Abstract
In the setting of normal kidney function, iron deficiency is associated with increased FGF23 production and cleavage, altering circulating FGF23 levels. Our objective was to determine how chronic kidney disease (CKD) and dietary iron intake affect FGF23 production and metabolism in wild-type (WT) and hepcidin knockout (HKO) mice. For 8 wk, the mice were fed diets that contained adenine (to induce CKD) or no adenine (control group), with either low-iron (4 ppm) or standard-iron (335 ppm) concentrations. The low-iron diet induced iron deficiency anemia in both the WT and HKO mice. Among the WT mice, in both the control and CKD groups, a low-iron compared with a standard-iron diet increased bone Fgf23 mRNA expression, C-terminal FGF23 (cFGF23) levels, and FGF23 cleavage as manifested by a lower percentage intact FGF23 (iFGF23). Independent of iron status, CKD was associated with inhibition of FGF23 cleavage. Similar results were observed in the HKO control and CKD groups. Dietary iron content was more influential on FGF23 parameters than the presence or absence of hepcidin. In the CKD mice (WT and HKO, total n = 42), independent of the effects of serum phosphate, iron deficiency was associated with increased FGF23 production but also greater cleavage, whereas worse kidney function was associated with increased FGF23 production but decreased cleavage. Therefore, in both the WT and HKO mouse models, dietary iron content and CKD affected FGF23 production and metabolism.
Keywords: fibroblast growth factor 23, iron deficiency anemia, hepcidin, chronic kidney disease
early stages of chronic kidney disease (CKD) are characterized by the development of progressive anemia (15) and concurrent elevation of fibroblast growth factor 23 (FGF23) levels (17), with subsequent reductions in 1,25(OH)2 vitamin D that precipitate secondary hyperparathyroidism (14). FGF23 is a hormone secreted by osteocytes that induces phosphaturia and decreases renal 1α-hydroxylase expression (33), physiologically functioning as a homeostatic regulator of phosphate and a counterregulatory hormone to 1,25(OH)2 D3. FGF23 levels increase early in the course of CKD and continue to rise as the glomerular filtration rate decreases (12, 17, 22, 30). Although elevated FGF23 levels help to maintain normophosphatemia until late in the CKD course, they have been associated with CKD progression (11, 18, 31) and increased cardiovascular morbidity and mortality (13, 18). Anemia also occurs early in CKD and is multifactorial in etiology, contributed to by iron deficiency, elevated hepcidin levels that mediate iron-restricted erythropoiesis, and inadequate erythropoietin synthesis (4). As with FGF23, CKD-related anemia is associated with CKD progression (28, 43), excess cardiovascular morbidity, and all-cause mortality (21, 42).
Although FGF23 levels increase early in the course of CKD, physiological regulation of FGF23 remains incompletely understood. Phosphate (2, 32), 1,25(OH)2 D3 (32, 44), parathyroid hormone (PTH) (23, 25), and calcium (35) have been implicated, but such factors are within normal ranges when bone and circulating FGF23 levels are already elevated (17, 29). Recent studies suggest that iron may also play a role in FGF23 regulation. Observational studies in humans with normal renal function have demonstrated an inverse relationship between iron status and circulating C-terminal FGF23 (6, 9, 16, 45). In mature wild-type mice with normal renal function, iron deficiency results in increased bone Fgf23 mRNA expression, increased circulating C-terminal FGF23, but normal levels of the intact, bioactive form of the hormone (10). These observations suggest that, while iron deficiency may increase FGF23 production, the activity of intracellular proteolytic cleavage mechanisms may be concurrently increased, resulting in elevated C-terminal levels, but normal intact FGF23 levels, thus maintaining normophosphatemia. However, in autosomal dominant hypophosphatemic rickets, which is characterized by a stabilizing FGF23 mutation that renders the molecule more resistant to cleavage, iron deficiency causes high levels of both intact and C-terminal FGF23, as seen in mice (10) and humans (16).
Thus iron status may play a critical role in FGF23 regulation. Iron deficiency anemia, which is highly prevalent in CKD and occurs early in the CKD course (15), may represent a novel mechanism contributing to CKD-associated elevations of FGF23 levels. A recent study by David et al. (8) demonstrated the acute effects of inflammation on iron and FGF23 parameters in a mouse CKD model; however, the effects of primary, chronic changes in dietary iron status on FGF23 in CKD are unknown. Furthermore, as levels of the iron-regulatory hormone hepcidin increase in CKD (3, 46), hepcidin-mediated iron sequestration may potentially affect FGF23 levels in CKD. To investigate the chronic effects of CKD, iron status, and hepcidin on FGF23 metabolism, we placed wild-type (WT) and hepcidin knockout (HKO) mice on 8-wk diets with low or standard iron concentrations, without or with adenine, which induces CKD (Table 1).
Table 1.
C57BL/6 mouse groups and diets
| Genotype | Dietary Adenine | Dietary Iron |
|---|---|---|
| Wild-type | No | Low (4 ppm) |
| Wild-type | No | Standard (335 ppm) |
| Wild-type | Yes | Low (4 ppm) |
| Wild-type | Yes | Standard (335 ppm) |
| Hepcidin knockout | No | Low (4 ppm) |
| Hepcidin knockout | No | Standard (335 ppm) |
| Hepcidin knockout | Yes | Low (4 ppm) |
| Hepcidin knockout | Yes | Standard (335 ppm) |
Wild-type and hepcidin knockout mice were placed on 8-wk diets with low or standard iron concentrations, without or with adenine, which induces chronic kidney disease (CKD).
METHODS
Animal studies.
Experiments were conducted in accordance with UCLA Division of Laboratory Animal Medicine guidelines, and the study protocol was approved by the UCLA Office of Animal Research Oversight. Mice were housed at UCLA, in standard cages with wood chip bedding that was changed twice weekly. Animal housing rooms were temperature and humidity controlled, with a 12:12-h light-dark cycle.
