Abstract
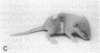
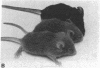
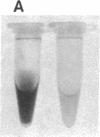
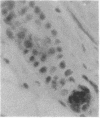
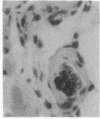
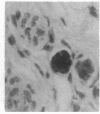
Transgenic mice of an inbred black strain were previously produced with the Tyr-SV40E transgene, comprising simian virus 40 transforming sequences driven by the tyrosinase promoter, in order to obtain melanomas; the animals were found to be lighter than normal in coat color, to various degrees. As described here, hypopigmentation resulted from diminished differentiation of melanized pigment granules in the melanocytes of the hair bulbs in vivo and occurred autonomously in cultured melanocytes. Whereas some of the mice had single-color coats, most (7/13) had coats of two or three colors; in addition, one single-color founder produced a two-color descendant. These eight mice had patterns seen in natural genotypes; the most striking were transversely striped to various extents, with regions of left-right asymmetry on either side of the dorsal midline. The patterns visualized the same clonal developmental territories of coat melanocytes displayed in allophenic mice that are formed from conjoined early embryo cells of different color genotypes. Some of the Tyr-SV40E transgenics were also cellular genotypic mosaics, probably arising by late integration of the transgene. However, one transgenic founder with a completely striped coat proved to be true-breeding, with autosomal inheritance of the pattern. The inherited striped pattern thus exemplifies the formation of phenotypically different but genetically identical developmental clones, or phenoclones, among cells of the same type. This line of transgenic mice provides exceptional material for experimental analysis of the molecular basis for clonal variation in gene expression and of the fate of oncogenic phenoclones of melanocytes occurring in the same individual.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradl M., Klein-Szanto A., Porter S., Mintz B. Malignant melanoma in transgenic mice. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):164–168. doi: 10.1073/pnas.88.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattanach B. M. Position effect variegation in the mouse. Genet Res. 1974 Jun;23(3):291–306. doi: 10.1017/s0016672300014932. [DOI] [PubMed] [Google Scholar]
- Eisinger M., Marko O. Selective proliferation of normal human melanocytes in vitro in the presence of phorbol ester and cholera toxin. Proc Natl Acad Sci U S A. 1982 Mar;79(6):2018–2022. doi: 10.1073/pnas.79.6.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein-Szanto A., Bradl M., Porter S., Mintz B. Melanosis and associated tumors in transgenic mice. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):169–173. doi: 10.1073/pnas.88.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larue L., Mintz B. Pigmented cell lines of mouse albino melanocytes containing a tyrosinase cDNA with an inducible promoter. Somat Cell Mol Genet. 1990 Jul;16(4):361–368. doi: 10.1007/BF01232464. [DOI] [PubMed] [Google Scholar]
- Mintz B. Clonal basis of mammalian differentiation. Symp Soc Exp Biol. 1971;25:345–370. [PubMed] [Google Scholar]
- Mintz B., Cronmiller C., Custer R. P. Somatic cell origin of teratocarcinomas. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2834–2838. doi: 10.1073/pnas.75.6.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz B. Gene control of mammalian differentiation. Annu Rev Genet. 1974;8:411–470. doi: 10.1146/annurev.ge.08.120174.002211. [DOI] [PubMed] [Google Scholar]
- Mintz B. Gene control of mammalian pigmentary differentiation. I. Clonal origin of melanocytes. Proc Natl Acad Sci U S A. 1967 Jul;58(1):344–351. doi: 10.1073/pnas.58.1.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz B. Gene expression in neoplasia and differentiation. Harvey Lect. 1978;71:193–246. [PubMed] [Google Scholar]
- Mintz B., Silvers W. K. Histocompatibility antigens on melanoblasts and hair follicle cells. Cell-localized homograft rejection in allophenic skin grafts. Transplantation. 1970 May;9(5):497–505. doi: 10.1097/00007890-197005000-00009. [DOI] [PubMed] [Google Scholar]
- Morse H. C., 3rd, Yetter R. A., Stimpfling J. H., Pitts O. M., Fredrickson T. N., Hartley J. W. Greying with age in mice: relation to expression of murine leukemia viruses. Cell. 1985 Jun;41(2):439–448. doi: 10.1016/s0092-8674(85)80017-9. [DOI] [PubMed] [Google Scholar]
- Tamura A., Halaban R., Moellmann G., Cowan J. M., Lerner M. R., Lerner A. B. Normal murine melanocytes in culture. In Vitro Cell Dev Biol. 1987 Jul;23(7):519–522. doi: 10.1007/BF02628423. [DOI] [PubMed] [Google Scholar]