Abstract
Pericytes are tissue-resident mesenchymal progenitor cells anatomically associated with the vasculature that have been shown to participate in tissue regeneration. Here, we tested the hypothesis that kidney pericytes, derived from FoxD1+ mesodermal progenitors during embryogenesis, are necessary for postnatal kidney homeostasis. Diphtheria toxin delivery to FoxD1Cre::RsDTR transgenic mice resulted in selective ablation of >90% of kidney pericytes but not other cell lineages. Abrupt increases in plasma creatinine, blood urea nitrogen, and albuminuria within 96 h indicated acute kidney injury in pericyte-ablated mice. Loss of pericytes led to a rapid accumulation of neutral lipid vacuoles, swollen mitochondria, and apoptosis in tubular epithelial cells. Pericyte ablation led to endothelial cell swelling, reduced expression of vascular homeostasis markers, and peritubular capillary loss. Despite the observed injury, no signs of the acute inflammatory response were observed. Pathway enrichment analysis of genes expressed in kidney pericytes in vivo identified basement membrane proteins, angiogenic factors, and factors regulating vascular tone as major regulators of vascular function. Using novel microphysiological devices, we recapitulated human kidney peritubular capillaries coated with pericytes and showed that pericytes regulate permeability, basement membrane deposition, and microvascular tone. These findings suggest that through the active support of the microvasculature, pericytes are essential to adult kidney homeostasis.
Keywords: pericyte, acute kidney injury, endothelium, microfluidics
Key Points
Pericytes are essential for adult kidney function
In the absence of pericytes, tubular epithelial cells become metabolically challenged and die
In the absence of pericytes, early indicators of inflammation are not detected after kidney injury
Pericytes support the kidney vasculature through the synthesis of basement membrane and factors that control vascular tone and angiogenesis
mesenchymal progenitor cells are important for the maintenance of tissue homeostasis at postnatal stages. This is evident in tissues with a high rate of cell turnover, like the bone marrow, where mesenchymal cells support the proliferation and differentiation of other cell types in the niche. In tissues with slow cell turnover, the focus has been placed on the role and response of mesenchymal progenitor cells in the context of tissue injury (39), and, consequently, there is a paucity of evidence for the role of resident mesenchymal progenitor cells in organ homeostasis.
In recent years, pericytes have emerged as a ubiquitous population of mesenchymal progenitor cells that reside in the stromal compartment of multiple organs, but attached to the microvasculature and fully or partially embedded in the capillary basement membrane (7). Pericytes possess the ability to generate the three main mesenchymal lineages: namely, cartilage, bone, and adipose tissue (7), and, despite constituting a small percentage of the organ's stromal cells, these cells can proliferate promptly in response to tissue injury and expand to cover the area of damage as cells known as fibroblasts. Stromal progenitor cells with such characteristics have been identified by means of lineage tracing (1, 12, 28, 51). In the kidney, we have previously traced the origin of pericytes to a layer of metanephric mesenchyme that activates the forkhead transcription factor (FoxD1) specifically during embryonic specification (14, 20). Consequently, pericytes in the normal adult kidney are FoxD1-derived cells that can be identified by membrane marker signature PDGF receptor (PDGFR)β+, CD73+, collagen (Col)1a1+, CD248+ CD45− cells (11).
Pericytes are characteristically embedded within the basement membrane of blood vessels and form intimate anatomical connections with endothelial cells through processes that end in peg-socket junctions (6, 19). On a functional level, pericytes have been shown to stabilize capillaries in vitro, an activity that depends partly on the inhibition of metalloproteases (41). Vasculature support becomes very important in the context of kidney disease. In both chronic kidney disease and end-stage kidney disease, progressive rarefaction of the capillaries surrounding the nephrons of the kidney (peritubular capillaries) causes kidney ischemia and accelerates disease progression, leading to further nephron injury and interstitial fibrosis (13, 33, 36).
A recent study (38) of embryonic development of the kidney has implicated pericytes in patterning of the kidney capillaries and regulating their diameter. In addition, pericytes have been identified as the major cell population to sense blood oxygen tension and respond by the production of erythropoietin (3, 34).
In the present study, we generated a FoxD1Cre::RsDTR transgenic mouse that permits the genetic ablation of kidney pericytes upon the systemic delivery of diphtheria toxin (DT). Using this effective tool, we assessed the relevance of pericytes for kidney function under healthy physiological conditions. Having identified specific functions for pericytes through ablation, we used novel microphysiological devices to interrogate and validate these functions in human peritubular capillaries.
METHODS
Animals.
Female Foxd1+/Cre (20) mice were bred with Gt(ROSA)26Sortm1(HBEGF)Awai mice to generate mice expressing the simian DT receptor (DTR) in FoxD1+ progenitor cells and their descendants (Foxd1Cre::RsDTR) in development and littermate controls (RsDTR). In some experiments, female Foxd1+/Cre mice were crossed with Rosa26+/(STOP)-tdTomato [B6;129S6-Gt(ROSA)26Sortm9(CAG-tdTomato)Hze] mice. To ablate pericytes, 25 ng/g DT (List Biological Laboratories) was injected intraperitoneally into Foxd1Cre::RsDTR mice or littermate controls. Blood samples and urine were collected on days 1, 2, and 3 after DT injection. Col1-green fluorescent protein (GFP) mice (28) were used to detect pericytes in live imaging analysis. All experiments were performed under protocols approved by the Department of Comparative Medicine, University of Washington, and the Institutional Animal Care and Use Committee at the University of Southern California and Biogen.
Pathway enrichment analysis.
Analysis of pericyte translatome from healthy kidneys by translated ribosomal affinity purification (TRAP) has been previously reported (16), and the data sets were deposited to GEO DataSets. Microarray data were normalized by robust multichip average using GeneSpring software (Agilent Technologies) (17). Microarrays have been deposited in the GEO database (Geo IDGSM1219324 through GSM1219338). Pathway enrichment analysis was performed using Ingenuity Pathway Analysis (Qiagen) software.
Human cell purification.
Human pericytes and human kidney microvascular endothelial cells (HKMECs) were purified from human kidneys obtained after voluntary pregnancy interruptions (day 90 to day 120 of gestation) performed at the University of Washington Medical Center (IRB447773EA University of Washington) or through a sponsored research agreement with New England Medical Center using methods previously described (15, 27). In brief, a single cell preparation, excluding the capsule and glomeruli, was generated by digestion with a collagenase cocktail (Liberase, Roche). Epithelial cells were depleted using anti-epithelial cell adhesion molecule- and lotus lectin-conjugated beads in magnetic columns (Dynabeads). The remaining portion of the sample was used to prospectively purify CD45−:CD31+ endothelial cells and PDGFRβ+:neural/glial antigen (NG)2+ pericytes by flow cytometry using directly conjugated anti-CD45 (clone HI30, Biolegend), anti-PECAM (clone WM59, BD Pharmingen), anti-PDGFRβ (clone 18A2, eBioscience), and anti-NG2 (clone LHM-2, R&D Systems). Only samples with >98% purity were used in the experiments.
Microphysiological device fabrication, culture, and experimental assays.
Microfluidic devices were generated as previously described (27, 48, 53). In brief, a neutralized liquid collagen solution was prepared at 4 mg/ml from a high-density (8–11 mg/ml) rat tail collagen type I stock solution (no. 354249, Corning). The collagen was injected into an oxygen plasma-sterilized PDMS device to create a microvessel network (27) or a single channel built in a PAR-V 3D microfluidic device and perfusion system (Nortis, Seattle, WA). The collagen was allowed to gel for 30 min at room temperature followed by an overnight incubation at 37°C. In coculture experiments, pericytes were added to the liquid collagen at a density of 5 × 104 cells/ml before injection. After gelation, endothelial cell culture medium (ScienCell ECM) was added to the reservoirs, and flow at a rate of 0.5 μl/min was initiated for 2 h before cell seeding. To seed the vessels, flow through the device was stopped, and 2-μl injections of human kidney microvascular endothelial cells (HKMECs) or from P1-P5 at a density of 7 × 106 cells/ml were delivered to the inlet port. Cells were allowed to adhere for 30 min under static conditions before flow was recommenced. Cells were maintained under a flow of 0.5 μl/min for 3–5 days before experimentation.
