
|
p53fl/fl p53fl/fl p53fl/flHras+ p53fl/flPtenfl/fl |
UPII UPKII UPII Adeno-Cre |
Hyperplasia and dysplasia No phenotype High grade, invasive UCC Invasive, metastatic UCC |
Gao, 200449 Ayala de la Peña, 201150 Gao, 200449 Puzio-Kuter, 200951 |
|
Ptenfl/fl Ptenfl/fl Ptenfl/fl Ptenfl/fl |
Fabp-Cre Ksp-Cre Adeno-Cre UPII |
Low grade UCC UCC of renal pelvis No phenotype No phenotype |
Yoo, 2006,52 Tsuruta, 200653 Qian, 200954 Puzio-Kuter, 200951 Ahmad, 201134 |
|
Rb-/- Rb-/-Ptenfl/fl Rb-/-p53fl/fl |
UPII Adeno-Cre UPII |
No phenotype No phenotype Invasive UCC after chemical carcinogenesis |
He, 200955 Puzio-Kuter, 200951 He, 200955 |
|
SV40 T antigen (low copy) SV40 T antigen (high copy) SV40 T antigen |
UPII UPII UPKII |
Carcinoma in situ Invasive, metastatic UCC Carcinoma in situ |
Zhang, 199956 Zhang, 199956 Ayala de la Peña, 201150 |
|
β-cateninexon3/+ β-cateninexon3/+Ptenfl/fl β-cateninexon3/+HrasQ61L |
UPII UPII UPII |
Hyperplasia Low grade, non-invasive UCC Low grade, non-invasive UCC |
Ahmad, 201134 Ahmad, 201134 Ahmad, 201135 |
| UCC = urothelial cell carcinoma |
The role of WNT signalling in urothelial cell carcinoma
Human urothelial cell carcinoma
The Wnt pathway is conserved widely throughout many species including Caenorhabditis elegans, Drosophila, Xenopus and higher order mammals. It was discovered originally in Drosophila where the gene wingless demonstrated the ability to influence segment polarity during larval development.12 The WNT pathway is now known to control many events during embryonic development and regulates homeostatic self-renewal in a number of adult tissues. As a result, mutations in this pathway are associated with the onset of various cancers, owing to the influence of the WNT pathway on processes such as proliferation, motility and cell fate at the cellular level.13
In colorectal cancer, it is now well established that germline and somatic mutations of the APC gene, a key component of the WNT pathway, are found in most cases.14–16 However, regarding UCC, there was still dubiety surrounding whether the disease was associated with somatic mutations of members of the WNT signalling pathway, including adenomatous polyposis coli (APC)17–20 and β-catenin.21–23 Loss of heterozygosity has been reported at the APC locus in 6–50% of UCC tumours analysed, with no APC mutations identified in tumours (n=24) or cell lines (n=4).18–20 Subsequently, evidence of missense (13%) and frameshift (3%) deletions in the APC protein adjacent to the β-catenin binding sites was reported.24 Interestingly, the authors were able to demonstrate that either APC mutations or β-catenin accumulation was associated with shorter disease free interval and shorter disease specific survival in multivariate analysis.
Earlier studies have demonstrated an upregulation of β-catenin by immunohistochemistry.25–29 In addition, CpG hypermethylation silencing of the region encoding WNT inhibitory factor 1, an inhibitor of the WNT signalling pathway, has been reported as a frequent event in UCC.30 In another study, epigenetic silencing of the four secreted frizzled receptor proteins, antagonists of the WNT signalling pathway, has been demonstrated as an independent predictor of invasive bladder cancer.31
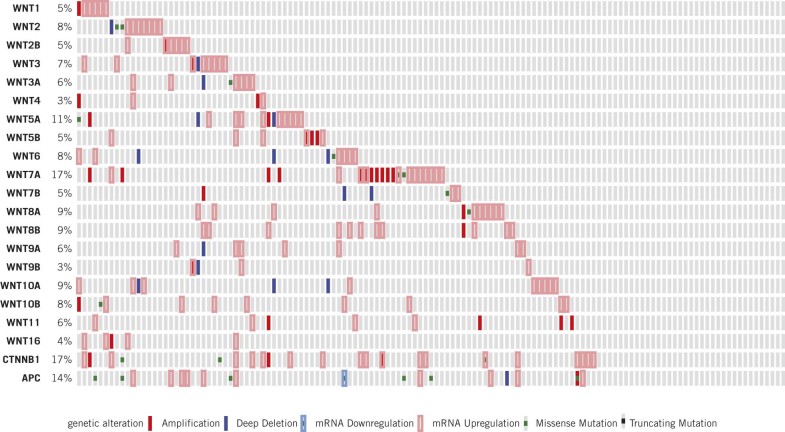
Recent sequencing studies demonstrate alteration of WNT family members in up to 73% of 131 chemotherapy naive, muscle invasive, HG bladder tumours (Fig 2).32
Figure 2.
