Abstract
Proteinuria is a major risk factor for chronic kidney disease progression. Furthermore, exposure of proximal tubular epithelial cells to excess albumin promotes tubular atrophy and fibrosis, key predictors of progressive organ dysfunction. However, the link between proteinuria and tubular damage is unclear. We propose that pathological albumin exposure impairs proximal tubular autophagy, an essential process for recycling damaged organelles and toxic intracellular macromolecules. In both mouse primary proximal tubule and immortalized human kidney cells, albumin exposure decreased the number of autophagosomes, visualized by the autophagosome-specific fluorescent markers monodansylcadaverine and GFP-LC3, respectively. Similarly, renal cortical tissue harvested from proteinuric mice contained reduced numbers of autophagosomes on electron micrographs, and immunoblots showed reduced steady-state LC3-II content. Albumin exposure decreased autophagic flux in vitro in a concentration-dependent manner as assessed by LC3-II accumulation rate in the presence of bafilomycin, an H+-ATPase inhibitor that prevents lysosomal LC3-II degradation. In addition, albumin treatment significantly increased the half-life of radiolabeled long-lived proteins, indicating that the primary mechanism of degradation, autophagy, is dysfunctional. In vitro, mammalian target of rapamycin (mTOR) activation, a potent autophagy inhibitor, suppressed autophagy as a result of intracellular amino acid accumulation from lysosomal albumin degradation. mTOR activation was demonstrated by the increased phosphorylation of its downstream target, S6K, with free amino acid or albumin exposure. We propose that excess albumin uptake and degradation inhibit proximal tubule autophagy via an mTOR-mediated mechanism and contribute to progressive tubular injury.
Keywords: proteinuria, albumin, reabsorption, autophagy, mTOR
chronic kidney disease (CKD) is often a progressive disease and is presently a worldwide threat to public health. In the United States, ∼23 million adults have physiological evidence of CKD (2) and nearly 80% exhibit proteinuria (3, 13). The degree of proteinuria in patients with kidney disease positively correlates with more rapid progression to end-stage renal disease (29, 45). Progressive CKD is partly caused by exposure of proximal tubular epithelial cells (PTEC) to excess proteins present on the luminal surface that promote tubular atrophy and fibrosis (3). Overwhelming evidence from diverse clinical trials supports the concept that proteinuria-reducing medications (e.g., angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, spironolactone) slow CKD progression and significantly delay renal replacement therapy (1, 13, 20, 25, 40). However, the absence of a clear biochemical understanding of the pathological effect of proteinuria on PTEC currently limits the development of targeted drug therapies.
In proteinuric states, the proximal tubule cell is exposed to excess albumin, the principal urinary protein contained in glomerular filtrate. The exact concentration of albumin in the proximal tubule in healthy kidneys is presently debated but is estimated to be between 20 (51) and 1,000 μg/ml of filtrate (42). In proteinuric states, albuminuria increases by 200- to 300-fold (14, 28, 36, 41, 42). This albuminuria results from an imbalance between markedly increased glomerular albumin filtration and limited tubular albumin reabsorption.
Normally, filtered albumin is reabsorbed by the proximal tubule via an endocytotic mechanism involving two surface-expressed glycoprotein receptors: megalin and cubulin (5, 50, 58). The reabsorbed albumin is degraded in lysosomes whereas megalin and cubulin are recycled to the apical membrane (8). Lysosomes are also essential for functional autophagy, a constitutive cellular process responsible for the removal of damaged organelles and macromolecules. Macroautophagy (hereafter referred to as autophagy) is a highly conserved and essential survival mechanism that continuously recycles valuable macromolecules, maintains homeostasis, even in the absence of external stressors, and has a key role in cell differentiation and remodeling. Under starvation conditions, autophagy plays a critical role in salvaging and recycling basic biochemical building blocks (amino acids, lipids, etc.) that support cell and tissue viability. A key regulator of autophagy is the mechanistic mammalian target of rapamycin (mTOR), a nutrient-sensing protein kinase that inhibits autophagy (10, 33, 34, 46). Autophagy also plays a crucial role in the degradation and turnover of dysfunctional organelles including depolarized mitochondria (35, 43).
Although exposure of PTEC to excess albumin causes cell toxicity (3, 5, 7, 12, 16, 41), neither the sensor nor the signal transduction cascade involved in albumin-induced toxicity has been identified. In the present study, we tested the hypothesis that impaired autophagy contributes to albumin overload-mediated proximal tubule cell toxicity. We suggest that albumin exposure typical of proteinuric states decreases autophagasome number and disrupts autophagasome function via an mTOR-dependent mechanism.
MATERIALS AND METHODS
Cell culture.
Primary proximal tubule cells were prepared and cultured as previously described (47). Briefly, proximal tubule cells were cultured from dissociated (by gentleMACS dissociator), collagenase-digested renal cortex from C57BL/6 mice (Charles River). Primary cells were grown in serum-free conditions. Cells were then imaged using confocal microscopy after treatment with or without albumin in nutritionally complete or starvation media (14155063, supplemented with 50 U/ml penicillin and 50 μg/ml streptomycin, EBSS, Life Technologies).
HK-2 green fluorescent protein-LC3 cell line.
HK-2 cells were ordered from ATCC and maintained in DMEM supplemented with 10% FBS, 50 U/ml penicillin, and 50 μg/ml streptomycin. Green fluorescent protein (GFP)-LC3 lentivirus was kindly obtained from Orian Shirihai's lab (Boston University School of Medicine). HK-2 cells were infected with GFP-LC3 and then sorted using a MoFlo Cell Sorter (BU Flow Cytometry Core) according to GFP intensity. Images were analyzed using ImageJ software (National Institutes of Health).
Immunoblot analysis and antibodies.
Protein samples were prepared and run as previously described (15). Commercially available antibodies were used to detect LC3 (L7543 1:1,000 dilution, Sigma), β-actin (A2228, 1:1,000 dilution, Sigma), Phospho p70/85 S6 kinase (9205, Thr389, 1:1,000 dilution, Cell Signaling), p62 (ab91526, 1:500 dilution, Abcam), and albumin (4929, 1:1,000 dilution, Cell Signaling). Secondary antibodies conjugated to horseradish peroxidase (Jackson ImmunoResearch) were used with enhanced chemiluminescence.
Autophagosome visualization in primary cells.
Mouse primary PTECs were stained with 0.05 mmol/l monodansylcadaverine (30432, Sigma) at 37°C for 1 h and then imaged using confocal microscopy (Olympus DSU microscope, BU Imaging Core). Cell fluorescence was analyzed with ImageJ software.
Long-lived protein degradation.
Primary PTECs were cultured with primary cell culture media (47) supplemented with l-[14C]phenylalanine (Phe) for 48 h. Media was then replaced with either primary cell culture media or starvation media (14155063, supplemented with 50 U/ml penicillin and 50 μg/ml streptomycin, EBSS, Life Technologies) with 2 mM nonradioactive phenylalanine. Media was removed and replaced for a washout period of 12 h to remove [14C]Phe released from short-lived protein degradation. Then, [14C]Phe release from long-lived proteins was calculated from radioactivity in the tricarboxylic acid-soluble form relative to total cell radioactivity. Cells were lysed, protein was isolated, and measurements were performed using a scintillation counter.
Albumin degradation assay using dye-quenched-albumin.
Dye-quenched (DQ)-BSA (DQ-albumin; Molecular Probes, Eugene, OR) is a BSA conjugate labeled to such a high degree with BODIPY dyes that the dye is self-quenching and therefore displays no detectable fluorescence activity. However, upon proteolysis in the lysosomes the quenching effect is abolished, resulting in highly fluorescent albumin fragments. DQ-albumin fluorescence was determined using a Tecan Infinite multimode microplate reader at 550-nm excitation and 620-nm emission wavelengths. DQ-albumin degradation was adjusted for background and normalized for total cellular protein (48).
Proteinuric mouse model.
The in vivo experiments were done according to the animal protocol approved by the Institutional Animal Care and Use Committee of the Boston University School of Medicine. C57BL/6 mice were injected with 1 ml of saline or 1 mg of the immunoglobulin fraction of sheep nephrotoxic serum (against glomerular antigens) that causes acute immune-complex glomerulonephritis with massive proteinuria by 24–48 h (44). Daily urine was collected from mice. Mice were euthanized after 7 days, and kidneys and blood were collected. Albuminuria was estimated by the urine albumin-to-creatinine ratio using an albumin ELISA and Creatinine Companion kit (Exocell). Tissue for histology was embedded in paraffin, and tissue for electron microscopy was placed in Karnovsky fixative. Periodic acid-Schiff (PAS) staining was performed according to the manufacturer's instructions (395B, Sigma). Electron microscopy was performed using a Jeol 1011 Transmission electron microscope with a GATAN Erlagshen ES1000W camera. A blinded investigator using the uniform random sampling method counted the autophagosomes (4). Vesicles were counted as autophagosomes if they had two membranes with a narrow electron lucent space between the double membrane.
Statistical analysis.
Data are expressed as means ± SE. Comparisons between groups were performed using unpaired Student's t-test. Statistical significance was determined at P < 0.05. All images and densitometry were analyzed using ImageJ.
RESULTS
Albumin inhibits autophagic flux.
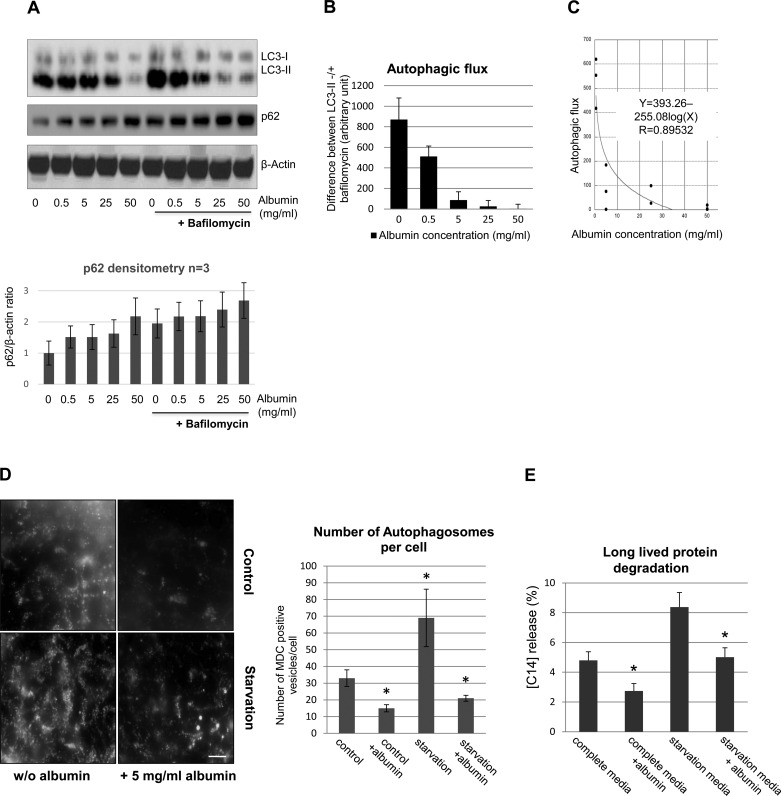
We first examined the effect of albumin overload on autophagy activity in a cell culture model of proteinuria. Cultured primary PTEC in serum-free media were exposed to recombinant human albumin at concentrations ranging from 0 to 50 mg/ml, which includes normal and pathological levels detected in the glomerular filtrate (14, 28, 36, 41, 42). Short-term primary cell viability was not affected by albumin treatment (data not shown). LC3-II, a component of the autophagosomal membrane, was measured as a marker of autophagy. In primary cells grown in serum-free media, the LC3-I-to-LC3-II ratio is low at baseline. In PTEC, albumin exposure for 5 days decreased LC3-II protein level in a concentration-dependent manner (Fig. 1A). The level of p62 protein, a substrate of autophagolysosomal proteolysis, increased with albumin treatment, consistent with accumulation of this protein due to autophagy inhibition. To estimate autophagic flux, LC3 was measured in the presence of bafilomycin, an H+-ATPase inhibitor (24) for 2 h before cell harvesting. The difference between LC3-II content in bafilomycin-treated cells and their bafilomycin null counterparts reflects autophagic flux, i.e., the number of autophagosomes formed during this time period. Increasing albumin exposure progressively reduced autophagic flux in PTEC (Fig. 1, B and C). Therefore, both total LC3-II content and autophagic flux are reduced by albumin exposure. To determine whether changes in extracellular osmolality per se depressed autophagy, LC3 content was measured in the presence of 70-kDa dextran, a macromolecule with a similar molecular weight as albumin. Importantly, dextran approximates a pure osmotic effect since it is nonmetabolized (9). In contrast to albumin, minimal uptake of fluorescent dextran into PTEC was detected at 24 h, and dextran did not suppress autophagy (data not shown).
Fig. 1.
