Abstract
Chondrogenic polypeptide growth factors influence articular chondrocyte functions that are required for articular cartilage repair. Sox9 is a transcription factor that regulates chondrogenesis, but its role in the growth factor regulation of chondrocyte proliferation and matrix synthesis is poorly understood. We tested the hypotheses that selected chondrogenic growth factors regulate sox9 gene expression and protein production by adult articular chondrocytes and that sox9 modulates the actions of these growth factors. To test these hypotheses, we delivered insulin-like growth factor-I (IGF-I), fibroblast growth factor-2 (FGF-2), bone morphogenetic protein-2 (BMP-2) and/or bone morphogenetic protein-7 (BMP-7), or their respective transgenes to adult bovine articular chondrocytes, and measured changes in sox9 gene expression and protein production. We then knocked down sox9 gene expression with sox9 siRNA, and measured changes in the expression of the genes encoding aggrecan and types I and II collagen, and in the production of glycosaminoglycan, collagen and DNA. We found that FGF-2 or the combination of IGF-I, BMP-2, and BMP-7 increased sox9 gene expression and protein production and that sox9 knockdown modulated growth factor actions in a complex fashion that differed both with growth factors and with chondrocyte function. The data suggest that sox9 mediates the stimulation of matrix production by the combined growth factors and the stimulation of chondrocyte proliferation by FGF-2. The mitogenic effect of the combined growth factors and the catabolic effect of FGF-2 appear to involve sox9-independent mechanisms. Control of these molecular mechanisms may contribute to the treatment of cartilage damage.
Keywords: sox9, growth factors, articular chondrocytes, small inhibitory RNA
Articular cartilage provides a gliding surface that enables pain-free motion of diarthrodial joints. Articular cartilage formation, maintenance, and repair depend on the chondrocytes embedded in the cartilage extracellular matrix. These cells have a poor intrinsic healing capability after cartilage damage or loss from injury or osteoarthritis [Buckwalter and Mankin, 1998]. As a result, the tissue loss is generally persistent and progressive. Polypeptide growth factors play a central role in articular cartilage function [Trippel, 1995]. Several growth factors have been identified that promote mitogenic and anabolic activities by articular chondrocytes. These growth factors include insulin-like growth factor-I (IGF-I), fibroblast growth factor-2 (FGF-2), bone morphogenetic protein-2 (BMP-2) and/or bone morphogenetic protein-7 (BMP-7). IGF-I is considered a candidate for articular cartilage repair because it stimulates both cell proliferation and the synthesis of the key cartilage matrix components, aggrecan and type II collagen [Luyten et al., 1988; Sah et al., 1994; Fortier et al., 2002; Loeser et al., 2003]. FGF-2 is a potent mitogen for chondrocytes and modulates both chondrocyte anabolic and catabolic activities [Bradham et al., 1994; Sah et al., 1994; Fujimoto et al., 1999; Madry et al., 2004; Henson et al., 2005; Im et al., 2007; Shi et al., 2012]. BMP-2 [Sailor et al., 1996; Grunder et al., 2004] and BMP-7 [Flechtenmacher et al., 1996; Chubinskaya et al., 2007] each promote chondrocyte matrix synthesis. All these factors have been shown to augment articular cartilage repair in ex vivo and in vivo models [Madry et al., 2001, 2005; Yokoo et al., 2005; Goodrich et al., 2007].
Sox9 is a master transcription factor that is required for cartilage formation during embryogenesis [Akiyama and Lefebvre, 2011] and contributes to articular cartilage development postnatally [Henry et al., 2012]. The sox9 gene (sox9) is expressed in chondroprogenitor cells in mouse embryos [Wright et al., 1995] and mouse sox9−/− embryonic stem cells are unable to differentiate into chondrocytes [Bi et al., 1999]. Expression of sox9 is detected in mouse articular cartilage up to nine months of age and sox9 protein up to 15 months [Salminen et al., 2001] but its gene expression is completely shut off in mature hypertrophic chondrocytes [de Crombrugghe et al., 2000]. Sox9 is essential for the expression of the type II collagen gene (col2α1) [Bell et al., 1997; Zhou et al., 1998], a marker of the chondrocyte phenotype. In human articular chondrocytes, transfer of the human sox9 gene (SOX9) increased the synthesis of type II collagen and proteoglycan [Cucchiarini et al., 2007], and both the rate and capacity of the synthesis of proteoglycan glycosaminoglycans [Tew et al., 2008].
Despite the shared roles of chondrogenic growth factors and sox9 on cartilage, few studies have investigated their potential interdependence, particularly in adult articular chondrocytes. Some of the growth factors that regulate chondrocyte activities also regulate sox9. IGF-I has been shown to increase SOX9 expression during redifferentiation of dedifferentiated articular chondrocytes and to up-regulate SOX9 expression [Shakibaei et al., 2006]. In adult canine articular chondrocytes, IGF-I increased sox9 protein production and countered the inhibitory effect of IL-1 on type II collagen production [Montaseri et al., 2011]. Recently, IGF-I-mediated COL2α1 expression in human articular chondrocytes has been shown to be associated with increased binding of sox9 to its cis element in a COL2α1 enhancer [Renard et al., 2012]. FGF-2 has been reported to increase sox9 expression in mouse primary costal chondrocytes and to increase the activity of a sox9-dependent chondrocyte-specific enhancer in col2α1 [Murakami et al., 2000]. When human osteoarthritic chondrocytes were treated with the sox9 and/or the FGF-2 transgenes, the combination preserved the mitogenic properties of FGF-2 alone and added the anabolic effects of sox9 alone [Cucchiarini et al., 2009]. BMP-2 has been found to stimulate sox9 expression through a CCAAT box in the sox9 promoter region in mouse embryonic fibroblasts during BMP-2 induced differentiation to chondrocytes [Pan et al., 2008]. To our knowledge, the role, if any, of sox9 in mediating the diverse actions of the growth factors that regulate articular chondrocytes remains unknown.
In prior studies, we found that delivery of the genes encoding IGF-I, FGF-2, BMP-2, and BMP-7, individually and in combination, to adult articular chondrocytes both differentially and similarly regulated chondrocyte reparative activities [Sailor et al., 1996; Shi et al., 2012, 2013]. These differences and similarities were particularly marked between the FGF-2 transgene and the combination of the IGF-I, BMP-2, and BMP-7 transgenes. Specifically, overexpression of FGF-2 inhibited, and the combined overexpression of IGF-I, BMP-2, and BMP-7 stimulated, type II collagen gene expression and protein production. Both treatments augmented aggrecan gene expression, but only overexpression of IGF-I, BMP-2, and BMP-7 increased its deposition as matrix. Both treatments similarly stimulated chondrocyte proliferation [Shi et al., 2013]. Due to the separation and overlap of their actions, FGF-2 and the combination of IGF-I, BMP-2, and BMP-7 were chosen to test the hypothesis that sox9 is involved in determining the specific actions of these growth factors. We found that sox9 plays widely divergent roles in mediating the growth factor regulation of articular chondrocytes.
MATERIALS AND METHODS
CHONDROCYTE CULTURE
Dulbecco’s minimum essential medium (DMEM), fetal bovine serum (FBS), penicillin, streptomycin and glutamine were from Life Technologies (Carlsbad, CA). Ascorbic acid and bovine serum albumin (BSA) were from Sigma (Saint Louis, MO). Basal medium was prepared with DMEM, 50 µg/ml ascorbic acid, 100 U/ml penicillin, 100 µg/ml streptomycin and 2mM glutamine. Complete medium was prepared with basal medium supplemented with 10% FBS. Recombinant human IGF-I was from Peprotech, Inc. (Rocky Hill, NJ). Recombinant human FGF-2, BMP-2 and BMP-7 were from R&D Systems (Minneapolis, MN). Articular chondrocytes from skeletally mature (physes closed) bovids were isolated and placed in primary culture as previously described [Shi et al., 2012].
DELIVERY OF GROWTH FACTOR TRANSGENES
Growth factor transgenes were delivered using the plasmid vectors pAAV-FGF-2, pAAV-IGF-I, pAAV-BMP-2, and pAAV-BMP-7 as previously described [Shi et al., 2012]. To improve the readability of the data presented, the transgenes carried by these vectors are designated tFGF-2, tIGF-I, tBMP-2, and tBMP-7 respectively. After 3 days in culture, chondrocytes were transfected using FuGENE 6 (Roched Applied Science) and pAAV plasmid DNA expressing the designated growth factors. Empty vector pAAV-MCS was used as control. For single transfections, 2 µg of plasmid DNA per well was used. For two- and three-transgene combinations, 2 µg of each plasmid DNA per well was used together. After 4–6 h, transfection was stopped by replacing the medium with 4ml fresh complete medium. On days 2 and 4 after transfection, conditioned medium (CM) was collected and replaced by 4ml basal medium. On day 6, CM was collected, and cell culture was terminated and cell layer was harvested.