Mouse models and diets.
WT C57BL/6 mice (Jackson Laboratories, Bar Harbor, ME) and HKO C57BL/6 mice (bred in the laboratory) of both sexes were used. Mouse diets were obtained from Harlan Teklad (Indianapolis, IN). For groups of mice in which CKD was induced, the diets contained 0.2% wt/wt adenine (19, 40, 41). Dietary iron concentrations were 4 ppm (low) or 335 ppm (standard). Diets contained standard phosphate concentrations. Diets were started postweaning, at 4 wk of age, and provided ad libitum. Mice remained on the diets for 8 wk, until the experimental end point. At the time of euthanasia, we collected whole blood, plasma, and serum and harvested livers and tibias, from which we flushed the bone marrow. Blood and tissue samples were processed immediately and stored at −80°C. Five mice died before the experimental end point: three WT CKD low-iron mice after 5 wk on the diet, one WT CKD standard iron mouse after 7 wk on the diet, and one HKO CKD standard iron mouse after 2 wk on the diet.
Biochemical parameters.
Complete blood counts were measured by a Hemavet 950 automated processor (Drew Scientific, Oxford, CT). Serum urea nitrogen, creatinine, phosphate, and calcium were assayed by colorimetric methods (Alfa-Wasserman ACE Alera and Axcel Systems, West Caldwell, NJ). Serum hepcidin was measured by ELISA, as previously described (20). Serum 1,25(OH)2 D3 was determined by enzyme immunoassay (Immunodiagnostics Systems, Boldon, Tyne & Wear, UK). Serum IL-6 was measured using Luminex bead-based assays (ThermoFisher, Waltham, MA). Serum intact parathyroid hormone (iPTH), plasma C-terminal FGF23 (cFGF23), and plasma intact FGF23 (iFGF23) concentrations were assayed using rodent-specific ELISA kits (Immutopics, San Clemente, CA and Quidel, San Diego, CA). Whereas the cFGF23 assay detects both the full-length, intact hormone and its inactive C-terminal proteolytic fragments, thus functioning as a surrogate measure of overall FGF23 production, the iFGF23 assay detects only the full-length, biologically active form. The percentage of iFGF23 was calculated by dividing the iFGF23 values (measured in pg/ml) by the cFGF23 values (also measured in pg/ml) and multiplying by 100.
Renal histological analysis.
Harvested kidneys were fixed in 4% paraformaldehyde, embedded in paraffin, and sectioned. Deparaffinized kidney tissue sections were stained with hematoxylin and eosin and assessed with light microscopy at ×10 and ×40 magnification.
Quantitative hepatic iron concentration.
Harvested livers were snap-frozen in liquid nitrogen and stored at −80°C. Small pieces of the livers (~100 mg) were weighed and homogenized. Protein precipitation solution (0.53N HCl and 5.3% trichloroacetic acid in ddH2O) was added, and the samples were boiled and centrifuged. Iron concentrations in the supernatants were measured by a colorimetric assay (Genzyme, Cambridge, MA), then normalized to the weights of the original samples to yield liver iron concentration.
Quantitative real-time PCR.
Marrow-free tibias were homogenized in TRIzol (Invitrogen, Life Technologies). RNA was then isolated according to the manufacturer’s protocol. We performed quantitative RT-PCR using the iScript RT-PCR kit (Bio-Rad, Hercules, CA) and primers specific for mouse Fgf23. We used the following PCR conditions: initial denaturation at 95°C for 2 min, followed by 40 cycles of denaturation at 94°C for 30 s, annealing at 58°C for 30 s, extension at 72°C for 1 min, and final extension at 72°C for 10 min. Fgf23 gene expression was normalized to that of hypoxanthine-guanine phosphoribosyltransferase (Hprt), and data are expressed as dCt = Ct(Hprt) – Ct(Fgf23) (24). Liver samples were processed and analyzed in a similar fashion to assess Saa (serum amyloid A) mRNA expression. Each RNA sample was analyzed in duplicate.
Mouse primer sequences used were as follows: Fgf23 forward: 5′-ACAGGAGCCATGACTCGAAG-3′; Fgf23 reverse: 5′-GCAATTCTCTGGGCTGAAGT-3′; Saa forward: 5′-AGTCTGGGCTGCTGAGAAAA-3′; Saa reverse: 5′-ATGTCTGTTGGCTTCCTGGT-3′; Hprt forward: 5′-CTGGTTAAGCAGTACAGCCCCAA-3′; Hprt reverse: 5′-CAGGAGGTCCTTTTCACCAGC-3′.
Statistical analysis.
Statistical analysis was performed using SigmaPlot 12.5 (San Jose, CA). mRNA expression data are presented as means ± SE; all other biochemical parameters are presented as medians and interquartile ranges. For the mRNA expression data, which were normally distributed with equal variances, parametric group comparisons were performed using t-tests. For the biochemical parameter data, which were not normally distributed or had unequal variances, nonparametric group comparisons were performed using Mann-Whitney rank sum tests. For each outcome variable (e.g., urea nitrogen, cFGF23, etc.), the following 10 pairwise comparisons were performed: low vs. standard iron within the WT control, WT CKD, HKO control, and HKO CKD groups; control vs. CKD within the WT low-iron, WT standard-iron, HKO low-iron, and HKO standard-iron groups; and WT vs. HKO within the CKD low-iron and CKD standard-iron groups. (WT vs. HKO comparisons within the control groups were not performed, as these pairwise comparisons were deemed to be less informative.) A P value of <0.05 was considered to be statistically significant; however, given multiple comparison testing, a Benjamini-Hochberg correction was used.
RESULTS
Effects of dietary iron content in WT control mice.