Mutational profile of 131 individual cases of muscle invasive urothelial cell carcinoma (http://www.cbioportal.org/public-portal/)
It has also been demonstrated recently that urothelial cancer associated 1 ribonucleic acid (which actives WNT signalling in a WNT6 dependent manner) increases the cisplatin resistance of bladder cancer cells in vitro.33
Indeed, in our own studies, we have demonstrated that WNT signalling is activated in approximately a third of clinical UCC samples.34,35 A significant correlation was observed between activation of the WNT pathway and either phosphoinositide 3-kinase (PI3K) or mitogen activated protein kinase (MAPK) activation. Interestingly, both groups were mutually exclusive with almost no overlap between the WNT/PI3K and the WNT/MAPK tumours (p<0.0001), suggesting a different molecular basis for each tumour subtype.
Modelling mutations of the WNT pathway in mouse models
Our findings in the human tissue microarray led us to hypothesise that activation of WNT signalling could lead to human UCC but that it required activation of an additional second mitogenic pathway (either PI3K or MAPK), leading to distinct subsets of WNT driven tumours. Our investigation of the mechanistic basis of WNT pathway signalling in UCC prompted us to examine whether dysregulation of this pathway could induce disease in a murine model.
In the first instance, to activate Wnt signalling, we used transgenic mice that carry a dominant allele of the β-catenin gene in which exon 3 is flanked by loxP sequences.36 When a urothelial specific Cre recombinase (UPII-Cre) is added, exon 3 is deleted, stopping β-catenin from being targeted for degradation, allowing accumulation in the urothelial cells and thus activating the Wnt signalling in a bladder specific manner.37 Overexpression of nuclear β-catenin led to the formation of localised hyperproliferative lesions in the bladder epithelium by 3 months, which did not progress to malignancy despite the mice being aged to 18 months of age.34 Furthermore, the UPII-Cre+β-cateninexon3/+ mice showed a marked upregulation of the phosphatase and tensin homolog (Pten) tumour suppressor protein that appeared to be a direct consequence of activating Wnt signalling in the bladder, suggesting a potential compensatory mechanism that could prevent progression to invasive disease.
In order to investigate this potential block to tumourigenesis, we interbred the UPII-Cre+β-cateninexon3/+ mice with mice engineered to activate either the PI3K (Ptenfl/fl) or MAPK (HrasQ61L) pathways specifically in the urothelium. In both cases, this overcame the block to tumourigenesis and induced a non-invasive UCC phenotype in the murine bladders. The UPII-Cre+β-cateninexon3/+Ptenfl/fl tumours contained increased activation of the PI3K pathway and their growth was dependent on mechanistic target of rapamycin (mTOR) signalling (ie regression occurred with rapamycin treatment, an inhibitor of mTOR signalling). In direct contrast, the tumours in UPII-Cre+β-cateninexon3/+HrasQ61L mice, although phenotypically similar, were driven by MAPK signalling, with no activation of the PI3K pathway (ie regression occurred with mitogen activated protein kinase kinase inhibition, an inhibitor of MAPK signalling, but not with rapamycin).34,35
Consequently, we were able to demonstrate that by using transgenic models of either Wnt/PI3K or Wnt/MAPK pathway activation, we can induce non-invasive UCC in vivo, leading to tumours with differing molecular and treatment profiles (Fig 3). These tumours mirror their human counterparts with respect to pathway activation secondary to mutations associated with human UCC.
Figure 3.