DELIVERY OF SOX9 SIRNA AND EXOGENOUS GROWTH FACTOR PROTEINS
Bovine sox9 siRNA (ON-TARGETplus Custom SMARTpool) was synthesized by Dharmacon (Table I). To determine whether this sox9 siRNA efficiently silences sox9 expression, adult bovine articular chondrocytes were transfected with sox9 siRNA or non-targeting siRNA (Dharmacon) according to manufacturer’s instructions. After 3 days in culture, the medium was replaced with 2ml complete medium and cells were transfected using 4 µl/well DharmaFECT3 (Dharmacon) and 10 µl of 20 µM sox9 siRNA. ON-TARGETplus Non-targeting siRNA was used for mock-transfection. After 16 h, siRNA transfection was stopped by replacing the medium with 4ml of fresh complete medium supplemented with the designated growth factor(s), 50 ng/ml FGF-2 or the combination of 200 ng/ml IGF-I with 100 ng/ml BMP-2 and 100 ng/ml BMP-7. Fresh complete medium without growth factors was used as control. On days 2 and 4 after transfection, conditioned medium was harvested and replaced with basal medium containing 0.1% BSA and the designated growth factor(s) as described above. Basal medium containing 0.1% BSA without growth factors was used as control. On day 6 after transfection, conditioned medium and cell layer were harvested separately.
TABLE I.
siRNAs and Primers and Probes for Real Time PCR
| Gene | ACC. No. | siRNA, primer or probe | Sequence |
|---|---|---|---|
| Sox9 | AF278703 | siRNA S1 | 5′-GCGTCAACGGCTCGAGCAA-3′ |
| siRNA S2 | 5′-TGACCGACGAGCAGGAGAA-3′ | ||
| siRNA S3 | 5′-GGAAGTCGGTGAAGAACGG-3′ | ||
| siRNA S4 | 5′-GACTGCTGAACGAGAGCGA-3′ | ||
| Not applicable | Not applicable | Non-targeting siRNA | 5′-TGGTTTACATGTCGACTAA-3′ |
| Sox9 | AF278703 | Forward primer | 5′-AATCTCCTGGACCCCTTCATG-3′ |
| Reverse primer | 5′-GGCGGACAGGCCCTTCT-3′ | ||
| Probe | 5′-AGATGACCGACGAGCAG-3′ | ||
| Col1α1 | NM_001034039 | Forward primer | 5′-AAAAGAGGCACGTCTGGTAC-3′ |
| Reverse primer | 5′-CGGATTCCAGTTCGAGTATGGCGG-3′ | ||
| Probe | 5′-GTGGTAGGTGATGTTCTGGG-3′ |
PROTEIN SAMPLE PREPARATION AND WESTERN BLOTTING
The cell layer was harvested in 0.5 ml lysis buffer [20mMTris (pH 7.6), 120 mM NaCl, 10 mM EDTA, 10% glycerol, 1% NP-40, 100 mM NaF, 10 mM Na4P2O7, 1 mM PMSF, 2 mM Na3VO4, 40 µg/ml leupeptin, 1 µM pepstatin A, and 10 µg/ml aprotinin]. Cell lysates were sonicated on ice and protein concentration was determined by Bio-Rad Protein Assay (Bio-Rad). Cell lysates containing 20 µg protein were electrophoresed on a 10% SDS–polyacrylamide gel under reducing condition, and the proteins were transferred to nitrocellulose membranes. The membranes were blocked with 5% fat-free milk (Bio-Rad) in Tris-buffered saline/Tween (TBST) for 1 h at room temperature and were then separately probed with primary antibodies to sox9 (R&D Systems) and β-actin (Sigma) overnight at 4°C.
RNA ISOLATION AND REAL-TIME PCR ANALYSIS
Total RNA was prepared from the chondrocyte cell layer using the RNeasy Mini kit (Qiagen, MD) and real-time PCR was performed as previously described [Shi et al., 2012]. Briefly, sox9 mRNA, aggrecan mRNA, col1α1 mRNA, col1α2 mRNA, col2α1 mRNA, and 18S rRNA were measured using a Prism 7000 Sequence Detector System and TaqMan Universal Master Mix (Applied Biosystems, Foster City, CA). Primers and probes for aggrecan mRNA, col1α2 mRNA, col2α1 mRNA, and 18S rRNA analysis were previously described [Shi et al., 2012]. Primers and probes for sox9 and col1α1 mRNA analysis were synthesized by Invitrogen and Applied Biosystems respectively (Table I). Target gene mRNA levels were normalized to 18S rRNA levels. Changes of target gene expression are expressed as the ratio of growth factor gene transfected cells to cells transfected with empty vector (mock-transfected control) or as the ratio of growth factor treatment and or sox9 siRNA transfected cells to the cells tranfected by non-targeting siRNA. Three independent experiments were performed using different batches of articular chondrocytes obtained from different bovine joints at different times. Data are presented as the average of fold changes.
GLYCOSAMINOGLYCAN, COLLAGEN AND DNA ANALYSIS
Glycosaminoglycan (GAG) and collagen were measured by dimethylmethlyene blue assay and hydroxyproline assay respectively, and DNA content as an index of cell proliferation was assessed by Picogreen dsDNA assay (Invitrogen) as previously described [Shi et al., 2013]. GAG and collagen in the medium (released) and retained with the chondrocytes (cell-associated) were analyzed separately [Shi et al., 2013].
RESULTS
EFFECT OF GROWTH FACTOR TRANSGENES ON SOX9 GENE EXPRESSION AND PROTEIN PRODUCTION
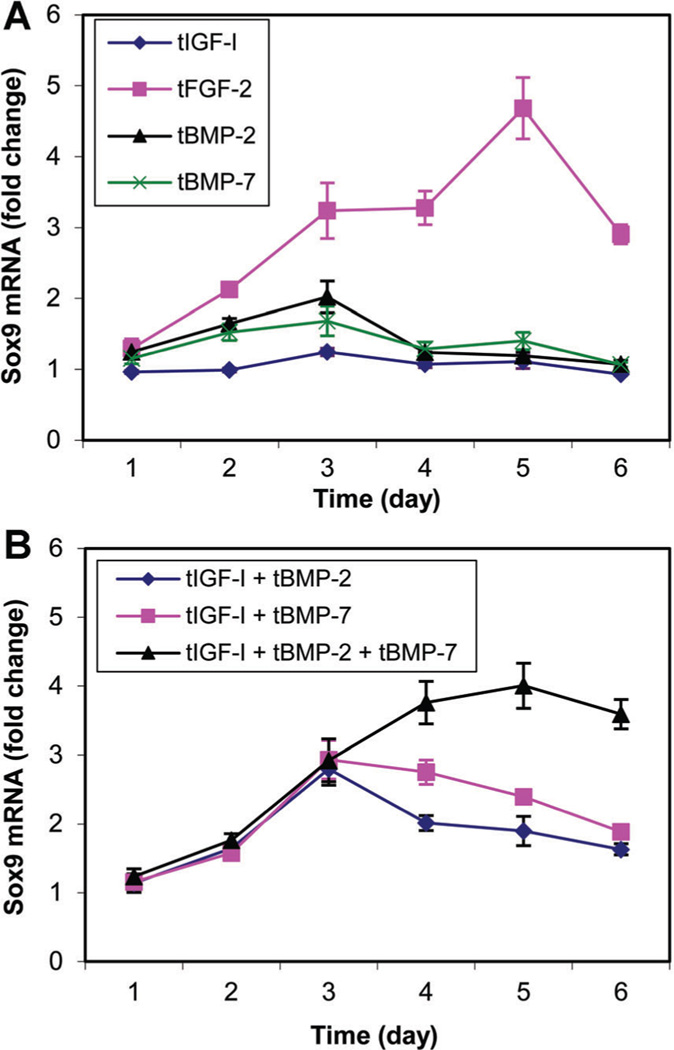
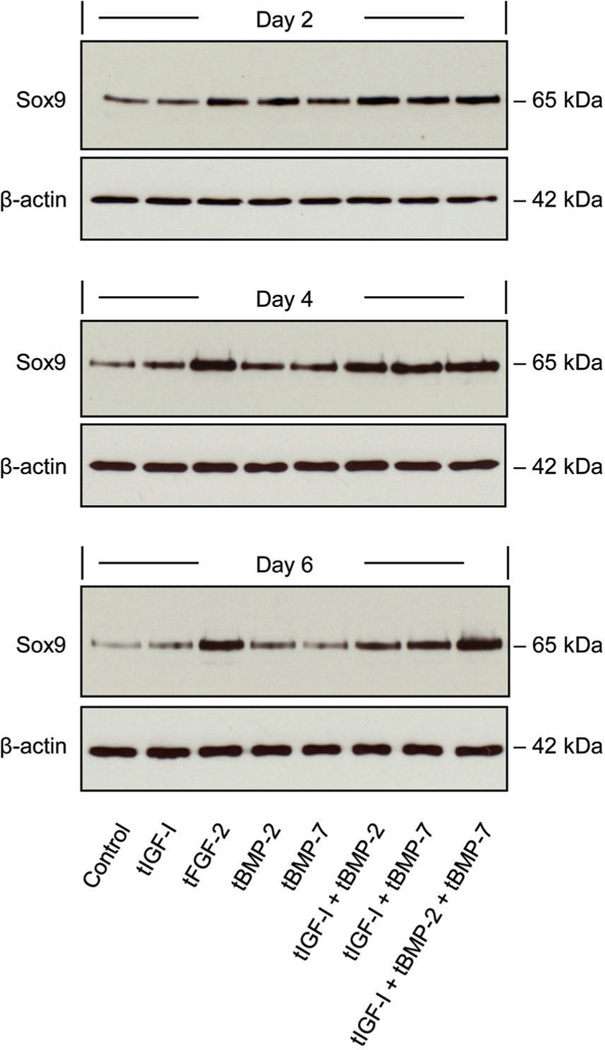
Delivery of tFGF-2 or [tIGF-I + tBMP-2 + tBMP-7] increased sox9 expression to a greater degree than the other individual growth factors or growth factor combinations tested (Fig. 1). Growth factor transgene regulation of sox9 protein production closely corresponded to sox9 expression (Fig. 2). These findings supported the selection of these two treatments for further investigation.
Fig. 1.
Sox9 mRNA changes in response to the designated growth factor transgenes (A) or transgene combinations (B). Transfected chondrocytes were cultured for the designated time periods. Sox9 mRNA was normalized to 18S RNA. Data are expressed as sox9 mRNA fold change to the respective control for each time point ± SD for three independent experiments. Maximal stimulation was 4.68-fold (P = 0.0046) and 4.00-fold (P = 0.0039) by tFGF-2 and [tIGF-I + tBMP-2 + tBMP-7, respectively.
Fig. 2.
Western blot of sox9 protein in response to the designated individual and combinations of growth factor transgenes. Cell lysate was prepared from transfected chondrocytes following culture for the designated time periods. Samples were separately probed for β-actin.
EFFECT OF SOX9 SIRNA ON GROWTH FACTOR REGULATION OF SOX9 GENE EXPRESSION
Growth factor proteins, rather than growth factor transgenes were selected for sox9 knockdown studies because the sox9 siRNA and growth factor transgenes required different transfection reagents, and we found that these failed to achieve satisfactory transfection efficiency when used in combination (data not shown).
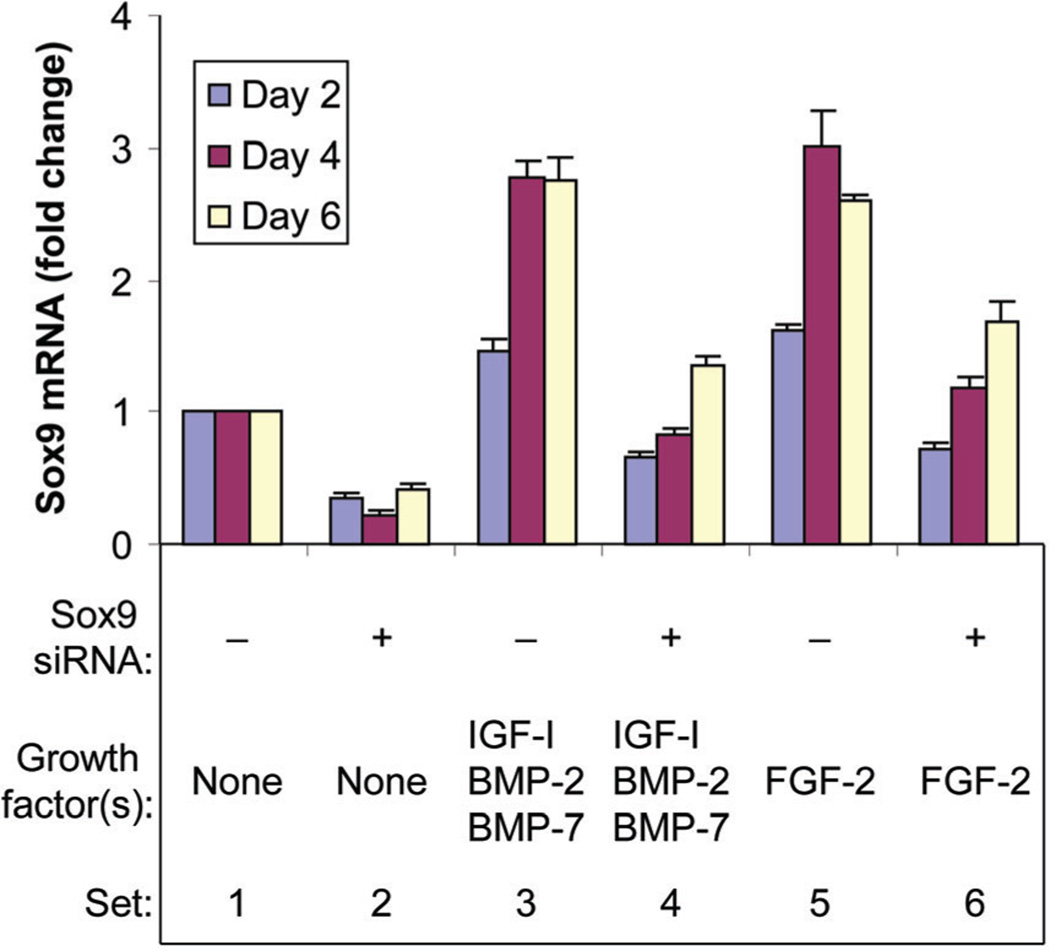
Chondrocytes were treated with or without FGF-2 or [IGF-I + BMP-2 + BMP-7] in the presence or absence of sox9 siRNA, and sox9 mRNA levels were quantified 2, 4, and 6 days following treatment. In the absence of growth factor(s), sox9 siRNA reduced sox9 mRNA levels compared to non-targeting siRNA. FGF-2 stimulated sox9 expression at all time points and sox9 siRNA reduced these augmented sox9 mRNA levels. The combination [IGF-I + BMP-2 + BMP-7] also stimulated sox9 expression at all time points and sox9 siRNA similarly reduced these augmented sox9 mRNA levels (Fig. 3). These data indicate that sox9 siRNA efficiently knocked down sox9 gene expression in chondrocytes with and without stimulation by FGF-2 or [IGF-I + BMP-2 + BMP-7].
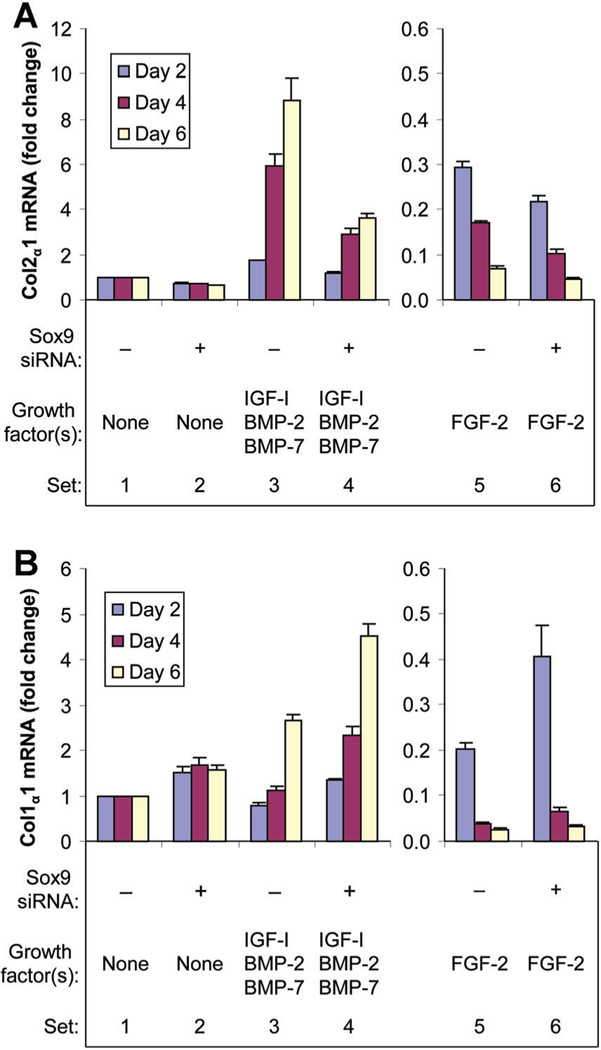
Fig. 3.
Knockdown of sox9 gene expression by sox9 siRNA. Chondrocytes were transfected by sox9 siRNA (+) or by non-targeting siRNA (−) and treated with no growth factor (None), FGF-2 or the combination of IGF-I, BMP-2, and BMP-7 proteins, then cultured for the designated time periods. Sox9 mRNA was normalized to 18S RNA. Data are expressed as sox9 mRNA change compared to the respective control for each time point ± SD for three independent experiments. Comparison groups are illustrated as data sets. Ranges in values are over the three time points. Sets 1 versus 2: range 59–77% (all P ≤ 0.0027). Sets 1 versus 3: range 1.47- to 2.77-fold (all P ≤ 0.0106). Sets 3 versus 4: range 51–70%, (all P < 0.0001). Sets 1 versus 5: range 1.61- to 3.02-fold (all P ≤ 0.0054), Sets 5 versus 6: range 35–61%, (all P < 0.0001).