For basement membrane assays, the gel was removed after 72 h of flow, fixed overnight in formalin, washed, and sectioned using a cryostat. Sections were labeled for basement membrane proteins and endothelial markers following common immunofluorescence procedures. Vessel permeability was measured using a dextran leak assay. Devices were removed from flow and transferred to a 37°C heated enclosure on a Zeiss inverted microscope. A ×10 water lens (W N-Apo 10×/0.3, Zeiss) was used to focus on an area of the vessel, and FITC-conjugated 40-kDa dextran (no. 46945, Sigma) at a density of 25 μg/ml in ScienCell ECM was perfused through the channel at a rate of 1 μl/min. Fluorescence images were captured every 3 s for 10 min. From the fluorescence increase in the collagen matrix, the permeability of the vessel was calculated using the following equation: permeability of the vessel = (1/ΔI0)(δI/δt)(r/2), where ΔI0 is the increase in fluorescent intensity in the vessel, δI/δt, is the rate of increase in fluorescent intensity, and r is the radius of the vessel, as previously described (52). Images were analyzed using a custom Matlab script to find the best fit for the permeability of each vessel. For the vascular reactivity assay, the microfluidic devices were placed on an inverted Zeiss microscope in a 37°C heated chamber, and DIC contrast images were taken with a ×10 water lens (W N-Apo 10×/0.3, Zeiss) every 3 s for 10 min. A solution of 1 μg/ml ANG II (A9525, Sigma) in ScienCell ECM was perfused through the vessel at a rate of 1 μl/min. Vessel diameter was measured using ImageJ at time 0, the start of the flow of ANG II through the vessel, and at a time of 10 min. For electron microscopy, 2.5% glutaraldehyde and 2.0% paraformaldehyde in 0.2M cacodylate buffer was flowed through the devices for 2 h at 1 μl/min. After a wash with cacodylate buffer, samples were processed for standard electron microscopy.
Tissue preparation and histology.
Mouse tissues were prepared and stained as previously described (16, 28). Primary antibodies against the following proteins were used for immunolabeling: PDGFRβ (1:200, eBioscience), CD31 (1:200, BD), F4/80 (1:200, Invitrogen), CD45 (eBioscience), podoplanin (1:100, eBioscience), and ki67 (1:200, Vector). Slides were incubated with fluorescence (Cy3 or FITC)-conjugated secondary antibodies (1:400-1:800, Jackson ImmunoResearch) and mounted with Vectashield/4′,6-diamidino-2-phenylindole, and images were captured using a Nikon TiE Inverted Widefield Fluorescence microscope. Areas of positive fluorescence in ×200 magnification of 10 randomly selected images per mouse were quantified using ImageJ software (http://rsbweb.nih.gov/ij/). Cell apoptosis was detected on paraffin sections by TUNEL assay using an In Situ Death detection kit (Roche) according to the manufacturer's protocol. Histological analysis was performed on paraffin-embedded sections stained with periodic acid-Schiff. Analysis of kidney injury included evaluation of vacuolization, debris, edema, tubule dilation, necrosis, and glomeruli morphology. Sections were semiquantitatively scored on a validated scale of 0-4 (where 0 = no changes, 1 = <25% of parenchyma affected, 2 = 25–50% of parenchyma affected, 3 = 50–75% of parenchyma affected, and 4 = >75% of parenchyma affected) (29). To stain for lipid accumulation, cryosections were stained for 10 min with a freshly prepared working solution of 60% oil red O (vol/vol; in distilled water) from stock (oil red O saturated in isopropanol). Slides were rinsed in distilled water, counterstained in Gill hematoxylin (Sigma), and mounted with aqueous medium (Sigma). Areas of positive stain in ×100 magnification of 10 randomly selected images per mouse were quantified using Adobe Photoshop software.
Morphometric glomerular analysis.
Glomerular area and volume were calculated using a previously reported computer-based morphometric analysis method (32). Kidney cortical section (3.5 μm in thickness) stained with periodic acid-Schiff were examined by light microscopy (Zeiss, Jena, Germany). For each section, ∼40 glomeruli were systematically digitized (glomeruli were consecutively encountered moving the microscope stage following an S-shape path) using a ×63 objective, and digital images were acquired. Estimation of glomerular area and volume was performed on digital images of cortical tissue using image-analysis tools (ImageJ; http://rsb.info.nih.gov/ij). Volume was estimated assuming a spherical conformation.
Multiphoton imaging of the kidney.
Intravital imaging of the intact, living mouse kidney was performed as previously described (18, 21). Briefly, mice were anesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg). The right carotid artery and/or jugular vein were cannulated for dye infusion. A 70-kDa Texas red dextran or Alexa 594 BSA was injected to label the vasculature. The kidney was exteriorized through a flank incision, and the animal placed on the microscope stage. Body temperature was maintained with a homeothermic blanket system (Harvard Apparatus). Time lapse images were acquired using a Leica TCS SP5 multiphoton confocal fluorescence imaging system with a ×63 objective powered by a Chameleon Ultra-II MP laser at 860 nm and a DMI 6000 inverted microscope's external nondescanned detectors using TRITC/FITC filters.
Renal function.
Plasma creatinine was measured using an enzymatic assay kit (Diazyme). Blood urea nitrogen (BUN) was measured using a colorimetric kit (Pointe Scientific). Urinary albumin concentration was measured using an ELISA kit (Albuwell M, Exocell).
Quantitative PCR.
RNA was isolated from kidney tissue samples using TRIzol reagent (Invitrogen) according to standard protocol. First-strand cDNA was synthesized using an iScript kit (Bio-Rad). Real-time PCR was performed using iTaq SYBR green supermix with ROX (Bio-Rad) and 7900HT ABI detection system (Applied Biosystems). Target genes were normalized by hypoxanthine phosphoribosyltransferase expression. mRNA expression was calculated using the 2−ΔΔCt method (where Ct is threshold cycle) and expressed as the n-fold difference relative to the control group.
Statistical analysis.
Statistical evaluation was carried out using one-way ANOVA followed by a Tukey posttest using GraphPad Prism (GraphPad Software) for multiple analyses and Student's t-test for single paired analyses. P values of <0.05 were considered to be significant. Error bars indicate SDs unless otherwise stated.
RESULTS
Kidney pericytes are localized to branching points of the vasculature.
To establish the anatomic location of pericytes in vivo, we performed live multiphoton imaging of whole kidneys from anesthetized Col1-GFP mice, which selectively labels pericytes (28). Administration of 70-kDa dextran-rhodamine to the arterial circulation enabled visualization of the kidney peritubular capillaries (see Supplemental Movie S1 in the Supplemental Material).1 Strikingly, pericytes were concentrated at areas of branching points in the microvascular network, had long processes that terminated at branch points where shear stress was maximal, and had cell bodies downstream of branching points. Over the course of 2 min, pericyte migration was not observed, but regular oscillations in individual peritubular capillary blood flow and vessel diameter were visualized.
Ablation of kidney pericytes causes tubular epithelial cell damage.