Albumin overload inhibits autophagy in cultured primary proximal tubular epithelial cells (PTEC). A: representative immunoblot analysis of LC3 and p62 content in whole cell lysates in primary PTEC treated with increasing concentrations of human recombinant albumin for 5 days. +Bafilomycin, cells treated with 100 nM bafilomycin A1 for 2 h before harvesting and lysis. β-Actin served as a loading control. Quantitative densitometry of p62 expression (n = 3) is also shown. B: autophagic flux calculated using densitometry from Western blots containing LC3-II levels before and after bafilomycin treatment. Values were normalized to β-actin. The change in autophagosome mass, measured by the difference in LC3-II content before and after complete inhibition of fusion for 2 h with bafilomycin is shown. Values are means ± SE; n = 3 independent experiments. C: the magnitude of autophagic flux declines in relation to the concentration of albumin in the apical media. Autophagic flux was determined as shown in Fig. 1B, and individual values were plotted vs. the apical medium albumin concentration. The curve fit was determined by regression analysis, Y = 393.26-255.08log(X), R = 0.89532, n = 12, P < 0.0001. D: the no. of autophagosomes was assessed in live PTEC with monodansylcadaverine (MDC), a fluorescent dye that accumulates in double membrane-bound autophagosomes. Cells were grown in complete primary culture media (control; top) or in EBSS overnight (starvation; bottom) in the absence (left) or presence of albumin (right). MDC-labeled vesicles are induced by starvation, while albumin treatment decreased the no. of MDC-labeled vacuoles. Images were taken with a Nikon Deconvolution Microscope; ×60. Scale bar = 10 μm. Bar graph indicates the average no. of MDC-labeled vesicles/cell ± SD determined by analyzing 20 cells/sample. Three independent experiments were performed obtaining similar results. *P < 0.05 indicates statistical significance compared with control. E: effect of albumin exposure and altered autophagy on the degradation of long-lived proteins. Proteolysis of long-lived proteins was measured in primary mouse PTEC labeled for 48 h in medium containing l-[14C]phenylalanine (Phe) followed by a 48-h chase period in complete or starvation media. After labeling, a 12-h washout period was performed to remove l-[14C]Phe that is released from short-lived proteins. [14C]Phe release was calculated from radioactivity in the tricarboxylic acid-soluble form relative to total cell radioactivity. Data represent 1 of 3 experiments. Values are means ± SE. *P < 0.05, complete media vs. complete media+albumin; starvation media vs. starvation media+albumin.
Albumin decreases the number of fluorescent labeled autophagosomes.
To further investigate the effect of albumin overload on autophagy, autophagosomes were visualized using monodansylcadaverine (MDC) in primary PTEC that were either incubated in complete cell culture media or starvation media, an autophagy-inducing condition (53, 54). Treatment with 5 mg/ml of albumin decreased the number of MDC-positive punctuate structures that represent autophagosomes in both media conditions by >60% (Fig. 1D). Although representative data are shown for the effect of 5 mg/ml albumin, higher albumin concentrations also decreased autophagy (data not shown). Interestingly, larger MDC-positive vesicles were observed (see Fig. 1D, bottom right), especially at earlier time points of albumin exposure, in albumin-treated cells maintained in starvation media. This observation raised the possibility that albumin initially inhibits the fusion step between autophagosomes and lysosomes without impairing the maturation of small, punctate autophagosomes to larger ones. However, at later time points of albumin exposure (3–5 days) and despite initial upregulation of autophagy by starvation, autophagosomes disappear almost completely from albumin-exposed cells.
Albumin decreases long-lived protein degradation.
Since autophagy is the main degradation pathway for long-lived proteins, we examined the effect of albumin-mediated inhibition of autophagy on the degradation rate of long-lived radiolabeled proteins. In primary PTEC maintained in complete media, albumin exposure increased the half-life of long-lived proteins by 27 ± 11% (Fig. 1E). As expected, the baseline half-life of long-lived proteins was shorter in PTEC maintained in starvation media, while albumin exposure increased the half-life of long-lived proteins by 48 ± 25% in starved cells (Fig. 1E).
Albumin decreases the number GFP-LC3-labeled autophagosomes in HK-2 cells.
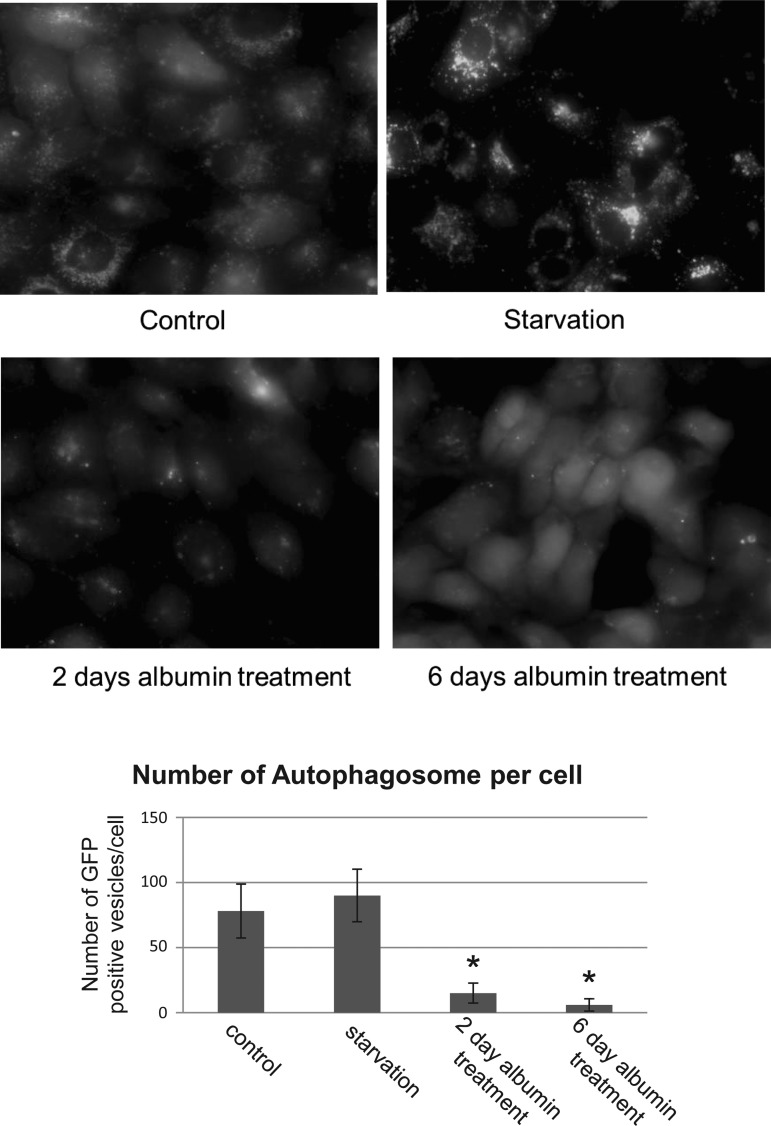
In addition to primary cells, we tested the effect of albumin exposure on autophagy in a human kidney cell line (HK-2) transfected with a lentivirus containing a GFP-LC3 fusion construct. In these GFP-LC3-expressing HK-2 cells, albumin overload decreases autophagosomes formation (Fig. 2). After 6 days of albumin treatment, the LC3-II-positive autophagosomes are nearly absent, whereas GFP-LC3-I is diffusely distributed throughout the cytosol. Importantly, overexpression of GFP-LC3 does not affect autophagy (31).
Fig. 2.
Proteinuria inhibits LC3-green fluorescent protein (GFP)-labeled autophagosome formation in HK-2 cells. Representative images are shown of direct fluorescence of HK-2 cells transfected with GFP-LC3, an autophagic marker, at baseline (Control), after overnight starvation in EBSS (Starvation), and after albumin treatment for either 2 or 6 days (bottom right); albumin concentration = 5 mg/ml. Images represent random fields from 3 independent experiments. In control cells, abundance of autophagic vesicles was observed, while albumin treatment resulted in a loss of GFP-positive autophagosomes. Bar graph indicates the average no. of GFP-labeled vesicles/cell ± SD determined by analyzing 20 cells/sample. Three independent experiments were performed obtaining similar results. Images were taken with a Nikon Deconvolution Microscope (×60). Scale bar = 10 μm. *P < 0.05, statistically significant compared with control.
Endocytosed albumin colocalizes with lysosomes and undergoes rapid degradation.
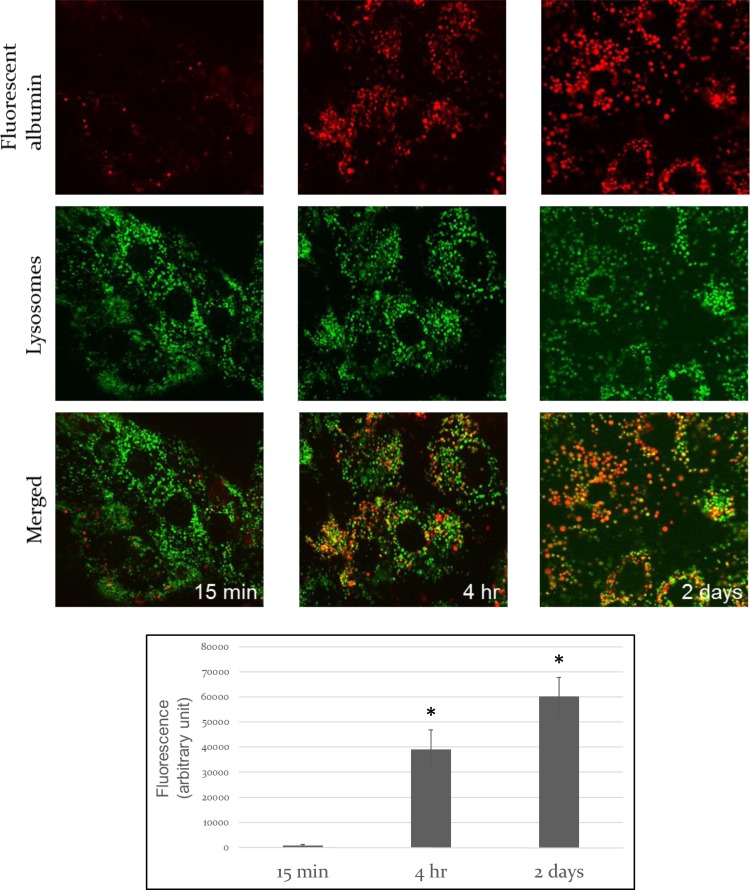
Albumin exposure results in albumin uptake into PTEC by endocytosis as evidenced by fluorescently labeled albumin containing endocytic vesicles after 15 min and clear colocalization between fluorescent-labeled albumin and a lysosomal fluorescent marker at later time points, respectively (Fig. 3, A–C). Albumin endocytosis is followed by lysosomal degradation measured by increasing fluorescent activity in cells that were treated with DQ-albumin. This fluorescent-labeled albumin is self-quenching at baseline and upon proteolysis in the lysosomes the quenching effect is abolished, resulting in highly fluorescent albumin fragments. Fluorescent activity is proportionate to albumin degradation (Fig. 3D).
Fig. 3.
Time course of endocytosed albumin localization in lysosomes and degradation in vitro. Representative images are shown of direct fluorescence of red fluorescent-labeled albumin and Lysotracker green in live primary PTEC following 15-min albumin exposure (A), 4-h albumin exposure (B), or 2-day albumin exposure (C). Endocytosed albumin colocalizes with lysosomes; albumin concentration = 5 mg/ml. Fluorescent albumin-to-unlabeled albumin ratio = 1:800. Images were taken with an Olympus DSU spinning disk Microscope (×60). Scale bar = 10 μm. D: albumin degradation in primary proximal tubule cells using dye-quenched (DQ)-albumin. The fluorescent dye is self-quenching at baseline, and upon proteolysis in the lysosomes the quenching effect is abolished, resulting in highly fluorescent albumin fragments. Primary proximal tubule cells were exposed to DQ-albumin (5 mg/dl) for 15 min, 4 h, and 2 days. Fluorescence was measured in cell lysates using a microplate reader at 550-nm excitation and 620-nm emission wavelengths. Labeled albumin-to-unlabeled albumin ratio = 1:400. Error bars represent means ± SE of at least 3 independent experiments. *P < 0.05, statistically significant difference.
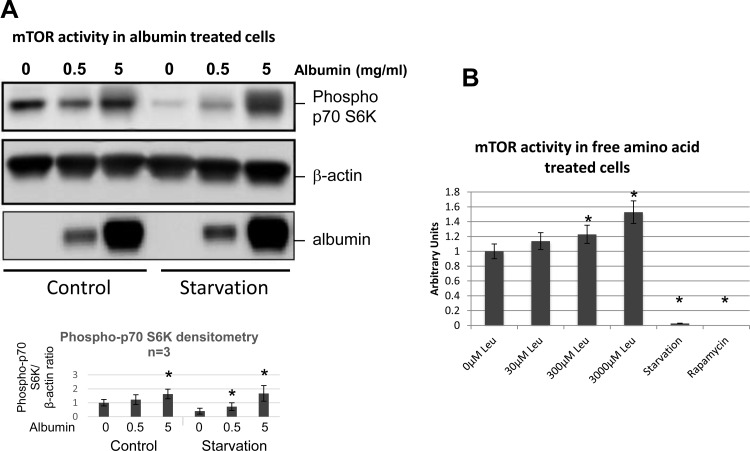
Albumin uptake or amino acid loading increases mTOR activity.
Albumin has been shown to undergo lysosomal degradation, resulting in the release of free amino acids into the cytosol (5, 17). Since intracellular amino acids activate mTOR, a primary inhibitor of autophagy (33, 34), amino acids generated by albumin catabolism could inhibit autophagy via an mTOR-dependent mechanism. To test this hypothesis, phospho-p-P70/85 s6 kinase, a downstream substrate of mTOR, was measured in control and in albumin-exposed primary PTEC. Increased activation of p-P70/85 s6 kinase was detected in albumin-treated cells (Fig. 4A). Similar results were observed when primary PTEC were treated with the exogenous amino acid leucine to mimic the increase in amino acid availability that results from lysosomal albumin degradation (Fig. 4B). Total s6 kinase protein level does not change with albumin or amino acid treatment (data not shown).
Fig. 4.
Albumin or amino acid loading increases mammalian target of rapamycin (mTOR) activity in primary cells. A: representative immunoblot of p-P70/85 s6 kinase (top; a downstream substrate of mTOR) in primary tubular cells harvested at baseline (Control) and after overnight starvation in EBSS (Starvation) with or without albumin treatment. Albumin-exposed cells received either 0.5 or 5 mg/ml albumin for 48 h. β-Actin (middle) serves as a loading control; n = 3 independent experiments. Bar graph represents quantitative densitometry of phospho-pS6K expression (n = 3). *P < 0.05 indicates statistically significant difference without vs. with albumin. B: addition of the amino acid leucine, a potent mTOR activator, caused a dose-dependent increase in the phosphorylation of p70 S6K, a target of mTOR, whereas starvation and rapamycin, as controls, potently inhibited mTOR activity. Bar graph represents densitometry from separate Western blot studies containing p-P70/85 S6 kinase in primary tubular cells treated with increasing leucine concentrations. Values were normalized to β-actin. Values are means ± SE; n = 3 independent experiments. *P < 0.05, statistically significant difference vs. control.
Albumin uptake does not result in lysosomal dysfunction.
We then tested whether albumin overload caused lysosomal dysfunction. However, no significant difference in lysosomal function was detected by acid phosphatase or cathepsin D activity in albumin-exposed PTEC vs. control (data not shown). Total lysosomal mass was not affected by excess albumin exposure as demonstrated by the absence of a significant change in Lamp-2, a lysosomal membrane protein (data not shown).
Autophagy is inhibited in proteinuric mice.
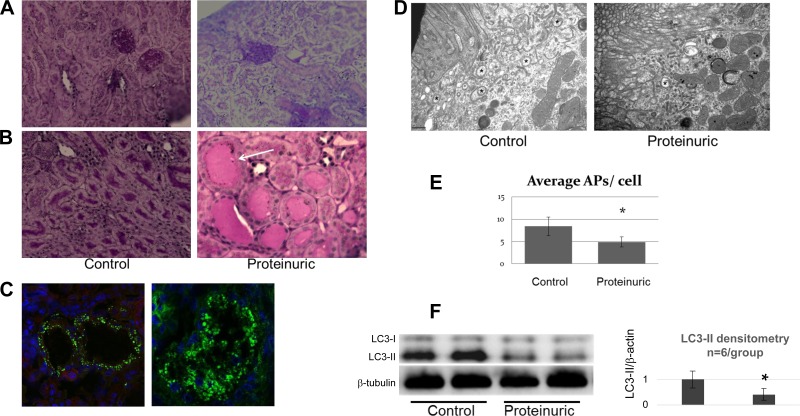
To test the effect of proteinuria on proximal tubular autophagy in vivo, proteinuria was induced in mice by injecting 1 mg of the gamma immunoglobulin fraction of sheep nephrotoxic serum. Exposure to this nephrotoxic serum causes acute immune complex-mediated glomerulonephritis with massive proteinuria within 24–48 h as assessed by the urinary albumin-to-creatinine ratio. Three days after nephrotoxic serum injection, animals were found to have an estimated proteinuria of 10 g as assessed by the spot urine albumin-to-creatinine ratio. PAS-stained kidney sections demonstrated abundant proteinaceous casts in the tubules within the renal cortex and medulla (Fig. 5, A and B). Immunofluorescence showed albumin reabsorption granules in the tubules of proteinuric animals (Fig. 5C). Compared with control, the renal cortexes of proteinuric animals contained significantly fewer autophagosomes as detected by electron microscopy (Fig. 5, D and E) and exhibited lower LC3-II protein content (Fig. 5F).
Fig. 5.
Autophagy inhibition in a proteinuric mouse model (n = 6 control and n = 6 proteinuric animals). A and B: representative light microscopy images of renal cortex (A) and medulla (B) of control (left) and proteinuric (right) animals with periodic acid-Schiff (PAS) staining. Proteinuric animals exhibited 4+ protein on dipstick. Images show proteinaceous casts (arrow) in proteinuric animals. Scale bar = 50 μm. C: albumin reabsorption granules in the proximal tubule. Immunofluorescence of albumin (red) in paraffin sections of cortex from proteinuric mouse kidney. Nuclei are stained with DAPI (blue). Images represent typical fields; ×60 (left) and ×100 (right) magnification. Scale bar = 25 μm. D: electron microscopy of double membrane autophagosomes (*) in proximal tubules from control and proteinuric mice. Image representative of 10 cells/sample is shown; ×30,000 magnification. Scale bar = 0.5 μm. E: no. of autophagosomes in the cytoplasm estimated from electron micrographs is shown; 10 cells/sample; n = 6 animals/group. Data represent means ± SE. *P < 0.05. F: immunoblot analysis of LC3 content from renal cortexes of 2 control and 2 proteinuric mice. β-Tubulin served as a loading control. Quantitative densitometry of LC3-II content from 6 animals/group is shown. *P < 0.05.
DISCUSSION
Recent data suggest that autophagy is involved in various renal diseases including ischemia, aging, toxic injury, glomerular diseases, and diabetic nephropathy (11, 19, 21–23, 27, 30, 49, 52). Autophagy also plays a key role in maintaining proximal tubule health (21–23, 27, 30, 49, 57). Regardless of the initial insult, progression to end-stage renal disease with extensive tubular atrophy and interstitial fibrosis on renal biopsy is the final common pathway of many forms of glomerular disease. Most progressive glomerulopathies are associated with persistently high levels of urinary protein excretion along with tubulointerstitial lesions on renal biopsy. Therefore, protein overload of tubules is a primary candidate for causing tubular injury and interstitial fibrosis in proteinuric states.