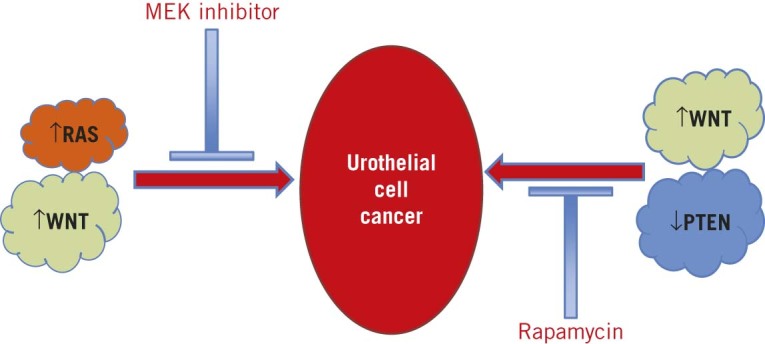
Divergent pathways of WNT driven urothelial cell carcinoma: Our work has suggested that Wnt activated/Pten inactivated tumours are non-invasive tumours that fill the bladders of the mice. They are dependent on mechanistic target of rapamycin (mTOR) signalling, as highlighted by responsiveness to the mTOR inhibitor rapamycin. By contrast, Wnt activated/Ras activated tumours are non-invasive tumours that are dependent on mitogen activated protein kinase signalling, as demonstrated by their responsiveness to MEK inhibition but not rapamycin treatment.
Summary and outlook
Despite transgenic models providing researchers with new insights into the molecular mechanisms in UCC, many limitations remain. First, recapitulating murine metastatic models of UCC continues to be challenging with a lack of spontaneous osseous lesions. The reasons for this could be multiple, including differences in the pelvic venous drainage of the mouse, our inability to detect small metastasis in mouse’s skeleton or the fact that the combinations of genetic defects are insufficient to initiate bone metastasis. In addition, one should not disregard the relatively short lifespan of the mouse, which together with the aggressive nature of the tumours we are engineering may not allow enough time for metastatic lesions to develop before the animals must be sacrificed owing to primary tumour burden.
Surprisingly, there remains a paucity of preclinical trials using these mouse models.35,38,39 As we discover additional biomarkers and ‘driver’ mutations through sequencing studies of human UCC, one would hope that we can develop new mouse models based on these findings.
An exciting development in the transgenic field involves using unbiased somatic mutagenesis to search for these ‘driver’ genes that contribute to the initiation and/or progression of UCC. These in vivo transposon-based methods could help us to identify biologically relevant genetic lesions in tumourigenesis, allowing identification of events that collaborate and/or synergise with the common mutations found in UCC.40,41
Despite the emergence of clinical biobanks, next-generation sequencing and other ‘omic’ approaches, the heterogeneity and multifocal nature of clinical UCC means that only limited numbers of these studies using clinical materials have produced mature data to inform on disease outcome in bacillus Calmette–Guérin and chemotherapy resistant disease, with large sample numbers required to give statistical significance. However, the information revealed by cancer genome sequencing has also highlighted the simplicity of currently available mouse models: it is no longer enough to compare a mouse model and a primary human tumour at the level of tissue and cellular phenotypes. Nevertheless, mouse models of UCC will continue to provide invaluable information on the biology of the disease, with the hope that this can be ultimately translated to earlier diagnosis and therefore better treatment of patients with UCC.
Given the critical roles of WNT pathway activation in the pathophysiology of many human diseases including bladder cancer, interest in the development of WNT signalling inhibitors has intensified (reviewed by Kahn, and Anastas and Moon).42,43 Different components of the WNT pathway can be targeted, with multiple agents (including small molecules, peptides and blocking antibodies) currently in clinical trials.
We have demonstrated that the WNT pathway is only active in a third of bladder cancers so patient stratification will be essential. Importantly, as with other cancers, inhibiting WNT signalling pathways in bladder cancer alone is unlikely to result in a substantial improvement in disease progression owing to the coactivation of additional oncogenic pathways. Studies aimed at identifying the genetic factors and biomarkers that can predict responses to treatment with WNT pathway modulators (either alone or in combination with other therapies) will be an important next step in determining the utility of these potential new therapies.
Acknowledgement
IA was funded by a Medical Research Council clinical research training fellowship and an Academy of Medical Sciences clinical lecturer starter grant. I am indebted to my supervisors, Professor Owen J Sansom and Professor Hing Y Leung, at the Beatson Institute.
References
- 1.Bladder Cancer Incidence Statistics. Cancer Research UK. http://www.cancerresearchuk.org/cancer-info/cancerstats/types/bladder/incidence/ (cited June 2015).
