Abstract
Zinc is an essential metal that serves as a cofactor in a variety of cellular processes, including meiotic maturation. Cellular control of zinc uptake, availability and efflux is closely linked to meiotic progression in rodent and primate reproduction where large fluctuations in zinc levels are critical at several steps in the oocyte-to-embryo transition. Despite these well-documented roles of zinc fluxes during meiosis, only a few of the genes encoding key zinc receptors, membrane-spanning transporters, and downstream signaling pathway factors have been identified to date. Furthermore, little is known about analogous roles for zinc fluxes in the context of a whole organism. Here, we evaluate whether zinc availability regulates germline development and oocyte viability in the nematode Caenorhabditis elegans, an experimentally flexible model organism. We find that similar to mammals, mild zinc limitation in C. elegans profoundly impacts the reproductive axis: the brood size is significantly reduced under conditions of zinc limitation where other physiological functions are not perturbed. Zinc limitation in this organism has a more pronounced impact on oocytes than sperm and this leads to the decrease in viable embryo production. Moreover, acute zinc limitation of isolated zygotes prevents extrusion of the second polar body during meiosis and leads to aneuploid embryos. Thus, the zinc-dependent steps in C. elegans gametogenesis roughly parallel those described in meiotic-to-mitotic transitions in mammals.
1. Introduction
Zinc is a transition metal that serves as a cofactor and structural regulator in a variety of proteins that participate in numerous cellular processes (Beyersmann and Haase, 2001; Bohnsack and Hirschi, 2004; Haase and Maret, 2010). We have shown that fluctuations in total cellular zinc levels play central regulatory roles controlling meiosis in mouse, non-human primate, and human oocytes before and after fertilization (Bernhardt et al., 2011; Duncan et al., 2016; Kong et al., 2012, 2014; Que et al., 2015; Zhang et al., 2016). In the mouse oocyte, total zinc levels increase by over 50% during meiotic maturation and this accumulation of zinc is required for the oocyte to progress properly to metaphase of meiosis II (Kim et al., 2010). Previous work has shown that fertilization and parthenogenesis initiate zinc exocytosis from zinc loaded cortical vesicles (Que et al., 2015; Kim et al., 2011) into the extracellular space through a series of coordinated events known as ‘zinc sparks’ (Kim et al., 2011). If zinc levels are not reduced, the egg cannot complete meiosis, and the zygote is unable to initiate the mitotic divisions. Therefore, zinc fluxes are fundamental events at several steps in the oocyte-egg-embryo transition, and are critical for mammalian reproduction. Despite these well-defined roles of zinc fluxes during meiosis (Suzuki et al., 2010a, 2010b), only a few of the genes encoding zinc receptors have been identified as mediating these switching events in mammals or other model systems to date. Zinc receptors, i.e., macromolecules defined by their ability to move or bind zinc, with known roles in meiosis include the cation transporters ZIP6 and ZIP10 and downstream signaling pathway factors, such as Emi2 (Lints and Hall, 2009d; Tian et al., 2014; Tian and Diaz, 2012, 2013). The nematode C. elegans would be an ideal model system for identifying pathway members, especially if readily triggered meiotic phenotypes of zinc depletion can be established. Given that zinc availability has already been established to regulate proper meiotic progression in mammalian oocytes, we test here whether a similar type of inorganic regulation of egg biology might extend further into the phylogenetic tree using the invertebrate, C. elegans.
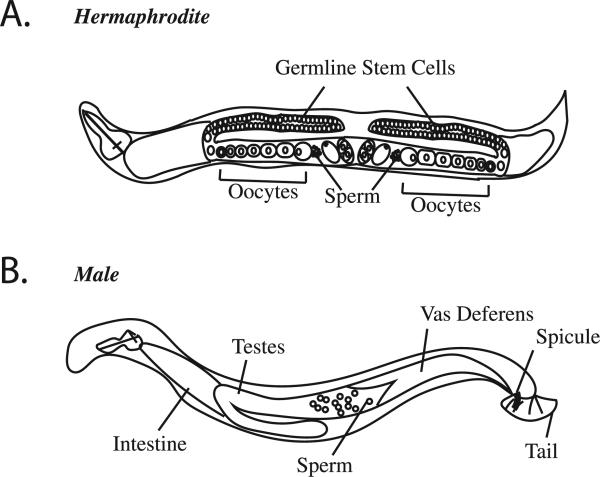
C. elegans exist as two sexes, hermaphrodites and males (L'Hernault, 2006; Lints and Hall, 2009c, 2009d). These worms develop through four larval stages (L1–L4), entering adulthood in approximately 3 days (Corsi et al., 2015). In self-fertilizing hermaphrodites, gonadogenesis completes and meiosis begins in the L4 stage. They first produce sperm and store these gametes in a compartment called the spermatheca, but upon becoming adults, there is a switch to oocyte production (Kimble and Crittenden, 2007). At this stage, the remaining meiotic cells in the germline begin maturing into oocytes, which are then fertilized as they pass through the spermatheca, becoming embryos that are laid and hatch (Greenstein, 2005). Each hermaphrodite produces approximately 300 self-progeny before running out of sperm, but they can produce many more offspring if mated with a male and sperm availability is not limited.
Many features of this system make it ideal for evaluating the regulatory roles of zinc fluxes in reproduction. The hermaphrodite gonad has two arms and each is arranged as a production line, with a population of germline stem cells in the distal region differentiating to enter meiosis, and then forming oocytes in the proximal region, as they move toward the spermatheca (McCarter et al., 1999; Von Stetina and Orr-Weaver, 2011) (Fig. 1). Therefore, this spatial-temporal gradient means that all stages of meiosis can be visualized simultaneously within the same worm. Moreover, C. elegans are transparent, allowing live imaging of the meiotic and mitotic divisions of the oocyte and embryo, and they are amenable to a wide variety of experimental manipulations. Based on these advantages, we assessed whether zinc availability impacts germline development or oocyte viability in a whole animal model.
Fig. 1.
C. elegans hermaphrodite and male anatomy. (A) Adult hermaphrodites have two gonad arms. The distal region of each arm contains germline stem cells that differentiate to enter meiosis and then move through the gonad as they progress through meiosis, forming oocytes in the proximal region of the gonad. Oocytes are then fertilized in the spermatheca, where the sperm are stored, triggering completion of the meiotic divisions and the beginning of the mitotic divisions of the embryo. (B) Males only contain sperm, and can mate with hermaphrodites.
Here, we test the hypothesis that growth of the worm under zinc deficient conditions will impair oocyte function and fertility. Zinc availability to both the worm and its food, E. coli, can be attenuated by addition of the metal chelator N,N,N′,N′-tetrakis(2-pyridylmethyl)ethylenediamine (TPEN) to the growth medium. TPEN has been employed in a number of studies of meiosis in isolated mammalian oocytes (Bernhardt et al., 2011; Kim et al., 2011; Suzuki et al., 2010a, 2010b). In order to establish mild zinc limitation in which there is no observable impact on the general physiological status of C. elegans, progeny counts were evaluated as a function of TPEN concentration in the growth medium. We found that growth of adults under mild zinc depletion leads to a statistically significant drop in the number oocytes produced. Moreover, acute zinc limitation of isolated zygotes prevents the extrusion of the second polar body and causes chromosome segregation defects, resulting in aneuploid embryos. These results establish a zinc-dependent phenotype in the reproductive axis of C. elegans and support the idea that zinc-regulated pathways in meiosis are evolutionarily conserved.