In the present studies, we show that albumin, the most prominent protein in the glomerular filtrate of proteinuric patients at risk for progressive CKD, inhibits PTEC autophagy. We show that albumin endocytosis leads to slower autopathic flux in vitro and lower numbers of autophagosomes in vitro and in vivo, suggesting that albumin reabsorption has a detrimental effect on tubular cell autophagy and function. The osmotic effect of albumin is not a causal factor as evidenced by the observation that 70-kDa dextran, unlike albumin, does not impair autophagy. Interestingly, we observed a relatively high basal autophagy level in proximal tubular cells especially in primary cells. The level of basal autophagy has been shown to vary between different cell types with terminally differentiated cell types, such as neurons and some cancer cells, having very high levels of autophagic flux (18, 32, 55, 56). Autophagy also seems to be higher in young animals (18, 56). Based on our studies, we propose to add primary proximal tubule cells from young mice to the list of cells with a high basal autophagy level.
Since several larger than usual autophagosomes were observed within hours of albumin exposure, we propose that autophagasome fusion with lysosomes might be impaired at these early time points. This failure of autophagosome-lysosome fusion could result in a continued expansion of autophagosomes, increasing their size above normal. However, with prolonged albumin treatment, the overall number of autophagosomes compared with control was clearly decreased. We also found that albumin treatment decreases long-lived protein degradation in primary proximal tubular cells, indicating that autophagy, their primary mechanism of degradation, is dysfunctional.
To elucidate the mechanism of dysfunctional autophagy as a result of albumin overload, we examined mTOR activity, a potent suppressor of autophagy. We observed that mTOR signaling is upregulated in albumin-overloaded cells by looking at the activation of one of its downstream effectors. This is consistent with our hypothesis that albumin degradation produces abundant intracellular amino acids that reduce autophagy by activating mTOR. Among the cellular mechanisms that may determine progression regardless of CKD etiology, the reabsorption of excess proteins filtered from the glomerulus into the renal tubule may have functional importance by initiating and maintaining tubular injury, leading to interstitial pathology. It remains to be seen whether the impairment in autophagy that results from excess albumin exposure also compromises mitophagy and mitochondrial health.
In contrast to other studies, we did not find a significant difference in lysosomal function in albumin-treated primary tubular cells (data not shown) (30, 38, 39). In fact, cathepsin D activity in PTEC did not significantly change in our albumin-exposed PTEC vs. control. It is possible that lysosomal membrane permeabilization during cell stress causes cathepsins to leak from the lysosomes into the cytoplasmic compartment as has been shown in other experimental models (6, 26, 37). If true, then lysosomal cathepsin availability for degrading lysosomal contents would be diminished and cytosolic cathepsin could result in progressive cellular damage, but total cathepsin per cell would remain unchanged.
Our study has potential limitations. First, human recombinant albumin was intentionally used in our experiments to avoid contamination and confounding by nonalbumin compounds. Albumin-bound molecules (e.g., fatty acids) or bioactive compounds (e.g., advanced glycation end products, immunoglobulins, transferrin, complement) present in the glomerular filtrate in proteinuric states could also contribute to PTEC toxicity. In culture, albumin could potentially promote cell injury by binding to, and facilitating the uptake of, biologically active molecules present in the medium, therefore altering experimental results. Finally, the relatively short lifespan of primary proximal tubules in culture limits the duration of albumin exposure in our model.
In a recent animal study, intraperitoneal injection of a free fatty acid and albumin mixture induced autophagy, an effect that was blunted by a high-fat diet and obesity (57). In our opinion, these results are difficult to interpret because the mechanism by which intraperitoneal injection of albumin causes proteinuria is not known and its consequences are likely to differ from those detected in our study of glomerular proteinuria.
In a prior report, the diabetic milieu leads to inhibition of mitophagy and causes an accumulation of dysfunctional mitochondria in the cytoplasm (59). In contrast, in another study, hyperglycemia promoted autophagy (60). This discrepancy could be due to the duration of the experimental insult on autophagy. Also, in in vivo experiments, the increase in autophagic vacuoles may be due to an increased formation and/or decreased clearance of autophagosomes. Given these experimental realities, the etiology of discrepancies between prior reports remains to be determined.
In summary, we show that albumin uptake and degradation by the proximal tubules decrease autophagy both in vitro and in vivo. Dysfunctional autophagy results in the accumulation of long-lived intracellular proteins with the potential to cause proteotoxicity. Reduced or dysfunctional autophagy may contribute to progressive cell injury and organ failure in proteinuric states. These observations suggest that rescuing autophagy during proteinuric states may be a rational approach for preventing or slowing the progression of chronic kidney disease.
GRANTS
The BUSM Medical Student Summer Research Program and Carolyn L. Kuckein Student Research Fellowship (from Alpha Omega Alpha Honor Medical Society) was awarded to A.C. Nolin; National Institutes of Health (NIH) Grant KO8DK090143 and AHA Scientist Development Grant to A. Havasi; and NIH Grants DK53387 (to S. C. Borkan), DK087910 (to M. V. Panchenko); and DK074778 and DK099618 (O. Shirihai).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.C.N., R.M.M., M.V.P., A.P.-H., and Z.W. performed experiments; A.C.N. and A.H. analyzed data; A.C.N., S.C.B., and A.H. interpreted results of experiments; A.C.N. and A.H. prepared figures; A.C.N. and A.H. drafted manuscript; A.C.N., M.V.P., O.S.S., S.C.B., and A.H. edited and revised manuscript; A.C.N., R.M.M., M.V.P., A.P.-H., Z.W., O.S.S., S.C.B., and A.H. approved final version of manuscript; A.H. provided conception and design of research.
REFERENCES
- 1.ACE Inhibitors in Diabetic Nephropathy Trialist Group. Should all patients with type 1 diabetes mellitus, and microalbuminuria receive angiotensin-converting enzyme inhibitors? A meta-analysis of individual patient data. Ann Intern Med 134: 370–379, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Anonymous. National Health and Nutrition Examination Survey (http://kidney.niddk.nih.gov/kudiseases/pubs/kustats/index.htm). [Google Scholar]
- 3.Abbate M, Zoja C, Remuzzi G. How does proteinuria cause progressive renal damage? J Am Soc Nephrol 17: 2974–2984, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Biazik J, Vihinen H, Anwar T, Jokitalo E, Eskelinen EL. The versatile electron microscope: an ultrastructural overview of autophagy. Methods 75: 44–53, 2015. [DOI] [PubMed] [Google Scholar]
- 5.Birn H, Christensen EI. Renal albumin absorption in physiology and pathology. Kidney Int 69: 440–449, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Boya P. Lysosomal function and dysfunction: mechanism and disease. Antioxid Redox Signal 17: 766–774, 2012. [DOI] [PubMed] [Google Scholar]
- 7.Chana RS, Sidaway JE, Brunskill NJ. Statins but not thiazolidinediones attenuate albumin-mediated chemokine production by proximal tubular cells independently of endocytosis. Am J Nephrol 28: 823–830, 2008. [DOI] [PubMed] [Google Scholar]
- 8.Christensen EI, Birn H. Megalin and cubilin: multifunctional endocytic receptors. Nat Rev Mol Cell Biol 3: 256–266, 2002. [DOI] [PubMed] [Google Scholar]
- 9.Christensen EI, Maunsbach AB. Effects of dextran on lysosomal ultrastructure and protein digestion in renal proximal tubule. Kidney Int 16: 301–311, 1979. [DOI] [PubMed] [Google Scholar]
- 10.Corradetti MN, Guan KL. Upstream of the mammalian target of rapamycin: do all roads pass through mTOR? Oncogene 25: 6347–6360, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Decuypere JP, Pirenne J, Jochmans I. Autophagy in renal ischemia-reperfusion injury: friend or foe? Am J Transplant 14: 1464–1465, 2014. [DOI] [PubMed] [Google Scholar]
- 12.Diwakar R, Pearson AL, Colville-Nash P, Brunskill NJ, Dockrell ME. The role played by endocytosis in albumin-induced secretion of TGF-β1 by proximal tubular epithelial cells. Am J Physiol Renal Physiol 292: F1464–F1470, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Dykeman-Sharpe J. Proteinuria, a modifiable risk factor: angiotensin converting enzyme inhibitors (ACEIs) and angiotensin II receptor blockers (ARBs). CANNT J 13: 34–36; quiz 37–38, 2003. [PubMed] [Google Scholar]
- 14.Erkan E, Devarajan P, Schwartz GJ. Apoptotic response to albumin overload: proximal vs. distal/collecting tubule cells. Am J Nephrol 25: 121–131, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Gall JM, Wong V, Pimental DR, Havasi A, Wang Z, Pastorino JG, Bonegio RG, Schwartz JH, Borkan SC. Hexokinase regulates Bax-mediated mitochondrial membrane injury following ischemic stress. Kidney Int 79: 1207–1216, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gekle M. Renal tubule albumin transport. Annu Rev Physiol 67: 573–594, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Gudehithlu KP, Pegoraro AA, Dunea G, Arruda JA, Singh AK. Degradation of albumin by the renal proximal tubule cells and the subsequent fate of its fragments. Kidney Int 65: 2113–2122, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Gump JM, Staskiewicz L, Morgan MJ, Bamberg A, Riches DW, Thorburn A. Autophagy variation within a cell population determines cell fate through selective degradation of Fap-1. Nat Cell Biol 16: 47–54, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huber TB, Edelstein CL, Hartleben B, Inoki K, Jiang M, Koya D, Kume S, Lieberthal W, Pallet N, Quiroga A, Ravichandran K, Susztak K, Yoshida S, Dong Z. Emerging role of autophagy in kidney function, diseases and aging. Autophagy 8: 1009–1031, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jafar TH, Stark PC, Schmid CH, Landa M, Maschio G, Marcantoni C, de Jong PE, de Zeeuw D, Shahinfar S, Ruggenenti P, Remuzzi G, Levey AS. Proteinuria as a modifiable risk factor for the progression of non-diabetic renal disease. Kidney Int 60: 1131–1140, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Jiang M, Wei Q, Dong G, Komatsu M, Su Y, Dong Z. Autophagy in proximal tubules protects against acute kidney injury. Kidney Int 82: 1271–1283, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaushal GP. Autophagy protects proximal tubular cells from injury and apoptosis. Kidney Int 82: 1250–1253, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kimura T, Takabatake Y, Takahashi A, Kaimori JY, Matsui I, Namba T, Kitamura H, Niimura F, Matsusaka T, Soga T, Rakugi H, Isaka Y. Autophagy protects the proximal tubule from degeneration and acute ischemic injury. J Am Soc Nephrol 22: 902–913, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klionsky DJ, Elazar Z, Seglen PO, Rubinsztein DC. Does bafilomycin A1 block the fusion of autophagosomes with lysosomes? Autophagy 4: 849–950, 2008. [DOI] [PubMed] [Google Scholar]
- 25.Klotman PE. Early treatment with ACE inhibition may benefit HIV-associated nephropathy patients. Am J Kidney Dis 31: 719–720, 1998. [DOI] [PubMed] [Google Scholar]
- 26.Kroemer G, Jaattela M. Lysosomes and autophagy in cell death control. Nat Rev Cancer 5: 886–897, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Kume S, Uzu T, Maegawa H, Koya D. Autophagy: a novel therapeutic target for kidney diseases. Clin Exp Nephrol 16: 827–832, 2012. [DOI] [PubMed] [Google Scholar]
- 28.Lazzara MJ, Deen WM. Model of albumin reabsorption in the proximal tubule. Am J Physiol Renal Physiol 292: F430–F439, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Levin A. Identification of patients and risk factors in chronic kidney disease—evaluating risk factors and therapeutic strategies. Nephrol Dial Transplant 16, Suppl 7: 57–60, 2001. [DOI] [PubMed] [Google Scholar]
- 30.Liu S, Hartleben B, Kretz O, Wiech T, Igarashi P, Mizushima N, Walz G, Huber TB. Autophagy plays a critical role in kidney tubule maintenance, aging and ischemia-reperfusion injury. Autophagy 8: 826–837, 2012. [DOI] [PubMed] [Google Scholar]
- 31.Mizushima N. Methods for monitoring autophagy. Int J Biochem Cell Biol 36: 2491–2502, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature 451: 1069–1075, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nicklin P, Bergman P, Zhang B, Triantafellow E, Wang H, Nyfeler B, Yang H, Hild M, Kung C, Wilson C, Myer VE, MacKeigan JP, Porter JA, Wang YK, Cantley LC, Finan PM, Murphy LO. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell 136: 521–534, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nobukuni T, Joaquin M, Roccio M, Dann SG, Kim SY, Gulati P, Byfield MP, Backer JM, Natt F, Bos JL, Zwartkruis FJ, Thomas G. Amino acids mediate mTOR/raptor signaling through activation of class 3 phosphatidylinositol 3OH-kinase. Proc Natl Acad Sci USA 102: 14238–14243, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okamoto K, Kondo-Okamoto N. Mitochondria and autophagy: critical interplay between the two homeostats. Biochim Biophys Acta 1820: 595–600, 2012. [DOI] [PubMed] [Google Scholar]
- 36.Oken DE, Cotes SC, Mende CW. Micropuncture study of tubular transport of albumin in rats with aminonucleoside nephrosis. Kidney Int 1: 3–11, 1972. [DOI] [PubMed] [Google Scholar]
- 37.Ono K, Kim SO, Han J. Susceptibility of lysosomes to rupture is a determinant for plasma membrane disruption in tumor necrosis factor alpha-induced cell death. Mol Cell Biol 23: 665–676, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Osicka TM, Houlihan CA, Chan JG, Jerums G, Comper WD. Albuminuria in patients with type 1 diabetes is directly linked to changes in the lysosome-mediated degradation of albumin during renal passage. Diabetes 49: 1579–1584, 2000. [DOI] [PubMed] [Google Scholar]
- 39.Osicka TM, Panagiotopoulos S, Jerums G, Comper WD. Fractional clearance of albumin is influenced by its degradation during renal passage. Clin Sci (Lond) 93: 557–564, 1997. [DOI] [PubMed] [Google Scholar]
- 40.Pedersen MM, Christensen CK, Hansen KW, Christiansen JS, Mogensen CE. ACE-inhibition and renoprotection in early diabetic nephropathy. Response to enalapril acutely and in long-term combination with conventional antihypertensive treatment. Clin Invest Med 14: 642–651, 1991. [PubMed] [Google Scholar]
- 41.Pollock CA, Poronnik P. Albumin transport and processing by the proximal tubule: physiology and pathophysiology. Curr Opin Nephrol Hypertens 16: 359–364, 2007. [DOI] [PubMed] [Google Scholar]
- 42.Russo LM, Sandoval RM, McKee M, Osicka TM, Collins AB, Brown D, Molitoris BA, Comper WD. The normal kidney filters nephrotic levels of albumin retrieved by proximal tubule cells: retrieval is disrupted in nephrotic states. Kidney Int 71: 504–513, 2007. [DOI] [PubMed] [Google Scholar]
- 43.Ryter SW, Cloonan SM, Choi AM. Autophagy: a critical regulator of cellular metabolism and homeostasis. Mol Cells 36: 7–16, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salant DJ, Belok S, Madaio MP, Couser WG. A new role for complement in experimental membranous nephropathy in rats. J Clin Invest 66: 1339–1350, 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sancho A, Gavela E, Avila A, Morales A, Fernandez-Najera JE, Crespo JF, Pallardo LM. Risk factors and prognosis for proteinuria in renal transplant recipients. Transplant Proc 39: 2145–2147, 2007. [DOI] [PubMed] [Google Scholar]
- 46.Sarbassov DD, Ali SM, Sabatini DM. Growing roles for the mTOR pathway. Curr Opin Cell Biol 17: 596–603, 2005. [DOI] [PubMed] [Google Scholar]
- 47.Sinha D, Bannergee S, Schwartz JH, Lieberthal W, Levine JS. Inhibition of ligand-independent ERK1/2 activity in kidney proximal tubular cells deprived of soluble survival factors up-regulates Akt and prevents apoptosis. J Biol Chem 279: 10962–10972, 2004. [DOI] [PubMed] [Google Scholar]
- 48.Slattery C, Lee A, Zhang Y, Kelly DJ, Thorn P, Nikolic-Paterson DJ, Tesch GH, Poronnik P. In vivo visualization of albumin degradation in the proximal tubule. Kidney Int 74: 1480–1486, 2008. [DOI] [PubMed] [Google Scholar]
- 49.Takabatake Y, Kimura T, Takahashi A, Isaka Y. Autophagy and the kidney: health and disease. Nephrol Dial Transplant 29: 1639–1647, 2014. [DOI] [PubMed] [Google Scholar]
- 50.Thelle K, Christensen EI, Vorum H, Orskov H, Birn H. Characterization of proteinuria and tubular protein uptake in a new model of oral l-lysine administration in rats. Kidney Int 69: 1333–1340, 2006. [DOI] [PubMed] [Google Scholar]
- 51.Tojo A, Endou H. Intrarenal handling of proteins in rats using fractional micropuncture technique. Am J Physiol Renal Fluid Electrolyte Physiol 263: F601–F606, 1992. [DOI] [PubMed] [Google Scholar]
- 52.Wang Z, Choi ME. Autophagy in kidney health and disease. Antioxid Redox Signal 20: 519–537, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang Z, Havasi A, Gall J, Bonegio R, Li Z, Mao H, Schwartz JH, Borkan SC. GSK3beta promotes apoptosis after renal ischemic injury. J Am Soc Nephrol 21: 284–294, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang Z, Havasi A, Gall JM, Mao H, Schwartz JH, Borkan SC. Beta-catenin promotes survival of renal epithelial cells by inhibiting Bax. J Am Soc Nephrol 20: 1919–1928, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wen X, Klionsky DJ. Autophagy is a key factor in maintaining the regenerative capacity of muscle stem cells by promoting quiescence and preventing senescence. Autophagy 12: 617–618, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wong PM, Feng Y, Wang J, Shi R, Jiang X. Regulation of autophagy by coordinated action of mTORC1 and protein phosphatase 2A. Nat Commun 6: 8048, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yamahara K, Kume S, Koya D, Tanaka Y, Morita Y, Chin-Kanasaki M, Araki H, Isshiki K, Araki S, Haneda M, Matsusaka T, Kashiwagi A, Maegawa H, Uzu T. Obesity-mediated autophagy insufficiency exacerbates proteinuria-induced tubulointerstitial lesions. J Am Soc Nephrol 24: 1769–1781, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhai XY, Nielsen R, Birn H, Drumm K, Mildenberger S, Freudinger R, Moestrup SK, Verroust PJ, Christensen EI, Gekle M. Cubilin- and megalin-mediated uptake of albumin in cultured proximal tubule cells of opossum kidney. Kidney Int 58: 1523–1533, 2000. [DOI] [PubMed] [Google Scholar]
- 59.Zhan M, Usman IM, Sun L, Kanwar YS. Disruption of renal tubular mitochondrial quality control by Myo-inositol oxygenase in diabetic kidney disease. J Am Soc Nephrol 26: 1304–1321, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhao X, Liu G, Shen H, Gao B, Li X, Fu J, Zhou J, Ji Q. Liraglutide inhibits autophagy and apoptosis induced by high glucose through GLP-1R in renal tubular epithelial cells. Int J Mol Med 35: 684–692, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]