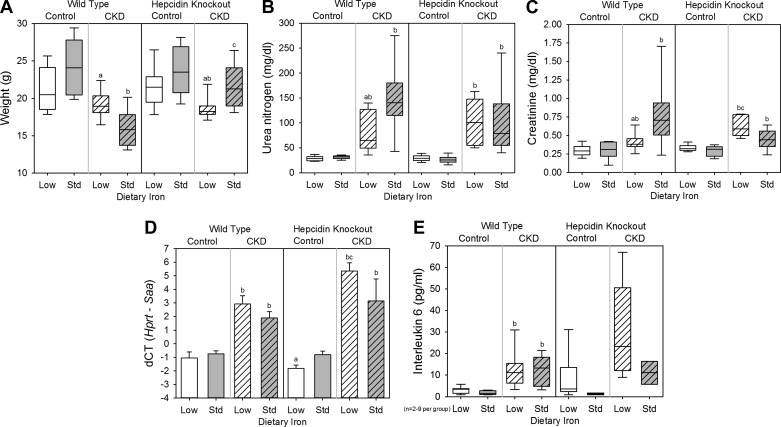
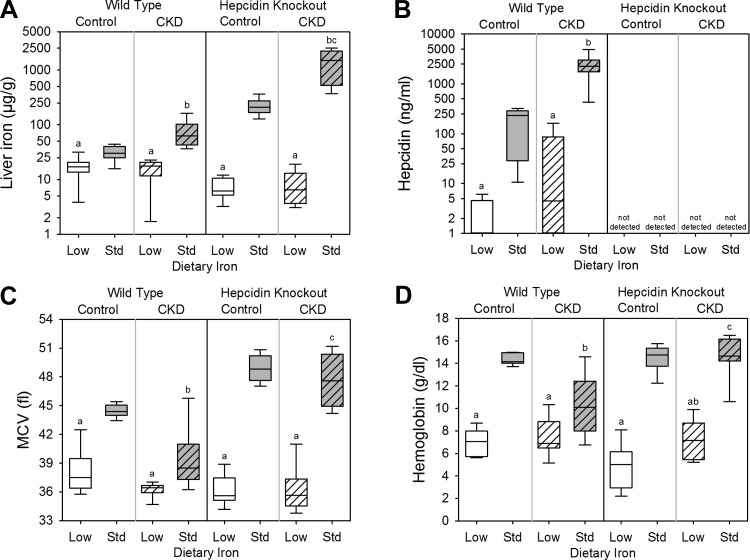
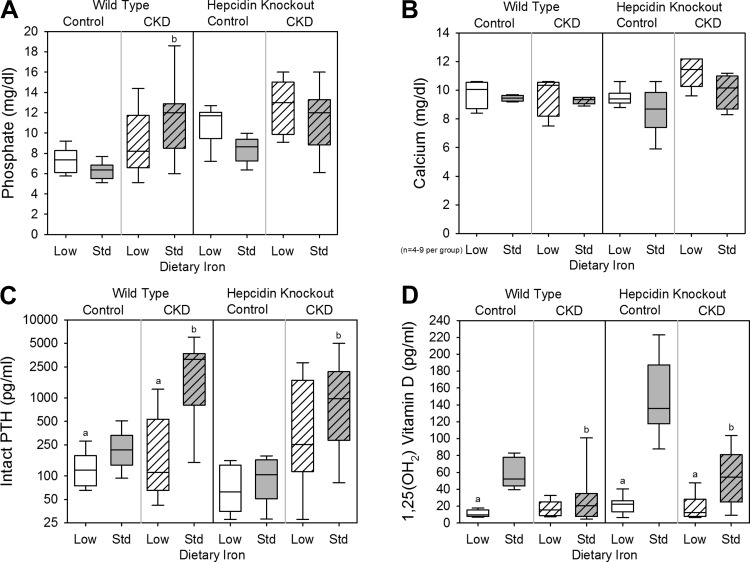
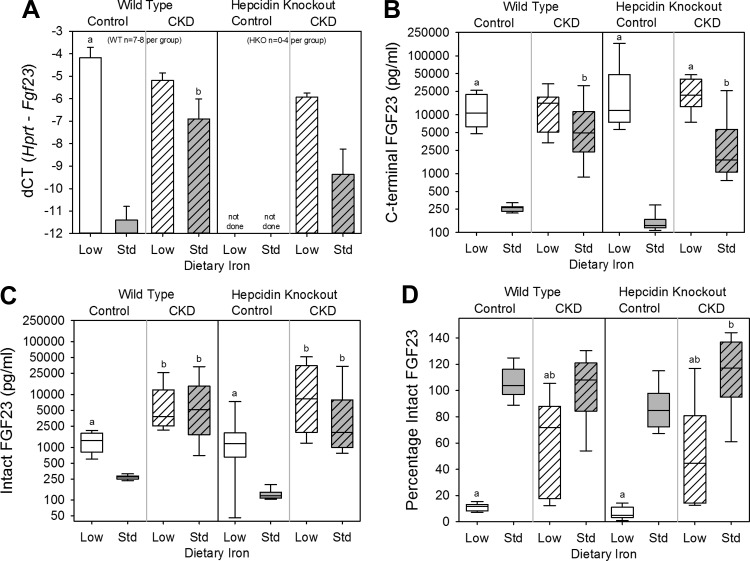
To assess the effects of dietary iron content in WT control mice, we compared the WT control low-iron group with the WT control standard-iron group. Body weight (Fig. 1A), kidney function (as assessed by serum urea nitrogen and creatinine; Fig. 1, B and C), and inflammation (as quantified by liver Saa mRNA expression and serum IL-6; Fig. 1, D and E) did not differ between the groups. The low-iron group had lower liver iron concentration (a measure of total body iron stores; Fig. 2A), serum hepcidin (Fig. 2B), mean corpuscular volume (MCV; Fig. 2C), and hemoglobin (Fig. 2D). Serum phosphate (Fig. 3A) and calcium (Fig. 3B) did not differ between the groups, but the low-iron group had lower iPTH (Fig. 3C) and 1,25(OH)2 D3 (Fig. 3D). The low-iron group had much higher bone Fgf23 mRNA expression (Fig. 4A), cFGF23 (Fig. 4B), and iFGF23 (Fig. 4C). However, the percentage of circulating FGF23 that was intact (Fig. 4D) was very low in this group, suggesting increased activity of cleavage mechanisms to offset increased expression. In the low-iron group, the higher iFGF23 levels likely explain the lower 1,25(OH)2 D3 levels. Also, the lower iPTH levels in the low-iron group, despite lower 1,25(OH)2 D3 concentrations, suggest possible FGF23-induced PTH suppression (5).