2. Materials and methods
2.1. Worm strains
EU1067: unc-119(ed3)ruIs32[unc-119(+)pie-1promoter::GFP::H2B] III; ruIs57[unc-119(+) pie-1promoter::GFP::tubulin] was used for fluorescence imaging of the meiotic spindle, gamete count, and for generating males (Wignall and Villeneuve, 2009). N2 (Bristol) wild type strain was used in all brood size experiments, food aversion, and pharyngeal contraction experiments (Wormbase, 2016b). The mutant strain fog-1(q253) (Wormbase, 2016a) was used in the mating experiment.
2.2. Growth media
Animals were grown on bacterial lawns plated on 6 cm agar plates following standard methods (Stiernagle, 2006) with the following modifications. Plates containing growth media were prepared with a final concentration of 10 μM TPEN (Sigma Aldrich, St. Louis, MO) to Nematode Growth Media (NGM) (Stiernagle, 2006) prior to pouring the plates. After the plates solidified, 200 μl of an overnight growth of E. coli strain OP50 in Luria Broth (Stiernagle, 2006) was added and allowed to dry at room temperature. Alternatively, a final concentration of 10 μM of the copper-specific chelators Neocuproine (Sigma) or Ammonium tetrathiomolybdate (Sigma) were dissolved in ethanol or H2O, respectively, and were added to the NGM as described above. For rescue experiments, plates were further supplemented with 20 μM metal salts CuSO4·5H2O (Sigma), ZnSO4·7H2O (Sigma), and FeSO4·7H2O (Sigma).
2.3. Brood size experiments
L4 stage hermaphrodites were picked onto control NGM or zinc insufficient NGM plates (1 worm/plate). Every 24 h, the worm was transferred to a fresh plate. Progeny were counted 48 h after the adult was removed from the plate. Progeny were scored from control hermaphrodites (day 1, n = 50, day 2, n = 43, day 3, n = 40, day 4, n = 33, day 5, n = 30) and zinc insufficient hermaphrodites (day 1, n = 52, day 2, n = 52, day 3, n = 25, day 4, n = 22, day 5, n = 18). Similarly, brood size experiments were conducted on plates with bacteria killed by UV irradiation, heat exposure (75 °C for 1 h) and 250 μg/ml Kanamycin (Sigma) (MacNeil et al., 2013). For DMSO vehicle: day 1, n = 20, day 2, n = 19, day 3, n = 10, day 4, and n = 18, and day 5, n = 19. For TPEN in DMSO: day 1, n = 25, day 2, n = 20, day 3, n = 10, day 4, n = 16, and day 5, n = 15. For methanol vehicle: day 1, n = 19, day 2, n = 19, day 3, n = 18, day 4, n = 15, day 5, n = 15. For TPEN in methanol: day 1, n = 19, day 2, n = 11, day 3, n = 20, day 4, n = 22, and day 5, n = 11. In the case of the TPEN in the methanol group, some worms were stuck between the agar and the plate wall on day 1 and were liberated for later egg laying in the experiment, others were caught in water on the side wall and were also liberated.
2.4. Hatching experiment
N2 animals were synchronized at the L4 stage and placed on either control plates (n = 14 adults) or plates with 10 μM TPEN (n = 12 adults). Each group was incubated at 20 °C for 24 h to allow for egg laying. After incubating, the adults were removed and allowed an additional 24 h for the embryos to hatch. After this time, the number of embryos that hatched was quantified.
2.5. Eating behavior experiments
Young adult eating behavior was quantified by counting the number of pharyngeal contractions per 30 s in control (n = 31) and zinc insufficient groups (n = 29). Food avoidance was quantified as the percent of worms eating (worms on the plate eating / total worms on plate × 100) for control (n = 35) and zinc insufficient groups (n = 37).
2.6. Metal rescue experiments
L4 stage hermaphrodites were picked onto control or TPEN plates and incubated at 20 °C for 6 h. From the TPEN group, some were selected to remain on the TPEN plates, while single adults were placed onto the rescue plates, supplemented as described above, and transferred to a fresh plate every 24 h. Progeny were counted 48 h after the adult was removed from the plate. For controls: n = 30, 18 h post rescue, and n = 21, 42 h post-rescue. For the 10 μM TPEN group: n = 32, 18 h post rescue, and n = 25, 42 h post-rescue. For the Cu rescue group: n = 30, 18 h post rescue, and n = 17, 42 h post-rescue. For the Zn rescue group: n = 25, 18 h post rescue, and n = 18, 42 h post-rescue. Finally, for the Fe rescue group: n = 27, 18 h post rescue, and n = 20, 42 h post-rescue.
2.7. Copper chelation
L4 hermaphrodites were picked onto plates supplemented with 10 μM Neocuproine or 10 μM ammonium tetrathiomolybdate (AT) and compared to control worms picked onto standard NGM. For Neocuproine we scored progeny for controls on day 1, n = 10, day 2 n = 10, day 3, n = 10, day 4, n = 8, and day 5, n = 8, and for the chelated group day 1, n = 20, day 2, n = 19, day 3, n = 18, day 4, n = 17, and day 5, n = 15. For ammonium tetrathiolmolybdate, we scored progeny for controls on day 1, n = 20, day 2, n = 19, and day 3, n = 18, and for the chelated group day 1, n = 23, day 2, n = 27, and day 3, n = 27. Every 24 h a single worm was transferred to a fresh plate to deposit their offspring. After 2 days the offspring were counted. The ammonium tetrathiolmolybdate experiment on days 2 and 3 had more worms because four worms on day 1 were stuck crawling between the plate and the agar and did not lay eggs on day 1. We liberated these worms and allowed them to keep laying eggs for the rest of the experiment.
2.8. Gamete counts
L4 stage EU1067 hermaphrodites and adult males were picked onto control and TPEN plates and incubated for 24 h at 20 °C. They were then ethanol fixed and mounted using Vectashield containing 10 μg/ml Hoechst. Fluorescence images were captured on a Leica DM5500 epifluorescence microscope. Oocytes were scored based on the presence of 6 chromosome bivalents within a distinct nuclear envelope and cellular membrane (Greenstein, 2005; Lints and Hall, 2009b). For the control, we scored n = 71 and for hermaphrodites grown on TPEN plates we scored n = 78 at L4, and n = 32 at L3. Sperm inside the spermatheca were identified with Hoechst (L'Hernault, 2006; Lints and Hall, 2009a). We scored n = 40 for the control group, and for the hermaphrodites grown on TPEN plates we scored n = 35 at L4, and n = 32 at L3. For the male sperm counts, we scored n = 19 control males, and n = 22 males grown on TPEN.
2.9. Production of males
Males were generated by heat shock in EU1067 animals. Approximately 5 late L4 stage hermaphrodites per plate were incubated at 30 °C for 3 h. After this incubation, worms were returned to 20 °C and allowed to produce F1 progeny. Some males were developed from this F1 generation, which were then used to set up matings to produce additional males.
2.10. Mating experiment
2.10.1. Hermaphrodites versus males
Early L4 stage hermaphrodites and males were picked onto either control or zinc insufficient plates to incubate for 24 h at 20 °C. After incubating, the following mating pairs were allowed to mate on control plates for 24 h:
Control hermaphrodites × control males (n = 56 matings)
Control hermaphrodites × zinc insufficient males (n = 51 matings)
Zinc insufficient hermaphrodites × control males (n = 61 matings)
Zinc insufficient hermaphrodites × zinc insufficient males (n = 46 matings)
After mating, adults were removed from the plates and the progeny were counted 24 h later.
2.10.2. Feminized versus male worms
Early L4 stage fog-1(q253) worms were picked onto either control or zinc insufficient plates to incubate for 24 h at 25 °C to produce the feminized phenotype. At this restrictive temperature, fog-1(q253) mutants do not produce sperm (Wormbase, 2016a), so we refer to them as “females” for simplicity. At the same time, males were also picked onto separate control or zinc insufficient plates to incubate for 24 h at 20 °C. After incubating, the following mating pairs were allowed to mate for 24 h:
Control females × control males (n = 26 matings)
Control females × zinc insufficient males (n = 18 matings)
Zinc insufficient females × control males (n = 16 matings)
Zinc insufficient females × zinc insufficient males (n = 20 matings)
After mating, adults were removed from the plates and the progeny were counted 24 h later.
2.11. Meiotic progression time-lapse imaging
2.11.1. Sample preparation
Adult EU1067 hermaphrodites were picked into an 8 μl drop of egg buffer (Zhang and Kuhn, 2013) supplemented with 10 μM TPEN within a 35 mm culture dish with a 10 mm well (World Precision Instruments, Sarasota FL). Zygotes were cut out of the hermaphrodites with a 20-gauge needle (BD). The dish was placed on the microscope stage and embryos in Meiosis II were selected for imaging.
2.11.2. Imaging
Zygotes were imaged on a SP5 II Laser Scanning Confocal Microscope located in the Biological Imaging Facility at Northwestern University. We used the 63× objective with the 488 laser to detect GFP and the HyD detector. Single plane images were captured at 45 s intervals until the zygotes reached the 2-cell stage. We imaged 5 control, and 6 zinc insufficient maturing oocytes. Movies were allowed to progress until cellular features moved out of focus. We then stopped the movies, refocused and resumed filming immediately, thus splitting the movies into separate files. Listed video time frames were based on the start of filming of each segment.
2.12. Metal content of NGM plates
Inductively coupled plasma mass spectrometry (ICP MS) was performed at the Quantitative Bio-element Imaging Center (QBIC) at Northwestern University. The full content of an NGM plate (10 ml) was dissolved in 5 ml of 3% nitric acid (Sigma Aldrich, St. Louis, MO) diluted with ultra pure H2O (18.2 MΩ·cm) for 1 h in triplicate. A multi-element internal standard (CLISS-1, Spex Certiprep, Metuchen, NJ, USA) was then added to produce a final solution of 3.0% nitric acid (v/v) with 5.0 ng/ml internal standard in a total sample volume of 10 ml.
ICP-MS was performed on a computer-controlled (Plasmalab software) Thermo XSeries II ICP-MS (Thermo Fisher Scientific, Waltham, MA, USA) operating in standard mode and equipped with a CETAC 260 autosampler (Omaha, NE, USA). Each sample was acquired using one survey run (10 sweeps) and three main (peak jumping) runs (100 sweeps). The isotopes selected for internal standard analysis were 59Co, 101,102Ru, 89Y, 115In, and 165Ho. Total iron, copper and zinc was determined using average values for the respective isotopes (57Fe, 63,65Cu, and 64,66,68Zn). Instrument performance was optimized daily through autotuning followed by verification via a performance report (passing the manufacturer's specifications).
2.13. Statistical analysis
The results were depicted as mean and standard error of mean (SEM). The Student's t-test, and 2-way analysis of variance (ANOVA) were used to evaluate significant differences between control and treatment groups. All statistical analysis was conducted using Graph Pad software from Prism. Values considered statistically significant were below p < 0.05.
3. Results
3.1. Zinc insufficiency impacts C. elegans reproduction
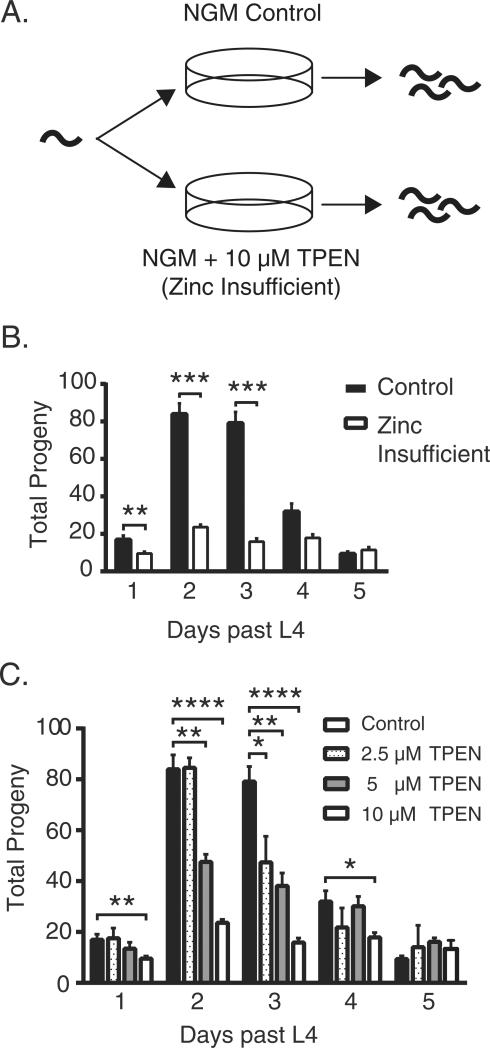
To determine if zinc insufficiency impacts C. elegans fertility, we picked L4 stage hermaphrodites onto plates containing 10 μM of TPEN, in excess of the chelating agent relative to the total zinc, copper and iron content of the plate (Fig. S1). Hermaphrodites grown on these plates produced smaller broods from days 1 through 3 (Fig. 2B) compared to those grown on control plates. The dose dependence of TPEN exposure was determined by titrating TPEN in the growth media at 2.5 μM, 5 μM and 10 μM concentrations. As the TPEN concentration incrementally increased, the brood size decreased correspondingly (Fig. 2C). At TPEN concentrations above 20 μM, adult worms crawled off the plates. We tested the viability of the eggs that were laid on the TPEN plates by quantifying the number that hatched. Within 24 h of egg laying by a young adult hermaphrodite, all of the embryos hatched in both control and TPEN groups (Fig. S2).
Fig. 2.
Metal insufficiency in C. elegans causes fertility defects. (A) Experimental setup: worms were grown on either control plates or on plates containing 10 μM TPEN to sequester available transition metal ions. (B) Total number of progeny produced by either untreated or 10 μM TPEN treated animals. Increased TPEN concentration incrementally reduces brood size from day 1 to day 5 (day 1 **p = 0.0024, day 2 **p = 0.0033, ****p < 0.0001, day 3 *p = 0.0279, **p = 0.0023, ****p < 0.0001, day 4 *p < 0.0153). (C) The concentration of TPEN in the growth media was varied to observe dose response effects. Fertility was measured for the first 5 days after L4 molt.
Since C. elegans are typically grown on plates with live bacteria as a food source, we next sought to determine if the observed brood size reduction following exposure to 10 μM TPEN might be the result of general effects on the adult worm or the bacteria. First, we scored the pharyngeal pumping rate of TPEN treated worms and also tested for food avoidance behaviors; in these assays we found no significant differences with control worms (Figs. S3, S4). Moreover, we observed similar effects on brood size when we triple-killed the bacteria prior to providing them as a food source to the worms (Fig. S5), demonstrating that it is unlikely that the effects on worm viability resulted from effects of TPEN treatment on the bacterial food source. We conclude that these experimental conditions, i.e., bacterial and worm growth on medium containing 10 μM TPEN, constitutes a mild zinc limitation condition that has little impact on the general health of the worm. This condition is nonetheless sufficient to induce a reduction in brood size by primarily impairing the reproductive axis of the worm.
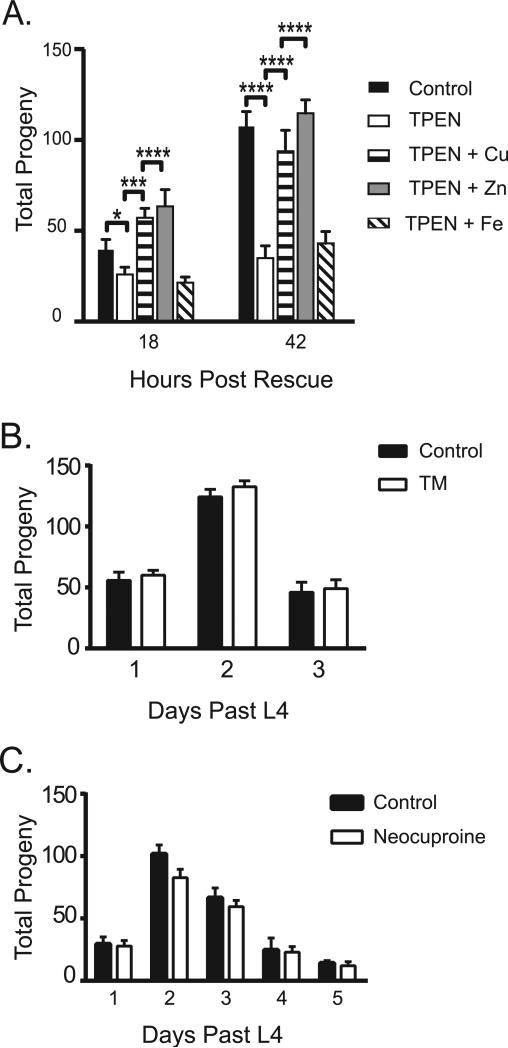
Given that TPEN can bind copper, zinc and iron ions with high affinity (Martell and Smith, 1998a), we next tested which of these essential transition metal ions gave rise to the limitation phenotype using rescue experiments as described previously (Kim et al., 2010). After incubating L4 stage hermaphrodites for 6 h on TPEN-containing plates, we moved the worms to TPEN plates supplemented with either copper, zinc, or iron salts, and scored the resulting brood size. While copper or zinc supplementation returned the brood size to near normal levels 48 h after rescue, iron supplementation did not restore the brood size to control levels at any point, indicating that the TPEN phenotype at this concentration does not result from iron-limitation (Fig. 3A). While the observed rescue upon copper addition could indicate that the effects of TPEN are due to copper limitation, since TPEN has a higher affinity for Cu(II) (Martell and Smith, 1998b) (10−19 M compared to 10−16 M for Zn), an alternative possibility is that the added copper is preferentially sequestering TPEN in the media and thus blocking its ability to reduce zinc availability in the rescue experiment. To distinguish between these possibilities, the effect of copper limitation on brood size was directly evaluated using two copper-specific chelators (10 μM ammonium tetrathiolmolybdate or 10 μM Neocuproine; Martell and Smith, 1998b). Neither treatment led to reduced brood size relative to controls (Fig. 3B,C), consistent with the hypothesis that the effects we observed following TPEN treatment were due to zinc limitation.
Fig. 3.
Fertility defects are likely due to zinc insufficiency. (A) Total number of progeny produced by untreated worms or worms treated with 10 μM TPEN for 6 h, and then supplemented with either Cu, Zn, or Fe in the growth media. (B–C) The total number of progeny measured from worms treated with known copper-specific chelators ammonium tetrathiomolybdate (b) or Neocuproine (c). Fertility was measured daily after the L4 molt. Error bars represent the standard error of mean of measurements. **p < 0.005, ***p < 0.0001 by Student's t-test.
3.2. Oocytes are more vulnerable to zinc insufficiency than sperm
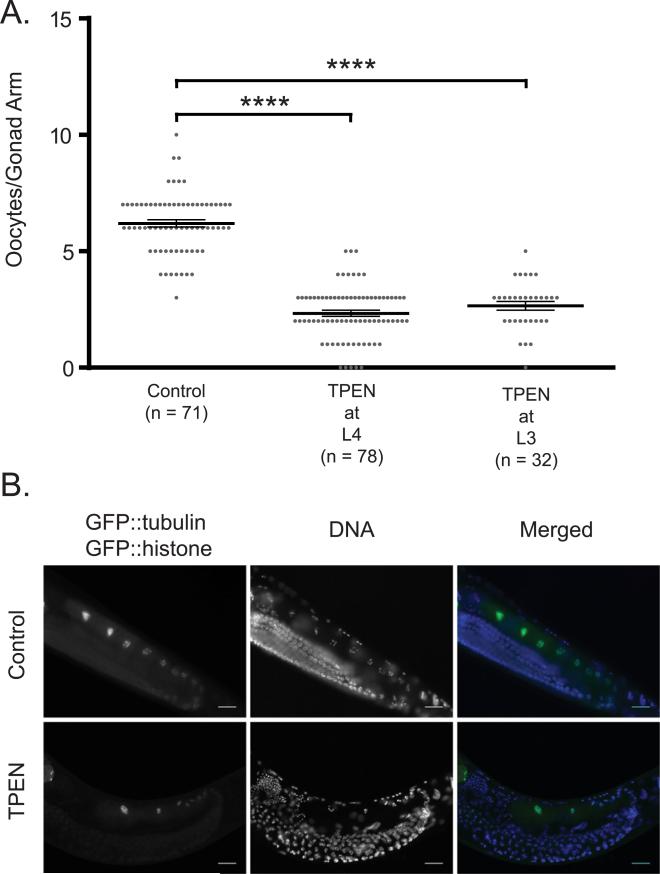
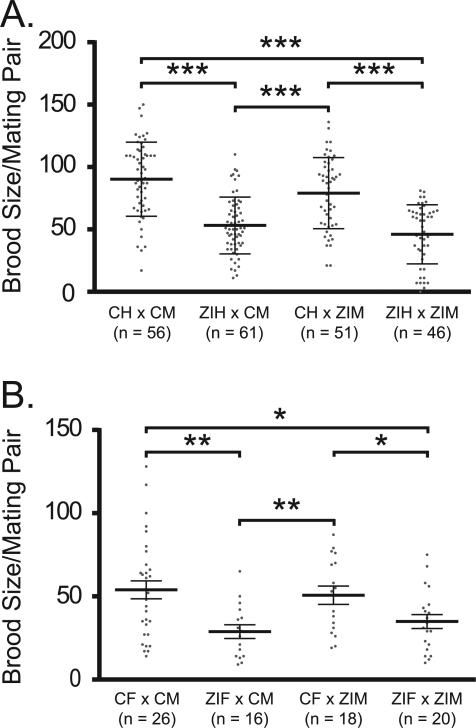
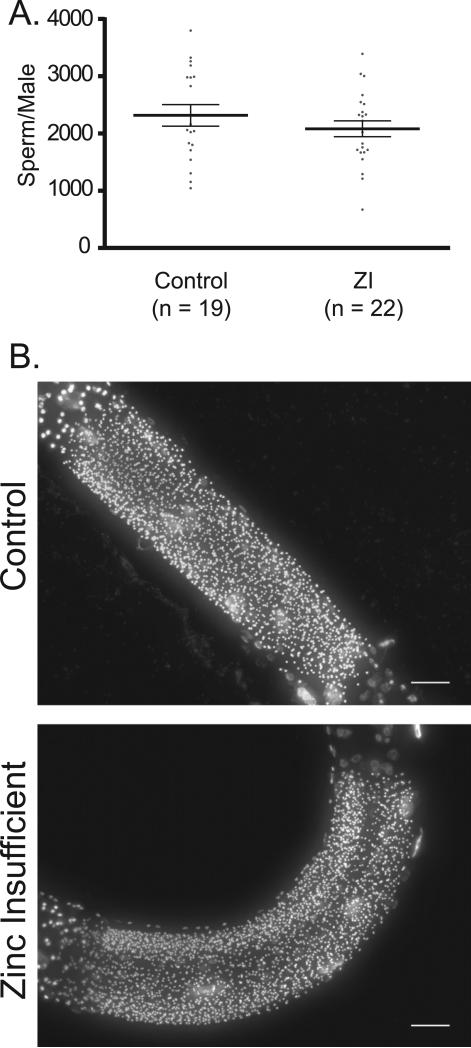
To establish whether the brood size defect under this zinc insufficiency condition is the result of impaired development of sperm, oocytes, or both, hermaphrodites were subjected to zinc insufficient conditions prior to oocyte formation (McCarter et al., 1999). Hermaphrodites exposed to zinc insufficient conditions in either L3 or L4 and then scored as young adults exhibit a significant reduction in the number of oocytes compared to the controls (Fig. 4), consistent with the interpretation that reduced zinc availability impacts oocyte production. In contrast, under the same conditions sperm production showed smaller differences that vary with stage (Fig. S6). While hermaphrodites exposed to zinc insufficient conditions in L3 show a small reduction in the number of sperm per gonad arm, exposure to 10 μM TPEN in late L4 did not cause a reduction in sperm number (Fig. S6). These results show that the large decrease in brood size of animals grown on 10 μM TPEN is the result of effects on oocyte rather than sperm production. To more directly test this idea, control and TPEN treated worms were mated in pairs in which each gamete (either oocyte or sperm) was exposed to zinc insufficient conditions prior to mating (Fig. 5). For these experiments, males were mated with either WT L4 hermaphrodites (prior to the time when they would begin to generate self-progeny) or with feminized fog-1(q253) mutant animals that do not produce sperm. A significant brood size reduction was measured each time the oocyte-bearing animals were exposed to zinc insufficiency. To determine whether zinc insufficiency has any effects on sperm production in males, male sperm was counted in control and zinc insufficient groups; no significant difference was observed (Fig. 6).
Fig. 4.
C. elegans oocyte production is sensitive to perturbations in zinc levels. (A) Distribution of oocytes in gonad arms of untreated worms or worms treated with 10 μM TPEN at either the L3 or L4 stage and then scored as young adults. N represents the number of gonad arms measured. p < 0.0001 for L3 and L4. (B) Fluorescence images of worms expressing GFP::histone and GFP::tubulin, showing the number of oocytes in a gonad arm of either untreated or TPEN treated worms. The left column shows the GFP signal, the center column is DNA stained with Hoechst, and the right column is a colored merge. Note that GFP expression increases near the proximal end of the gonad, highlighting the fully-formed oocytes, while Hoechst labels all nuclei in the gonad and in the somatic cells of the worm. Scale bar is 20 μm.
Fig. 5.
Oocytes are significantly more vulnerable to zinc insufficiency than sperm. (A) C. elegans hermaphrodites untreated (control hermaphrodite = CH) or treated with 10 μM TPEN (zinc insufficient hermaphrodite = ZIH) were mated with untreated (control male = CM) or TPEN treated males (zinc insufficient male = ZIM). Analysis by 2-way ANOVA p value was p < 0.00001. (B) Feminized C. elegans (fog-1(q253)) either untreated (control female = CF) or TPEN treated (zinc insufficient female = ZIF) were mated with untreated (CM) or TPEN treated (ZIM) males. One hermaphrodite or female was paired with one male per mating plate, mated for 24 h, and the total progeny was counted 24 h later. N represents number of breeding pairs examined. Analysis by 2-way ANOVA: p = 0.0003. Also shown are results of post hoc pairwise analysis by Student's t-test, where *p < 0.05, **p < 0.001, ***p < 0.00001. Bars in the graph and error bars represent the mean and standard deviation of the distribution.
Fig. 6.
Mild zinc insufficiency has a small impact on C. elegans male sperm counts. (A) Distribution of sperm counts in gonad arms of either control males or males treated with 10 μM TPEN (zinc insufficient = ZI). N represents the number of gonad arms measured. Bars and error bars represent the mean and standard deviation of the distribution, respectively. The difference in the distributions was found to be statistically insignificant by Student's t-test. (B) Representative images of fixed C. elegans males stained with Hoechst, demarking the sperm. Control or zinc insufficient males (10 μM TPEN) do not have visually different numbers of sperm. Scale bar is 20 μm.
3.3. Zinc insufficient oocytes experience spindle defects and hyperploidy during the meiosis to mitosis transition
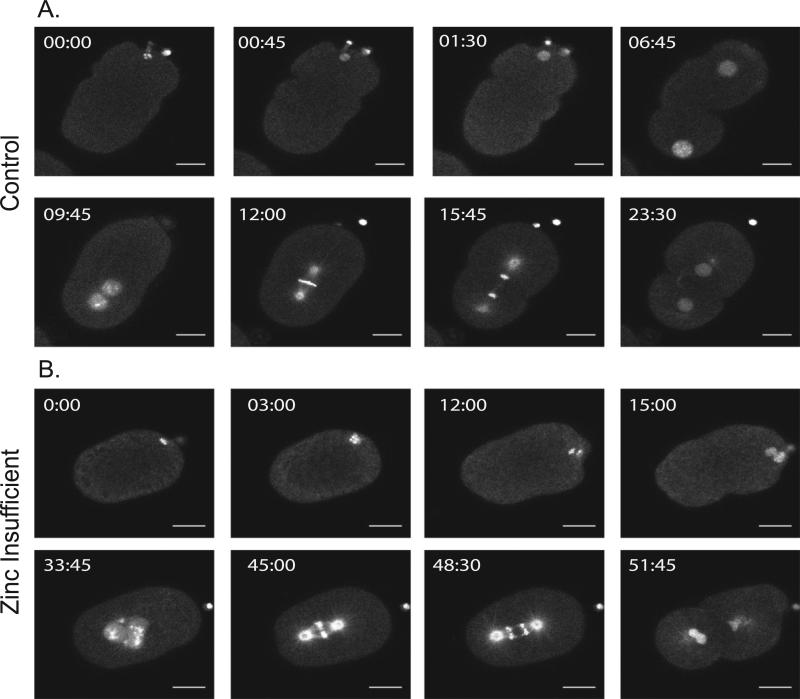
To assess whether zinc insufficiency has effects on the meiotic and mitotic divisions, independent of effects on events earlier in the germline, zygotes from untreated worms were dissected into media containing 10 μM TPEN. This treatment profoundly altered several steps in the transition from meiosis to mitosis after fertilization. Time lapse images of embryos expressing GFP::tubulin and GFP::histone (to label microtubules and chromosomes, respectively) revealed that zygotes treated with 10 μM TPEN often did not extrude a second polar body at the end of Meiosis II but instead formed an extra female pronucleus (Fig. 7B and Supplemental movies 7B.1, 7B.2); these two female pronuclei then migrated toward the male pronucleus and upon nuclear envelope breakdown, a spindle formed. However, these spindles exhibited defects. In control embryos (Fig. 7A and supplemental movies 7A.1, and 7A.2), spindles orient along the long axis of the embryo and then initiate anaphase (Gonczy et al., 1999; Oegema and Hyman, 2006). However, in the TPEN treated embryos, we observed spindles that initiated anaphase but exhibited lagging chromosomes, demonstrating that TPEN affects the fidelity of chromosome segregation (Fig. 7B and supplemental movies 7B.1,7B.2). TPEN treatment of three additional zygotes revealed similar effects. In one occasion, a zygote did not extrude the second polar body with normal timing during Telophase II. Instead, the zygote later pinched off the second large polar body within the extra embryonic space, between the cytoplasm and the eggshell (approximately 8 min after the beginning of pronuclear migration; Supplemental movies S7B.1-S7B.6), suggesting that it is sometimes possible for the cell to overcome polar body retention. In an extreme case, another zygote successfully extruded both polar bodies, but they were later pulled back into the cytoplasm, and the zygote arrested during pseudocleavage (Supplemental Movie S8B). The zygote formed a “popcorn” shape, the pronuclei never migrated properly, and the two polar bodies remained near the edge of the cytoplasm (Figs. S8B, C, and supplemental movies S8B.1-S8B.6). In contrast, the control zygote was able to undergo pseudocleavage during a similar time frame and the pronuclei were able to migrate normally (Fig. S8A, and supplemental movies S8A.1-S8A.6). Therefore, TPEN treatment has severe effects on the completion of the meiotic divisions and results in mitotic defects.
Fig. 7.
Acute zinc insufficiency causes defects in the meiotic and mitotic divisions. Example time lapse movies of embryos dissected from untreated worms into media containing 10 μM TPEN; worms are expressing GFP::tubulin and GFP::histone to visualize spindle dynamics. Acute exposure to TPEN causes dramatic defects in meiosis and mitosis. In (A), the second polar body is properly extruded at 45 s. One maternal and one paternal pronucleus are apparent (06:45). They properly migrate, fuse and the metaphase spindle forms (12:00). The cell then successfully divides to the 2-cell stage. In (B), the second polar body fails to extrude (15:00). This is followed by the appearance of two maternal pronuclei, which join the male pronucleus (33:45). The mitotic spindle forms (45:00) and chromosomes segregate (48:30), but exhibit lagging chromosomes. Scale bar = 10 μm, n = 5 in the control group, and n = 6 in the TPEN treated group.
4. Discussion
Zinc fluxes are known to both regulate oocyte maturation and the egg-to-embryo transition in mammals (Kim et al., 2011, 2010), however little is known about whether zinc availability plays any role in invertebrate germ cell function. Guided by results showing that TPEN-induced zinc insufficiency controls meiotic progression in oocytes as well as the egg-to-embryo transition in mouse (Kim et al., 2011; Kong et al., 2012), we show that C. elegans reproductive function is similarly sensitive to zinc limitation. We found that this reproductive phenotype was robust and showed that it most likely arises from the ability of TPEN to restrict zinc, as opposed to copper and iron availability. We also find that acute treatment of isolated zygotes with TPEN disrupts polar body extrusion, affects chromosome segregation, and potentially affects cell cycle progression. The observed defects lead to severe aneuploidy in the embryos, pointing to a crucial role for zinc in meiotic and mitotic fidelity. Specifically, the late meiotic and early mitotic divisions in the C. elegans embryo are highly sensitive to physiologically- or pharmacologically-induced changes in zinc availability, consistent with zinc limitation experiments in mouse oocyte development and fertilization. Aneuploidy in mammals is already linked to impairment of gamete development (Hall et al., 2006; Hunt and Hassold, 2008), and we demonstrate here that aneuploidy occurring in C. elegans impairs gamete development as well. The fact that eggs laid by zinc-deficient animals are able to hatch, whereas isolated embryos dissected into TPEN-media have severe defects, highlights an interesting difference between these two conditions. In the case of an intact worm, the fact that they are still able to produce viable progeny (albeit fewer than wildtype) suggests that the adult worms are still able to utilize zinc either from the food or from zinc stores within the body. Isolated embryos cannot do this, so we suspect that the defects in polar body extrusion and mitosis arise from the inability of the isolated egg to acquire zinc from another source. Finally, we demonstrate how this approach to zinc limitation can be exploited in mating experiments, a critical feature for future experiments that will exploit the powerful genetic opportunities in this model organism to identify pathway members that mediate zinc signaling events.
Perhaps most significantly, these studies expand our understanding of inorganic regulatory pathways across evolution. Our prior work provides insights into mouse, monkey and human meiotic progression where egg development occurs during embryonic development and terminal meiosis is triggered in the adult. These early studies of zinc limitation in the worm (Davis et al., 2009; Roh et al., 2013, 2012, 2015) enable the design of more mechanistic studies that can resolve how putative zinc signaling events operate in an organism that depends on stem cell generation of oocytes. Our results confirm that zinc uptake is necessary for the generation of quality oocytes in this context as well.
Canonical zinc trafficking and zinc-responsive transcription mechanisms are known in single cell organisms (Bird et al., 2000, 2004, 2003, 2006; Evans-Galea et al., 2003; Frey et al., 2011; Gilston et al., 2014; Herbig et al., 2005; Wu et al., 2009) but are just beginning to be mapped in multicellular organisms. Many signs point to specialized zinc-responsive pathways in the early stages of development in mammals such as those involving Emi2 control of APC/C activity in meiosis (Bernhardt et al., 2011; Suzuki et al., 2010a). Given our new results showing zinc-dependence at related stages in invertebrate meiotic and mitotic cell cycles, we plan to test the speculative idea that zinc was coopted very early in evolution as a meiotic transition signal. The ease of scoring these zinc-dependent phenotypes in the worm is important for future genetic screens that probe the mechanistic basis of this regulation. An inorganic signal, such as zinc fluxes across membranes or changes in availability inside the cell during a time of reductive division and in the absence of appreciable transcription, is an elegant solution to the advancement of the meiotic cell cycle. Additional studies will illuminate the specific receptors, transporters and mechanisms by which zinc is mobilized and deployed in a stage specific manner.
Supplementary Material
Acknowledgements
We acknowledge C. Schiffer and S. Cheung for technical assistance. We thank A. Sue and S. Siepka for assistance in data analysis and manuscript preparation, and the Morimoto lab for providing the worm strain fog-1(q253). Some strains were provided by the CGC, which is funded by the NIH Office of Research Infrastructure Programs (P40 OD010440). Imaging work was performed at the Northwestern University Biological Imaging Facility generously supported by the Chemistry of Life Processes Institute and the NU Office for Research. Confocal microscopy was performed on a Leica TCS SP5 laser scanning confocal microscope system purchased with funds from the NU Office for Research. Metal analysis was performed at the Northwestern University Quantitative Bio-element Imaging Center with support from NASA Ames Research Center Grant NNA04CC36G.
Funding
This research was supported by National Institutes of Health grants GM038784 (TVO) and GM115848 (TVO & TKW), the Chicago Biomedical Consortium with support from the Searle Funds at The Chicago Community Trust (SMW, AM), and training grants T32CA009560 and F31GM112478 in support of AM.
Abbreviations
- TPEN
N,N,N′,N′-tetrakis(2-pyridylmethyl)ethylenediamine
- NGM
Nematode growth media
- ZI
Zinc insufficient
- TM
Ammonium tetrathiomolybdate
- CH
Control Hermaphrodite
- CM
Control male
- CF
Control female
- ZIH
zinc insufficient hermaphrodite
- ZIM
zinc insufficient male
- ZIF
zinc insufficient female
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.cbpc.2016.09.007.
References
- Bernhardt ML, Kim AM, O'Halloran TV, Woodruff TK. Zinc requirement during meiosis I-meiosis II transition in mouse oocytes is independent of the MOS-MAPK pathway. Biol. Reprod. 2011;84:526–536. doi: 10.1095/biolreprod.110.086488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyersmann D, Haase H. Functions of zinc in signaling, proliferation and differentiation of mammalian cells. Biometals. 2001;14:331–341. doi: 10.1023/a:1012905406548. [DOI] [PubMed] [Google Scholar]
- Bird A, Evans-Galea MV, Blankman E, Zhao H, Luo H, Winge DR, Eide DJ. Mapping the DNA binding domain of the Zap1 zinc-responsive transcriptional activator. J. Biol. Chem. 2000;275:16160–16166. doi: 10.1074/jbc.M000664200. [DOI] [PubMed] [Google Scholar]
- Bird AJ, McCall K, Kramer M, Blankman E, Winge DR, Eide DJ. Zinc fingers can act as Zn2+ sensors to regulate transcriptional activation domain function. EMBO J. 2003;22:5137–5146. doi: 10.1093/emboj/cdg484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird AJ, Blankman E, Stillman DJ, Eide DJ, Winge DR. The Zap1 transcriptional activator also acts as a repressor by binding downstream of the TATA box in ZRT2. EMBO J. 2004;23:1123–1132. doi: 10.1038/sj.emboj.7600122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird AJ, Swierczek S, Qiao W, Eide DJ, Winge DR. Zinc metalloregulation of the zinc finger pair domain. J. Biol. Chem. 2006;281:25326–25335. doi: 10.1074/jbc.M600655200. [DOI] [PubMed] [Google Scholar]
- Bohnsack BL, Hirschi KK. Nutrient regulation of cell cycle progression. Annu. Rev. Nutr. 2004;24:433–453. doi: 10.1146/annurev.nutr.23.011702.073203. [DOI] [PubMed] [Google Scholar]
- Corsi AK, Wightman B, Chalfie M. WormBook, editor. A transparent window into biology: a primer on Caenorhabditis elegans. The C.Elegans Research Community. 2015 doi: 10.1895/wormbook.1.177.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis DE, Roh HC, Deshmukh K, Bruinsma JJ, Schneider DL, Guthrie J, Robertson JD, Kornfeld K. The cation diffusion facilitator gene cdf-2 mediates zinc metabolism in Caenorhabditis elegans. Genetics. 2009;182:1015–1033. doi: 10.1534/genetics.109.103614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan FE, Que EL, Zhang N, Feinberg EC, O'Halloran TV, Woodruff TK. The zinc spark is an inorganic signature of human egg activation. Sci. Rep. 2016;6:24737. doi: 10.1038/srep24737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans-Galea MV, Blankman E, Myszka DG, Bird AJ, Eide DJ, Winge DR. Two of the five zinc fingers in the Zap1 transcription factor DNA binding domain dominate site-specific DNA binding. Biochemistry. 2003;42:1053–1061. doi: 10.1021/bi0263199. [DOI] [PubMed] [Google Scholar]
- Frey AG, Bird AJ, Evans-Galea MV, Blankman E, Winge DR, Eide DJ. Zinc-regulated DNA binding of the yeast Zap1 zinc-responsive activator. PLoS One. 2011;6:e22535. doi: 10.1371/journal.pone.0022535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilston BA, Wang S, Marcus MD, Canalizo-Hernandez MA, Swindell EP, Xue Y, Mondragon A, O'Halloran TV. Structural and mechanistic basis of zinc regulation across the E. coli Zur regulon. PLoS Biol. 2014;12:e1001987. doi: 10.1371/journal.pbio.1001987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonczy P, Schnabel H, Kaletta T, Amores AD, Hyman T, Schnabel R. Dissection of cell division processes in the one cell stage Caenorhabditis elegans embryo by mutational analysis. J. Cell Biol. 1999;144:927–946. doi: 10.1083/jcb.144.5.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenstein D. Control of oocyte meiotic maturation and fertilization. WormBook; 2005. pp. 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase H, Maret W. The regulatory and signaling functions of zinc ions in human cellular physiology. Cell. Mol. Biol. Met. 2010:181–212. [Google Scholar]
- Hall H, Hunt P, Hassold T. Meiosis and sex chromosome aneuploidy: how meiotic errors cause aneuploidy; how aneuploidy causes meiotic errors — commentary. Curr. Opin. Genet. Dev. 2006;16:323–329. doi: 10.1016/j.gde.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Herbig A, Bird AJ, Swierczek S, McCall K, Mooney M, Wu CY, Winge DR, Eide DJ. Zap1 activation domain 1 and its role in controlling gene expression in response to cellular zinc status. Mol. Microbiol. 2005;57:834–846. doi: 10.1111/j.1365-2958.2005.04734.x. [DOI] [PubMed] [Google Scholar]
- Hunt PA, Hassold TJ. Human female meiosis: what makes a good egg go bad? Trends Genet. 2008;24:86–93. doi: 10.1016/j.tig.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Kim AM, Vogt S, O'Halloran TV, Woodruff TK. Zinc availability regulates exit from meiosis in maturing mammalian oocytes. Nat. Chem. Biol. 2010;6:674–681. doi: 10.1038/nchembio.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim AM, Bernhardt ML, Kong BY, Ahn RW, Vogt S, Woodruff TK, O'Halloran TV. Zinc sparks are triggered by fertilization and facilitate cell cycle resumption in mammalian eggs. ACS Chem. Biol. 2011;6:716–723. doi: 10.1021/cb200084y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimble J, Crittenden SL. Controls of germline stem cells, entry into meiosis, and the sperm/oocyte decision in Caenorhabditis elegans. Annu. Rev. Cell Dev. Biol. 2007;23:405–433. doi: 10.1146/annurev.cellbio.23.090506.123326. [DOI] [PubMed] [Google Scholar]
- Kong BY, Bernhardt ML, Kim AM, O'Halloran TV, Woodruff TK. Zinc maintains prophase I arrest in mouse oocytes through regulation of the MOS-MAPK pathway. Biol. Reprod. 2012;87(11):11–12. doi: 10.1095/biolreprod.112.099390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong BY, Duncan FE, Que EL, Kim AM, O'Halloran TV, Woodruff TK. Maternally-derived zinc transporters ZIP6 and ZIP10 drive the mammalian oocyteto-egg transition. Mol. Hum. Reprod. 2014;20:1077–1089. doi: 10.1093/molehr/gau066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- L'Hernault SW. Spermatogenesis. WormBook; 2006. pp. 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lints R, Hall DH. Male Reproductive System, Germ Line. WormAtlas; 2009a. [Google Scholar]
- Lints R, Hall DH. Reproductive System, Germ Line. WormAtlas; 2009b. [Google Scholar]
- Lints R, Hall DH. Male Introduction. WormAtlas; 2009c. [Google Scholar]
- Lints R, Hall DH. Reproductive System, Germline. WormAtlas; 2009d. [Google Scholar]
- MacNeil LT, Watson E, Arda HE, Zhu LJ, Walhout AJ. Diet-induced developmental acceleration independent of TOR and insulin in C. elegans. Cell. 2013;153:240–252. doi: 10.1016/j.cell.2013.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martell AE, Smith RM. Plenum. New York: 1998a. NIST Critical Stability Constants of Metal Compexes. [Google Scholar]
- Martell AE, Smith RM. Plenum. New York: 1998b. NIST critical stability constants of metal complexes. NIST Standard Reference Database 46, v. 5.0. [Google Scholar]
- McCarter J, Bartlett B, Dang T, Schedl T. On the control of oocyte meiotic maturation and ovulation in Caenorhabditis elegans. Dev. Biol. 1999;205:111–128. doi: 10.1006/dbio.1998.9109. [DOI] [PubMed] [Google Scholar]
- Oegema K, Hyman AA. Cell division. WormBook; 2006. pp. 1–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Que EL, Bleher R, Duncan FE, Kong BY, Gleber SC, Vogt S, Chen S, Garwin SA, Bayer AR, Dravid VP, Woodruff TK, O'Halloran TV. Quantitative mapping of zinc fluxes in the mammalian egg reveals the origin of fertilization-induced zinc sparks. Nat. Chem. 2015;7:130–139. doi: 10.1038/nchem.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh HC, Collier S, Guthrie J, Robertson JD, Kornfeld K. Lysosome-related organelles in intestinal cells are a zinc storage site in C. elegans. Cell Metab. 2012;15:88–99. doi: 10.1016/j.cmet.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh HC, Collier S, Deshmukh K, Guthrie J, Robertson JD, Kornfeld K. ttm-1 encodes CDF transporters that excrete zinc from intestinal cells of C. elegans and act in a parallel negative feedback circuit that promotes homeostasis. PLoS Genet. 2013;9:e1003522. doi: 10.1371/journal.pgen.1003522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh HC, Dimitrov I, Deshmukh K, Zhao G, Warnhoff K, Cabrera D, Tsai W, Kornfeld K. A modular system of DNA enhancer elements mediates tissue-specific activation of transcription by high dietary zinc in C. elegans. Nucleic Acids Res. 2015;43:803–816. doi: 10.1093/nar/gku1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiernagle T. Maintenance of C. elegans. WormBook; 2006. pp. 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Suzuki E, Yoshida N, Kubo A, Li H, Okuda E, Amanai M, Perry AC. Mouse Emi2 as a distinctive regulatory hub in second meiotic metaphase. Development. 2010a;137:3281–3291. doi: 10.1242/dev.052480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Yoshida N, Suzuki E, Okuda E, Perry AC. Full-term mouse development by abolishing Zn2+-dependent metaphase II arrest without Ca2+ release. Development. 2010b;137:2659–2669. doi: 10.1242/dev.049791. [DOI] [PubMed] [Google Scholar]
- Tian X, Diaz FJ. Zinc depletion causes multiple defects in ovarian function during the periovulatory period in mice. Endocrinology. 2012;153:873–886. doi: 10.1210/en.2011-1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian X, Diaz FJ. Acute dietary zinc deficiency before conception compromises oocyte epigenetic programming and disrupts embryonic development. Dev. Biol. 2013;376:51–61. doi: 10.1016/j.ydbio.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian X, Anthony K, Neuberger T, Diaz FJ. Preconception zinc deficiency disrupts postimplantation fetal and placental development in mice. Biol. Reprod. 2014;90:83. doi: 10.1095/biolreprod.113.113910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Stetina JR, Orr-Weaver TL. Developmental control of oocyte maturation and egg activation in metazoan models. Cold Spring Harb. Perspect. Biol. 2011;3:a005553. doi: 10.1101/cshperspect.a005553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wignall SM, Villeneuve AM. Lateral microtubule bundles promote chromosome alignment during acentrosomal oocyte meiosis. Nat. Cell Biol. 2009;11:839–844. doi: 10.1038/ncb1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wormbase T, H., editor. fog-1. 2016a [Google Scholar]
- Wormbase Strain. 2016b;N2 [Google Scholar]
- Wu CY, Roje S, Sandoval FJ, Bird AJ, Winge DR, Eide DJ. Repression of sulfate assimilation is an adaptive response of yeast to the oxidative stress of zinc deficiency. J. Biol. Chem. 2009;284:27544–27556. doi: 10.1074/jbc.M109.042036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Kuhn JR. Cell isolation and culture. WormBook; 2013. pp. 1–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Duncan FE, Que EL, O'Halloran TV, Woodruff TK. The fertilization-induced zinc spark is a novel biomarker of mouse embryo quality and early development. Sci. Rep. 2016;6:22772. doi: 10.1038/srep22772. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.