- 2.Falke J, Witjes JA. Contemporary management of low-risk bladder cancer. Nat Rev Urol 2011; : 42–49. [DOI] [PubMed] [Google Scholar]
- 3.Knowles MA. What we could do now: molecular pathology of bladder cancer.Mol Pathol 2001; : 215–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steinberg GD, Trump DL, Cummings KB. Metastatic bladder cancer. Natural history, clinical course, and consideration for treatment.Urol Clin North Am 1992; : 735–746. [PubMed] [Google Scholar]
- 5.Williams SG,Stein JP. Molecular pathways in bladder cancer. Urol Res 2004; : 373–385. [DOI] [PubMed] [Google Scholar]
- 6.Bladder Cancer Statistics and Outlook. Cancer Research UK. http://www.cancerresearchuk.org/about-cancer/type/bladder-cancer/treatment/bladder-cancer-statistics-and-outlook (cited June 2015). [Google Scholar]
- 7.Wu XR. Urothelial tumorigenesis: a tale of divergent pathways. Nat Rev Cancer 2005; : 713–725. [DOI] [PubMed] [Google Scholar]
- 8.Koss LG. Bladder cancer from a perspective of 40 years.J Cell Biochem Suppl 1992; : 23–29. [DOI] [PubMed] [Google Scholar]
- 9.Lauss M, Ringnér M, Höglund M.Prediction of stage, grade, and survival in bladder cancer using genome-wide expression data: a validation study.Clin Cancer Res 2010; :4,421–4,433. [DOI] [PubMed] [Google Scholar]
- 10.Lindgren D,Frigyesi A,Gudjonsson S , et al. Combined gene expression and genomic profiling define two intrinsic molecular subtypes of urothelial carcinoma and gene signatures for molecular grading and outcome. Cancer Res 2010; : 3,463–3,472. [DOI] [PubMed] [Google Scholar]
- 11.Lindgren D, Gudjonsson S,Jee KJ, et al. Recurrent and multiple bladder tumors show conserved expression profiles.BMC Cancer 2008; : 183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nüsslein-Volhard C,Wieschaus E. Mutations affecting segment number and polarity in Drosophila.Nature 1980; : 795–801. [DOI] [PubMed] [Google Scholar]
- 13.Polakis P. Wnt signaling and cancer.Genes Dev 2000; : 1,837–1,851. [PubMed] [Google Scholar]
- 14.Rubinfeld B,Souza B,Albert I, et al. Association of the APC gene product with beta-catenin.Science 1993; : 1,731–1,734. [DOI] [PubMed] [Google Scholar]
- 15.Cottrell S, Bicknell D,Kaklamanis L,Bodmer WF.Molecular analysis of APC mutations in familial adenomatous polyposis and sporadic colon carcinomas.Lancet 1992; : 626–630. [DOI] [PubMed] [Google Scholar]
- 16.Kinzler KW, Nilbert MC,Su LK, et al. Identification of FAP locus genes from chromosome 5q21.Science 1991; : 661–665. [DOI] [PubMed] [Google Scholar]
- 17.Urakami S, Shiina H,Enokida H, et al. Combination analysis of hypermethylated Wnt-antagonist family genes as a novel epigenetic biomarker panel for bladder cancer detection.Clin Cancer Res 2006; : 2,109–2,116. [DOI] [PubMed] [Google Scholar]
- 18.Stoehr R, Krieg RC,Knuechel R, et al. No evidence for involvement of beta-catenin and APC in urothelial carcinomas.Int J Oncol 2002; : 905–911. [PubMed] [Google Scholar]
- 19.Böhm M,Kirch H,Otto T, et al. Deletion analysis at the DEL-27, APC and MTS1 loci in bladder cancer: LOH at the DEL-27 locus on 5p13–12 is a prognostic marker of tumor progression.Int J Cancer 1997; : 291–295. [DOI] [PubMed] [Google Scholar]
- 20.Miyamoto H, Shuin T,Ikeda I, et al. Loss of heterozygosity at the p53, RB, DCC and APC tumor suppressor gene loci in human bladder cancer.J Urol 1996; : 1,444–1,447. [PubMed] [Google Scholar]
- 21.Burger M, van der Aa MN,van Oers JM, et al. Prediction of progression of non-muscle-invasive bladder cancer by WHO 1973 and 2004 grading and by FGFR3 mutation status: a prospective study.Eur Urol 2008; : 835–843. [DOI] [PubMed] [Google Scholar]
- 22.Shiina H,Igawa M,Shigeno K, et al.β-catenin mutations correlate with over expression of C-myc and cyclin D1 Genes in bladder cancer.J Urol 2002; :2,220–2,226. [DOI] [PubMed] [Google Scholar]