Fig. 1.
General, renal, and inflammatory parameters in wild-type and hepcidin knockout mice. Included are body weight (A), serum urea nitrogen (B), serum creatinine (C), liver Saa mRNA expression (D), and serum IL-6 (E) for all groups. a: Statistically significant pairwise comparison of low- vs. standard-iron groups within the wild-type control, wild-type chronic kidney diseae (CKD), hepcidin knockout control, and/or hepcidin knockout CKD cohorts; b: statistically significant pairwise comparison of control vs. CKD groups within the wild-type low-iron, wild-type standard-iron, hepcidin knockout low-iron, and/or hepcidin knockout standard-iron cohorts; c: statistically significant pairwise comparison of wild-type vs. hepcidin knockout groups within the CKD low-iron and/or CKD standard-iron cohorts. Given multiple comparison testing, the Benjamini-Hochberg correction was used. Data in A, B, C, and E are presented as medians and interquartile ranges, with box plot whiskers representing the 10th and 90th percentiles. Data in D are presented as means and SE; n = 6–14 mice/group, except where denoted on the graphs.
Fig. 2.
Hematological parameters in wild-type and hepcidin knockout mice. Included are liver iron (A), serum hepcidin (B), mean corpuscular volume (MCV; C), and hemoglobin (D) for all groups. a: Statistically significant pairwise comparison of low- vs. standard-iron groups within the wild-type control, wild-type CKD, hepcidin knockout control, and/or hepcidin knockout CKD cohorts. b: Statistically significant pairwise comparison of control vs. CKD groups within the wild-type low-iron, wild-type standard-iron, hepcidin knockout low-iron, and/or hepcidin knockout standard-iron cohorts; c: statistically significant pairwise comparison of wild-type vs. hepcidin knockout groups within the CKD low-iron and/or CKD standard-iron cohorts. Given multiple comparison testing, the Benjamini-Hochberg correction was used. Data are presented as medians and interquartile ranges, with box plot whiskers representing the 10th and 90th percentiles; n = 6–14 mice/group.
Fig. 3.
Mineral metabolism parameters in wild-type and hepcidin knockout mice. Included are serum phosphate (A), serum calcium (B), serum intact parathyroid hormone (PTH; C), and serum 1,25(OH)2 vitamin D (D) for all groups. a: Statistically significant pairwise comparison of low- vs. standard-iron groups within the wild-type control, wild-type CKD, hepcidin knockout control, and/or hepcidin knockout CKD cohorts; b: statistically significant pairwise comparison of control vs. CKD groups within the wild-type low-iron, wild-type standard-iron, hepcidin knockout low-iron, and/or hepcidin knockout standard-iron cohorts. Given multiple comparison testing, the Benjamini-Hochberg correction was used. Data are presented as medians and interquartile ranges, with box plot whiskers representing the 10th and 90th percentiles; n = 6–14 mice/group, except where denoted on the graphs.
Fig. 4.
FGF23 parameters in wild-type and hepcidin knockout mice. Included are bone Fgf23 mRNA expression (A), plasma C-terminal FGF23 (B), plasma intact FGF23 (C), and percentage of intact FGF23 (D) for all groups. a: Statistically significant pairwise comparison of low- vs. standard-iron groups within the wild-type control, wild-type CKD, hepcidin knockout control, and/or hepcidin knockout CKD cohorts; b: statistically significant pairwise comparison of control vs. CKD groups within the wild-type low-iron, wild-type standard-iron, hepcidin knockout low-iron, and/or hepcidin knockout standard-iron cohorts. Given multiple comparison testing, the Benjamini-Hochberg correction was used. Data in A are presented as means and SE. Data in B, C, and D are presented as medians and interquartile ranges, with box plot whiskers representing the 10th and 90th percentiles; n = 8–14 mice/group, except where denoted on the graphs. In A, bone Fgf23 mRNA expression was not able to be performed in the hepcidin knockout control mice secondary to sample degradation.
Effects of dietary iron content in WT CKD mice.
To assess the effects of dietary iron content in WT CKD mice, we compared the WT CKD low-iron group with the WT CKD standard-iron group. The low-iron group had higher body weight (Fig. 1A) and lower urea nitrogen and creatinine (Fig. 1, B and C); liver Saa mRNA expression and serum IL-6 did not differ between the groups (Fig. 1, D and E). As with the WT control mice, the WT CKD low-iron group had lower liver iron concentration (Fig. 2A), serum hepcidin (Fig. 2B), MCV (Fig. 2C), and hemoglobin (Fig. 2D). Serum phosphate (Fig. 3A), calcium (Fig. 3B), and 1,25(OH)2 D3 levels (Fig. 3D) did not differ between the groups, but the low-iron group had lower iPTH (Fig. 3C). Despite better kidney function, the low-iron group tended to have higher bone Fgf23 mRNA expression and cFGF23 (P = 0.080 and P = 0.068, respectively; Fig. 4, A and B). However, iFGF23 levels were identical in the two groups (Fig. 4C), secondary to increased activity of cleavage mechanisms in the low-iron group, as indicated by the lower percentage iFGF23 (Fig. 4D). As iFGF23 did not differ between the groups, 1,25(OH)2 D3 levels were similar. The lower iPTH levels in the low-iron group, despite no differences in iFGF23, 1,25(OH)2 D3, phosphate, and calcium levels, may reflect better kidney function.