SOX9 KNOCKDOWN MODULATES GROWTH FACTOR REGULATION OF CHONDROCYTE MATRIX GENE EXPRESSION
Aggrecan gene expression
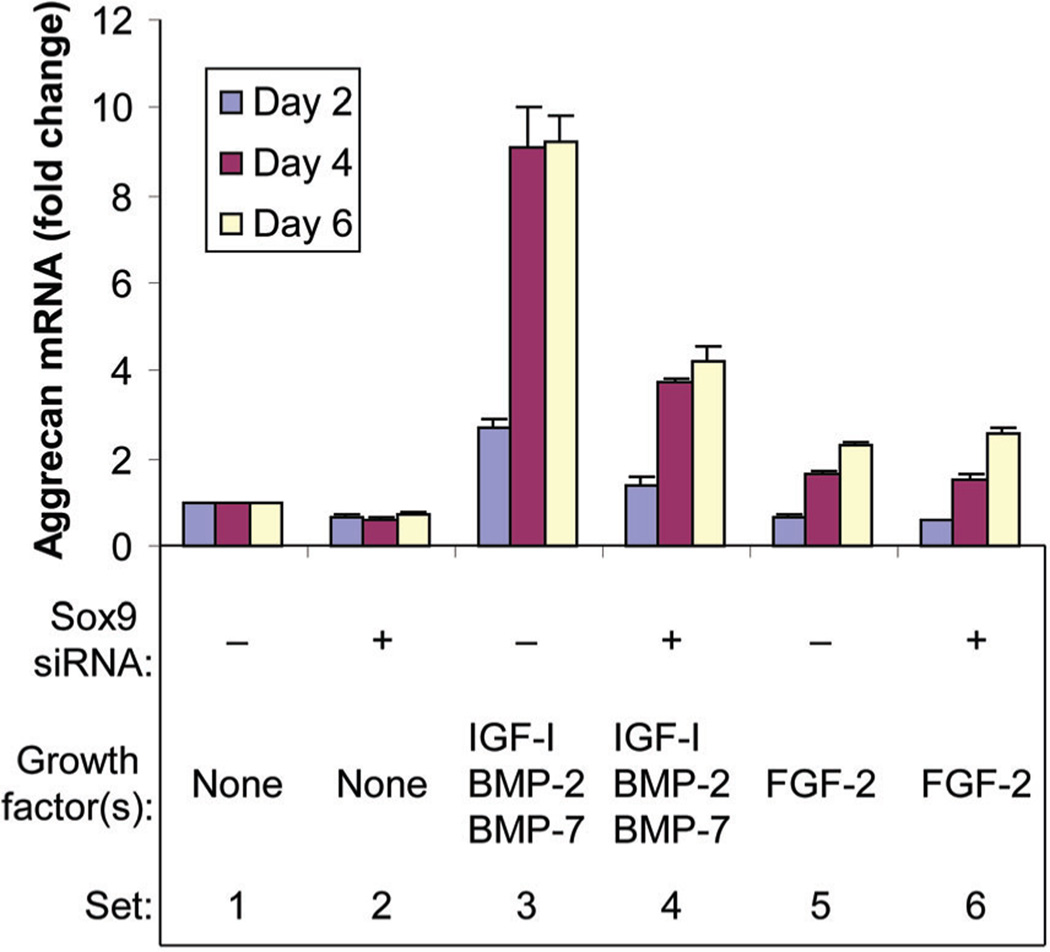
In the absence of growth factor(s), sox9 knockdown inhibited aggrecan gene (acan) expression at all time points. FGF-2 increased acan expression over time, but sox9 knockdown had minimal effect on this FGF-2 stimulation. The combination [IGF-I + BMP-2 + BMP-7] progressively stimulated acan expression and sox9 knockdown reduced this augmented expression at all time points (Fig. 4).
Fig. 4.
Effect of sox9 knockdown on aggrecan gene (acan) expression. Chondrocytes were transfected by sox9 siRNA (+) or by non-targeting siRNA (−) and treated with no growth factor (None), FGF-2 or the combination of IGF-I, BMP-2 and BMP-7 proteins, then cultured for the designated time periods. Aggrecan mRNA was normalized to 18S RNA. Data are expressed as sox9 mRNA change compared to the respective control for each time point ± SD for three independent experiments. Comparison groups are illustrated as data sets. Ranges in values are over the three time points. Sets 1 versus 2: range 29–38%, (all P < 0.012). Sets 1 versus 3: range 2.72- to 9.23-fold (P ≤ 0.0040). Sets 3 versus 4: range 49–59% (all P ≤ 0.0001). Sets 1 versus 5: 32% (P = 0.0062) at day 2, 1.68-fold (P = 0.0016) at day 4 to 2.33-fold (P = 0.0007) at day 6. Sets 5 versus 6: range 16%, (P = 0.0015) at day 2, no significant difference (NS) at day 4 or day 6.
Collagen gene expression
Col2α1 expression was inhibited by sox9 knockdown at all time points in the absence of growth factor(s). FGF-2 progressively decreased col2α1 expression and sox9 knockdown further decreased this col2α1 expression. In contrast to FGF-2, [IGF-I + BMP-2 + BMP-7] progressively stimulated col2α1 expression. As for FGF-2, sox9 knockdown progressively decreased this col2α1 expression (Fig. 5A). Col1α1 expression was stimulated by sox9 knockdown at all time points in the absence of growth factor(s). FGF-2 decreased col1α1 expression and sox9 siRNA partly reversed this inhibition at all time points. The combination [IGF-I + BMP-2 + BMP-7] initially decreased col1α1 expression and then progressively increased it over time. Sox9 siRNA further stimulated this col1α1 expression at all time points (Fig. 5B). Regulation of col1α2 was similar to that of col1α1 (Supplemental Fig. S1).
Fig. 5.
Effect of sox9 knockdown on type II collagen gene (col2α1) A: and type I collagen α1 gene (col1α1) B: expression. Chondrocytes were transfected by sox9 siRNA (+) or by non-targeting siRNA (−) were treated with no growth factor (None), FGF-2 or the combination of IGF-I, BMP-2 and BMP-7 proteins, then cultured for the designated time periods. Col2α1 mRNA and col1α1 mRNA were normalized to 18S RNA respectively. Data are expressed as col2α1 mRNA (A) and col1α1 mRNA (B) changes compared to the respective control for each time point ± SD for three independent experiments. Comparison groups are illustrated as data sets. Ranges in values are over the three time points. A: Sets 1 versus 2: range 29–37%, (all P ≤ 0.0097). Sets 1 versus 3: range 1.76-fold (P = 0.0018) to 8.85-fold (P = 0.0050). Sets 3 versus 4: range 32% (P < 0.0001) to 59% (P < 0.0001). Sets 1 versus 5: range 71% (P = 0.0001) to 93% (P ≤ 0.0001). Sets 5 versus 6: range 25–41% (all P ≤ 0.0001). B: Sets 1 versus 2: range 1.49- to 1.68-fold (all P ≤ 0.0238). Sets 1 versus 3: range 22% (P = 0.0222) at day 2, NS at day 4 to 2.67-fold (P = 0.0015) at day 6. Sets 3 versus 4: range 1.70- to 2.09-fold (all P < 0.0001). Sets 1 versus 5: range 80% (P = 0.0001) to 97% (P < 0.0001). Sets 5 versus 6: range 1.21- to 2.00-fold (all P ≤ 0.0147).
SOX9 KNOCKDOWN MODULATES GROWTH FACTOR REGULATION OF CHONDROCYTE EXTRACELLULAR MATRIX PRODUCTION
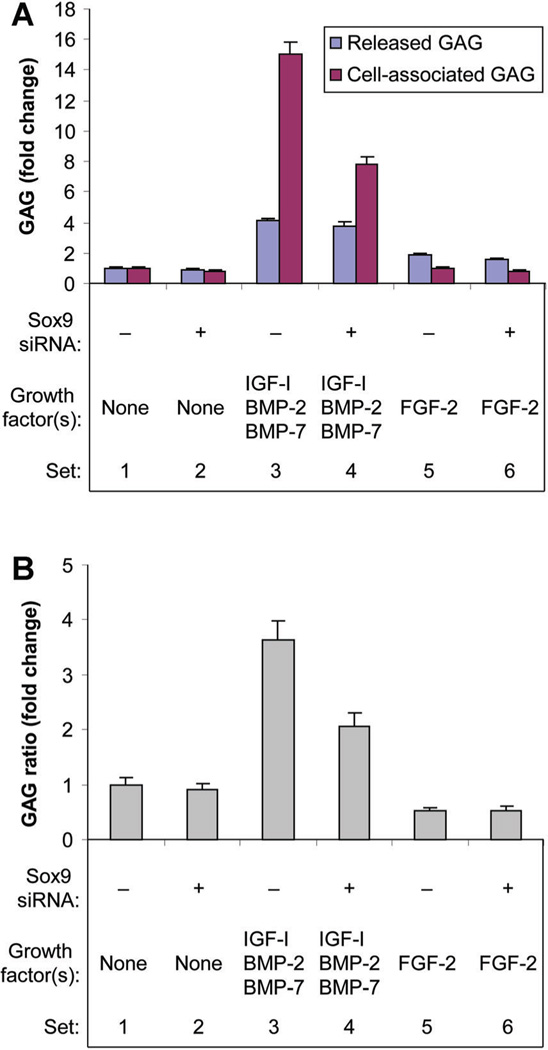
Glycosaminoglycan production
In the absence of growth factor(s), sox9 knockdown did not significantly affect released GAG production, and only modestly inhibited cell-associated GAG production. FGF-2 stimulated released GAG, but did not change cell-associated GAG. In the presence of FGF-2, sox9 knockdown inhibited both forms of GAG. In contrast, [IGF-I + BMP-2 + BMP-7] stimulated both forms of GAG and this effect was greater for cell-associated (15.00-fold) than for released (4.14-fold) GAG. Sox9 knockdown did not change the effect of [IGF-I + BMP-2 + BMP-7] on released GAG, but reduced the stimulation of cell associated GAG (Fig. 6A). To quantify the effect of these treatments on the fate of the GAG produced by the cells, the ratio of cell-associated to released GAG (GAG distribution ratio) was calculated for each treatment condition. These results demonstrate that, although FGF-2 increased total GAG production, it decreased the proportion of GAG that led to matrix formation. In contrast, [IGF-I + BMP-2 + BMP-7] increased both total GAG production and the GAG distribution ratio (Fig. 6B). Sox9 knockdown did not influence the action of FGF-2, but decreased the GAG distribution ratio in the presence of [IGF-I + BMP-2 + BMP-7].
Fig. 6.
Effect of sox9 knockdown on glycosaminoglycan production (A) and glycosaminoglycan distribution between the medium and the cells (B). Chondrocytes were transfected by sox9 siRNA (+) or by non-targeting siRNA (−) and treated with no growth factor (None), FGF-2 or the combination of IGF-I, BMP-2, and BMP-7 proteins. Culture medium was harvested at the designated time points and the cells harvested at 6 days. Glycosaminoglycan in the medium (released GAG) and cell layer (cell-associated GAG) were measured separately. Data are expressed as changes in released GAG and cell-associated GAG (A), and change in the ratio of cell-associated GAG to released GAG (B) for the treatment group compared to the respective control (GAG distribution ratio) ± SD for three independent experiments. Comparison groups are illustrated as data sets. A: Sets 1 versus 2: released GAG (NS), cell-associated GAG 18% (P = 0.0067). Sets 1 versus 3: released GAG 4.14-fold (P < 0.0001), cell associated GAG 15.00-fold (P < 0.0001). Sets 3 versus 4: released GAG (NS), cell associated GAG 48% (P < 0.0001). Sets 1 versus 5: released GAG 1.91-fold (P < 0.0001), cell-associated GAG (NS). Sets 5 versus 6: released GAG 18% (P = 0.0018), cell-associated GAG 17% (P = 0.0091). B: Sets 1 versus 2: NS. Sets 1 versus 3: 3.63-fold (P < 0.0001). Sets 3 versus 4: 43% (P < 0.0001). Sets 1 versus 5: 47% (P < 0.0001). Sets 5 versus 6: NS.
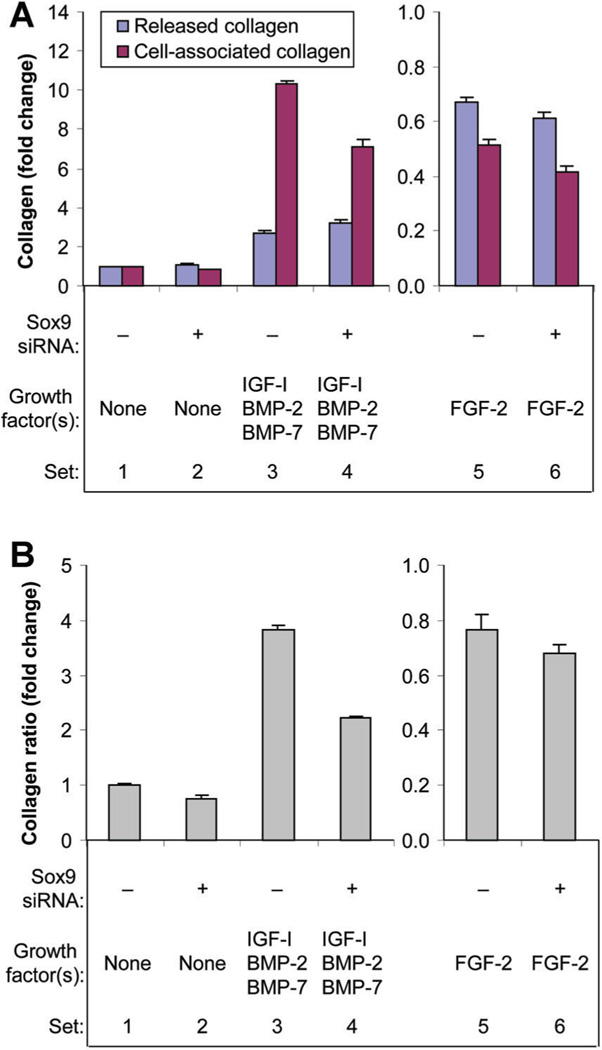
Collagen production
In absence of growth factor(s), sox9 knockdown stimulated released collagen and inhibited cell-associated collagen. FGF-2 inhibited both released and cell-associated collagen and sox9 knockdown further inhibited both of them. In contrast, the combination [IGF-I + BMP-2 + BMP-7] stimulated both released and cell associated collagen. Sox9 knockdown further increased released collagen, but reduced the stimulation of cell associated collagen (Fig. 7A). In addition to decreasing the total amount of collagen, FGF-2 decreased the proportion of that collagen that was retained by the cells, while [IGF-I + BMP-2 + BMP-7] increased both the amount of collagen and its distribution ratio (Fig. 7B). Sox9 knockdown had minimal effect on the action of FGF-2, but decreased the collagen distribution ratio in the presence of [IGF-I + BMP-2 + BMP-7].
Fig. 7.
Effect of sox9 knockdown on collagen production (A) and collagen distribution between the medium and the cells (B). Chondrocytes were transfected by sox9 siRNA (+) or by non-targeting siRNA (−) and treated with no growth factor (None), FGF-2 or the combination of IGF-I, BMP-2, and BMP-7 proteins. Culture medium was harvested at the designated time points and the cells harvested at 6 days. Collagen in the medium (released collagen) and cell layer (cell-associated collagen) were measured separately. Data are expressed as changes in released collagen and cell-associated collagen (A), and change in the ratio of cell-associated collagen to released collagen (B) for the treatment group compared to the respective control (collagen distribution ratio) ± SD for three independent experiments. Comparison groups are illustrated as data sets. A: Sets 1 versus 2: released collagen 1.08-fold (P = 0.0270), cell-associated collagen 18% (P < 0.0001). Sets 1 versus 3: released collagen 2.69-fold (P < 0.0001), cell associated collagen 10.31-fold (P < 0.0001). Sets 3 versus 4: released collagen 1.19-fold (P < 0.0001), cell associated collagen 31% (P < 0.0001). Sets 1 versus 5: released collagen 33%, cell-associated collagen 49% (both P < 0.0001). Sets 5 versus 6: released collagen 9% (P = 0.0157), cell associated collagen 19% (P < 0.0001). B: Sets 1 versus 2: 24% (P < 0.0001). Sets 1 versus 3: 3.83-fold (P < 0.0001). Sets 3 versus 4: 42% (P < 0.0001). Sets 1 versus 5: 23% (P < 0.0001). Sets 5 versus 6: 11% (P = 0.0167).
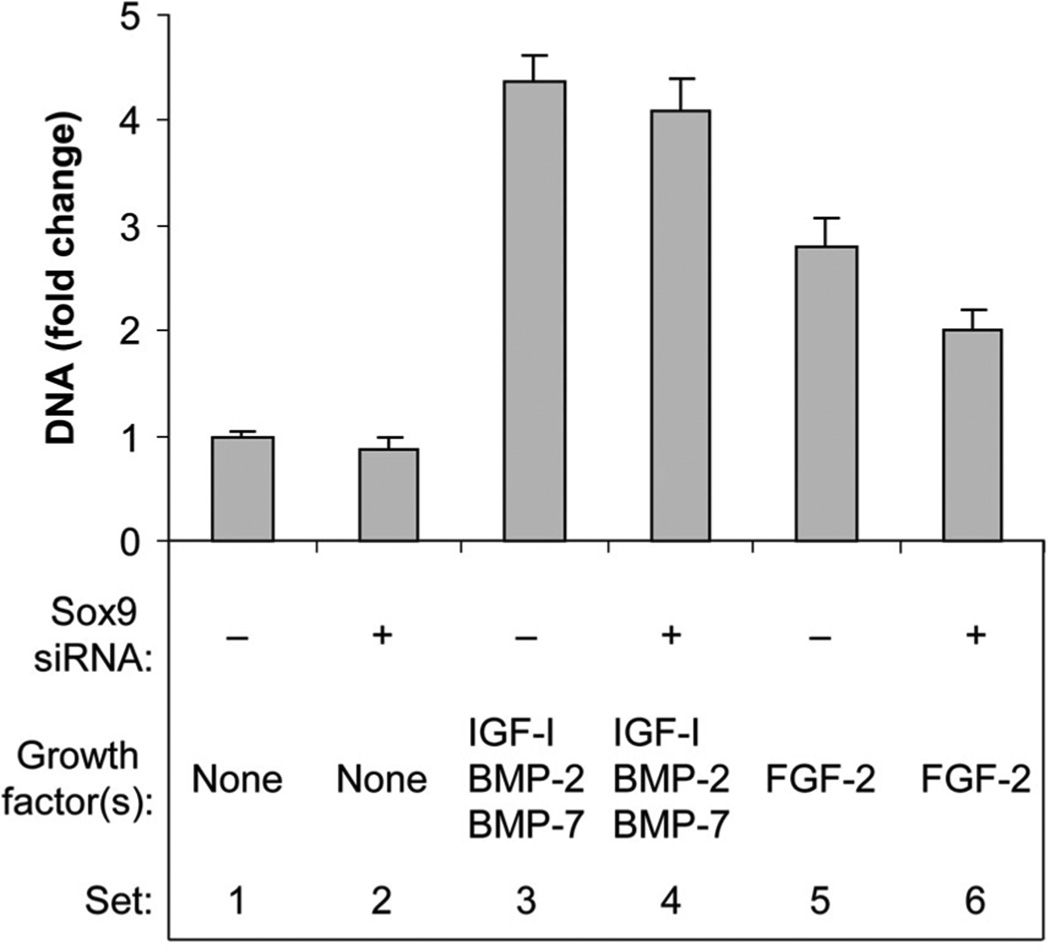
EFFECT OF SOX9 KNOCKDOWN ON GROWTH FACTOR REGULATION OF CELL PROLIFERATION
In the absence of growth factor(s), sox9 knockdown had little effect on chondrocyte proliferation. FGF-2 increased DNA content, and sox9 siRNA attenuated this effect. The combination [IGF-I + BMP-2 + BMP-7] increased DNA content, but sox9 siRNA did not significantly influence this action (Fig. 8).
Fig. 8.
Effect of sox9 knockdown on cell proliferation. Chondrocytes were transfected by sox9 siRNA (+) or by non-targeting siRNA (−) and treated with no growth factor (None), FGF-2 or the combination of IGF-I, BMP-2, and BMP-7 proteins, then cultured for 6 days. DNA content was measured in the cell layer. Changes in DNA content serve as an index of cell proliferation. Data are expressed as DNA change compared to the control ± SD for three independent experiments. Comparison groups are illustrated as data sets. Sets 1 versus 2: NS. Sets 1 versus 3: 4.37-fold (P < 0.0001). Sets 3 versus 4: NS. Sets 1 versus 5: 2.81-fold (P < 0.0001). Sets 5 versus 6: 29% (P = 0.0003).
DISCUSSION
To our knowledge, this is the first demonstration that sox9 plays distinct roles in the growth factor regulation of adult articular chondrocytes. The present data are consistent with the prior observation that tFGF-2 modestly increased aggrecan gene expression and GAG production, while markedly inhibiting type II collagen and type I collagen gene expression and collagen protein production, and that [tIGF-I + tBMP-2 + tBMP-7] markedly stimulated aggrecan and type II collagen gene expression, and the production of both GAG and collagen protein. These data contrast with the current findings that FGF-2 and [IGF-I + BMP-2 + BMP-7], delivered either as transgenes or as proteins stimulated sox9 expression and protein production. The differential effects on chondrocyte function contrast with the similar effects of these treatments on sox9 and suggest that sox9 plays different roles in the actions of these growth factors.
Prior studies reported a relation between sox9 and aggrecan in a cartilage-derived cell line, TC6 [Sekiya et al., 2000], and a correlation between human SOX9 and ACAN expression in FGF-2 stimulated human ear chondrocytes [Mandl et al., 2004]. In the present studies, FGF-2 stimulated both acan and sox9 expression in bovine articular chondrocytes. However, sox9 knockdown failed to influence FGF-2 stimulated acan expression. For this reason, the correlation between sox9 and acan expression in our studies may not be causal. The combination [IGF-I + BMP-2 + BMP-7] also stimulated both acan and sox9 expression, but, unlike its effect on FGF-2 regulation, sox9 knockdown substantially mitigated the increase in acan expression. Sox9 knockdown also inhibited acan expression in the absence of growth factor(s). Taken together, these data suggest that FGF-2, [IGF-I + BMP-2 + BMP-7] and constitutive sox9 each promote acan expression. The data further suggest that [IGF-I + BMP-2 + BMP-7] regulation of acan appears to be mediated, at least in part, through sox9 upregulation, while that of FGF-2 is predominantly through sox9-independent mechanisms.
Type II collagen is the principal collagen in articular cartilage and a major structural component of cartilage matrix. It is found in few other tissues and is a phenotypic marker of chondrocytes. In prior gene transfer studies, tFGF-2 was shown to markedly inhibit, and [tIGF-I + tBMP-2 + tBMP-7] to markedly stimulate, col2α1 expression [Shi et al., 2012]. In the present investigation using growth factor proteins, FGF-2 almost completely abrogated col2α1 expression, while [IGF-I + BMP-2 + BMP-7] increased it to over eightfold control levels. Yet, FGF-2 and [IGF-I + BMP-2 + BMP-7] increased sox9 expression 3.0-fold and 2.8-fold, respectively, both in a time-dependent fashion. The opposite effects of FGF-2 and [IGF-I + BMP-2 + BMP-7] on col2α1, coupled with their similar stimulation of sox9 expression suggest that col2α1 expression is dissociated from sox9 expression. However, the siRNA data suggest an alternative explanation. In the absence of growth factor(s), sox9 knockdown repressed col2α1 expression. Sox9 knockdown also progressively attenuated by 32% to 59% (all P < 0.0001) the marked stimulation of col2α1 expression by [IGF-I + BMP-2 + BMP-7] over time, and further inhibited by 25–41% (all P < 0.001) an already profound reduction in col2α1 expression by FGF-2. Taken together, these results suggest that sox9 promotes col2α1 expression under all three treatment conditions tested. However, while [IGF-I + BMP-2 + BMP-7] stimulation of col2α1 expression is largely sox9-dependent, this role of sox9 is minimal compared to the marked sox9-independent inhibition of col2α1 expression by FGF-2.
The type I collagen genes, col1α1 and col1α2 are typically not expressed in adult articular chondrocytes [Eyre, 2002] and are minimally expressed in short term primary chondrocyte culture [von der Mark et al., 1977]. In marked contrast to col2α1, sox9 knockdown increased col1α1 and col1α2 expression in the absence of growth factor(s) and in [IGF-I + BMP-2 + BMP-7] treated chondrocytes. In the presence of FGF-2, sox9 knockdown initially produced a similar increase in col1α1 and col1α2 expression but this disappeared with the essentially complete (97%) inhibition by FGF-2 by the end of culture. To our knowledge, this is first study to demonstrate that sox9 represses col1α1 and col1α2 expression in adult articular chondrocytes.
Taken together, these findings indicate that sox9 plays opposite roles in the regulation of type I and type II collagen gene expression. Sox9 appears to stimulate col2α1 expression and to suppress col1α1 and col1α2 expression. Thus, these data suggest that sox9 supports the chondrocyte phenotype in these cells. The specific pathways responsible for the differential sox9 regulation of different collagen genes remain to be elucidated.
The distribution of new GAG and collagen between cell layer (cell-associated matrix molecules) and medium (released matrix molecules) is of considerable importance to articular cartilage repair. GAG and collagen that is retained with the cells contribute to the formation of new cartilage. When lost into the medium, they do not provide structural benefit. The production and retention of these matrix molecules may reflect chondrocyte anabolic activities, while release of the matrix molecules may reflect chondrocyte catabolic activities [Sah et al., 1994; Im et al., 2007; Shi et al., 2013].
The findings that sox9 knockdown reduced the marked stimulation by [IGF-I + BMP-2 + BMP-7] of total GAG production and of the GAG distribution, but did not affect released GAG, suggest that sox9 increases the GAG distribution ratio by increasing cell-associated GAG rather than by decreasing released GAG. Although FGF-2 modestly increased total GAG production, it decreased the GAG distribution ratio. Interestingly, sox9 knockdown did not alter this ratio, even though FGF-2 increased sox9 expression and sox9 knockdown reduced this FGF-2 stimulation by as much as 61%. Taken together, these data suggest that sox9 mediates, at least in part, the regulation of GAG distribution by [IGF-I + BMP-2 + BMP-7], but that sox9 is not involved in the regulation of GAG distribution by FGF-2.
Treatment with [IGF-I + BMP-2 + BMP-7] increased both total collagen and the collagen distribution ratio. Sox9 knockdown had little effect on total collagen production, but decreased the collagen distribution ratio 42%. This effect reflects both a further increase in released collagen (2.69- to 3.22-fold), and a repression of the [IGF-I + BMP-2 + BMP-7] stimulation of cell-associated collagen (10.31- to 7.13-fold) by sox9 knockdown. Taken together, these data suggest that sox9 plays an important role in directing the newly synthesized collagen to the cell layer. These actions were similar in both direction and magnitude to those on GAG.
In contrast to [IGF-I + BMP-2 + BMP-7], FGF-2 markedly inhibited total collagen production and decreased the collagen distribution ratio. Sox9 knockdown had little effect on this FGF-2 regulation of total collagen production or collagen distribution. Coupled with the finding that FGF-2 stimulated sox9 expression and sox9 protein production, and that sox9 knockdown reduced this stimulation, these results suggest that the marked reduction in collagen production and collagen ratio by FGF-2 are not mediated by sox9.
Few studies have investigated a potential role for sox9 role in the proliferation of adult articular chondrocytes. Although FGF-2 and [IGF-I + BMP-2 + BMP-7] each increased both chondrocyte proliferation and sox9 expression, sox9 knockdown reduced the proliferative effect of FGF-2, but did not influence that of [IGF-I + BMP-2 + BMP-7]. These results suggest that the mechanism of FGF-2 stimulation of chondrocyte proliferation involves sox9, while this effect of [IGF-I + BMP-2 + BMP-7] is mediated by other pathways.
Taken together, these data suggest that the role of sox9 differs for the anabolic and the mitogenic functions regulated by these growth factors. Specifically, sox9 appears to participate in the regulation of matrix production by [IGF-I + BMP-2 + BMP-7], but not by FGF-2, and in the mitogenic activity of FGF-2, but not of [IGF-I + BMP-2 + BMP-7].
Although the importance of sox9 in mesenchymal cells [Wright et al., 1995] and embryonic chondrocytes in endochondral skeletal development [Lefebvre and Smits, 2005] and chondrocyte-derived dedifferentiated cells [Shakibaei et al., 2006], is well-established, its role in adult articular chondrocytes is controversial. In adult human and osteoarthritic articular chondrocytes, SOX9 expression did not correlate with COL2α1 [Aigner et al., 2003; Yagi et al., 2005] or with ACAN expression [Yagi et al., 2005]. In adult mouse articular cartilage, sox9 protein was only faintly detectable in comparison to growth plate chondrocytes [Davies et al., 2002]. On the other hand, SOX9 overexpression has been shown to restore extracellular matrix production in primary human osteoarthritic chondrocytes [Cucchiarini et al., 2007] and to promote the repair of articular cartilage defects in an in vivo rabbit knee cartilage repair model [Cucchiarini et al., 2013]. The present studies found a strong correlation between sox9 expression and both col2α1 and acan expression only in the chondrocytes treated with the combination of IGF-I, BMP-2, and BMP-7. The seeming inconsistency in these reports with respect to type II collagen gene expression may reflect sox9 binding to different col2α1 regulatory elements. In primary rabbit articular chondrocytes, low levels of sox9 overexpression increased col2α1 expression through its specific intronic enhancer, while high levels of sox9 overexpression inhibited col2α1 expression through the −266 bp promoter [Kypriotou et al., 2003]. The specific pathways responsible for the observed growth factor actions remain to be elucidated.
A limitation of these studies is our finding that the siRNA and growth factor transgenes required different transfection reagents, and that these failed to achieve satisfactory transfection efficiency when used in concert. We resolved this limitation by employing growth factor proteins to perform the sox9 knockdown studies. We found that the growth factor transgenes and growth factor proteins generated similar effects on sox9 regulation and chondrocyte function.
Both FGF-2 and [IGF-I + BMP-2 + BMP-7] increased cell proliferation. For this reason, the observed changes in matrix production may, in part, reflect changes in the number of cells. Because the changes in cell proliferation were similar for both treatments, this effect on the comparisons is likely to be small. An additional limitation is that, as with any knockdown study employing siRNA, blockade of target transcripts is incomplete. The siRNA data is likely to underestimate the result that would be obtained by eliminating sox9 expression.
Growth factors regulate articular chondrocyte functions through complex signaling networks and, subsequently, by transcription factors. While many of the signaling pathways employed by the tested growth factors have been well-described [Chen et al., 2004; Werner et al., 2008; Ellman et al., 2013], the role of the central chondrogenic transcription factor, sox9, in their mechanism of action is less well investigated. The present data suggest that sox9 mediates, at least in part, the action of certain growth factors, but not of others. The data further indicate that the role of sox9 also differs with respect to different chondrocyte functions regulated by these growth factors, including pro-anabolic, anti-catabolic, and mitogenic activity. Despite these differences, sox9 consistently supported the chondrocyte phenotype. Interventions that target sox9 in the mechanisms of action of these growth factors may help improve the results of articular cartilage repair.
Supplementary Material
Acknowledgments
The authors thank Cathy Summerlot for assistance in the preparation of the manuscript.
Grant sponsor: National Institutes of Health; Grant number: AR047702; Grant sponsor: United States Veterans Administration; Grant number: BX000447.
Footnotes
Conflict of interest: The authors have no conflict of interest related to this work.
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article at the publisher’s web-site.
REFERENCES
- Aigner T, Gebhard PM, Schmid E, Bau B, Harley V, Poschl E. SOX9 expression does not correlate with type II collagen expression in adult articular chondrocytes. Matrix Biol. 2003;22:363–372. doi: 10.1016/s0945-053x(03)00049-0. [DOI] [PubMed] [Google Scholar]
- Akiyama H, Lefebvre V. Unraveling the transcriptional regulatory machinery in chondrogenesis. J Bone Miner Metab. 2011;29:390–395. doi: 10.1007/s00774-011-0273-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell DM, Leung KK, Wheatley SC, Ng LJ, Zhou S, Ling KW, Sham MH, Koopman P, Tam PP, Cheah KS. SOX9 directly regulates the type-II collagen gene. Nat Genet. 1997;16:174–178. doi: 10.1038/ng0697-174. [DOI] [PubMed] [Google Scholar]
- Bi W, Deng JM, Zhang Z, Behringer RR, de Crombrugghe B. Sox9 is required for cartilage formation. Nat Genet. 1999;22:85–89. doi: 10.1038/8792. [DOI] [PubMed] [Google Scholar]
- Bradham DM, der Wiesche B, Precht P, Balakir R, Horton W. Transrepression of type II collagen by TGF-beta and FGF is protein kinase C dependent and is mediated through regulatory sequences in the promoter and first intron. J Cell Physiol. 1994;158:61–68. doi: 10.1002/jcp.1041580109. [DOI] [PubMed] [Google Scholar]
- Buckwalter JA, Mankin HJ. Articular cartilage repair and transplantation. Arthritis Rheum. 1998;41:1331–1342. doi: 10.1002/1529-0131(199808)41:8<1331::AID-ART2>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Chen D, Zhao M, Mundy GR. Bone morphogenetic proteins. Growth Factors. 2004;22:233–241. doi: 10.1080/08977190412331279890. [DOI] [PubMed] [Google Scholar]
- Chubinskaya S, Hurting M, Rueger DC. OP-1/BMP-7 in cartilage repair. Int Orthop. 2007;31:773–781. doi: 10.1007/s00264-007-0423-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cucchiarini M, Orth P, Madry H. Direct rAAV SOX9 administration for durable articular cartilage repair with delayed terminal differentiation and hypertrophy in vivo. J Mol Med (Berl) 2013;91:625–636. doi: 10.1007/s00109-012-0978-9. [DOI] [PubMed] [Google Scholar]
- Cucchiarini M, Terwilliger EF, Kohn D, Madry H. Remodelling of human osteoarthritic cartilage by FGF-2, alone or combined with Sox9 via rAAV gene transfer. J Cell Mol Med. 2009;13:2476–2488. doi: 10.1111/j.1582-4934.2008.00474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cucchiarini M, Thurn T, Weimer A, Kohn D, Terwilliger EF, Madry H. Restoration of the extracellular matrix in human osteoarthritic articular cartilage by overexpression of the transcription factor SOX9. Arthritis Rheum. 2007;56:158–167. doi: 10.1002/art.22299. [DOI] [PubMed] [Google Scholar]
- Davies SR, Sakano S, Zhu Y, Sandell LJ. Distribution of the transcription factors Sox9, AP-2, and [delta]EF1 in adult murine articular and meniscal cartilage and growth plate. J Histochem Cytochem. 2002;50:1059–1065. doi: 10.1177/002215540205000808. [DOI] [PubMed] [Google Scholar]
- de Crombrugghe B, Lefebvre V, Behringer RR, Bi W, Murakami S, Huang W. Transcriptional mechanisms of chondrocyte differentiation. Matrix Biol. 2000;19:389–394. doi: 10.1016/s0945-053x(00)00094-9. [DOI] [PubMed] [Google Scholar]
- Ellman MB, Yan D, Ahmadinia K, Chen D, An HS, Im HJ. Fibroblast growth factor control of cartilage homeostasis. J Cell Biochem. 2013;114:735–742. doi: 10.1002/jcb.24418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyre D. Collagen of articular cartilage. Arthritis Res. 2002;4:30–35. doi: 10.1186/ar380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flechtenmacher J, Huch K, Thonar EJ, Mollenhauer JA, Davies SR, Schmid TM, Puhl W, Sampath TK, Aydelotte MB, Kuettner KE. Recombinant human osteogenic protein 1 is a potent stimulator of the synthesis of cartilage proteoglycans and collagens by human articular chondrocytes. Arthritis Rheum. 1996;39:1896–1904. doi: 10.1002/art.1780391117. [DOI] [PubMed] [Google Scholar]
- Fortier LA, Mohammed HO, Lust G, Nixon AJ. Insulin-like growth factor-I enhances cell-based repair of articular cartilage. J Bone Joint Surg Br. 2002;84:276–288. doi: 10.1302/0301-620x.84b2.11167. [DOI] [PubMed] [Google Scholar]
- Fujimoto E, Ochi M, Kato Y, Mochizuki Y, Sumen Y, Ikuta Y. Beneficial effect of basic fibroblast growth factor on the repair of full-thickness defects in rabbit articular cartilage. Arch Orthop Trauma Surg. 1999;119:139–145. doi: 10.1007/s004020050377. [DOI] [PubMed] [Google Scholar]
- Goodrich LR, Hidaka C, Robbins PD, Evans CH, Nixon AJ. Genetic modification of chondrocytes with insulin-like growth factor-1 enhances cartilage healing in an equine model. J Bone Joint Surg Br. 2007;89:672–685. doi: 10.1302/0301-620X.89B5.18343. [DOI] [PubMed] [Google Scholar]