- 23.Shiina H, Igawa M,Urakami S, et al.Alterations of beta- and gamma-catenin in N-butyl-N-(-4-hydroxybutyl)nitrosamine-induced murine bladder cancer.Cancer Res 2001; :7,101–7,109. [PubMed] [Google Scholar]
- 24.Kastritis E,Murray S,Kyriakou F, et al.Somatic mutations of adenomatous polyposis coli gene and nuclear b-catenin accumulation have prognostic significance in invasive urothelial carcinomas: evidence for Wnt pathway implication.Int J Cancer 2009; : 103–108. [DOI] [PubMed] [Google Scholar]
- 25.Kashibuchi K,Tomita K,Schalken JA, et al.The prognostic value of E-cadherin, alpha-, beta-, and gamma-catenin in urothelial cancer of the upper urinary tract.Eur Urol 2006; : 839–845. [DOI] [PubMed] [Google Scholar]
- 26.Nakopoulou L,Zervas A,Gakiopoulou-Givalou H , et al.Prognostic value of E-cadherin, beta-catenin, P120ctn in patients with transitional cell bladder cancer.Anticancer Res 2000; :4,571–4,578. [PubMed] [Google Scholar]
- 27.Zhu X, Kanai Y,Saito A, et al.Aberrant expression of beta-catenin and mutation of exon 3 of the beta-catenin gene in renal and urothelial carcinomas.Pathol Int 2000; : 945–952. [DOI] [PubMed] [Google Scholar]
- 28.Garcia del Muro X,Torregrosa A,Muñoz J, et al.Prognostic value of the expression of E-cadherin and beta-catenin in bladder cancer. Eur J Cancer 2000; : 357–362. [DOI] [PubMed] [Google Scholar]
- 29.Shimazui T, Schalken JA,Giroldi LA , et al.Prognostic value of cadherin-associated molecules (alpha-, beta-, and gamma-catenins and p120cas) in bladder tumors.Cancer Res 1996; : 4,154–4,158. [PubMed] [Google Scholar]
- 30.Urakami S, Shiina H,Enokida H , et al.Epigenetic inactivation of Wnt inhibitory factor-1 plays an important role in bladder cancer through aberrant canonical Wnt/beta-catenin signaling pathway.Clin Cancer Res 2006; : 383–391. [DOI] [PubMed] [Google Scholar]
- 31.Marsit CJ,Karagas MR,Andrew A, et al.Epigenetic inactivation of SFRP genes and TP53 alteration act jointly as markers of invasive bladder cancer.Cancer Res 2005; :7,081–7,085. [DOI] [PubMed] [Google Scholar]
- 32.Cancer Genome Atlas Research Network. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature 2014; : 315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fan Y,Shen B, Tan M , et al. Long non-coding RNA UCA1 increases chemoresistance of bladder cancer cells by regulating Wnt signaling. FEBS J 2014; : 1,750–1,758. [DOI] [PubMed] [Google Scholar]
- 34.Ahmad I,Morton JP, Singh LB et al. . β-Catenin activation synergizes with PTEN loss to cause bladder cancer formation. Oncogene 2011; : 178–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahmad I,Patel R, Liu Y et al. . Ras mutation cooperates with β-catenin activation to drive bladder tumourigenesis. Cell Death Dis 2011; : e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harada N,Tamai Y,Ishikawa T et al.. Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene.EMBO J 1999; : 5,931–5,942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moon RT,Kohn AD, De Ferrari GV, Kaykas A. . WNT and beta-catenin signalling: diseases and therapies. Nat Rev Genet 2004; : 691–701. [DOI] [PubMed] [Google Scholar]
- 38.Seager CM,Puzio-Kuter AM, Patel T et al. . Intravesical delivery of rapamycin suppresses tumorigenesis in a mouse model of progressive bladder cancer. Cancer Prev Res 2009; :1,008–1,014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kinkade CW,Castillo-Martin M, Puzio-Kuter A et al. . Targeting AKT/mTOR and ERK MAPK signaling inhibits hormone-refractory prostate cancer in a preclinical mouse model.J Clin Invest 2008; : 3,051–3,064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Collier LS,Carlson CM, Ravimohan S et al. . Cancer gene discovery in solid tumours using transposon-based somatic mutagenesis in the mouse. Nature 2005; : 272–276. [DOI] [PubMed] [Google Scholar]