Effects of CKD in WT mice.
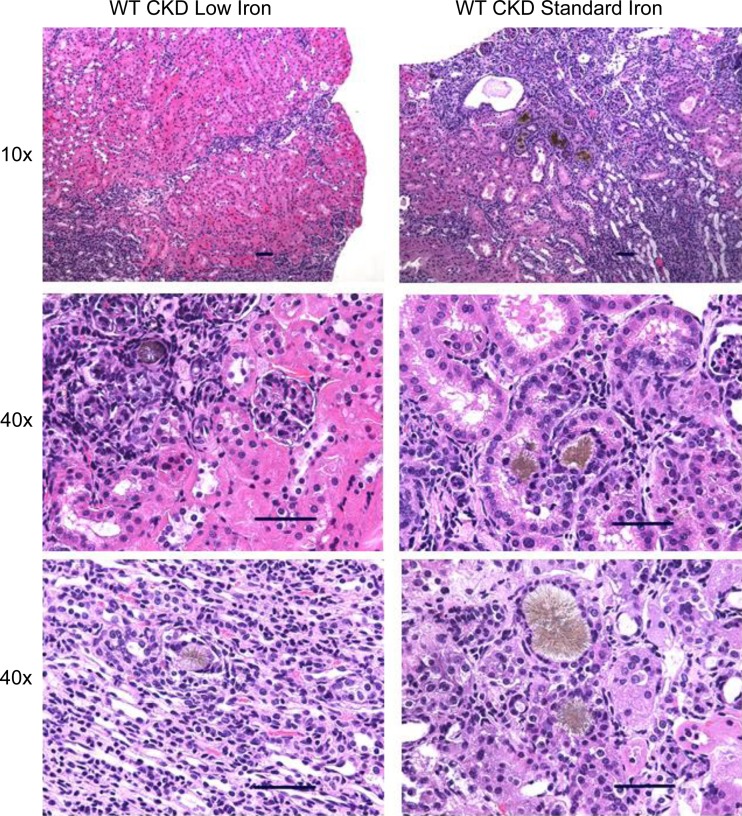
To assess the effects of CKD in WT mice, we compared the WT CKD groups with their corresponding WT control groups. The adenine diet induced significant peritubular leukocyte infiltration, deposition of crystalloid structures in the tubular lumina, and tubular dilatation; representative renal histology is shown in Fig. 5. Compared with the WT control low-iron group, the WT CKD low-iron group had similar body weight (Fig. 1A), but higher urea nitrogen (Fig. 1B), higher creatinine (Fig. 1C), and more inflammation (Fig. 1, D and E). Liver iron (Fig. 2A), serum hepcidin (Fig. 2B), MCV (Fig. 2C), hemoglobin (Fig. 2D), phosphate (Fig. 3A), calcium (Fig. 3B), iPTH (Fig. 3C), 1,25(OH)2 D3 (Fig. 3D), bone Fgf23 mRNA expression (Fig. 4A), and cFGF23 (Fig. 4B) did not differ between the two groups. Despite similar iron, hematological, and mineral metabolism parameters, the CKD group had higher iFGF23 (Fig. 4C) and percentage of iFGF23 (Fig. 4D), suggesting iron-independent inhibition of FGF23 cleavage in CKD.
Fig. 5.
Renal histopathological analysis of mice on the adenine diets. Kidney tissue sections were stained with hematoxylin and eosin and assessed with light microscopy at ×10 and ×40 magnification. The adenine diet induced significant peritubular leukocyte infiltration, deposition of crystalloid structures in the tubular lumina, and tubular dilatation. Scale bar = 50 μm.
Among the WT mice on standard-iron diets, the CKD group had lower body weight (Fig. 1A), higher urea nitrogen (Fig. 1B), higher creatinine (Fig. 1C), and more inflammation (Fig. 1, D and E). The CKD group had higher liver iron (Fig. 2A), higher serum hepcidin (Fig. 2B), lower MCV (Fig. 2C), and lower hemoglobin (Fig. 2D), consistent with CKD-associated, hepcidin-mediated iron-restricted erythropoiesis. As expected, the CKD group also had higher serum phosphate (Fig. 3A), higher iPTH (Fig. 3C), lower 1,25(OH)2 D3 (Fig. 3D), higher bone Fgf23 mRNA expression (Fig. 4A), and higher cFGF23 and iFGF23 levels (Fig. 4, B and C).
Effects of dietary iron content in HKO control mice.
To assess the effects of dietary iron content in HKO control mice, we compared the HKO control low-iron group with the HKO control standard-iron group, allowing for a maximally dichotomized comparison of iron status. HKO mice on low-iron diets become iron deficient, like WT mice; however, unlike WT mice, HKO mice on standard-iron diets become iron loaded, as the absence of hepcidin allows for maximal enteral iron absorption.
The HKO control low (iron-deficient)- and standard-iron (iron-loaded) groups had similar body weight (Fig. 1A) and kidney function (Fig. 1, B and C). The low-iron group had slightly less liver Saa mRNA expression (Fig. 1D); however, this result was not reflected by serum IL-6 (Fig. 1E). The low-iron group had lower liver iron concentration (Fig. 2A), MCV (Fig. 2C), and hemoglobin (Fig. 2D); serum hepcidin levels were undetectable in both groups (Fig. 2B). Serum phosphate (Fig. 3A), calcium (Fig. 3B), and iPTH (Fig. 3C) did not differ between the groups, but 1,25(OH)2 D3 levels were much lower in the low-iron group (Fig. 3D). The low-iron group had much higher cFGF23 and iFGF23 (Fig. 4, B and C); however, the percentage iFGF23 (Fig. 4D) was very low, again suggesting increased cleavage coupled with increased production. In the low-iron group, the higher iFGF23 levels likely explain the lower 1,25(OH)2 D3 levels.
Effects of dietary iron content in HKO CKD mice.
To assess the effects of dietary iron content in HKO CKD mice, we compared the HKO CKD low-iron group to the HKO CKD standard-iron group. The low-iron group had lower body weight (Fig. 1A), but kidney function did not differ between the groups (Fig. 1, B and C), and differences in inflammation did not reach statistical significance (Fig. 1, D and E). The low-iron group had lower liver iron concentration (Fig. 2A), MCV (Fig. 2C), and hemoglobin (Fig. 2D); serum hepcidin was undetectable in both groups (Fig. 2B). Serum phosphate (Fig. 3A), calcium (Fig. 3B), and iPTH (Fig. 3C) did not differ between the groups, but the low-iron group had lower 1,25(OH)2 D3 levels (Fig. 3D). The low-iron group tended to have higher bone Fgf23 mRNA expression (P = 0.11; Fig. 4A) and had much higher cFGF23 levels (Fig. 4B), potentially demonstrating the differential effects of iron deficiency vs. iron loading on FGF23 production in this CKD model. Yet, the effects of somewhat higher levels of inflammation in the low-iron group may also have been contributory. Despite large differences in cFGF23 levels, iFGF23 concentrations (Fig. 4C) did not statistically differ between the groups, secondary to increased activity of cleavage mechanisms in the low-iron group, as indicated by the lower percentage iFGF23 (Fig. 4D). Although differences in iFGF23 levels did not reach statistical significance, slightly higher iFGF23 in the low-iron group may have contributed to the lower 1,25(OH)2 D3 levels.
Effects of CKD in HKO mice.
To assess the effects of CKD in HKO mice, we compared the HKO CKD groups to their corresponding HKO control groups. Compared with the HKO control low-iron group, the HKO CKD low-iron group had lower body weight (Fig. 1A), higher urea nitrogen (Fig. 1B), higher creatinine (Fig. 1C), more liver Saa mRNA expression (Fig. 1D), and tended to have higher serum IL-6 (Fig. 1E). Liver iron (Fig. 2A) and MCV (Fig. 2C) did not differ between the two groups, although the CKD group was slightly less anemic (Fig. 2D). Serum phosphate (Fig. 3A), calcium (Fig. 3B), iPTH (Fig. 3C), 1,25(OH)2 D3 (Fig. 3D), and cFGF23 (Fig. 4B) did not differ between the two groups. However, the CKD group had higher iFGF23 (Fig. 4C) and percentage of iFGF23 (Fig. 4D), again suggesting iron-independent inhibition of FGF23 cleavage in CKD.
Among the HKO mice on standard-iron diets, the control and CKD groups had similar body weight (Fig. 1A), although the CKD group had higher urea nitrogen (Fig. 1B), higher creatinine (Fig. 1C), more liver Saa mRNA expression (Fig. 1D), and tended to have higher serum IL-6 (Fig. 1E). The CKD group had higher liver iron (Fig. 2A) and, despite worse kidney function and more inflammation, similar MCV and hemoglobin (Fig. 2, C and D), demonstrating the important contribution of hepcidin to CKD-associated anemia. Serum phosphate tended to be higher in the CKD group (Fig. 3A) and, as expected, the CKD group had higher iPTH (Fig. 3C), lower 1,25(OH)2 D3 (Fig. 3D), and higher cFGF23 and iFGF23 levels (Fig. 4, B and C).
Effects of hepcidin on FGF23 parameters in CKD.
To assess the effects of hepcidin on FGF23 parameters in CKD, we first compared the WT CKD low-iron group with the HKO CKD low-iron group. The HKO CKD low-iron group had undetectable serum hepcidin, but the WT group, despite CKD, had significant iron deficiency-induced hepcidin suppression (Fig. 2B). These two groups had similar liver iron (Fig. 2A), MCV (Fig. 2C), hemoglobin (Fig. 2D), mineral metabolism parameters (Fig. 3), and FGF23 levels (Fig. 4). We next compared the WT CKD standard-iron group with the HKO CKD standard-iron group, which allowed for a more dichotomized comparison of hepcidin status in CKD, as the WT CKD group had dramatically increased hepcidin levels, whereas the HKO CKD group had undetectable hepcidin (Fig. 2B). The HKO group had higher liver iron (Fig. 2A), MCV (Fig. 2C), and hemoglobin (Fig. 2D), again demonstrating the contribution of hepcidin to CKD-associated anemia. However, bone Fgf23 mRNA expression (Fig. 4A) and circulating FGF23 levels (Fig. 4, B and C) did not statistically differ between the WT and HKO groups, suggesting that, in our CKD model, dietary iron content (low vs. standard) was more influential than the presence or absence of hepcidin per se.
Regression modeling.
In the CKD cohort, which included both WT and HKO mice (n = 42 mice total), we assessed the associations among biochemical parameters. In multiple linear regression analysis, independent of phosphate and urea nitrogen, log-transformed liver iron was inversely associated with log-transformed cFGF23 and positively associated with percentage iFGF23 (Table 2). These results suggest that, in CKD, iron deficiency is independently associated with increased cFGF23 (increased FGF23 production) and concurrently a decreased percentage of iFGF23 (increased FGF23 cleavage). Urea nitrogen was positively associated with both log-transformed cFGF23 and the percentage of iFGF23, suggesting that, independent of iron and phosphate concentrations, worsening kidney function is associated with increased cFGF23 (increased FGF23 production) and an increased percentage of iFGF23 (decreased FGF23 cleavage).
Table 2.
Multiple linear regression modeling: association of independent variables with log cFGF23 and association of independent variables with percentage of intact FGF23 in CKD mice
| Independent Variable | Standardized Coefficient (95% CI) | P Value | Adjusted R2 | n |
|---|---|---|---|---|
| log cFGF23 | ||||
| Log liver iron | −0.55 (−0.81, −0.28) | <0.001 | 0.37 | 42 |
| Phosphate | −0.24 (−0.56, 0.08) | 0.13 | ||
| Urea nitrogen | 0.64 (0.31, 0.96) | <0.001 | ||
| Percentage intact FGF23 | ||||
| Log liver iron | 0.55 (0.31, 0.79) | <0.001 | 0.49 | 42 |
| Phosphate | −0.12 (−0.40, 0.17) | 0.41 | ||
| Urea nitrogen | 0.41 (0.12, 0.70) | 0.007 |
Models evaluated the independent associations of iron, phosphate, and urea nitrogen with FGF23 parameters in the CKD cohort.
DISCUSSION
FGF23 is a critical hormone involved in mineral metabolism and cross talk between bone and kidney. As elevated FGF23 levels are associated with CKD progression and adverse cardiovascular outcomes (11, 13, 18, 31), there is a need to better understand FGF23 regulation to define optimal therapeutic approaches to reduce CKD-associated morbidity and mortality. Regulation of FGF23 may be controlled at both the transcriptional and posttranslational stages. Transcriptional regulation of FGF23 is complex, influenced by a host of systemic and local factors (26). Posttranslational regulation is achieved via intracellular cleavage mechanisms that determine what percentage of translated FGF23 is secreted in its intact, bioactive form (39). In the present study, we demonstrate that iron deficiency anemia is associated with concurrently increased FGF23 production and cleavage in mice with normal and impaired kidney function, and that, independent of iron status, CKD is associated with inhibition of FGF23 cleavage.
Previous experimental studies have revealed that iron deficiency upregulates bone FGF23 expression (7, 8, 10). In mature mice with normal kidney function, iron deficiency resulted in increased bone Fgf23 mRNA expression and increased circulating cFGF23, but normal levels of iFGF23 (10), suggesting concurrently increased FGF23 production and proteolytic cleavage. We confirmed these observations, as our 12-wk-old WT control mice fed an iron-deficient diet had high bone Fgf23 mRNA expression, high cFGF23 levels, but a very low percentage of iFGF23. Absolute iFGF23 levels were not normal, however, in this case likely secondary to massively increased FGF23 production overwhelming cleavage mechanisms. The effects of iron deficiency on FGF23 were even more dramatic in the HKO control group, which became the most iron-depleted control group, likely secondary to an inability to store any iron. This group had some of the highest cFGF23 levels, as well as the lowest median percentage of iFGF23 (5%). Of the control groups, the mice on the low-iron diets had the highest absolute iFGF23 levels, likely contributing to low 1,25(OH)2 D3 concentrations.
Although the effects of chronic iron deficiency on FGF23 parameters have been characterized in the setting of normal kidney function, how chronic changes in iron status affect FGF23 in the setting of impaired kidney function is unknown. Compared with the WT CKD standard-iron group, the WT CKD low-iron group tended to have higher bone Fgf23 mRNA expression and cFGF23 levels, but a significantly lower percentage of iFGF23, resulting in similar absolute iFGF23 levels. These observations suggest that, in CKD, iron deficiency is associated with both increased FGF23 production and cleavage, as has been observed in non-CKD cohorts. The CKD low-iron group had higher FGF23 production despite significantly better kidney function, suggesting potent iron deficiency-associated effects.
Whereas the difference in iron status between the WT CKD low- and standard-iron groups was moderate, the difference in iron status between the HKO CKD low- and standard-iron groups was much more pronounced, as the absence of hepcidin allows for maximal enteral iron loading with a standard-iron diet. With a larger difference in iron status between the HKO CKD groups, in which kidney function and phosphate were similar, the effects on bone Fgf23 mRNA expression, circulating FGF23 levels, and percentage of iFGF23 were greater. Although the HKO CKD low-iron group tended to have more inflammation than the HKO CKD standard-iron group, which may contribute to increased Fgf23 transcription (8), there is also evidence that FGF23 induces an inflammatory response in the liver (37). Therefore, the relationship between FGF23 and inflammation is bidirectional, as inflammation may increase FGF23, and FGF23 may increase inflammation.
As iron status affects FGF23 production and metabolism in CKD, we hypothesized that hepcidin status would also affect FGF23 parameters. In the setting of low dietary iron intake, both the WT CKD and HKO CKD groups developed iron deficiency anemia, with similar effects on FGF23 parameters. Therefore, when dietary iron intake is very low, the presence or absence of hepcidin in CKD does not affect FGF23. However, in the setting of standard dietary iron intake, the HKO CKD group accumulated ~10 times more liver iron than the WT CKD group and did not become anemic. Despite very large differences in liver iron and hemoglobin between the WT CKD and HKO CKD standard-iron groups, differences in FGF23 parameters did not reach statistical significance. Therefore, when dietary iron intake is not limited, the presence or absence of hepcidin in CKD resulted in large changes in hematological parameters, but less pronounced changes in FGF23 measures. Our major conclusions are similar to those reported by Akchurin et al. (1) while our manuscript was under review. As in our study, hepcidin ablation in the setting of CKD did not affect serum phosphate or circulating FGF23 levels compared with wild-type CKD controls, but did normalize hemoglobin. These results strengthen the evidence that hepcidin status itself is not a major determinant of FGF23 levels in CKD.
In our experimental models, iron deficiency was associated with increased Fgf23 transcription and increased FGF23 posttranslational cleavage; however, the direct and/or indirect mechanisms by which iron deficiency effects these changes have yet to be fully characterized. Iron deficiency is associated with stabilization of hypoxia-inducible factor 1α (HIF1α), which increases Fgf23 transcription (8, 10) and also upregulates furin, which cleaves FGF23 (8, 27, 36). Therefore, HIF1α may contribute to iron deficiency-mediated coupling of increased FGF23 production and cleavage. Although in vitro studies have demonstrated that iron chelation and hypoxia increase cellular Fgf23 expression (7, 10), in animal models, iron deficiency may possibly elicit both direct and indirect effects on FGF23. For example, in our analyses, iron varied with hemoglobin. As such, we could not differentiate associations with iron deficiency from associations with other possible anemia-related factors. Therefore, we cannot rule out that other hematological factors related to anemia or erythropoiesis additionally mediated the effects of iron deficiency on FGF23 production.
Independent of iron status, CKD was associated with inhibition of FGF23 cleavage. In both the WT and HKO cohorts, the low-iron diet induced similar increases in cFGF23 levels in the control and CKD groups; however, the CKD groups had a much higher percentage of iFGF23. In our regression modeling, worse kidney function in CKD was associated with a higher percentage of iFGF23, independent of liver iron and serum phosphate concentrations, demonstrating the effects of more advanced CKD itself on FGF23 metabolism. Qualitatively similar data have recently been reported using another mouse CKD model (Col4a3 knockout) in which acute inflammation, characterized by decreased serum iron and increased ferritin, increased bone Fgf23 mRNA expression and cFGF23 levels similarly in WT and CKD mice, but increased iFGF23 levels to a much greater extent in the CKD mice (8). Furthermore, in end-stage renal disease, circulating FGF23 is almost exclusively intact (34, 38). The mechanisms by which FGF23 cleavage may be downregulated in CKD remain unknown.
In summary, the current results demonstrate that iron deficiency anemia independently affects FGF23 production and cleavage in CKD as it does in the setting of normal kidney function. In CKD, the effects of chronic dietary iron deficiency on FGF23 parameters seem to be more pronounced than the effects of hepcidin ablation. Also, worsening kidney function is independently associated with inhibition of FGF23 cleavage. Further studies are needed to elucidate the mechanisms by which iron deficiency anemia and CKD may directly or indirectly affect FGF23 production and metabolism.
GRANTS
This work was supported in part by USPHS Grants DK-67563, DK-35423, DK-80984; CTSI Grant UL1 TR-000124; a National Institutes of Health (NIH) K12 Child Health Research Career Development Award K12 HD-034610; NIH Training Grant T32 DK-07789; and funds from the UCLA Children’s Discovery and Innovation Institute.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.R.H., T.G., E.N., and I.B.S. provided conception and design of research; M.R.H. K.C., M.R., V.G. and E.V. performed experiments; M.R.H. K.C., M.R., and D.G. analyzed data; M.R.H., T.G., E.N., and I.B.S. interpreted results of experiments; M.R.H. prepared figures; M.R.H. drafted manuscript; M.R.H., T.G., E.N., and I.B.S. edited and revised manuscript; M.R.H., T.G., E.N., and I.B.S. approved final version of manuscript.
ACKNOWLEDGMENTS
FGF23 and PTH kits were kindly provided by Quidel and Immutopics International.
REFERENCES
- 1.Akchurin O, Sureshbabu A, Doty SB, Zhu YS, Patino E, Cunningham-Rundles S, Choi ME, Boskey A, Rivella S. Lack of hepcidin ameliorates anemia and improves growth in an adenine-induced mouse model of chronic kidney disease. Am J Physiol Renal Physiol 311: F877–F889, 2016. doi: 10.1152/ajprenal.00089.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antoniucci DM, Yamashita T, Portale AA. Dietary phosphorus regulates serum fibroblast growth factor-23 concentrations in healthy men. J Clin Endocrinol Metab 91: 3144–3149, 2006. doi: 10.1210/jc.2006-0021. [DOI] [PubMed] [Google Scholar]
- 3.Ashby DR, Gale DP, Busbridge M, Murphy KG, Duncan ND, Cairns TD, Taube DH, Bloom SR, Tam FW, Chapman RS, Maxwell PH, Choi P. Plasma hepcidin levels are elevated but responsive to erythropoietin therapy in renal disease. Kidney Int 75: 976–981, 2009. doi: 10.1038/ki.2009.21. [DOI] [PubMed] [Google Scholar]
- 4.Babitt JL, Lin HY. Mechanisms of anemia in CKD. J Am Soc Nephrol 23: 1631–1634, 2012. doi: 10.1681/ASN.2011111078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ben-Dov IZ, Galitzer H, Lavi-Moshayoff V, Goetz R, Kuro-o M, Mohammadi M, Sirkis R, Naveh-Many T, Silver J. The parathyroid is a target organ for FGF23 in rats. J Clin Invest 117: 4003–4008, 2007. doi: 10.1172/JCI32409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braithwaite V, Prentice AM, Doherty C, Prentice A. FGF23 is correlated with iron status but not with inflammation and decreases after iron supplementation: a supplementation study. Int J Pediatr Endocrinol 2012: 27, 2012. doi: 10.1186/1687-9856-2012-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clinkenbeard EL, Farrow EG, Summers LJ, Cass TA, Roberts JL, Bayt CA, Lahm T, Albrecht M, Allen MR, Peacock M, White KE. Neonatal iron deficiency causes abnormal phosphate metabolism by elevating FGF23 in normal and ADHR mice. J Bone Miner Res 29: 361–369, 2014. doi: 10.1002/jbmr.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.David V, Martin A, Isakova T, Spaulding C, Qi L, Ramirez V, Zumbrennen-Bullough KB, Sun CC, Lin HY, Babitt JL, Wolf M. