Abstract
Within the past decade tremendous efforts have been made to understand the mechanism behind aquaporin-2 (AQP2) water channel trafficking and recycling, to open a path toward effective diabetes insipidus therapeutics. A recent study has shown that integrin-linked kinase (ILK) conditional-knockdown mice developed polyuria along with decreased AQP2 expression. To understand whether ILK also regulates AQP2 trafficking in kidney tubular cells, we performed in vitro analysis using LLCPK1 cells stably expressing rat AQP2 (LLC-AQP2 cells). Upon treatment of LLC-AQP2 cells with ILK inhibitor cpd22 and ILK-siRNA, we observed increased accumulation of AQP2 in the perinuclear region, without any significant increase in the rate of endocytosis. This perinuclear accumulation did not occur in cells expressing a serine-256-aspartic acid mutation that retains AQP2 in the plasma membrane. We then examined clathrin-mediated endocytosis after ILK inhibition using rhodamine-conjugated transferrin. Despite no differences in overall transferrin endocytosis, the endocytosed transferrin also accumulated in the perinuclear region where it colocalized with AQP2. These accumulated vesicles also contained the recycling endosome marker Rab11. In parallel, the usual vasopressin-induced AQP2 membrane accumulation was prevented after ILK inhibition; however, ILK inhibition did not measurably affect AQP2 phosphorylation at serine-256 or its dephosphorylation at serine-261. Instead, we found that inhibition of ILK increased F-actin polymerization. When F-actin was depolymerized with latrunculin, the perinuclear located AQP2 dispersed. We conclude that ILK is important in orchestrating dynamic cytoskeletal architecture during recycling of AQP2, which is necessary for its subsequent entry into the exocytotic pathway.
Keywords: aquaporin-2, integrin-linked kinase, kidney tubular cells, diabetes insipidus
maintaining tight control over body fluid homeostasis is critical for human health. The kidney collecting duct system is the primary component that regulates water reabsorption and final urinary concentration. One of the key players during this process is aquaporin-2 (AQP2), a water channel expressed in the principal cells (PCs) of kidney collecting ducts (CDs) (44). Diabetes insipidus (DI) is a condition characterized by a reduction in water reabsorption in the CDs, which leads to an excessive excretion of very dilute urine (polyuria). This is due either to lack of centrally secreted vasopressin, a condition referred to as central diabetes insipidus (central DI), or the lack of response of CD principal cells to vasopressin, nephrogenic diabetes insipidus (NDI) (20, 31, 45). Within the last few decades, there has been considerable progress in understanding the mechanism behind AQP2 trafficking and recycling during body fluid homeostasis. Binding of vasopressin to the vasopressin type 2 receptor (V2R) located on the basolateral membrane of CD principal cells activates adenosine 3′,5′-cyclic monophosphate (cAMP) production which, in turn, activates protein kinase A (PKA). AQP2 is phosphorylated at serine-256, 264 and 269 and dephosphorylated at serine-261. These events, combine to increase the duration of AQP2 residence in the apical membrane of principal cells to mediate water reabsorption (4, 25, 33).
Dynamic changes in cytoskeletal architecture are an important determinant of AQP2 trafficking (12, 20, 49). There are three types of cytosolic fibers: microtubules, microfilaments (F-actin), and intermediate filaments (10). The cytoskeletal architecture not only permits the trafficking of AQP2-bearing vesicles, but it also controls and limits their passage to the plasma membrane. Numerous studies have shown that vasopressin depolymerizes F-actin-containing fibers in parallel with an increased accumulation of AQP2 at the plasma membrane (7, 12, 19).
Integrin-linked kinase (ILK) is a multidomain β1-integrin tail-binding protein and a central component of cell–matrix adhesions that play an important role in cytoskeleton regulation and coupling of extracellular matrix (ECM) to the actin cytoskeleton and signaling complexes (11, 48). ILK associates with intracellular signaling cascades through binding partners such as AKT, a kinase phosphorylated directly by ILK at serine-473 (27, 35) that was previously reported to play a role in AQP2 regulation (15, 18, 36). A role for ILK in membrane trafficking pathways and cytoskeleton rearrangements has been well established. In particular, inhibition of ILK can affect cytoskeletal rearrangement, leading to mislocalization of cell cargo (26, 46).
We discovered quite recently that AQP2 and β1-integrin interact, and our study suggested, therefore, an association between the ECM and integrin signaling on AQP2 trafficking (6). However, it is not well understood how ECM-integrin modulates AQP2 trafficking. Interestingly, a recent study by Cano-Peñalver and coworkers (5) showed that ILK also plays a role in regulating water reabsorption in the kidney. They observed that ILK conditional knockdown (cKD-ILK) adult mice had polyuria and a decreased urine osmolality when compared with littermates with normal ILK expression. However, no changes were observed in the serum level of arginine-vasopressin (AVP) or the expression of V2R or AQP3 in the kidney, suggesting that the role ILK plays is likely downstream of the V2R receptor. Although an effect of ILK on AQP2 trafficking was suggested, the cellular mechanism involved was not investigated. Therefore, we have now performed a series of in vitro experiments to study the effect of ILK on the trafficking of AQP2 in LLCPK1 cells stably expressing rat AQP2 (LLC-AQP2 cells), a long-established in vitro system for AQP2 trafficking studies (1, 16, 17, 32, 38). Our data suggest that ILK regulates AQP2 recycling, specifically its subsequent entry into the exocytotic pathway, by modulating actin cytoskeletal architecture.
MATERIALS AND METHODS
Chemicals and reagents.
Unless otherwise stated, all chemicals and reagents were acquired from Sigma (St. Louis, MO). ILK inhibitor Calbiochem-Compound 22 (cpd22) (21) was purchased from EMD Chemicals (San Diego, CA) and ILK-siRNA-[5′-AAGGACACAUUCUGGAAGGGG-3′] was obtained from GE Dharmacon (Lafayette, CO). To modulate ILK expression with ILK-SiRNA, cells were transfected with ILK-SiRNA (80μM) + 3 μl Lipofectamine 2000 in 1 ml Opti-MEM for 5 h. Experiments were carried out 48 h after ILK-siRNA incubation. To modulate ILK function with cpd22, cells were serum-starved for 1 h following a 60 min cpd22 (2 μM) incubation in serum-free medium. Latrunculin B (428020, Calbiochem) was used at a 0.1 μM final concentration for 30 min. Methyl-β-cyclodextrin (MβCD) was used at (10 mM) final concentration for 30 min. Unless otherwise stated, lysine vasopressin (vasopressin) was used at 10 nM final concentration. Transferrin from human serum, Alexa Fluor 568 conjugate was obtained from Life Technologies (Carlsbad, CA). Rabbit polyclonal actin antibody (a4967) (1:20,000) and Alexa Fluor 555 phalloidin #8953 (1:1,000) were purchased from Cell Signaling Technology (Danvers, MA). Rabbit polyclonal anti- Rab11 antibody (1:200) was from BD Biosciences (San Jose, CA). AQP2 polyclonal goat antibody (sc-9882) (1:5,000) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit antibodies recognizing AQP2 phosphorylation sites serine-256 and 261 were gifts from Dr. Mark Knepper at National Institutes of Health (NIH) or purchased from PhosphoSolutions (Aurora, CO) and used at 1:3,000 final concentrations.
Animals.
All animal experiments conducted conform to the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Massachusetts General Hospital Institutional Committee on Research Animal Care and by the Institutional Animal Care and Use Committee of the University of Alcalá and conformed to Directive 2010/63/EU of the European Parliament. To generate cKD-ILK, C57Bl/6 mice homozygous for floxed ILK flanked by loxP (LOX) were mated to mice carrying tamoxifen (TX)-inducible CreER(T) recombinase (CRE) (34). Male CRE-LOX mice (8 wk old) were injected intraperitoneally with 1.5 mg of TX (Sigma-Aldrich, St. Louis, MO) or vehicle for 5 consecutive days, to induce ILK deletion. Three weeks after the injections, tail DNA was genotyped by PCR with primers corresponding to excised ILK gene (230 bp) or to nonexcised ILK (2100 bp): 5′-CCAGGTGGCAGAGGTAAGTA-3′ and 5′-CAAGGAATAAGGTGAGCTTCAGAA-3′ (26).
Kidney slice culture.
Kidney slice culture was performed as previously described (2). In brief, 3 mo old male wild-type C57BL/6 mice were anesthetized with isofluorane. Kidneys were harvested, and 0.5-1.0 mm slices were carefully cut with a Stadie-Riggs slicer (Thomas Scientific, Swedesboro, NJ), washed twice, and incubated at 37°C in Hanks' balanced salt solution (HBSS) (pH 7.4, with 5% CO2) for 15 min. After equilibration, the slices were incubated in HBSS containing chemicals: HBSS alone as a control for 75 min or 2.5 μM of cpd22 in HBSS for 75 min for ILK inhibition. A cocktail of 1 μM arginine-vasopressin (AVP) + 10 μM forskolin was added to some controls, and ILK inhibited slices for 30 min before the end of incubation. After incubation, all of the samples were immersed in 4% PLP (paraformaldehyde-lysine-periodate) fixative overnight. PLP-fixed kidney slices were washed three times for a total of 3 h in a rocker with PBS. Slices were incubated in PBS with 30% sucrose overnight at 4°C, embedded in Tissue-Tek OCT compound 4583 (Sakura Finetek USA, Torrance, CA). We used the CM3050S cryostat (Leica Microsystems, Bannockburn, IL) to cut 5 μm sections, collected them onto Superfrost Plus microscope slides (Fisher Scientific, Pittsburgh, PA), and stored them at −20°C until use.
Cells culture endocytosis and exocytosis assay.
LLC-PK1 cells stably expressing wild-type c-myc-tagged aquaporin-2 (LLC-AQP2 cells) (17), yellow fluorescent protein (ssYFP) (32), or neither were cultured at 37°C in a 5% CO2 atmosphere in DMEM, Corning/Cellgro (Manassas, VA) + 10% FBS + 2 mg/ml Glu + penicillin and streptomycin 100 IU/ml, with the addition of 1 mg/ml G418 where applicable. Unless otherwise stated, cells were grown either on coverslips or on standard P6 polystyrene falcon culture dishes (Corning Science) to a 70–80% confluence. All cell lines were free of mycoplasma and viruses; contamination was tested by PCR. For endocytosis assays, cells were grown on glass coverslips in a 12-well cell culture-treated plate (BD, Franklin Lakes, NJ) and grown to 80% confluence. To modulate ILK function, cells were serum-starved for 1 h and then treated with cpd22 (2 μM) for 1 h. To some groups vasopressin (10 nM) was added for 15 min before the end of incubation. To perform the fluid phase endocytosis assay, cells were incubated in serum-free DMEM for 1 h followed by serum-free DMEM containing 1.5 mg/ml dialyzed Alexa 555-dextran for no more than 15 min at 37°C. To perform the transferrin endocytosis assay, fluorescein-rhodamine-conjugated transferrin was prepared to a final concentration of 20–25 μg/ml in DMEM without phenol red, containing 1% BSA. Cells were washed two times with ice-cold PBS and incubated with fluorescein-rhodamine-conjugated transferrin for a maximum of 20 min at 37°C. After incubation cells were washed immediately with ice-cold PBS, fixed, and visualized under a fluorescence microscope (80i Nikon). The exocytosis assay was performed as described previously, by measuring secreted ssYFP using a multimode plate reader (32).
Immunofluorescence and confocal microscopy.
After treatment, cells grown on coverslips were fixed in 4% PFA in PBS + 50 g/l sucrose (PFS) for 20 min. Fixed coverslips were permeabilized in 0.1% Triton X-100 for 4 min before being washed three times with PBS. For tissues, cryosections on slides were rehydrated with PBS and treated with 1% SDS for 4 min for antigen retrieval. AQP2 was detected using mouse anti-c-myc (A-14) polyclonal antibody (1:100, Santa Cruz Biotechnology) and a secondary anti-mouse IgG conjugated to FITC (25 μg/ml, Jackson ImmunoResearch Laboratories). Immunofluorescence was performed as previously reported. Evaluation was done with a Nikon 80i or Nikon A1 confocal microscope (Nikon Instruments, Melville, NY). ImageJ (NIH; https://imagej.nih.gov/ij/) or Volocity 5.0 (Perkin Elmer, Waltham, MA) software was used for analysis of the images. Background corrections and contrast/brightness enhancement were performed identically for all images in the same experiment.
F-actin quantification assay.
F-actin quantification assay was performed as described previously (49). Briefly, 8×104 LLC-AQP2 cells were plated on 24-well plates and incubated in normal DMEM. After 2 days of incubation, the DMEM was replaced with HBSS, and cells were incubated for 2 h. Some cells were treated with vasopressin (10 nM) + forskolin (10 μM) for 30 min, latrunculin A (0.1 μM) for 30 min, or cpd22 (2 μM) for 1 h. After treatment, HBSS buffer was removed and cells were incubated in binding buffer containing phalloidin (20 mM KH2PO4, 10 mM PIPES, 5 mM EGTA, 2 mM MgCl2, 4% PFA, 0.1% TritonX-100, 250 nM rhodamine phalloidin) for 15 min at room temperature. Binding buffer was removed and cells were washed four times with PBS, incubated in methanol overnight at −20°C to extract bound rhodamine-phalloidin. The methanol containing extracted rhodamine-phalloidin was read using a Beckman DTX-880 multiplate reader (excitation 535 nm, emission 595 nm).
Cell lysate preparation and immunoblotting.
After treatments, cells were rinsed three times with ice-cold DPBS GIBCO (New York, NY) and lysed in 1X PBS lysis buffer (8 mM NaF, 0.5% NP-40, 4 mM Na3VO4, 0.1% Triton X-100) supplemented with protease and phosphatase inhibitor cocktail (Biotool, Houston, TX) for 20 min. Cells were then passed five times through a 27-gauge syringe followed by centrifugation at 6,000 for 10 min. The supernatant (protein lysate) concentration was measured and standardized according to the Pierce BCA Protein Assay Kit (Pierce Biotechnology, Rockford, IL). Immunoblotting was performed as previously reported here (24).
Statistical analysis.
Unless otherwise stated, data are means ± SE of a variable number of experiments, detailed in the figure legends. All statistics were analyzed with an unpaired Student’s t-test for two groups or one-way ANOVA for more using GraphPad Prism version 6.00 for MacIntosh, (San Diego, CA). In some datasets, multiple-comparison posttests were performed. P values < 0.05 were considered statistically significant (*P < 0.05. **P < 0.01, ***P < 0.001, ****P < 0.0001).
RESULTS
ILK inhibition leads to accumulation of AQP2 in the perinuclear region.
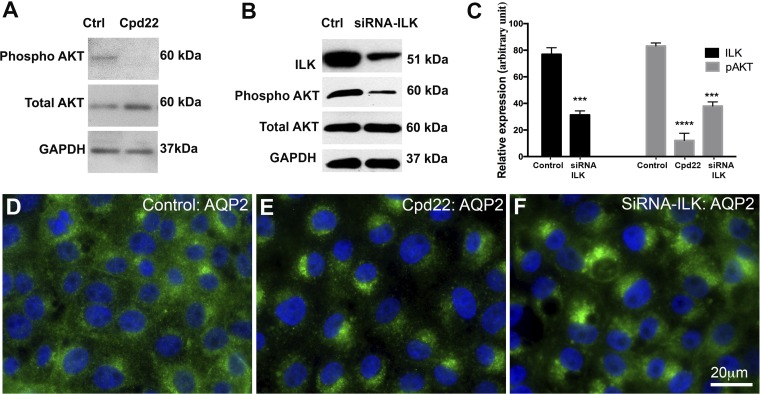
To mechanistically understand the role of ILK in AQP2 trafficking, we inhibited ILK using a chemical inhibitor (cpd22) and performed ILK-siRNA knock-down on an LLCPK-1 cell line stably expressing AQP2 (LLC-AQP2 cells). After 60 min of cpd22 treatment, AQP2 accumulated in the perinuclear region with almost no accumulation in the plasma membrane. We noticed that this patch accumulates only on one side of the nucleus. To further confirm this we transfected LLC-AQP2 cells with ILK-SIRNA. We observed AQP2 perinuclear accumulation 48 h after transfection with ~85% of cells showing partial or dense perinuclear patches (Fig. 1). Interestingly, under our acute treatment condition, AQP2 expression was not significantly altered in ILK-siRNA knockdown cells 48 h posttransfection. However, between 72 and 96 h after ILK-siRNA transfection, there was an ∼50% decrease in AQP2 protein expression (data not shown), which is consistent with data obtained from ILK conditional knockout animals (5).
Fig. 1.
Inhibition of integrin-linked kinase (ILK) with both chemical inhibitor cpd22 (2 μM) for 60 min and ILK-siRNA knockdown (48 h) leads to perinuclear accumulation of aquaporin-2 (AQP2). A: Akt phosphorylation at Ser-473 (pAKT serine-437) was reduced by cpd22 in our LLC-AQP2 cells. Inhibition of ILK suppresses the phosphorylation of the ILK substrate AKT at s473 (8, 34, 35). B: the expression of ILK in cells was reduced 48 h after ILK-siRNA transfection. Similar to cpd22, it reduced the phosphorylation of the ILK substrate AKT at serine-473. C: a bar graph quantification of A and B. D: immunofluorescence image showing baseline AQP2 expression and distribution in normal mock-treated (transfection medium + lipofectamine only) LLC-AQP2 cells. E: immunofluorescence image showing a perinuclear accumulation of AQP2 60 min after cpd22 treatment. F: immunofluorescence image showing a perinuclear accumulation of AQP2 48 h after ILK-siRNA treatment. Approximately 85% of the cells showed either partial or complete accumulation of AQP2 at the perinuclear, with ~70% of them showing a complete (tight) accumulation. This confirmed the results seen with chemical inhibitor cpd22. Scale = 20 μm; Green, AQP2; blue, nucleus. Bar values represent ± SE. ***P < 0.001, ****P < 0.0001, n ≥ 3, control vs. treatment. Statistics were done by unpaired Student’s t-test.
ILK inhibition blocks vasopressin-induced plasma membrane expression of AQP2.