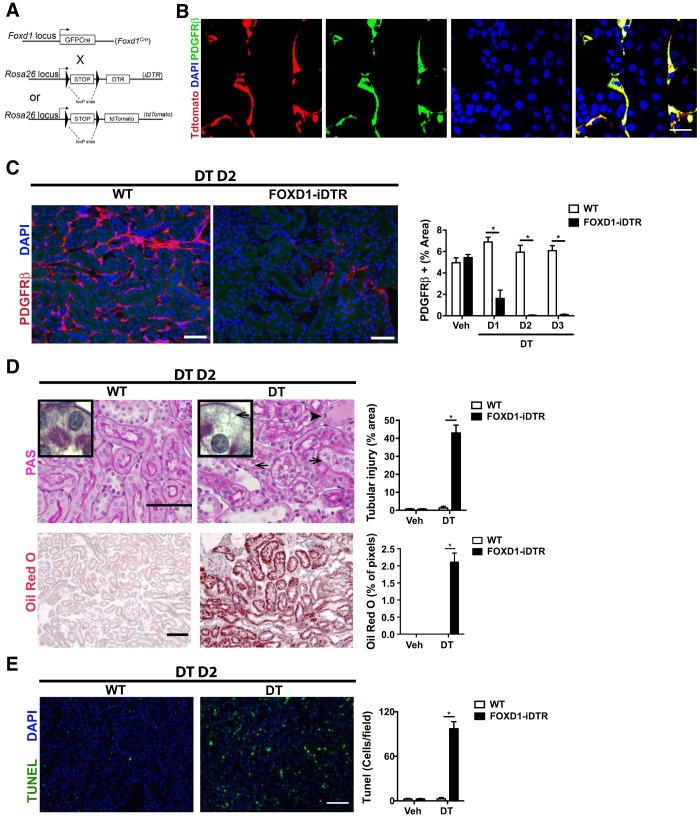
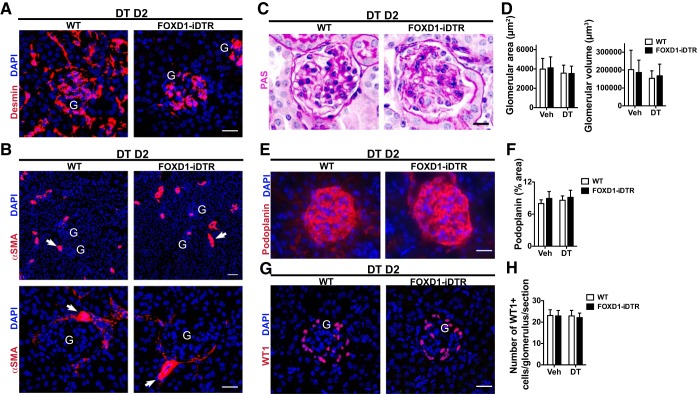
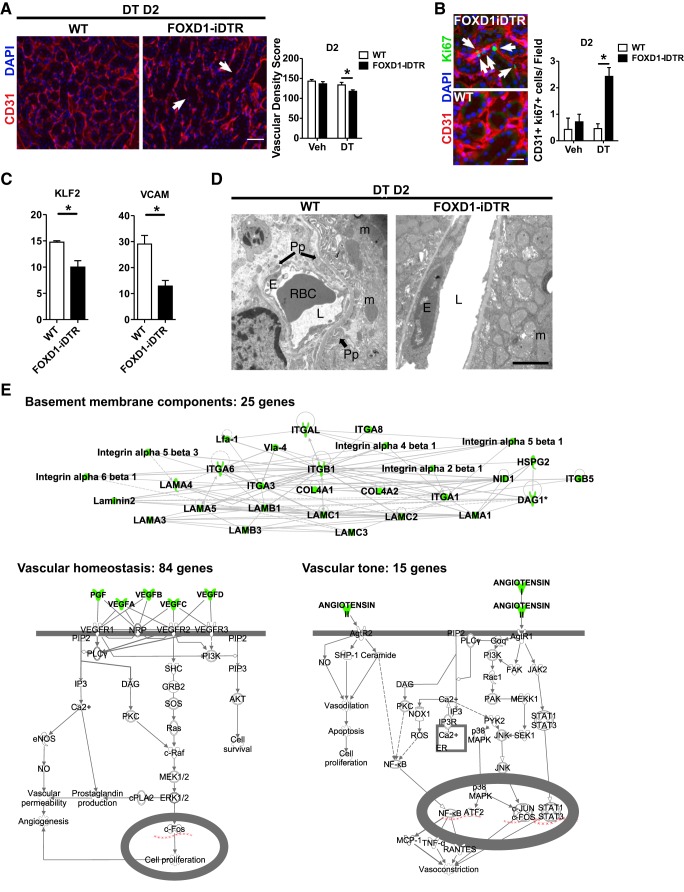
To explore the role of pericytes in kidney homeostasis, we generated conditional FoxD1+/Cre::Rosa26iDTR/tdTomato and FoxD1+/Cre::Rosa26+/iDTR mice, hereafter named FoxD1Cre::RsDTR mice, which express DTRs in cells of the FOXD1 progenitor cell lineage (Fig. 1A). Expression of the inducible DTR in pericytes was confirmed by detection of tdTomato fluorescence in cells coexpressing PDGFRβ, an established pericyte/mural cell marker (Fig. 1B). A dose-response analysis (data not shown) of DT administration determined that 25 ng/g was the most effective dose, ablating ∼90% of pericytes in the kidney by day 2 (Fig. 1C). Identical doses of DT to littermate controls lacking DTRs had no effect (Fig. 1C). Importantly, although mesangial and vascular smooth muscle cells also derive from the FoxD1 lineage, neither of the two cell populations was ablated by the treatment, indicating that the dose of DT used was not high enough to significantly affect those cell types (Fig. 2, A and B).
Fig. 1.
Characterization of in vivo ablation of kidney pericytes. A: schema showing simplified gene loci maps for the two alleles that result in FoxD1::iDTR mice expressing the diphtheria toxin (DT) receptor (DTR) in cells of the FoxD1 lineage as well as mice expressing tdTomato in cells of the FoxD1 lineage. B: split panel images showing the expression of tdTomato in adult kidney pericytes derived from FoxD1+ embryonic progenitors identified by coexpression of PDGF receptor (PDGFR)β. DAPI, 4′,6-diamidino-2-phenylindole. Bar = 25 μm. C: detection (left) and quantification (right) of kidney pericytes in FoxD1::iDTR mice that received a single intraperitoneal injection of either vehicle (Veh) or DT. D1–D3, days 1–3 posttreatment; WT, wild type. Values are means ± SD. *P < 0.05 by Student's t-test. Bar = 50 μm. D: determination of tubular injury by periodic acid-Schiff (PAS) staining of kidney sections from vehicle- and DT-recipient FoxD1::iDTR mice (top). Lipid vacuoles are indicated by arrows. The accumulation of proteinaceous fluid in proximal tubules is indicated by an arrowhead. Lipid accumulation was confirmed by oil red O staining (bottom). Values in bar graphs are means ± SD. *P < 0.05 by Student's t-test. Bar = 50 μm. E: TUNEL immunofluorescence showing increased cell death in the kidneys of FoxD1::iDTR mice after DT treatment compared with vehicle. Values are means ± SD; n = 6–7 mice/group. *P < 0.05 by Student's t-test. Bar = 50 μm.
Fig. 2.
Effects of DT administration on the glomerulus. A: detection of mesangial cells using anti-desmin antibody in kidney sections of WT and FOXD1-iDTR mice 2 days after DT administration. Bar = 25 μm. B: detection of vascular smooth muscle (arrows) using anti-smooth muscle actin (SMA) antibody. G, glomerulus. Bar in top image = 50 μm; bar in bottom image = 25 μm. C: images of PAS-stained kidney sections showing glomeruli. Bar = 25 μm. D: quantification of glomerular area and volume. E: detection of podocytes using anti-podoplanin antibody. Bar = 25 μm. F: quantification of podoplanin cells by morphometry. G: detection of podocytes using anti-Wilms' tumor 1 (WT1) antibody. Bar = 25 μm. H: quantification of WT1+ cells. Values are means ± SD; n = 3–5 mice/group.
Histological analysis revealed that pericyte ablation causes acute injury with the presence of protein casts, loss of the brush border and epithelial cell degeneration with intense vacuolization in proximal tubules, and increased tubular epithelial cell death (Fig. 1, D and E). The vacuoles observed in epithelial cells consisted of lipid droplets, potentially indicating dysfunctional fatty acid metabolism. These features are highly similar to human acute kidney injury (49). Interestingly, histological assessment of glomerular structures showed preserved morphology with no signs of glomerular damage (Fig. 2, C–F). Immunohistological analysis using the podocyte markers podoplanin and Wilms' tumor 1 revealed no significant changes in podocyte content (Fig. 2, E–H). These data indicated that the effect of pericyte ablation chiefly affects nonglomerular segments of the nephron.
Pericytes are essential for renal function in the adult.
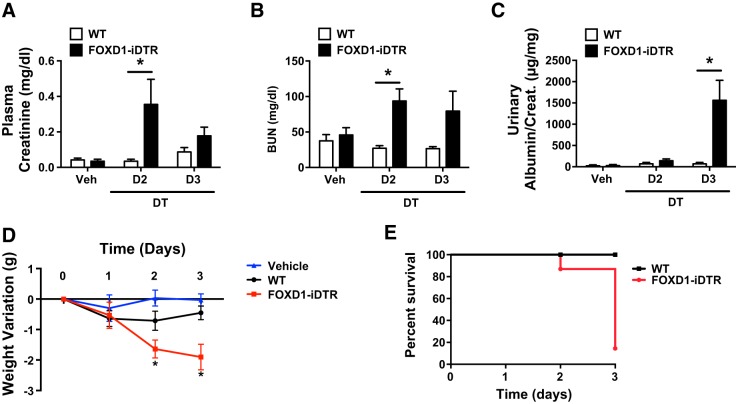
Having determined the profound impact of pericyte ablation on kidney histological integrity, we then proceeded to assess its impact on kidney function. Plasma creatinine and BUN levels were significantly increased by day 2 in pericyte-ablated mice compared with DT-treated control mice (Fig. 3, A and B). Levels of urinary albumin/creatinine were found to be significantly higher on day 3 in the ablated group compared with the DT-treated control group (Fig. 3C). Pericyte ablation led to steady and significant weight loss through posttreatment day 3 (Fig. 3D), when 90% of FoxD1Cre::RsDTR mice that received DT died (Fig. 3E). No significant weight loss was observed in RsDTR mice that received DT, ruling out potential side effects from DT administration (Fig. 3D).
Fig. 3.
Functional consequences of pericyte ablation. A–C: values corresponding to plasma creatinine (A), blood urea nitrogen (BUN; B), and the urinary albumin-to-creatinine ratio (C). Values are means ± SD. *P < 0.05 by Student's t-test. D: quantification of daily body weight changes in WT and FoxD1::iDTR mice that received daily intraperitoneal injections of either vehicle or DT. E: survival curves for WT and FoxD1::iDTR mice treated with DT. n = 6–8 mice/group.
Therefore, pericyte ablation leads to a rapid deterioration of kidney function, histological features of acute kidney injury resulting in death within 72 h.
In the absence of pericytes, tubular injury does not trigger an inflammatory response.
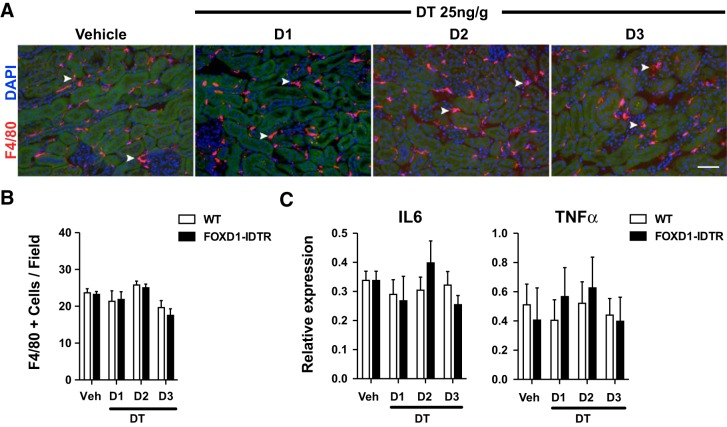
Kidney injury triggers an inflammatory response that can lead to further damage through the secretion of inflammatory cytokines. The initial stages of this process are characterized by infiltrating neutrophils and monocytes/macrophages that produce inflammatory cytokines. To study whether pericyte ablation triggers inflammatory cell recruitment, we assessed macrophage infiltration by immunofluorescence detection of F4/80, a well-established macrophage marker (Fig. 4A). We found no differences in the number of macrophages in the kidneys of DT-treated FoxD1Cre::RsDTR mice compared with DT-treated wild-type mice (Fig. 4B). Analysis of mRNA levels for TNF-α and IL-6, two major cytokines secreted during the inflammatory process, showed no significant changes in the pericyte-ablated group compared with the DT-treated control group with no kidney injury (Fig. 4C). In addition, no significant differences were observed for monocyte chemoattractant protein-1 (data not shown). These observations indicate that acute inflammatory cell recruitment is an unlikely cause of the epithelial damage observed in the absence of pericytes and suggest that pericytes are important in leukocyte recruitment and inflammatory cytokine production.
Fig. 4.
Effect of pericyte ablation on inflammation. Macrophages (arrowheads) in the kidneys of FoxD1::iDTR mice treated with DT were detected by F4/80 immunofluorescence (A) and quantified (B). Values are means ± SD. Bar = 50 μm. C: quantitative PCR analysis of IL-6 and TNF-α expression in whole kidneys of WT and FoxD1::iDTR mice treated with DT. Values are means ± SD; n = 6–8 mice/group.
Pericytes support adult kidney vasculature homeostasis.
Because pericytes contribute to different aspects of the microvasculature, including its development, maturation, and stabilization (2, 35, 43), we next examined the effect of pericyte ablation on vascular homeostasis in the adult kidney. Histological assessment indicated that DT-treated FoxD1Cre::RsDTR mice had disrupted peritubular capillaries by day 2 posttreatment, with reduced vascular density and increased endothelial cell proliferation (Fig. 5, A and B). Reduced expression levels for VCAM-1 and Krüppel-like factor 2, which are involved in the regulation of vascular tone (10), confirmed the loss of vascular integrity (Fig. 5C). Structural analysis of pericyte-ablated kidneys by electron microscopy showed dilation of peritubular capillaries with swollen endothelial cells, notable for a high ribosomal content. Tubular epithelial cells adjacent to these vessels displayed swollen mitochondria in which loss of cristae could be observed, an indicator of cell stress (Fig. 5D).
Fig. 5.
Role of pericytes in regulating capillary homeostasis. A, left: representative CD31 immunofluorescence images from FoxD1::iDTR mice at day 2 of either vehicle or DT treatment. Arrows indicate areas of capillary loss. Bar = 50 μm. Right, quantification of capillary density. B: representative CD31 and Ki67 immunofluorescence images (bar = 25 μm) and quantification of endothelial cell proliferation. Arrows indicate proliferating endothelial cells. C: quantitative PCR quantification of Krüppel-like factor (KLF)-2 and VCAM mRNA. Values are means ± SD. *P < 0.05 by Student's t-test. D: electron microscopy images of either vehicle- or DT-treated FoxD1::iDTR mouse kidneys. Pp, pericyte; L, lumen; E, endothelial cell; RBC, red blood cell; m, mitochondria. Bar = 2 μm. E: analysis of pericyte-specific translational profiles from healthy Col1a1::GFP-L10a mouse kidneys by translated ribosomal affinity purification (TRAP). Col, collagen; GFP, green fluorescent protein. Pathway enrichment analysis was performed using pathway analysis software.
Previous studies have identified discrete pericyte-secreted factors that regulate vasculature stability and angiogenesis, including angiopoietin 1 (9, 46), VEGF (8), and IL-6 (24). To capture the pericyte-specific transcriptome involved in vascular homeostasis in the normal kidney in vivo, we analyzed the translational profiles of whole Col1a1-L10aGFP mouse kidney medulla extracts that were subjected to TRAP (17). Pathway enrichment analysis of actively translated genes (Table 1) specifically enriched in pericytes compared with the remainder of the kidney revealed genes enriched in 3 major pathways, namely, 25 basement membrane components, 84 vascular homeostasis factors, and 15 vascular tone-regulating factors (Fig. 5E).
Table 1.
Genes identified in the translatome of healthy mouse kidney pericytes that affect vascular permeability, tone, and homeostasis
| Gene Symbol | Gene Name |
|---|---|
| Basement membrane | |
| COL4A1 | Collagen, type IV, α1 |
| COL4A2 | Collagen, type IV, α2 |
| COL4A4 | Collagen, type IV, α4 |
| DAG1 | Dystroglycan 1 |
| NID1 | Nidogen 1 |
| HSPG2 | Heparan sulfate proteoglycan 2 |
| ITGB8 | Integrin, β8 |
| ITGA3 | Integrin, α3 |
| ITGAL | Integrin, αL |
| ITGA5 | Integrin, α5 |
| ITGB5 | Integrin, β5 |
| ITGA1 | Integrin, α1 |
| ITGB1 | Integrin, β1 |
| ITGA6 | Integrin, α6 |
| ITGA8 | Integrin, α8 |
| ITGB8 | Integrin, β8 |
| LAMC1 | Laminin, γ1 |
| LAMA4 | Integrin, α4 |
| LAMA5 | Integrin, α5 |
| LAMA3 | Integrin, α3 |
| LAMB3 | Integrin, β3 |
| LAMC3 | Laminin, γ3 |
| LAMB1 | Integrin, β1 |
| LAMA1 | Integrin, α1 |
| LAMC2 | Laminin, γ2 |
| Vascular tone | |
| AGT | Angiotensinogen (serpin peptidase inhibitor, clade A, member 8) |
| GDF15 | Growth differentiation factor 15 |
| GMFB | Glia maturation factor-β |
| FGF2 | Fibroblast growth factor 2 (basic) |
| PGF | Placental growth factor |
| CMTM7 | CKLF-like MARVEL transmembrane domain containing 7 |
| EDN1 | Endothelin 1 |
| IL1B | Interleukin 1β |
| IL1RN | Interleukin 1 receptor antagonist |
| MIF | Macrophage migration inhibitory factor (glycosylation-inhibiting factor) |
| TGFB1 | Transforming growth factor-β1 |
| PF4 | Platelet factor 4 |
| SCG2 | Secretogranin II |
| TNFSF10 | Tumor necrosis factor (ligand) superfamily, member 10 |
| VAV3 | vav 3 guanine nucleotide exchange factor |
| Vascular homeostasis | |
| ANGPT1 | Angiopoietin 1 |
| ANGPT2 | Angiopoietin 2 |
| ANGPT4 | Angiopoietin 4 |
| ANGPTL3 | Angiopoietin-like 3 |
| BDNF | Brain-derived neurotrophic factor |
| BMP2 | Bone morphogenetic protein 2 |
| BMP4 | Bone morphogenetic protein 4 |
| BMP6 | Bone morphogenetic protein 6 |
| BTC | β-Cellulin |
| CTGF | Connective tissue growth factor |
| EGF | Epidermal growth factor |
| EREG | Epiregulin |
| FGF1 | Fibroblast growth factor 1 (acidic) |
| FGF2 | Fibroblast growth factor 2 (basic) |
| FGF3 | Fibroblast growth factor 3 |
| FGF4 | Fibroblast growth factor 4 |
| FGF7 | Fibroblast growth factor 7 |
| FGF9 | Fibroblast growth factor 9 |
| FGF18 | Fibroblast growth factor 18 |
| FIGF | c-Fos-induced growth factor (vascular endothelial growth factor D) |
| GAS6 | Growth arrest-specific 6 |
| GDNF | Glial cell-derived neurotrophic factor |
| GHRL | Ghrelin/obestatin prepropeptide |
| GRN | Granulin |
| HBEGF | Heparin-binding EGF-like growth factor |
| HGF | Hepatocyte growth factor (hepapoietin A, scatter factor) |
| IGF1 | Insulin-like growth factor 1 (somatomedin C) |
| IGF2 | Insulin-like growth factor 2 |
| INHBA | Inhibin-βA |
| JAG1 | Jagged 1 |
| KITLG | KIT ligand |
| LEFTY1 | Left-right determination factor 1 |
| LEP | Leptin |
| MDK | Midkine (neurite growth-promoting factor 2) |
| NDP | Norrie disease (pseudoglioma) |
| NGF | Nerve growth factor (β-polypeptide) |
| NODAL | Nodal growth differentiation factor |
| NRG2 | Neuregulin 2 |
| PDGFA | Platelet-derived growth factor α-polypeptide |
| PDGFB | Platelet-derived growth factor β-polypeptide |
| PDGFC | Platelet-derived growth factor C |
| PGF | Placental growth factor |
| PTN | Pleiotrophin |
| Vascular homeostasis | |
| TGFA | Transforming growth factor-α |
| TGFB1 | Transforming growth factor-β1 |
| TGFB2 | Transforming growth factor-β2 |
| VEGFA | Vascular endothelial growth factor A |
| VEGFB | Vascular endothelial growth factor B |
| VEGFC | Vascular endothelial growth factor C |
| AIMP1 | Aminoacyl tRNA synthetase complex-interacting multifunctional protein 1 |
| CCL2 | Chemokine (C-C motif) ligand 2 |
| CCL5 | Chemokine (C-C motif) ligand 5 |
| CCL11 | Chemokine (C-C motif) ligand 11 |
| CCL28 | Chemokine (C-C motif) ligand 28 |
| CSF1 | Colony stimulating factor 1 (macrophage) |
| CSF3 | Colony stimulating factor 3 (granulocyte) |
| CX3CL1 | Chemokine (C-X3-C motif) ligand 1 |
| CXCL2 | Chemokine (C-X-C motif) ligand 2 |
| CXCL6 | Chemokine (C-X-C motif) ligand 6 |
| CXCL10 | Chemokine (C-X-C motif) ligand 10 |
| CXCL12 | Chemokine (C-X-C motif) ligand 12 |
| CXCL14 | Chemokine (C-X-C motif) ligand 14 |
| Cxcl15 | Chemokine (C-X-C motif) ligand 15 |
| EDN1 | Endothelin 1 |
| IL6 | Interleukin 6 |
| IL13 | Interleukin 13 |
| IL15 | Interleukin 15 |
| IL18 | Interleukin 18 |
| IL17B | Interleukin 17B |
| IL1B | Interleukin 1β |
| IL1RN | Interleukin 1 receptor antagonist |
| LIF | Leukemia inhibitory factor |
| PF4 | Platelet factor 4 |
| SLURP1 | Secreted LY6/PLAUR domain containing 1 |
| SPP1 | Secreted phosphoprotein 1 |
| TIMP1 | Tissue inhibitor of metallopeptidase 1 |
| TNF | Tumor necrosis factor |
| TNFSF10 | Tumor necrosis factor (ligand) superfamily, member 10 |
| TNFSF12 | Tumor necrosis factor (ligand) superfamily, member 12 |
| VAV3 | vav 3 guanine nucleotide exchange factor |
| WNT2 | Wingless-type MMTV integration site family member 2 |
| WNT4 | Wingless-type MMTV integration site family, member 4 |
| WNT5A | Wingless-type MMTV integration site family, member 5A |
| WNT7A | Wingless-type MMTV integration site family, member 7A |
Taken together, these data showed that loss of pericyte function results in a rapid disruption of the kidney endothelial architecture likely by alterations in the basement membrane and vascular homeostasis.
Pericytes spontaneously invest in the kidney microvascular wall, where they regulate permeability and tone.
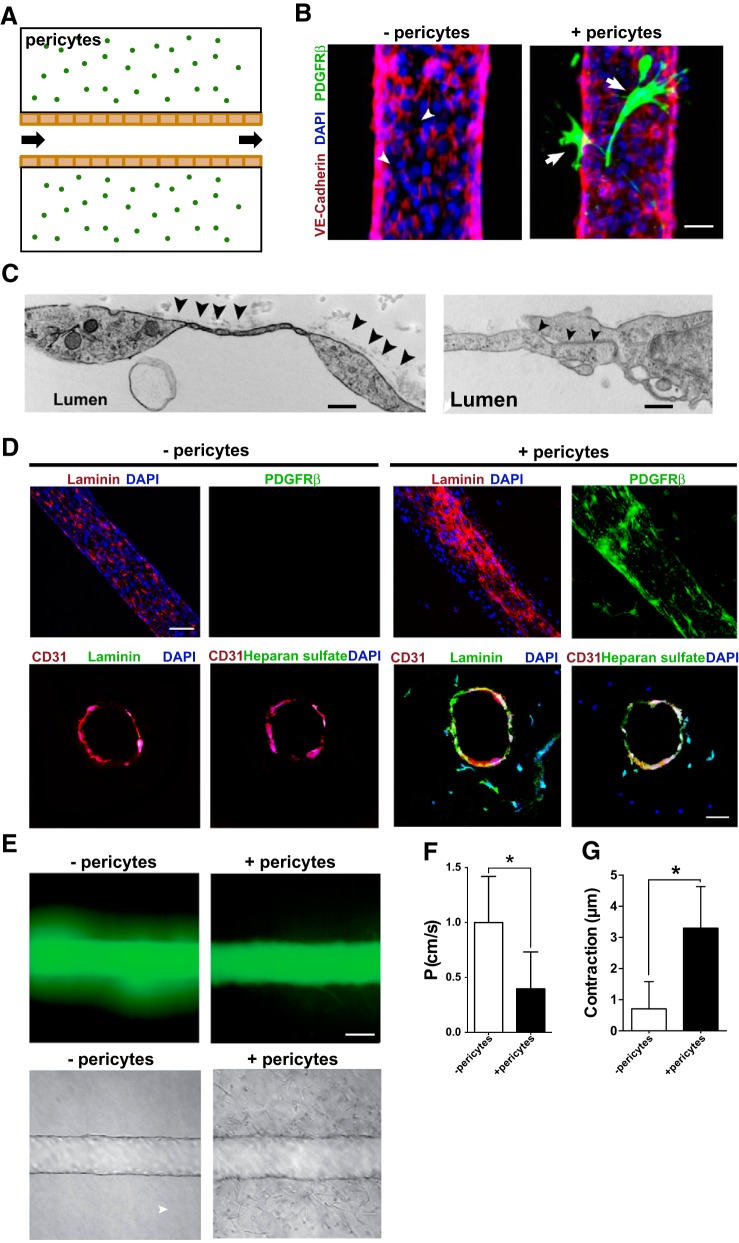
We have previously shown that purified mouse kidney pericytes bind to endothelial tubes derived from the umbilical vein (human umbilical vein endothelial cells) in three-dimensional gel assays in vitro, suppressing the activity of endothelial cell-secreted metalloproteinases and preventing the regression of capillaries in vitro (22, 41). Here, we tested the effect of pericytes on regulating microfabricated and functioning human kidney peritubular capillaries in vitro. HKMECs were purified, expanded, and then seeded into prefabricated channels in a collagen matrix. Unidirectional luminal flow of culture medium was driven by a pump through a single lumen (Fig. 6A). Twenty-four hours after their introduction into a branched network of channels (Fig. 6B) or a single prefabricated channel (Fig. 6C), HKMECs coated the walls of the fabricated channels, forming tight junctions and a barrier that mimicked a kidney peritubular capillary (Fig. 6, B and C) (27). The addition of purified human kidney pericytes randomly dispersed into the collagen matrix during gelation resulted in pericyte migration toward the capillaries and coating the abluminal surface of the vessels (Fig. 6B). This typical pericyte behavior indicated that our microfluidic devices were permissive for interactions between the two cell types and confirmed previous results using mouse cells (22, 41). Microstructural analysis by electron microscopy scanning of vessel walls indicated the presence of a basement membrane on the abluminal surface of capillaries with integrated pericytes (Fig. 6C). Since the basement membrane plays an important role in vessel stability, we examined the devices 72 h after the creation of the kidney capillaries for the deposition of laminin in single or branched channel capillaries. In the absence of pericytes, endothelial cells produced a limited amount of laminin and heparan sulfate proteoglycan. Both matrix components were deposited into an organized basement membrane when capillaries were coated by pericytes compared with the vessels alone (Fig. 6D).