- 41.Dupuy AJ,Akagi K, Largaespada DA , et al. Mammalian mutagenesis using a highly mobile somatic Sleeping Beauty transposon system. Nature 2005; : 221–226. [DOI] [PubMed] [Google Scholar]
- 42.Kahn M. Can we safely target the WNT pathway? Nat Rev Drug Discov 2014; : 513–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anastas JN,Moon RT. . WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer 2013; : 11–26. [DOI] [PubMed] [Google Scholar]
- 44.Zhang ZT,Pak J,Huang HY et al. . Role of Ha-ras activation in superficial papillary pathway of urothelial tumor formation. Oncogene 2001; : 1,973–1,980. [DOI] [PubMed] [Google Scholar]
- 45.Cheng J,Huang H, Zhang ZT , et al. Overexpression of epidermal growth factor receptor in urothelium elicits urothelial hyperplasia and promotes bladder tumor growth. Cancer Res 2002; : 4,157–4,163. [PubMed] [Google Scholar]
- 46.Ahmad I,Singh LB, Foth M et al. . K-Ras and β-catenin mutations cooperate with Fgfr3 mutations in mice to promote tumorigenesis in the skin and lung, but not in the bladder. Dis Model Mech 2011; : 548–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Foth M,Ahmad I, van Rhijn BW et al. . Fibroblast growth factor receptor 3 activation plays a causative role in urothelial cancer pathogenesis in cooperation with Pten loss in mice. J Pathol 2014; : 148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rampias T,Vgenopoulou P, Avgeris M , et al. A new tumor suppressor role for the Notch pathway in bladder cancer. Nat Med 2014; : 1,199–1,205. [DOI] [PubMed] [Google Scholar]
- 49.Gao J,Huang HY, Pak J et al.. p53 deficiency provokes urothelial proliferation and synergizes with activated Ha-ras in promoting urothelial tumorigenesis. Oncogene 2004; : 687–696. [DOI] [PubMed] [Google Scholar]
- 50.Ayala de la Peña F,Kanasaki K, Kanasaki M , et al.Loss of p53 and acquisition of angiogenic microRNA profile are insufficient to facilitate progression of bladder urothelial carcinoma in situ to invasive carcinoma. J Biol Chem 2011; : 20,778–20,787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Puzio-Kuter AM, Castillo-Martin M, Kinkade CW , et al.Inactivation of p53 and Pten promotes invasive bladder cancer. Genes Dev 2009; : 675–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yoo LI.Liu DW, Le Vu S , et al.Pten deficiency activates distinct downstream signaling pathways in a tissue-specific manner. Cancer Res 2006; : 1,929–1,939. [DOI] [PubMed] [Google Scholar]
- 53.Tsuruta H,Kishimoto H, Sasaki T , et al.Hyperplasia and carcinomas in Pten-deficient mice and reduced PTEN protein in human bladder cancer patients. Cancer Res 2006; : 8,389–8,396. [DOI] [PubMed] [Google Scholar]
- 54.Qian CN.Furge KA, Knol J , et al.Activation of the PI3K/AKT pathway induces urothelial carcinoma of the renal pelvis: identification in human tumors and confirmation in animal models. Cancer Res 2009; : 8,256–8,264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.He F.Mo L, Zheng XY , et al.Deficiency of pRb family proteins and p53 in invasive urothelial tumorigenesis. Cancer Res 2009; : 9,413–9,421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang ZT.Pak J, Shapiro E , et al.Urothelium-specific expression of an oncogene in transgenic mice induced the formation of carcinoma in situ and invasive transitional cell carcinoma. Cancer Res 1999; : 3,512–3,517. [PubMed] [Google Scholar]
- 57.Ahmad I.Sansom OJ, Leung HY.Exploring molecular genetics of bladder cancer: lessons learned from mouse models. Dis Model Mech 2012; : 323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]