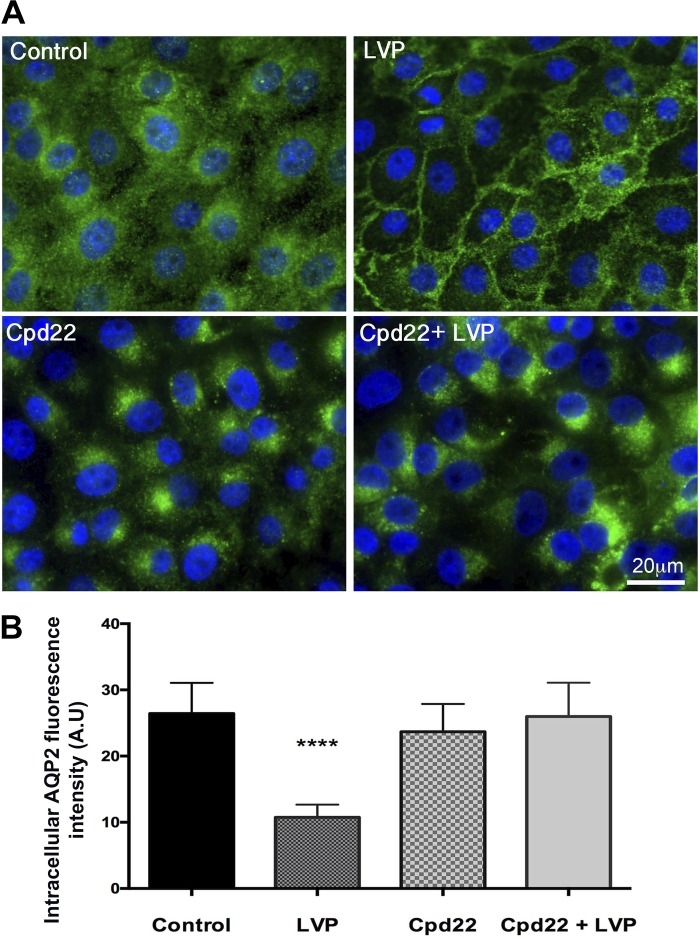
V2R activation by vasopressin normally induces the accumulation of AQP2 in the plasma membrane of target epithelial cells, including LLC-AQP2 cells (17). We asked, therefore, whether ILK inhibition has any effect on vasopressin-stimulated AQP2 trafficking. LLC-AQP2 cells were pretreated with cpd22 for 45 min, and then VP was added. In the control cells, vasopressin treatment caused significant membrane accumulation as previously reported (25). In contrast, vasopressin stimulation was not able to induce membrane accumulation of AQP2 after ILK inhibition, and the AQP2 remained in a perinuclear patch. This result shows that vasopressin signaling is not able to rescue the perinuclear-located AQP2 when ILK is inhibited (Fig. 2).
Fig. 2.
A: immunofluorescence staining showing the baseline distribution of AQP2 in control cells and the accumulation of AQP2 in the plasma membrane after 15 min of vasopressin treatment. ILK inhibition with Cpd22 blocked vasopressin-induced membrane accumulation of AQP2 and a perinuclear patch of AQP2 developed in these cells. Scale = 20 μm. Green, AQP2; blue, nucleus. B: intracellular AQP2 fluorescence intensity arbitrary units (AU), showing a significant decrease in intracellular AQP2 in LLC-AQP2 cells treated with vasopressin (LVP) but not in LLC-AQP2 cells treated with vasopressin after Cpd22 treatment. Bar values represent ± SE. ****P < 0.0001, n ≥ 3. Statistics were done by 1-way ANOVA. The mean of each column was compared with control mean.
Inhibition of ILK alters the distribution of clathrin-mediated endocytosed vesicles without an effect on total endocytosis.
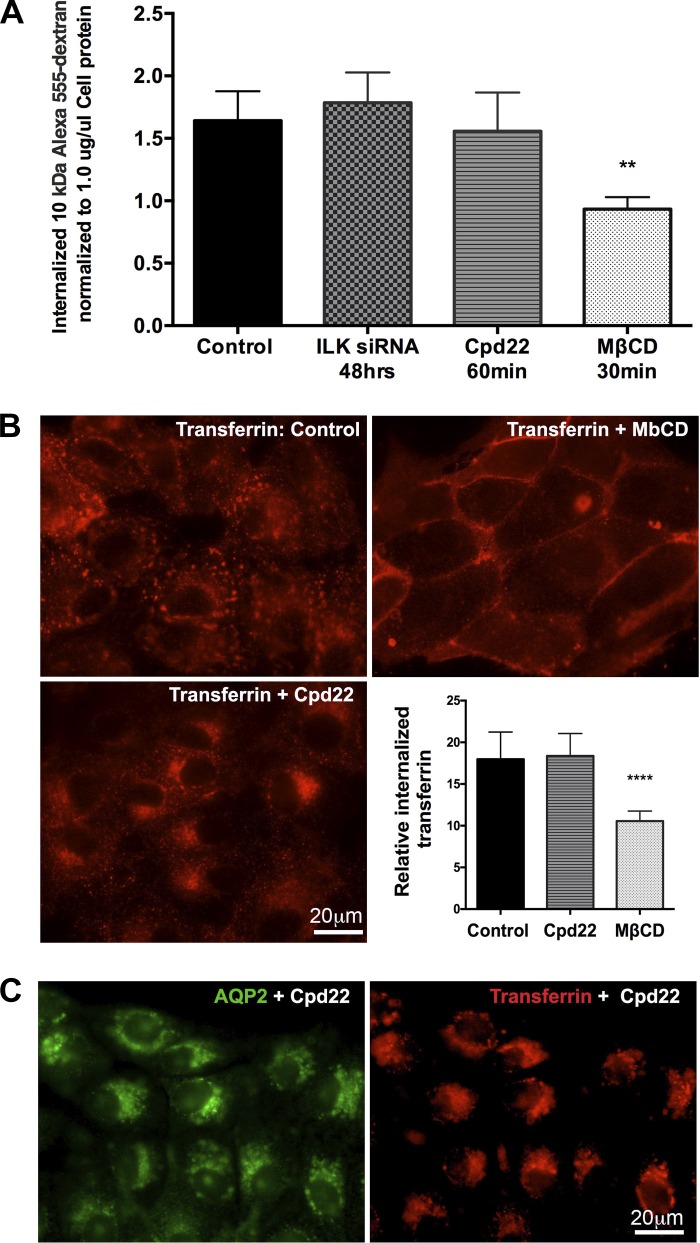
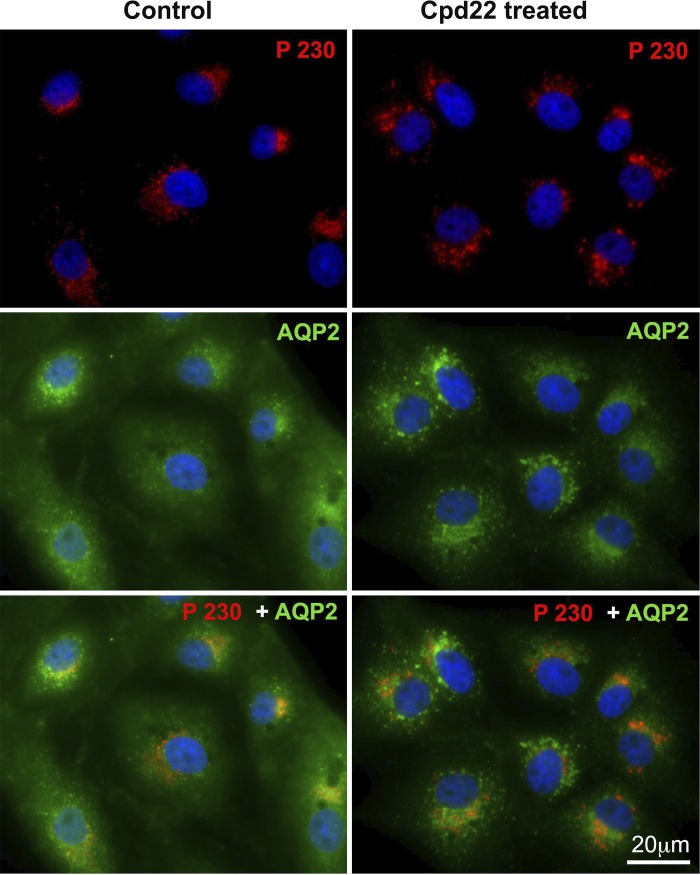
We then investigated whether the perinuclear accumulation of AQP2 after ILK inhibition was due to an increase in AQP2 endocytosis and/or blockade of the exocytosis pathway. We performed a fluorescence-based 10 kDa Alexa 555-dextran fluid phase endocytosis assay with MβCD as a positive control. MβCD is a cholesterol-depleting agent that blocks endocytosis (39). Our results showed no significant difference in the uptake of Alexa 555-dextran between control and cells treated with cpd22 or ILK-siRNA (Fig. 3A). Because we did not find any change in the bulk endocytosis rate after ILK inhibition, we followed clathrin-mediated endocytosis after ILK inhibition more specifically using a rhodamine-transferrin endocytosis assay. This assay takes the advantage that both AQP2 and transferrin receptor relocate from the plasma membrane into the intracellular vesicles via a dynamin-dependent, clathrin-mediated endocytosis pathway (14, 40). Despite no obvious difference in overall endocytosis in the presence or absence of ILK inhibition, the endocytosed rhodamine-transferrin also accumulated at the perinuclear region after ILK inhibition, similar to our findings with AQP2 (Fig. 3B). Moreover, double imaging of AQP2 and the endocytosed transferrin after ILK inhibition showed that both colocalized within the same perinuclear patch (Fig. 3C). To further assess whether ILK inhibition also affects AQP2 recycling vesicles, we performed a coimmunofluorescence of AQP2 and the recycling endosome marker Rab11 (29, 40). We found that the perinuclear patch was immunostained with Rab11 upon ILK inhibition by cpd22 or after ILK-siRNA knockdown (Fig. 4). We also found that the perinuclear trapped AQP2 colocalized poorly with the trans-Golgi network marker p230 (Fig. 5). Taken together, our data suggest that ILK inhibition likely redirects the clathrin-mediated, endocytotic vesicles with their cargoes (including AQP2 and /or transferrin) to a recycling compartment that accumulates in the perinuclear region.
Fig. 3.
A: a bar graph showing fluid phase endocytosis assay results of internalized fluorescence of 10 kDa Alexa 555-dextran. There was no significant difference in endocytosis of 10 kDa Alexa 555-dextran in LLC-AQP2 cells after ILK inhibition by both siRNA and cpd22. A block of endocytosis with methyl-β-cyclodextrin (MβCD) was used as a positive control and significantly inhibited 10 kDa Alexa 555-dextran endocytosis compared with any of the treatments. B: the clathrin endocytosis pathway was assessed by a rhodamine-conjugated transferrin endocytosis assay, which showed accumulation of transferrin at the perinuclear region in cells treated with ILK inhibitor cpd22 when compared with controls. However, quantification of the endocytosed transferrin revealed no difference in the overall endocytosis rate between controls and ILK inhibited cells. A block of endocytosis with MβCD was used as a positive control. C: double staining showing colocalization of AQP and endocytosed transferrin in the perinuclear patch after ILK inhibition, suggesting that clathrin-endocytosed cargo is accumulated at the perinuclear region upon ILK inhibition. Scale = 20 μm. Green, AQP2; red, transferrin. Bar values represent ± SE. **P < 0.01, **** P < 0.0001, n ≥ 3. Statistics were done by 1-way ANOVA. The mean of each column was compared with control mean.
Fig. 4.
Immunofluorescence staining showing that Rab11 also accumulates in the perinuclear region after ILK inhibition. Control cells stained with Rab11 or AQP2 or both show a baseline scattered distribution of both AQP2 and Rab11, while those treated with cpd22 or ILK siRNA show a colocalization of both AQP2 and Rab11 in the perinuclear region. Scale, 20 μm. Green, AQP2; red, Rab11.
Fig. 5.
Immunofluorescence staining of AQP2 and trans-Golgi network marker (p230) showing no changes in p230 distribution after ILK inhibition. The accumulated perinuclear AQP2 after ILK inhibition localized poorly with p230. Scale = 20 μm. Green, AQP2; red, p230.
ILK inhibition-associated perinuclear accumulation only targets endocytosed AQP2.
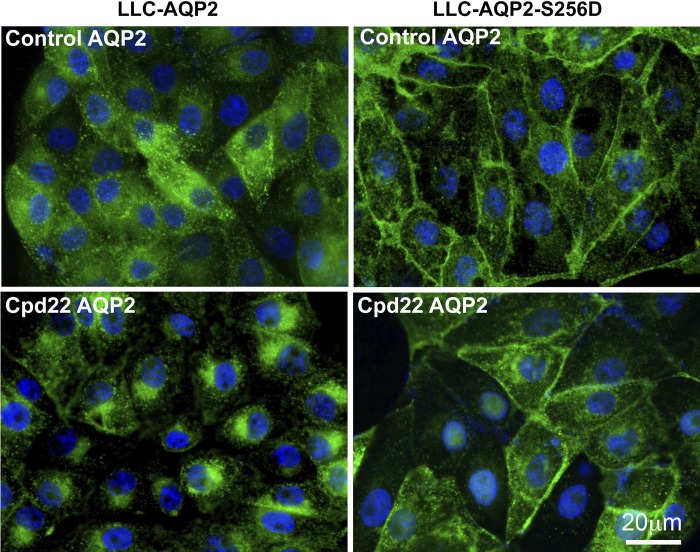
We then examined whether the perinuclear patch formation depends on AQP2 internalized by endocytosis from the cell surface. To achieve this we took advantage of LLCPK1 cells with a point mutation substituting aspartic acid (D) for serine (S) on the COOH terminus of AQP2 at serine-256, LLC-AQP2-S256D (38). In the baseline state, this cell line retains AQP2 at the plasma membrane because its rate of endocytosis is significantly reduced compared with wild-type LLC-AQP2. However, there is still an appreciable amount of AQP2 on vesicles inside the cytoplasm. In these LLC-AQP2-S256D-expressing cells, we did not find a similar perinuclear accumulation of AQP2 after ILK inhibition, even though some AQP2 was present on vesicles in these cells. Furthermore, ILK inhibition failed to increase endocytosis of the previously membrane-bound AQP2 in LLC-AQP2-S256D cells, which have a very low rate of endocytosis (38). Our data suggest, therefore, that ILK inhibition increases perinuclear accumulation of newly endocytosed AQP2, and not by recruiting all cellular AQP2-contining vesicles into the patch (Fig. 6).
Fig. 6.
The perinuclear accumulation of AQP2 after 60 min of cpd22 treatment was also reexamined and in LLC-AQP2 cells and LLCPK1 cells stably expressing a S256→256D phosphorylation mutant, LLC-AQP2-S256D. After 60 min of cpd22 treatment, immunofluorescence revealed that LLC-AQP2-S256D cells did not develop a perinuclear patch after ILK inhibition, in contrast to cells expressing wild-type AQP2. AQP2 was present on the plasma membrane and some intracellular structures in LLC-AQP2-S256D cells before and after cpd22 treatment. Scale = 20 μm. Green, AQP2; blue, nucleus.
Inhibition of ILK inhibits vasopressin-induced exocytosis without affecting baseline exocytosis.
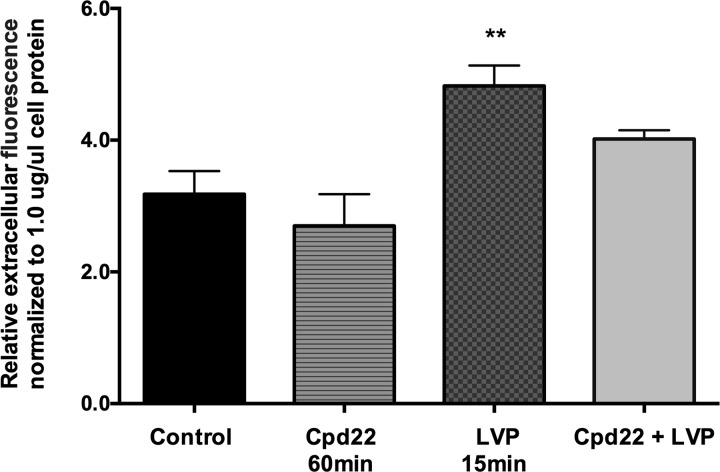
Since we found that ILK inhibition did not affect the rate of endocytosis, we next examined whether it affects exocytosis. To do this, we applied a fluorimetric exocytosis assay, as previously reported (32). By measuring secreted soluble YFP in cells (which is packaged into vesicles in the exocytotic pathway), we found no difference in the overall exocytosis at the baseline level between control cells and cells treated with ILK inhibitor. However, after vasopressin stimulation for 15 min, we observed a burst of exocytosis in control cells as shown previously, and this vasopressin triggered a burst of exocytosis was blocked by ILK inhibition (Fig. 7).
Fig. 7.
Effect of ILK inhibition on exocytosis. A graph shows the relative soluble, secreted yellow fluorescent protein ssYFP from LLCPK1 cell line that stably expresses a ssYFP (LLCPK1-AQP2-ssYFP). Relative ssYFP extracellular medium fluorescence values from each well were read immediately after collection using a multimode plate reader (model DTX880; Beckman-Coulter). Higher fluorescence values represent higher rates of exocytosis. There was a significant increase in exocytosis in cells treated with vasopressin; however, this vasopressin-stimulated exocytosis was blunted by ILK inhibitor cpd22. Bar values represent ± SE. ** P < 0.01, n ≥ 3. Statistics were done by 1-way ANOVA. The mean of each column was compared with control mean.
ILK inhibition does not prevent vasopressin-mediated phosphorylation of AQP2.
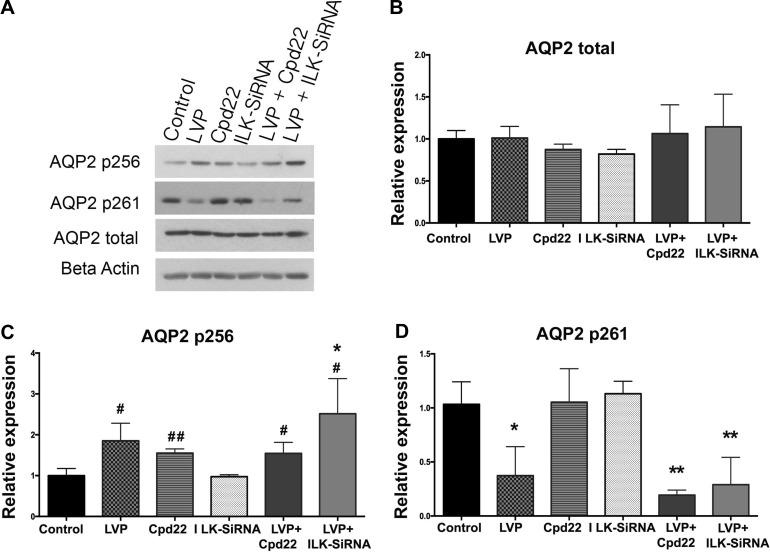
AQP2 phosphorylation is critically important for AQP2 trafficking and its regulation (9, 25, 47). Therefore, we examined the phosphorylation of AQP2 at the two well-studied key residues, serine-256 and serine-261, in the presence of ILK inhibition using AQP2 phosphorylation-specific antibodies, a generous gift from Dr. Mark A. Knepper. It has been shown that AQP2 serine-256 phosphorylation is both necessary and sufficient to cause membrane accumulation of AQP2. In addition, phosphorylation of residues serine-264 and serine-269 increases after vasopressin/forskolin stimulation and that of serine-261 decreases. It is believed that only phosphorylation of AQP2 at serine-256 and (perhaps) dephosphorylation of serine-261 are essential for AQP2 membrane accumulation and that serine-269 phosphorylation plays an additional role in retarding the endocytosis of plasma membrane AQP2 (1, 6, 9, 13, 28, 30, 37). Our data indicate that ILK inhibition by either cpd22 or IKL-siRNA does not impede vasopressin-induced serine-256 phosphorylation or serine-261 dephosphorylation after ILK inhibition. LLC-AQP2 cells treated with cpd22 or ILK-siRNA were still capable of increasing AQP2 serine-256 phosphorylation and serine-261 dephosphorylation in response to vasopressin (Fig. 8).
Fig. 8.
A: representative Western blotting showing no pattern changes in the levels of total AQP2 (25 kDa) and AQP2 phosphorylation residues serine-256 (25 kDa) and serine-261(25 kDa) in both cpd22 and ILK-siRNA LLC-AQP2 cells after vasopressin stimulation. Graphs show quantification of Western blotting results of total AQP2 (B), AQP2 p256 (C), AQP2 p261 (D). Bar values represent ± SE. *P < 0.05, **P < 0.01; n ≥ 3. Statistics were done by 1-way ANOVA, and the mean of each column was compared with control mean. Additional Student t-test analysis was performed to compare individual treated sample mean to the control mean. Bar values represent ± SE. #P < 0.05, ##P < 0.01; n ≥ 3.
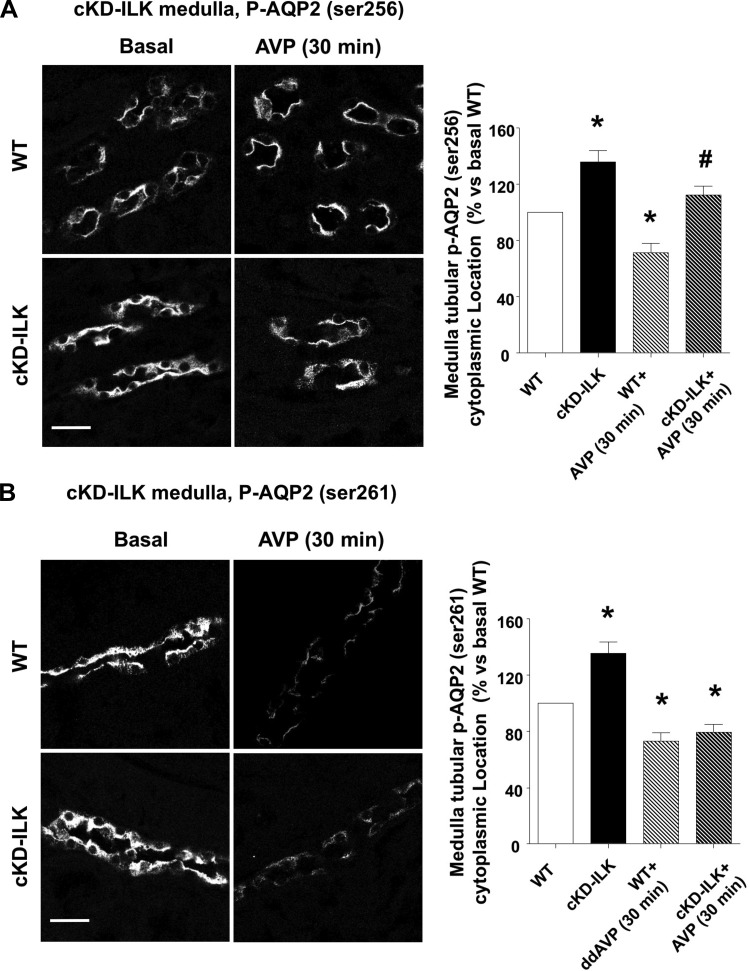
Furthermore, we wanted to reconfirm this phenomenon by specifically examining cKD-ILK mice, which we previously reported (5). We performed ex vivo AVP treatments in both cKD-ILK and wild-type mice and examined the expression and subcellular location of both phosphorylated AQP2 isoforms (serine-256 and serine-261). Under basal conditions, we found that both serine-256 and serine-261 phosphorylated AQP2 isoforms were relatively equally expressed in both cKD-ILK and wild-type CDs but more accumulated in the cytoplasm in cKD-ILK CDs as compared with wild type (Fig. 9, A and B). In addition, after 30 min of AVP treatment, only the wild-type CDs showed accumulation of the serine-256 phosphorylated AQP2 isoform in the apical membrane (Fig. 9A). Similar to what we observed in LLC-AQP2 cells treated with cpd22 or ILK-siRNA, both cKD-ILK and wild-type CDs showed a significant reduction of serine-261 phosphorylated AQP2 in response to vasopressin (Fig. 9B). In summary, while ILK inhibition prevents vasopressin-induced membrane accumulation of AQP2, it does not prevent vasopressin-induced phosphorylation of AQP2 at serine-256 or dephosphorylation at serine-261.
Fig. 9.
Subcellular location of phosphorylated AQP2 isoforms (serine-256 and serine-261) in ILK conditional-knockdown (cKD-ILK) adult mice. Renal slices from wild-type (WT) and cKD-ILK mice were treated ex vivo with arginine-vasopressin (AVP) (100 nM) or vehicle (basal) for 30 min. Representative immunofluorescence-labeled microscopy images from renal medullas and the analysis of the phosphorylated AQP2 isoforms distribution in the tubular cell cytoplasm are shown. Values are represented as means ± SE (% vs. basal WT). *P < 0.05 vs. basal WT, #P < 0.05 vs. AVP-treated WT; n ≥ 3. Scale bars = 20 μm.
F-actin depolymerization disperses the perinuclear accumulation of AQP2 formed after ILK inhibition.
The previous experiments suggest that ILK inhibition corrupts the AQP2 trafficking pathway after AQP2 endocytosis and prevents its entry into the usual exocytotic pathway leading to plasma membrane insertion of AQP2. However, this inhibition does not occur by altering the AQP2 phosphorylation state at two key residues. Multiple studies have shown that ILK is essential for actin cytoskeletal organization (3) and that F-actin depolymerization is necessary for AQP2 plasma membrane accumulation (7, 12, 19). We hypothesized that the perinuclear AQP2 accumulation after ILK inhibition might be associated with a disruption of the cytoskeleton that could involve a complex interplay of actin polymerization and depolymerization. We, therefore, performed immunofluorescence staining of F-actin on cells treated with the ILK inhibitor cpd22 using rhodamine-conjugated phalloidin. We observed an increase in F-actin polymerization after ILK inhibition in these treated cells. When we depolymerized F-actin using latrunculin B 30 min after the initiation ILK inhibition, AQP2 was dispersed throughout the cytoplasm and the perinuclear patch was not formed (Fig. 10, top). This suggests that F-actin, especially in its polymerized state, is necessary for the formation and/or maintenance of the perinuclear accumulation of AQP2 mediated by ILK inhibition. Actin depolymerization releases the “trapped” AQP2 from this patch.
Fig. 10.
Top: immunostaining of F-actin by phalloidin and AQP2 showing actin depolymerization by latrunculin disperses the cpd22-induced perinuclear accumulation of AQP2. A: control cells showing diffuse AQP2 staining. B: ILK inhibitor cpd22 treated cells showing a bright patch of AQP2 near the nucleus. C: cells treated with cpd22 showing the dispersion of the AQP2 perinuclear patch after a further 25 min treatment with latrunculin. D: control cells showing typical F-actin fibers. E: cells showing increased F-actin staining in the cortical cytoplasm after 60 min of cpd22 treatment. F: F-actin depolymerization after 25 min treatment with latrunculin B. Scale = 20 µm. Green, AQP2; blue, nucleus; red, F-actin. Bottom: graph showing results of the F-actin quantification assay. Before subjecting them to an F-actin quantification assay, we treated cells with or without vasopressin/forskolin or cpd22 or both. Some cells were treated with cpd22 for 30 or 60 min. The F-actin quantification assay revealed that F-actin content decreased significantly after 20 min of vasopressin/forskolin treatment as previously reported (49). F-actin polymerization was detectable after only 30 min of cpd22 treatment and reached statistical significance after 60 min. However, cpd22 did not rescue F-actin depolymerization induced by vasopressin/forskolin. These results represent 3 different F-actin assays. F-actin content values were expressed as relative fluorescence after subtraction of the negative control values. Data were adjusted depending on cell protein concentration. Each fluorescence value was expressed as relative fluorescence unit (RFU)/protein (µg). Latrunculin B was used as the F-actin depolymerization positive control. Bar values represents ± SE. *P < 0.05, ****P < 0.001, n ≥ 3. Statistics were done by 1-way ANOVA, and the mean of each column was compared with the control mean.
Cpd22 significantly increases F-actin formation.
We next examined whether inhibiting ILK directly affects F-actin polymerization (Fig. 10, bottom). Earlier studies have shown that vasopressin depolymerizes F-actin, and our group showed that this vasopressin-mediated F-actin depolymerization actually depends on the presence of AQP2 in cells (49). Using an F-actin assay that has been reported previously (49), we confirmed the assay’s validity by depolymerizing F-actin with latrunculin B as a positive control. We found a small but significant decrease in the F-actin content of cells treated with vasopressin/forskolin for 30 min as we previously reported (49), and this occurred even in the cells that were first treated with ILK inhibitor cpd22 for 30 min. However, cells treated with cpd22 alone for 60 min showed a significant increase of F-actin formation. The finding that vasopressin was able to depolymerize F-actin even after cpd22 treatment indicates that the interplay between actin polymerization and depolymerization is complex and will require further investigation.
ILK inhibitor cpd22 inhibits vasopressin-associated AQP2 plasma membrane trafficking in mouse CD PCs.
Since we know that cKD-ILK mice kidney CD PCs showed more AQP2 accumulation in the cytoplasm as compared with wild type (5), we wanted to examine whether the ILK inhibitor-cpd22 would have a similar effect in wild-type mouse PCs. We found that mouse kidney slices treated with cpd22 had similar perinuclear accumulated AQP2 as seen in cKD-ILK mice kidney CD PCs. Moreover, as expected, control kidney slices showed an increase level AQP2 in CD PC apical membranes after AVP, while cpd22 inhibited this effect (Fig. 11). This is equivalent to what we observed in ILK inhibited LLC-AQP2 cells treated with vasopressin. Altogether, this supports the previously postulated idea that the polyuria seen in cKD-ILK mice is primarily caused by ILK inhibition, and it is independent of the canonical V2R-associated AQP2 regulation.
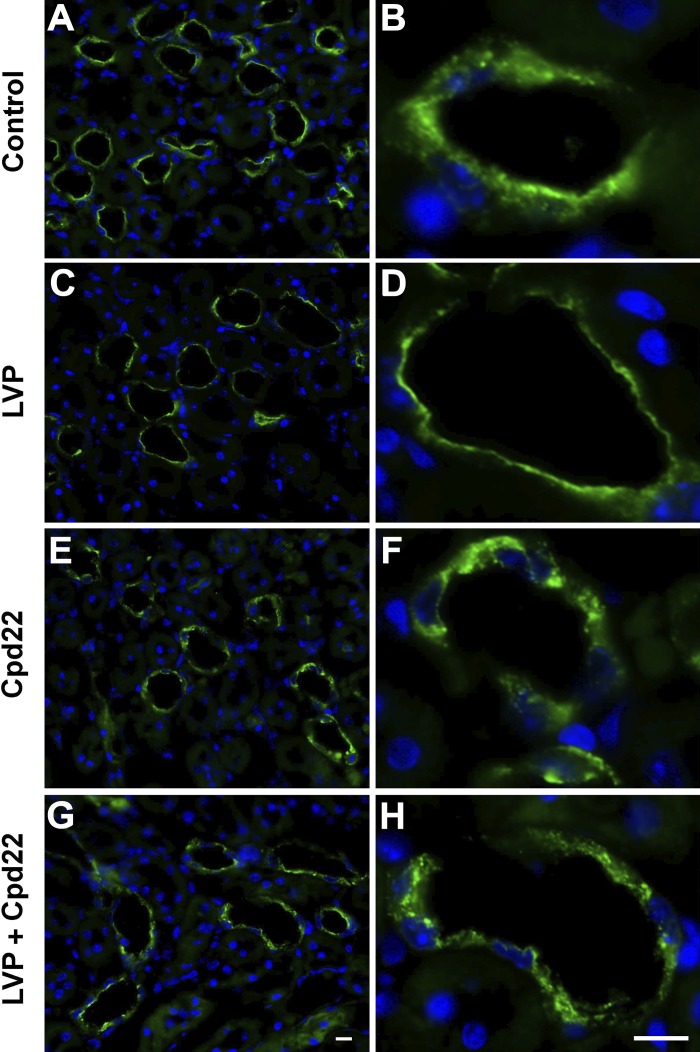
Fig. 11.
Immunofluorescence images showing cpd22 inhibits vasopressin-associated AQP2 plasma membrane accumulation in collecting ducts from wild-type mice kidney slices in vitro. A, B: control tissues treated with HBSS alone for 75 min. C, D: 45 min treatment of HBSS alone followed by 30 min AVP treatment, which caused accumulation of AQP2 on the apical plasma membrane. E, F: 75 min of cpd22 in HBSS treatment showing patches of AQP2 accumulation within principal cells. G, H: 30 min AVP in HBSS treatment after 45 min cpd22 treatment showing no accumulation of AQP2 in the membrane and the presence of large intracellular patches of AQP2. Scale = 15 µm. Green, AQP2; blue, nucleus.
DISCUSSION
We have demonstrated that inhibition of ILK function in LLC-AQP2 cells causes an accumulation of AQP2 in the perinuclear region and prevents the plasma membrane accumulation of AQP2 that is normally induced by vasopressin. Similar results were obtained by using the ILK inhibitor cpd22 or after ILK knockdown with siRNA. This perinuclear accumulation could be due to an increase in AQP2 endocytosis and a decrease in its exocytosis, leading to intracellular accumulation. Using our secreted yellow fluorescent protein (ssYFP) assay (32), we indeed found that the rate of exocytosis after ILK inhibition was reduced in these cells. However, a fluid phase endocytosis assay showed that the overall rate of endocytosis was not affected by ILK inhibition. Moreover, when we tracked the path of another endocytosed clathrin cargo molecule, transferrin, we found that it also accumulated in the perinuclear region after ILK inhibition. Because AQP2 is also internalized by clathrin-mediated endocytosis, this indicates that the intracellular trafficking of proteins that are internalized in this pathway is affected by ILK inhibition. Furthermore, Rab 11, which is known to interact with endocytosed AQP2 (41), also accumulated in the perinuclear region after ILK inhibition, indicating an effect on the recycling pathway. Importantly, the perinuclear accumulation of AQP2 after ILK inhibition appears to be mainly a result of the accumulation of newly endocytosed AQP2, since no such accumulation was seen after 60 min of ILK inhibition in cells expressing the S256→256D phosphorylation mutant of AQP2, which is internalized much more slowly than wild-type AQP2 (38). Altogether, this suggests ILK is involved in directing endocytosed AQP2 into the appropriate exocytotic recycling compartment from which it can access the plasma membrane either constitutively or after vasopressin stimulation.
In addition to inducing the perinuclear accumulation of AQP2, inhibition of ILK function also impaired the response to vasopressin both in cell cultures and in kidney tissue slices. Vasopressin is known to induce AQP2 apical membrane accumulation partly by increasing the phosphorylation of AQP2 serine residues, especially that of serine-256 (30). However, this failure of AQP2 to accumulate in the plasma membrane in response to vasopressin does not seem to be associated with its phosphorylation state, at least at the key residue serine-256, and also at serine-261. LLC-AQP2 cells treated with the ILK inhibitor cpd22 or ILK-siRNA both showed the appropriate increase in vasopressin-induced AQP2 serine-256 phosphorylation. Moreover, serine-261 dephosphorylation after vasopressin treatment occurred in both cpd22- and ILK-siRNA-treated cells. This was also observed in cKD-ILK mice kidneys. This implies that ILK inhibition did not function by preventing vasopressin-induced phosphorylation of AQP2 at serine-256 or dephosphorylation at serine-261, which prompted us to search for another potential mechanism to explain the effect of ILK inhibition.
Besides altering the phosphorylation state of AQP2 serine residues, vasopressin is also known to induce F-actin depolymerization, which is critically important in AQP2 trafficking (3, 7, 12, 19). Studies have shown that vasopressin inhibits RhoA-dependent actin polymerization, and this is necessary for AQP2 plasma membrane accumulation. Indeed, transfecting cells with dominant negative Rho A, or inhibiting its activity, induces AQP2 membrane accumulation even in the absence of vasopressin stimulation (19, 43). In addition, depolymerization of F-actin with statins also results in vasopressin-independent AQP2 membrane accumulation and an increase in urinary concentration in rodent models of diabetes insipidus (22, 42, 49). After ILK inhibition with cpd22, we found a significant increase in F-actin formation by phalloidin staining and with an F-actin polymerization assay. Thus, under baseline conditions, AQP2 may accumulate in a perinuclear compartment due to excessive actin polymerization after ILK inhibition. The perinuclear patch of AQP2 was completely dispersed following global actin depolymerization by latrunculin B.
However, and unexpectedly, vasopressin-induced F-actin depolymerization was not impaired by cpd22. Cells treated with cpd22 still responded to vasopressin by F-actin depolymerization, despite the fact that AQP2 did not accumulate at the cell surface. This result suggests that different F-actin pools are involved in AQP2 trafficking, one pool that is susceptible to depolymerization by vasopressin, perhaps involved in AQP2 exocytosis near the plasma membrane, while the second pool, which is increased after ILK inhibition, retains AQP2 in the perinuclear region and is not susceptible to vasopressin-induced depolymerization. This suggestion is supported by data showing that F-actin depolymerization induced by vasopressin occurs in the subplasma membrane cortical cytoskeleton, but that the bulk of cellular F-actin is resistant to vasopressin-induced depolymerization (12, 23). This local effect also explains why the effect of vasopressin in the F-actin depolymerization assay is relatively modest, with only ~10% of cellular actin being affected. Our data suggest, therefore, that ILK regulates AQP2 recycling after endocytosis and its subsequent entry into the exocytotic pathway by orchestrating changes in dynamic cytoskeletal architecture.
GRANTS
The Nephcure Foundation, NIH Grant R21 DK-092619, an ASN Gottschalk Research grant, and ECOR/MGH provided support for this work. The Microscopy Core Facility of the Program in Membrane Biology receives additional support from the Boston Area Diabetes and Endocrinology Research Center (NIH DK-57521) and the Center for the Study of Inflammatory Bowel Disease (NIH DK-43351). Additional material support was from NIH R01 DK-096586 (D. Brown). This work was also supported by grants from the Spanish National Program for Research, Development and Innovation and cofunded by the Instituto de Salud Carlos III and FEDER funds (PI/11/01630, PI/14/01939, PI/14/02075 and FEDER funds ISCIII RETIC REDinREN programs RD06/0016/0002 and RD12/0021/0006) and the Spanish Ministerio de Ciencia e Innovación (SAF 2010–16198). J. L. Cano-Peñalver was supported by a fellowship from the Spanish Ministerio de Ciencia e Innovación (BES 2011-045069).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
F.A.M., J.L.C.-P., D.B., and H.J.L. conception and design of research; F.A.M., J.L.C.-P., and W.L. performed experiments; F.A.M., J.L.C.-P., D.B., and H.J.L. analyzed data; F.A.M., J.L.C.-P., D.R.P., M.R.P., D.B., S.d.F., and H.J.L. interpreted results of experiments; F.A.M. and J.L.C.-P. prepared figures; F.A.M. and J.L.C.-P. drafted manuscript; F.A.M., D.B., S.d.F., and H.J.L. edited and revised manuscript; F.A.M., J.L.C.-P., M.R.P., D.B., S.d.F., and H.J.L. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Drs. Richard Bouley and Teodor G. Paunescu for scientific and technical insights and Dr. Anil V. Nair for confocal microscopy help.
REFERENCES
- 1.Arthur J, Huang J, Nomura N, Jin WW, Li W, Cheng X, Brown D, Lu HJ. Characterization of the putative phosphorylation sites of the AQP2 C terminus and their role in AQP2 trafficking in LLC-PK1 cells. Am J Physiol Renal Physiol 309: F673–F679, 2015. doi: 10.1152/ajprenal.00152.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bouley R, Breton S, Sun T, McLaughlin M, Nsumu NN, Lin HY, Ausiello DA, Brown D. Nitric oxide and atrial natriuretic factor stimulate cGMP-dependent membrane insertion of aquaporin 2 in renal epithelial cells. J Clin Invest 106: 1115–1126, 2000. doi: 10.1172/JCI9594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boulter E, Van Obberghen-Schilling E. Integrin-linked kinase and its partners: a modular platform regulating cell-matrix adhesion dynamics and cytoskeletal organization. Eur J Cell Biol 85: 255–263, 2006. doi: 10.1016/j.ejcb.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Brown D, Fenton R. The cell biology of vasopressin action. In: Brenner and Rector’s The Kidney, edited by Taal MW, Chertow GM, Marsden PA, Skorecki K, Yu ASL, Brenner BM. Philadelphia, PA: Elsevier, 2015, p. 302, 2015. [Google Scholar]
- 5.Cano-Peñalver JL, Griera M, Serrano I, Rodríguez-Puyol D, Dedhar S, de Frutos S, Rodríguez-Puyol M. Integrin-linked kinase regulates tubular aquaporin-2 content and intracellular location: a link between the extracellular matrix and water reabsorption. FASEB J 28: 3645–3659, 2014. doi: 10.1096/fj.13-249250. [DOI] [PubMed] [Google Scholar]
- 6.Chen Y, Rice W, Gu Z, Li J, Huang J, Brenner MB, Van Hoek A, Xiong J, Gundersen GG, Norman JC, Hsu VW, Fenton RA, Brown D, Lu HA. Aquaporin 2 promotes cell migration and epithelial morphogenesis. J Am Soc Nephrol 23: 1506–1517, 2012. doi: 10.1681/ASN.2012010079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ding GH, Franki N, Condeelis J, Hays RM. Vasopressin depolymerizes F-actin in toad bladder epithelial cells. Am J Physiol Cell Physiol 260: C9–C16, 1991. [DOI] [PubMed] [Google Scholar]
- 8.Edwards LA, Thiessen B, Dragowska WH, Daynard T, Bally MB, Dedhar S. Inhibition of ILK in PTEN-mutant human glioblastomas inhibits PKB/Akt activation, induces apoptosis, and delays tumor growth. Oncogene 24: 3596–3605, 2005. doi: 10.1038/sj.onc.1208427. [DOI] [PubMed] [Google Scholar]
- 9.Fenton RA, Moeller HB, Hoffert JD, Yu MJ, Nielsen S, Knepper MA. Acute regulation of aquaporin-2 phosphorylation at Ser-264 by vasopressin. Proc Natl Acad Sci U S A 105: 3134–3139, 2008. doi: 10.1073/pnas.0712338105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fletcher DA, Mullins RD. Cell mechanics and the cytoskeleton. Nature 463: 485–492, 2010. doi: 10.1038/nature08908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hannigan GE, Leung-Hagesteijn C, Fitz-Gibbon L, Coppolino MG, Radeva G, Filmus J, Bell JC, Dedhar S. Regulation of cell adhesion and anchorage-dependent growth by a new beta 1-integrin-linked protein kinase. Nature 379: 91–96, 1996. doi: 10.1038/379091a0. [DOI] [PubMed] [Google Scholar]
- 12.Hays RM, Condeelis J, Gao Y, Simon H, Ding G, Franki N. The effect of vasopressin on the cytoskeleton of the epithelial cell. Pediatr Nephrol 7: 672–679, 1993. doi: 10.1007/BF00852577. [DOI] [PubMed] [Google Scholar]
- 13.Hoffert JD, Nielsen J, Yu MJ, Pisitkun T, Schleicher SM, Nielsen S, Knepper MA. Dynamics of aquaporin-2 serine-261 phosphorylation in response to short-term vasopressin treatment in collecting duct. Am J Physiol Renal Physiol 292: F691–F700, 2007. doi: 10.1152/ajprenal.00284.2006. [DOI] [PubMed] [Google Scholar]
- 14.Hopkins CR, Miller K, Beardmore JM. Receptor-mediated endocytosis of transferrin and epidermal growth factor receptors: a comparison of constitutive and ligand-induced uptake. J Cell Sci Suppl 1985, Suppl 3: 173–186, 1985. doi: 10.1242/jcs.1985.Supplement_3.17. [DOI] [PubMed] [Google Scholar]
- 15.Jung HJ, Kwon TH. Membrane trafficking of collecting duct water channel protein AQP2 regulated by Akt/AS160. Electrolyte Blood Press 8: 59–65, 2010. doi: 10.5049/EBP.2010.8.2.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katsura T, Gustafson CE, Ausiello DA, Brown D. Protein kinase A phosphorylation is involved in regulated exocytosis of aquaporin-2 in transfected LLC-PK1 cells. Am J Physiol Renal Physiol 272: F817–F822, 1997. [PubMed] [Google Scholar]
- 17.Katsura T, Verbavatz JM, Farinas J, Ma T, Ausiello DA, Verkman AS, Brown D. Constitutive and regulated membrane expression of aquaporin 1 and aquaporin 2 water channels in stably transfected LLC-PK1 epithelial cells. Proc Natl Acad Sci U S A 92: 7212–7216, 1995. doi: 10.1073/pnas.92.16.7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim HY, Choi HJ, Lim JS, Park EJ, Jung HJ, Lee YJ, Kim SY, Kwon TH. Emerging role of Akt substrate protein AS160 in the regulation of AQP2 translocation. Am J Physiol Renal Physiol 301: F151–F161, 2011. doi: 10.1152/ajprenal.00519.2010. [DOI] [PubMed] [Google Scholar]
- 19.Klussmann E, Tamma G, Lorenz D, Wiesner B, Maric K, Hofmann F, Aktories K, Valenti G, Rosenthal W. An inhibitory role of Rho in the vasopressin-mediated translocation of aquaporin-2 into cell membranes of renal principal cells. J Biol Chem 276: 20451–20457, 2001. doi: 10.1074/jbc.M010270200. [DOI] [PubMed] [Google Scholar]
- 20.Kortenoeven ML, Fenton RA. Renal aquaporins and water balance disorders. Biochim Biophys Acta 1840: 1533–1549, 2014. doi: 10.1016/j.bbagen.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 21.Lee SL, Hsu EC, Chou CC, Chuang HC, Bai LY, Kulp SK, Chen CS. Identification and characterization of a novel integrin-linked kinase inhibitor. J Med Chem 54: 6364–6374, 2011. doi: 10.1021/jm2007744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li W, Zhang Y, Bouley R, Chen Y, Matsuzaki T, Nunes P, Hasler U, Brown D, Lu HA. Simvastatin enhances aquaporin-2 surface expression and urinary concentration in vasopressin-deficient Brattleboro rats through modulation of Rho GTPase. Am J Physiol Renal Physiol 301: F309–F318, 2011. doi: 10.1152/ajprenal.00001.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loo CS, Chen CW, Wang PJ, Chen PY, Lin SY, Khoo KH, Fenton RA, Knepper MA, Yu MJ. Quantitative apical membrane proteomics reveals vasopressin-induced actin dynamics in collecting duct cells. Proc Natl Acad Sci U S A 110: 17119–17124, 2013. doi: 10.1073/pnas.1309219110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu H, Sun TX, Bouley R, Blackburn K, McLaughlin M, Brown D. Inhibition of endocytosis causes phosphorylation (S256)-independent plasma membrane accumulation of AQP2. Am J Physiol Renal Physiol 286: F233–F243, 2004. doi: 10.1152/ajprenal.00179.2003. [DOI] [PubMed] [Google Scholar]
- 25.Lu HJ, Matsuzaki T, Bouley R, Hasler U, Qin QH, Brown D. The phosphorylation state of serine 256 is dominant over that of serine 261 in the regulation of AQP2 trafficking in renal epithelial cells. Am J Physiol Renal Physiol 295: F290–F294, 2008. doi: 10.1152/ajprenal.00072.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malan D, Elischer A, Hesse M, Wickström SA, Fleischmann BK, Bloch W. Deletion of integrin linked kinase in endothelial cells results in defective RTK signaling caused by caveolin 1 mislocalization. Development 140: 987–995, 2013. doi: 10.1242/dev.091298. [DOI] [PubMed] [Google Scholar]
- 27.McDonald PC, Oloumi A, Mills J, Dobreva I, Maidan M, Gray V, Wederell ED, Bally MB, Foster LJ, Dedhar S. Rictor and integrin-linked kinase interact and regulate Akt phosphorylation and cancer cell survival. Cancer Res 68: 1618–1624, 2008. doi: 10.1158/0008-5472.CAN-07-5869. [DOI] [PubMed] [Google Scholar]
- 28.Moeller HB, Knepper MA, Fenton RA. Serine 269 phosphorylated aquaporin-2 is targeted to the apical membrane of collecting duct principal cells. Kidney Int 75: 295–303, 2009. doi: 10.1038/ki.2008.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nedvetsky PI, Stefan E, Frische S, Santamaria K, Wiesner B, Valenti G, Hammer JA III, Nielsen S, Goldenring JR, Rosenthal W, Klussmann E. A role of myosin Vb and Rab11-FIP2 in the aquaporin-2 shuttle. Traffic 8: 110–123, 2007. doi: 10.1111/j.1600-0854.2006.00508.x. [DOI] [PubMed] [Google Scholar]
- 30.Nejsum LN, Zelenina M, Aperia A, Frøkiaer J, Nielsen S. Bidirectional regulation of AQP2 trafficking and recycling: involvement of AQP2-S256 phosphorylation. Am J Physiol Renal Physiol 288: F930–F938, 2005. doi: 10.1152/ajprenal.00291.2004. [DOI] [PubMed] [Google Scholar]
- 31.Nielsen S, Kwon TH, Frøkiaer J, Knepper MA. Key roles of renal aquaporins in water balance and water-balance disorders. News Physiol Sci 15: 136–143, 2000. [DOI] [PubMed] [Google Scholar]
- 32.Nunes P, Hasler U, McKee M, Lu HA, Bouley R, Brown D. A fluorimetry-based ssYFP secretion assay to monitor vasopressin-induced exocytosis in LLC-PK1 cells expressing aquaporin-2. Am J Physiol Cell Physiol 295: C1476–C1487, 2008. doi: 10.1152/ajpcell.00344.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park EJ, Kwon TH. A minireview on vasopressin-regulated aquaporin-2 in kidney collecting duct cells. Electrolyte Blood Press 13: 1–6, 2015. doi: 10.5049/EBP.2015.13.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peroukides S, Bravou V, Varakis J, Alexopoulos A, Kalofonos H, Papadaki H. ILK overexpression in human hepatocellular carcinoma and liver cirrhosis correlates with activation of Akt. Oncol Rep 20: 1337–1344, 2008. [PubMed] [Google Scholar]
- 35.Persad S, Attwell S, Gray V, Delcommenne M, Troussard A, Sanghera J, Dedhar S. Inhibition of integrin-linked kinase (ILK) suppresses activation of protein kinase B/Akt and induces cell cycle arrest and apoptosis of PTEN-mutant prostate cancer cells. Proc Natl Acad Sci U S A 97: 3207–3212, 2000. doi: 10.1073/pnas.97.7.3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pisitkun T, Jacob V, Schleicher SM, Chou CL, Yu MJ, Knepper MA. Akt and ERK1/2 pathways are components of the vasopressin signaling network in rat native IMCD. Am J Physiol Renal Physiol 295: F1030–F1043, 2008. doi: 10.1152/ajprenal.90339.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rice WL, Li W, Mamuya F, McKee M, Păunescu TG, Lu HA. Polarized trafficking of AQP2 revealed in three dimensional epithelial culture. PLoS One 10: e0131719, 2015. doi: 10.1371/journal.pone.0131719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rice WL, Zhang Y, Chen Y, Matsuzaki T, Brown D, Lu HA. Differential, phosphorylation dependent trafficking of AQP2 in LLC-PK1 cells. PLoS One 7: e32843, 2012. doi: 10.1371/journal.pone.0032843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Russo LM, McKee M, Brown D. Methyl-beta-cyclodextrin induces vasopressin-independent apical accumulation of aquaporin-2 in the isolated, perfused rat kidney. Am J Physiol Renal Physiol 291: F246–F253, 2006. doi: 10.1152/ajprenal.00437.2005. [DOI] [PubMed] [Google Scholar]
- 40.Sun TX, Van Hoek A, Huang Y, Bouley R, McLaughlin M, Brown D. Aquaporin-2 localization in clathrin-coated pits: inhibition of endocytosis by dominant-negative dynamin. Am J Physiol Renal Physiol 282: F998–F1011, 2002. doi: 10.1152/ajprenal.00257.2001. [DOI] [PubMed] [Google Scholar]
- 41.Takata K, Matsuzaki T, Tajika Y, Ablimit A, Hasegawa T. Localization and trafficking of aquaporin 2 in the kidney. Histochem Cell Biol 130: 197–209, 2008. doi: 10.1007/s00418-008-0457-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tamma G, Klussmann E, Oehlke J, Krause E, Rosenthal W, Svelto M, Valenti G. Actin remodeling requires ERM function to facilitate AQP2 apical targeting. J Cell Sci 118: 3623–3630, 2005. doi: 10.1242/jcs.02495. [DOI] [PubMed] [Google Scholar]
- 43.Valenti G, Procino G, Tamma G, Carmosino M, Svelto M. Minireview: aquaporin 2 trafficking. Endocrinology 146: 5063–5070, 2005. doi: 10.1210/en.2005-0868. [DOI] [PubMed] [Google Scholar]
- 44.van Lieburg AF, Knoers NV, Deen PM. Discovery of aquaporins: a breakthrough in research on renal water transport. Pediatr Nephrol 9: 228–234, 1995. doi: 10.1007/BF00860757. [DOI] [PubMed] [Google Scholar]
- 45.Wesche D, Deen PM, Knoers NV. Congenital nephrogenic diabetes insipidus: the current state of affairs. Pediatr Nephrol 27: 2183–2204, 2012. doi: 10.1007/s00467-012-2118-8. [DOI] [PubMed] [Google Scholar]
- 46.Wickström SA, Lange A, Hess MW, Polleux J, Spatz JP, Krüger M, Pfaller K, Lambacher A, Bloch W, Mann M, Huber LA, Fässler R. Integrin-linked kinase controls microtubule dynamics required for plasma membrane targeting of caveolae. Dev Cell 19: 574–588, 2010. doi: 10.1016/j.devcel.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wilson JL, Miranda CA, Knepper MA. Vasopressin and the regulation of aquaporin-2. Clin Exp Nephrol 17: 751–764, 2013. doi: 10.1007/s10157-013-0789-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu C, Dedhar S. Integrin-linked kinase (ILK) and its interactors: a new paradigm for the coupling of extracellular matrix to actin cytoskeleton and signaling complexes. J Cell Biol 155: 505–510, 2001. doi: 10.1083/jcb.200108077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yui N, Lu HJ, Bouley R, Brown D. AQP2 is necessary for vasopressin- and forskolin-mediated filamentous actin depolymerization in renal epithelial cells. Biol Open 1: 101–108, 2012. doi: 10.1242/bio.2011042. [DOI] [PMC free article] [PubMed] [Google Scholar]