Fig. 6.
Evaluation of human kidney pericyte function in microphysiological devices. A: schema showing the microfluidic device used. The flow was automated, constant, and unidirectional through a 150-μm lumen. B: representative immunofluorescence images showing capillaries formed by purified human kidney microvascular endothelial (HKMECs) labeled with vascular-endothelial cadherin (red) with (+) or without (−) pericytes (green). Bar = 25 μm. C: electron microscopy scanning of microfabricated human capillaries showing basement membrane matrix deposition on the abluminal face (left; bar = 1 μm) and tight junctions (right; bar = 500 nm). D: immunofluorescence images of fabricated kidney capillaries immunostained with antibodies against laminin and heparan sulfate in the absence or presence of pericytes (PDGFRβ). Top, longitudinal scanning; bottom, cross-sectional scanning. Bar = 50 μm. E, top: fluorescence imaging of dextran diffusion. Bottom, bright-field images of microfluidic channels coated with HKMECs in the absence or presence of pericytes. Bar = 50 μm. F: quantification of vessel permeability (P). Values in the bar graph are means ± SD. *P < 0.05 by Student's t-test. G: quantification of microfluidic vessel contraction. Values in the bar graph are means ± SD. *P < 0.05 by Student's t-test.
To test whether pericytes modified the functional properties of capillaries, we measured the diffusion coefficient after administration of 70-kDa dextran-FITC into the vessel lumen. We found that although HKMECs alone provided a barrier, equivalent to a 10-fold reduction (diffusion coefficient of 10−5 with no endothelial cells and 10−6 with endothelial cells) in permeability in their absence, there was nevertheless a significant leak of the dextran marker into the surrounding matrix, and this likely represents permeability at tight junctions (Fig. 6, E and F, and Supplemental Movie S2). Pericytes migrating to the vessel wall resulted in a further reduction in permeability of approximately threefold (Fig. 6, E and F, and Supplemental Movie S2, A and B). In the presence of pericytes, residual permeability appeared as a diffuse signal across the vessel as opposed to discontinuous leakage, suggesting that pericytes may enhance junctional integrity.
Since pericytes are a source of factors regulating tone, are enriched in nonmuscle myosins, and appeared to contract in live imaging of kidney capillaries (Supplemental Movie S1), we evaluated their role in controlling vascular tone in the single lumen device. ANG II, a widely recognized vasopressor that controls arteriolar diameter, was infused. Over a 10-min period of imaging, capillary diameter was continuously evaluated. In 5-day-old capillaries consisting of HKMECs only, there was a small but significant contraction of the microvessel measured (Fig. 6G and Supplemental Movie S3, A and B). However, in the presence of recruited pericytes, this contraction was much more robust, equating to a 5% reduction in diameter. The greatest contraction appeared to occur in areas where pericytes were in close contact, but the presence of pericytes resulted in enhanced contractility across the entire vessel.
DISCUSSION
Using a conditional genetic ablation method to study the contribution of resident pericytes to adult kidney homeostasis, we show that pericytes of the FoxD1 lineage play an essential role in the maintenance of renal function, since in their absence, characteristic features of acute kidney injury (including tubular cell vacuolation, tubular proteinaceous casts, and elevated levels of plasma creatinine and BUN) develop rapidly. The ablated mice also developed albuminuria in the absence of glomerular lessions. These findings are consistent with previously reported cases of albuminuria in patients with acute tubular necrosis that have no detectable glomerular damage (50). Although albuminuria is generally believed to be the result of increased transit of albumin through the glomerular basement membrane, it may also be attributed to altered proximal tubule receptor-mediated endocytosis of albumin (37).
Pericytes in the kidney express both PDGFRβ and PDGFRα and originate developmentally from a population of cap mesenchyme progenitor cells, which express the transcription factor FoxD1. These mesenchymal progenitors appear to originate from either odd-skipped related (OSR)1+ mesenchymal cells of the intermediate mesoderm or migrating neural crest progenitors (26). Fate mapping of these cells has indicated they represent a major population of cells that become pathological myofibroblasts that contribute to interstitial fibrosis and inflammation (14, 20). However, their function in organ homeostasis has not been previously explored. In nephrogenesis, the pericytes are referred to more generally as stromal cells. Mice deficient in either PDGF-B or the pericyte-restricted receptor PDGFRβ display abnormal kidney development with immature nephrons (25, 43), and more recent studies of FoxD1-derived stromal cells point to essential roles for these cells in vascular patterning and nephrogenesis (31, 35).
One natural assumption is that in adult organ homeostasis, pericytes become quiescent or dormant cells and serve little function. Our results, however, propose an active role for pericytes in supporting both vascular and tubular function. Tubular epithelial cells in the kidneys of pericyte-ablated mice were metabolically stressed, displaying lipid vacuoles and altered mitochondrial morphology. Lipid accumulation, seen as vacuolation, might be the result of deficient oxidation of fatty acids, for which proper mitochondrial function is necessary or as a result of acute nutrient deficiency. Such effects might occur in the absence of blood flow, but the results obtained did not show thrombosis, and although capillaries were lost, this loss was not matched by the extent of tubular injury. It is therefore likely that severe microvascular dysfunction from pericyte loss leads to secondary dysfunction of the tubules. Supporting this hypothesis, we show that pericyte ablation leads to a disruption of the kidney vasculature, where severe injury to endothelial cells with swelling was observed. This result fits well the role of pericytes as mural cells supporting the endothelium and closely resembles the severe vascular phenotype observed in PDGFRβ−/− and PDGF-B−/− mice in which the pericyte lineage is absent during development. Both mutant mice lines display abnormal capillaries, with brain and kidney aneurysms and hemorrhages that lead to perinatal death (25, 30, 43). It supports a critical nursing role for kidney pericytes in vascular development recently highlighted (31, 35).
In addition to establishing focal contacts with endothelial cells, pericytes regulate different aspects of vascular biology via paracrine signals. Through the secretion of angiopoietin 1, a glycoprotein that binds to the Tie2 receptor in endothelial cells, pericytes induce capillary diameter increases (46, 47). Our analysis of affinity-purified translating ribosomes showed that pericytes synthesize members of the VEGF family of angiogenic factors in the normal adult kidney. Those results validate previous studies that have reported VEGF expression in pericytes (4, 23) and support the view that pericytes can regulate vessel remodeling. Our results also showed that pericytes synthesize angiotensinogen, suggesting the existence of a local mechanism of angiotensin production. Aside from the main axis of angiotensinogen production, pericyte-derived angiotensinogen could act as an autocrine signal to rapidly exert vasoconstriction and fine tune blood flow. This would be in line with our findings and previous work showing that pericytes locally regulate the diameter of brain capillaries by means of vasoconstriction (38).
The pericyte translatome also comprised several basement membrane matrix components, and this confirms previous data reporting that pericytes contribute to the assembly of the vascular basement membrane (45). Importantly, our results confirm the observation that endothelial cells do produce some of the basement membrane proteins, namely, laminin, yet other components might be exclusive to the pericyte. For example, we could not detect heparin sulfate proteoglycan in microvessels formed by endothelial cells alone, but we did find it when pericytes were present.
We developed a novel micophysiological system to further study the supporting role of pericytes on human peritubular capillaries and, in doing so, demonstrated essential roles for pericytes in vascular homeostasis. Our in vitro experiments provide evidence that pericytes stabilize capillaries, reducing their permeability. Loss of barrier function of capillaries is associated with tissue edema and loss of oncotic gradients that permit flow of molecules from the tubule to the capillary and vice versa. Such an abrupt loss of barrier that may occur after pericyte ablation could explain the loss of organ function. Pericyte-derived angiopoietin-1, as well as loss of other factors of the Robo-Slit and S1P families, could be causal in this observation. In addition, abrupt loss of certain basement membrane factors may contribute to the barrier. Such an hypothesis is supported by previous findings that the basement membrane of leaky tumor-associated microvessels is thin or even absent in areas along the vessel (42).
The lack of a detectable acute inflammatory response after pericyte ablation, despite evidence of tubular cell death and the likely consequent release of damage-associated molecules, was an unexpected result. However, several lines of evidence support the hypothesis that pericytes are important contributors to the inflammatory process. We have previously shown that while Toll-like receptor (TLR)-2/TLR-4 signaling was dispensable in inflammatory myeloid cells after sterile kidney injury, TLR-2/TLR-4 and Myd88 signaling was necessary for fibrogenic gene expression and secretion of IL-6 and monocyte chemoattractant protein-1 by pericytes (5). Similar results have been reported by another group, showing that danger-associated molecular patterns (damage-associated molecules) released by tissue injury trigger an inflammatory response in pericytes by which those cells release cytokines that attract and activate leukocytes, directing their trafficking via macrophage migration inhibitory factor signaling (44). A growing body of evidence supports the notion that pericytes are major contributors to the acute inflammatory response triggered by tissue injury by producing cytokines and chemokines critical for the recruitment of leukocytes and also in guiding leukocytes across the vascular barrier (40).
The microfluidic systems presented here provide a novel platform to study the functional role of supporting cells in kidney function. The measurements performed using this device provide strong evidence that pericytes in the kidney capillary network are significant contributors to the regulation of renal blood flow, the formation of junctions, and the stability of microvascular networks. We are currently using these systems to elucidate the mechanisms through which pericytes provide this support.
In conclusion, our study provides evidence for an essential role of pericytes in postnatal tissue homeostasis highlighting the relevance of these cells to the preservation of vasculature, tubular epithelial cell health, and the inflammatory response.
GRANTS
This work was funded by Biogen, by National Institutes of Health Grants DK-087389, DK-093493, DK-094768, DK-64324, and TR-000504, and by American Heart Association grant 12040023.
DISCLOSURES
All employees of Biogen have stock in the company. J. S. Duffield has served on the scientific advisory board for Promedior Incorporated and Regulus Therapeutics, has stock options with Promedior Incorporated, is a cofounder of Muregen LLC, and has patents for the use of agents to treat kidney disease.
AUTHOR CONTRIBUTIONS
D.R.L., G.M., A.H., G.C., T.A., L.D., I.G.G., G.L., J.P.-P., and J.S.D. performed experiments; D.R.L., G.M., G.C., T.A., I.G.G., and J.S.D. analyzed data; D.R.L., G.C., L.D., and J.S.D. interpreted results of experiments; D.R.L., G.C., L.D., and J.S.D. prepared figures; D.R.L. and J.S.D. drafted manuscript; D.R.L., G.M., A.H., G.C., L.D., I.G.G., J.P.-P., and J.S.D. edited and revised manuscript; D.R.L., G.M., A.H., G.C., T.A., L.D., I.G.G., K.F., G.L., J.P.-P., and J.S.D. approved final version of manuscript; G.C., G.L., and J.S.D. conception and design of research.
ACKNOWLEDGMENTS
The authors thank the Lynn and Mike Garvey Microscopy Suite, the University of Washington Renal Pathology Core, Dr. Ying Zheng for advice, and James Burford and Kelly Hudkins for assistance with imaging. The authors also thank Dr. Ben Humphreys for kindly sharing the pericyte TRAP data set.
Footnotes
Supplemental Material for this article is available at the American Journal of Physiology-Renal Physiology website.
REFERENCES
- 1.Acharya A, Baek ST, Huang G, Eskiocak B, Goetsch S, Sung CY, Banfi S, Sauer MF, Olsen GS, Duffield JS, Olson EN, Tallquist MD. The bHLH transcription factor Tcf21 is required for lineage-specific EMT of cardiac fibroblast progenitors. Development 139: 2139–2149, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res 97: 512–523, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Bachmann S, Le Hir M, Eckardt KU. Co-localization of erythropoietin mRNA and ecto-5′-nucleotidase immunoreactivity in peritubular cells of rat renal cortex indicates that fibroblasts produce erythropoietin. J Histochem Cytochem 41: 335–341, 1993. [DOI] [PubMed] [Google Scholar]
- 4.Bai Y, Zhu X, Chao J, Zhang Y, Qian C, Li P, Liu D, Han B, Zhao L, Zhang J, Buch S, Teng G, Hu G, Yao H. Pericytes contribute to the disruption of the cerebral endothelial barrier via increasing VEGF expression: implications for stroke. PLos One 10: e0124362, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campanholle G, Mittelsteadt K, Nakagawa S, Kobayashi A, Lin SL, Gharib SA, Heinecke JW, Hamerman JA, Altemeier WA, Duffield JS. TLR-2/TLR-4 TREM-1 signaling pathway is dispensable in inflammatory myeloid cells during sterile kidney injury. PLos One 8: e68640, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caruso RA, Fedele F, Finocchiaro G, Pizzi G, Nunnari M, Gitto G, Fabiano V, Parisi A, Venuti A. Ultrastructural descriptions of pericyte/endothelium peg-socket interdigitations in the microvasculature of human gastric carcinomas. Anticancer Res 29: 449–453, 2009. [PubMed] [Google Scholar]
- 7.Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, Traas J, Schugar R, Deasy BM, Badylak S, Buhring HJ, Giacobino JP, Lazzari L, Huard J, Peault B. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3: 301–313, 2008. [DOI] [PubMed] [Google Scholar]
- 8.Darland DC, Massingham LJ, Smith SR, Piek E, Saint-Geniez M, D'Amore PA. Pericyte production of cell-associated VEGF is differentiation-dependent and is associated with endothelial survival. Dev Biol 264: 275–288, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Davis S, Aldrich TH, Jones PF, Acheson A, Compton DL, Jain V, Ryan TE, Bruno J, Radziejewski C, Maisonpierre PC, Yancopoulos GD. Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell 87: 1161–1169, 1996. [DOI] [PubMed] [Google Scholar]
- 10.Dekker RJ, van Thienen JV, Rohlena J, de Jager SC, Elderkamp YW, Seppen J, de Vries CJ, Biessen EA, van Berkel TJ, Pannekoek H, Horrevoets AJ. Endothelial KLF2 links local arterial shear stress levels to the expression of vascular tone-regulating genes. Am J Pathol 167: 609–618, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duffield JS. Cellular and molecular mechanisms in kidney fibrosis. J Clin Invest 124: 2299–2306, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dulauroy S, Di Carlo SE, Langa F, Eberl G, Peduto L. Lineage tracing and genetic ablation of ADAM12+ perivascular cells identify a major source of profibrotic cells during acute tissue injury. Nat Med 18: 1262–1270, 2012. [DOI] [PubMed] [Google Scholar]
- 13.Eardley KS, Kubal C, Zehnder D, Quinkler M, Lepenies J, Savage CO, Howie AJ, Kaur K, Cooper MS, Adu D, Cockwell P. The role of capillary density, macrophage infiltration and interstitial scarring in the pathogenesis of human chronic kidney disease. Kidney Int 74: 495–504, 2008. [DOI] [PubMed] [Google Scholar]
- 14.Gomez IG, Duffield JS. The FOXD1 lineage of kidney perivascular cells and myofibroblasts: functions and responses to injury. Kidney Int Suppl 4: 26–33, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gomez IG, MacKenna DA, Johnson BG, Kaimal V, Roach AM, Ren S, Nakagawa N, Xin C, Newitt R, Pandya S, Xia TH, Liu X, Borza DB, Grafals M, Shankland SJ, Himmelfarb J, Portilla D, Liu S, Chau BN, Duffield JS. Anti-microRNA-21 oligonucleotides prevent Alport nephropathy progression by stimulating metabolic pathways. J Clin Invest 125: 141–156, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grgic I, Campanholle G, Bijol V, Wang C, Sabbisetti VS, Ichimura T, Humphreys BD, Bonventre JV. Targeted proximal tubule injury triggers interstitial fibrosis and glomerulosclerosis. Kidney Int 82: 172–183, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grgic I, Krautzberger AM, Hofmeister A, Lalli M, DiRocco DP, Fleig SV, Liu J, Duffield JS, McMahon AP, Aronow B, Humphreys BD. Translational profiles of medullary myofibroblasts during kidney fibrosis. J Am Soc Nephrol 25: 1979–1990, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hackl MJ, Burford JL, Villanueva K, Lam L, Susztak K, Schermer B, Benzing T, Peti-Peterdi J. Tracking the fate of glomerular epithelial cells in vivo using serial multiphoton imaging in new mouse models with fluorescent lineage tags. Nat Med 19: 1661–1666, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayden MR, Karuparthi PR, Habibi J, Lastra G, Patel K, Wasekar C, Manrique CM, Ozerdem U, Stas S, Sowers JR. Ultrastructure of islet microcirculation, pericytes and the islet exocrine interface in the HIP rat model of diabetes. Exp Biol Med (Maywood) 233: 1109–1123, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Humphreys BD, Lin SL, Kobayashi A, Hudson TE, Nowlin BT, Bonventre JV, Valerius MT, McMahon AP, Duffield JS. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol 176: 85–97, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang JJ, Toma I, Sipos A, McCulloch F, Peti-Peterdi J. Quantitative imaging of basic functions in renal (patho)physiology. Am J Physiol Renal Physiol 291: F495–F502, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Kida Y, Ieronimakis N, Schrimpf C, Reyes M, Duffield JS. EphrinB2 reverse signaling protects against capillary rarefaction and fibrosis after kidney injury. J Am Soc Nephrol 24: 559–572, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim Y, Imdad RY, Stephenson AH, Sprague RS, Lonigro AJ. Vascular endothelial growth factor mRNA in pericytes is upregulated by phorbol myristate acetate. Hypertension 31: 511–515, 1998. [DOI] [PubMed] [Google Scholar]