- Grunder T, Gaissmaier C, Fritz J, Stoop R, Hortschansky P, Mollenhauer J, Aicher WK. Bone morphogenetic protein (BMP)-2 enhances the expression of type II collagen and aggrecan in chondrocytes embedded in alginate beads. Osteoarthritis Cartilage. 2004;12:559–567. doi: 10.1016/j.joca.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Henry SP, Liang S, Akdemir KC, de Crombrugghe B. The postnatal role of Sox9 in cartilage. J Bone Miner Res. 2012;27:2511–2525. doi: 10.1002/jbmr.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson FM, Bowe EA, Davies ME. Promotion of the intrinsic damage-repair response in articular cartilage by fibroblastic growth factor-2. Osteoarthritis Cartilage. 2005;13:537–544. doi: 10.1016/j.joca.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Im H-J, Muddasani P, Natarajan V, Schmid TM, Block JA, Davis F, van Wijnen AJ, Loeser RF. Basic fibroblast growth factor stimulates matrix metalloproteinase-13 via the molecular cross-talk between the mitogen-activated protein kinases and protein kinase C delta pathways in human adult articular chondrocytes. J Biol Chem. 2007;282:11110–11121. doi: 10.1074/jbc.M609040200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kypriotou M, Fossard-Demoor M, Chadjichristos C, Ghayor C, de Crombrugghe B, Pujol J, Galera P. SOX9 exerts a bifunctional effect on type II collagen gene (COL2A1) expression in chondrocytes depending on the differentiation state. DNA Cell Biol. 2003;22:119–129. doi: 10.1089/104454903321515922. [DOI] [PubMed] [Google Scholar]
- Lefebvre V, Smits P. Transcriptional control of chondrocyte fate and differentiation. Birth Defects Res C. 2005;75:200–212. doi: 10.1002/bdrc.20048. [DOI] [PubMed] [Google Scholar]
- Loeser RF, Pacione CA, Chubinskaya S. The combination of insulin-like growth factor 1 and osteogenic protein 1 promotes increased survival of and matrix synthesis by normal and osteoarthritic human articular chondrocytes. Arthritis Rheum. 2003;48:2188–2196. doi: 10.1002/art.11209. [DOI] [PubMed] [Google Scholar]
- Luyten FP, Hascall VC, Nissley SP, Morales TI, Reddi AH. Insulin-like growth factors maintain steady-state metabolism of proteoglycans in bovine articular cartilage explants. Arch Biochem Biophys. 1988;267:416–425. doi: 10.1016/0003-9861(88)90047-1. [DOI] [PubMed] [Google Scholar]
- Madry H, Emkey G, Zurakowski D, Trippel SB. Overexpression of human fibroblast growth factor 2 stimulates cell proliferation in an ex vivo model of articular chondrocyte transplantation. J Gene Med. 2004;6:238–245. doi: 10.1002/jgm.488. [DOI] [PubMed] [Google Scholar]
- Madry H, Kaul G, Cucchiarini M, Stein U, Zurakowski D, Remberger K, Menger MD, Kohn D, Trippel SB. Enhanced repair of articular cartilage defects in vivo by transplanted chondrocytes overexpressing insulin-like growth factor I (IGF-I) Gene Ther. 2005;12:1171–1179. doi: 10.1038/sj.gt.3302515. [DOI] [PubMed] [Google Scholar]
- Madry H, Zurakowski D, Trippel SB. Overexpression of human insulin-like growth factor-I promotes new tissue formation in an ex vivo model of articular chondrocyte transplantation. Gene Ther. 2001;8:1443–1449. doi: 10.1038/sj.gt.3301535. [DOI] [PubMed] [Google Scholar]
- Mandl EW, Jahr H, Koevoet JLM, van Leeuwen JPTM, Weinans H, Verhaar JAN, van Osch GJVM. Fibroblast growth factor-2 in serum-free medium is a potent mitogen and reduces dedifferentiation of human ear chondrocytes in monolayer culture. Matrix Biol. 2004;23:231–241. doi: 10.1016/j.matbio.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Montaseri A, Busch F, Mobasheri A, Buhrmann C, Aldinger C, Rad JS, Shakibaei M. IGF-1 and PDGF-bb suppress IL-1beta-induced cartilage degradation through down-regulation of NF-B signaling: Involvement of Src/PI-3K/AKT pathway. PLoS ONE. 2011;6:e28663. doi: 10.1371/journal.pone.0028663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami S, Kan M, McKeehan WL, de Crombrugghe B. Up-regulation of the chondrogenic Sox9 gene by fibroblast growth factors is mediated by the mitogen-activated protein kinase pathway. Proc Natl Acad Sci U S A. 2000;97:1113–1118. doi: 10.1073/pnas.97.3.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Q, Yu Y, Chen Q, Li C, Wu H, Wan Y, Ma J, Sun F. Sox9, a key transcription factor of bone morphogenetic protein-2-induced chondrogenesis, is activated through BMP pathway and a CCAAT box in the proximal promoter. J Cell Physiol. 2008;217:228–241. doi: 10.1002/jcp.21496. [DOI] [PubMed] [Google Scholar]
- Renard E, Poree B, Chadjichristos C, Kypriotou M, Maneix L, Bigot N, Legendre F, Ollitrault D, De Crombrugghe B, Mallein-Gerin F, Moslemi S, Demoor M, Boumediene K, Galera P. Sox9/Sox6 and Sp1 are involved in the insulin-like growth factor-I-mediated upregulation of human type II collagen gene expression in articular chondrocytes. J Mol Med. 2012;90:649–666. doi: 10.1007/s00109-011-0842-3. [DOI] [PubMed] [Google Scholar]
- Sah RL, Chen AC, Grodzinsky AJ, Trippel SB. Differential effects of bFGF and IGF-I on matrix metabolism in calf and adult bovine cartilage explants. Arch Biochem Biophys. 1994;308:137–147. doi: 10.1006/abbi.1994.1020. [DOI] [PubMed] [Google Scholar]
- Sailor LZ, Hewick Rm, Morris EA. Recombinant human bone morphogenetic protein-2 maintains the articular chondrocyte phenotype in long-term culture. J Orthop Res. 1996;14:937–945. doi: 10.1002/jor.1100140614. [DOI] [PubMed] [Google Scholar]
- Salminen H, Vuorio E, Saamanen AM. Expression of Sox9 and type IIA procollagen during attempted repair of articular cartilage damage in a transgenic mouse model of osteoarthritis. Arthritis Rheum. 2001;44:947–955. doi: 10.1002/1529-0131(200104)44:4<947::AID-ANR152>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Sekiya I, Tsuji K, Koopman P, Watanabe H, Yamada Y, Shinomiya K, Nifuji A, Noda M. SOX9 enhances aggrecan gene promoter/enhancer activity and is up-regulated by retinoic acid in a cartilage-derived cell line, TC6. J Biol Chem. 2000;275:10738–10744. doi: 10.1074/jbc.275.15.10738. [DOI] [PubMed] [Google Scholar]
- Shakibaei M, Seifarth C, John T, Rahmanzadeh M, Mobasheri A. Igf-I extends the chondrogenic potential of human articular chondrocytes in vitro: Molecular association between Sox9 and Erk1/2. Biochem Pharmacol. 2006;72:1382–1395. doi: 10.1016/j.bcp.2006.08.022. [DOI] [PubMed] [Google Scholar]
- Shi S, Mercer S, Eckert GJ, Trippel SB. Regulation of articular chondrocyte aggrecan and collagen gene expression by multiple growth factor gene transfer. J Orthop Res. 2012;30:1026–1031. doi: 10.1002/jor.22036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S, Mercer S, Eckert GJ, Trippel SB. Growth factor transgenes interactively regulate articular chondrocytes. J Cell Biochem. 2013;114:908–919. doi: 10.1002/jcb.24430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tew SR, Pothacharoen P, Katopodi T, Hardingham TE. SOX9 transduction increases chondroitin sulfate synthesis in cultured human articular chondrocytes without altering glycosyltransferase and sulfotransferase transcription. Biochem J. 2008;414:231–236. doi: 10.1042/BJ20080262. [DOI] [PubMed] [Google Scholar]
- Trippel SB. Growth factor actions on articular cartilage. J Rheumatol Suppl. 1995;43:129–132. [PubMed] [Google Scholar]
- von der Mark K, Gauss V, von der Mark H, Muller P. Relationship between cell shape and type of collagen synthesised as chondrocytes lose their cartilage phenotype in culture. Nature. 1977;267:531–532. doi: 10.1038/267531a0. [DOI] [PubMed] [Google Scholar]
- Werner H, Weinstein D, Bentov I. Similarities and differences between insulin and IGF-I: structures, receptors, and signalling pathways. Arch Physiol Biochem. 2008;114:17–22. doi: 10.1080/13813450801900694. [DOI] [PubMed] [Google Scholar]
- Wright E, Hargrave MR, Christiansen J, Cooper L, Kun J, Evans T, Gangadharan U, Greenfield A, Koopman P. The Sry-related gene Sox9 is expressed during chondrogenesis in mouse embryos. Nat Genet. 1995;9:15–20. doi: 10.1038/ng0195-15. [DOI] [PubMed] [Google Scholar]
- Yagi R, McBurney D, Laverty D, Weiner S, Horton WE., Jr Intrajoint comparisons of gene expression patterns in human osteoarthritis suggest a change in chondrocyte phenotype. J Orthop Res. 2005;23:1128–1138. doi: 10.1016/j.orthres.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Yokoo N, Saito T, Uesugi M, Kobayashi N, Xin KQ, Okuda K, Mizukami H, Ozawa K, Koshino T. Repair of articular cartilage defect by autologous transplantation of basic fibroblast growth factor gene-transduced chondrocytes with adeno-associated virus vector. Arthritis Rheum. 2005;52:164–170. doi: 10.1002/art.20739. [DOI] [PubMed] [Google Scholar]
- Zhou G, Lefebvre V, Zhang Z, Eberspaecher H, de Crombrugghe B. Three high mobility group-like sequences within a 48-base pair enhancer of the Col2a1 gene are required for cartilage-specific expression in vivo. J Biol Chem. 1998;273:14989–14997. doi: 10.1074/jbc.273.24.14989. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.