- 24.LaBarbera KE, Hyldahl RD, O'Fallon KS, Clarkson PM, Witkowski S. Pericyte NF-κB activation enhances endothelial cell proliferation and proangiogenic cytokine secretion in vitro. Physiol Rep 3: e12309, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leveen P, Pekny M, Gebre-Medhin S, Swolin B, Larsson E, Betsholtz C. Mice deficient for PDGF B show renal, cardiovascular, and hematological abnormalities. Genes Dev 8: 1875–1887, 1994. [DOI] [PubMed] [Google Scholar]
- 26.Levinson RS, Batourina E, Choi C, Vorontchikhina M, Kitajewski J, Mendelsohn CL. Foxd1-dependent signals control cellularity in the renal capsule, a structure required for normal renal development. Development 132: 529–539, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Ligresti G, Nagao RJ, Xue J, Choi YJ, Xu J, Ren S, Aburatani T, Anderson SK, MacDonald JW, Bammler TK, Schwartz SM, Muczynski A, Duffield JS, Himmelfarb J, Zheng Y. A novel three-dimensional human peritubular microvascular system. J Am Soc Nephrol; doi: 10.1681/ASN.2015070747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin SL, Kisseleva T, Brenner DA, Duffield JS. Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am J Pathol 173: 1617–1627, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin SL, Li B, Rao S, Yeo EJ, Hudson TE, Nowlin BT, Pei H, Chen L, Zheng JJ, Carroll TJ, Pollard JW, McMahon AP, Lang RA, Duffield JS. Macrophage Wnt7b is critical for kidney repair and regeneration. Proc Natl Acad Sci USA 107: 4194–4199, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lindahl P, Johansson BR, Leveen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science 277: 242–245, 1997. [DOI] [PubMed] [Google Scholar]
- 31.Little MH, McMahon AP. Mammalian kidney development: principles, progress, and projections. Cold Spring Harb Perspect Biol; doi: 10.1101/cshperspect.a008300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Macconi D, Sangalli F, Bonomelli M, Conti S, Condorelli L, Gagliardini E, Remuzzi G, Remuzzi A. Podocyte repopulation contributes to regression of glomerular injury induced by ACE inhibition. Am J Pathol 174: 797–807, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mallamaci F, Benedetto FA, Tripepi G, Cutrupi S, Pizzini P, Stancanelli B, Seminara G, Bonanno G, Rapisarda F, Fatuzzo P, Malatino LS, Zoccali C. Vascular endothelial growth factor, left ventricular dysfunction and mortality in hemodialysis patients. J Hypertens 26: 1875–1882, 2008. [DOI] [PubMed] [Google Scholar]
- 34.Maxwell PH, Osmond MK, Pugh CW, Heryet A, Nicholls LG, Tan CC, Doe BG, Ferguson DJ, Johnson MH, Ratcliffe PJ. Identification of the renal erythropoietin-producing cells using transgenic mice. Kidney Int 44: 1149–1162, 1993. [DOI] [PubMed] [Google Scholar]
- 35.Nakagawa N, Xin C, Roach AM, Naiman N, Shankland SJ, Ligresti G, Ren S, Szak S, Gomez IG, Duffield JS. Dicer1 activity in the stromal compartment regulates nephron differentiation and vascular patterning during mammalian kidney organogenesis. Kidney Int 87: 1125–1140, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ohashi R, Shimizu A, Masuda Y, Kitamura H, Ishizaki M, Sugisaki Y, Yamanaka N. Peritubular capillary regression during the progression of experimental obstructive nephropathy. J Am Soc Nephrol 13: 1795–1805, 2002. [DOI] [PubMed] [Google Scholar]
- 37.Osicka TM, Houlihan CA, Chan JG, Jerums G, Comper WD. Albuminuria in patients with type 1 diabetes is directly linked to changes in the lysosome-mediated degradation of albumin during renal passage. Diabetes 49: 1579–1584, 2000. [DOI] [PubMed] [Google Scholar]
- 38.Peppiatt CM, Howarth C, Mobbs P, Attwell D. Bidirectional control of CNS capillary diameter by pericytes. Nature 443: 700–704, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pretheeban T, Lemos DR, Paylor B, Zhang RH, Rossi FM. Role of stem/progenitor cells in reparative disorders. Fibrogenesis Tissue Repair 5: 20, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Proebstl D, Voisin MB, Woodfin A, Whiteford J, D'Acquisto F, Jones GE, Rowe D, Nourshargh S. Pericytes support neutrophil subendothelial cell crawling and breaching of venular walls in vivo. J Exp Med 209: 1219–1234, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schrimpf C, Xin C, Campanholle G, Gill SE, Stallcup W, Lin SL, Davis GE, Gharib SA, Humphreys BD, Duffield JS. Pericyte TIMP3 and ADAMTS1 modulate vascular stability after kidney injury. J Am Soc Nephrol 23: 868–883, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sennino B, Falcon BL, McCauley D, Le T, McCauley T, Kurz JC, Haskell A, Epstein DM, McDonald DM. Sequential loss of tumor vessel pericytes and endothelial cells after inhibition of platelet-derived growth factor B by selective aptamer AX102. Cancer Res 67: 7358–7367, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soriano P. Abnormal kidney development and hematological disorders in PDGF beta-receptor mutant mice. Genes Dev 8: 1888–1896, 1994. [DOI] [PubMed] [Google Scholar]
- 44.Stark K, Eckart A, Haidari S, Tirniceriu A, Lorenz M, von Bruhl ML, Gartner F, Khandoga AG, Legate KR, Pless R, Hepper I, Lauber K, Walzog B, Massberg S. Capillary and arteriolar pericytes attract innate leukocytes exiting through venules and ‘instruct’ them with pattern-recognition and motility programs. Nat Immunol 14: 41–51, 2013. [DOI] [PubMed] [Google Scholar]
- 45.Stratman AN, Davis GE. Endothelial cell-pericyte interactions stimulate basement membrane matrix assembly: influence on vascular tube remodeling, maturation, and stabilization. Microsc Microanal 18: 68–80, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sundberg C, Kowanetz M, Brown LF, Detmar M, Dvorak HF. Stable expression of angiopoietin-1 and other markers by cultured pericytes: phenotypic similarities to a subpopulation of cells in maturing vessels during later stages of angiogenesis in vivo. Lab Invest 82: 387–401, 2002. [DOI] [PubMed] [Google Scholar]
- 47.Thurston G, Suri C, Smith K, McClain J, Sato TN, Yancopoulos GD, McDonald DM. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science 286: 2511–2514, 1999. [DOI] [PubMed] [Google Scholar]
- 48.Tourovskaia A, Fauver M, Kramer G, Simonson S, Neumann T. Tissue-engineered microenvironment systems for modeling human vasculature. Exp Biol Med (Maywood) 239: 1264–1271, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Venkatachalam MA, Weinberg JM, Kriz W, Bidani AK. Failed tubule recovery, AKI-CKD transition, and kidney disease progression. J Am Soc Nephrol 26: 1765–1776, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.West BL, Picken MM, Leehey DJ. Albuminuria in acute tubular necrosis. Nephrol Dial Transplant 21: 2953–2956, 2006. [DOI] [PubMed] [Google Scholar]
- 51.Worthley DL, Churchill M, Compton JT, Tailor Y, Rao M, Si Y, Levin D, Schwartz MG, Uygur A, Hayakawa Y, Gross S, Renz BW, Setlik W, Martinez AN, Chen X, Nizami S, Lee HG, Kang HP, Caldwell JM, Asfaha S, Westphalen CB, Graham T, Jin G, Nagar K, Wang H, Kheirbek MA, Kolhe A, Carpenter J, Glaire M, Nair A, Renders S, Manieri N, Muthupalani S, Fox JG, Reichert M, Giraud AS, Schwabe RF, Pradere JP, Walton K, Prakash A, Gumucio D, Rustgi AK, Stappenbeck TS, Friedman RA, Gershon MD, Sims P, Grikscheit T, Lee FY, Karsenty G, Mukherjee S, Wang TC. Gremlin 1 identifies a skeletal stem cell with bone, cartilage, and reticular stromal potential. Cell 160: 269–284, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yuan W, Lv Y, Zeng M, Fu BM. Non-invasive measurement of solute permeability in cerebral microvessels of the rat. Microvasc Res 77: 166–173, 2009. [DOI] [PubMed] [Google Scholar]
- 53.Zheng Y, Chen J, Craven M, Choi NW, Totorica S, Diaz-Santana A, Kermani P, Hempstead B, Fischbach-Teschl C, Lopez JA, Stroock AD. In vitro microvessels for the study of angiogenesis and thrombosis. Proc Natl Acad Sci USA 109: 9342–9347, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]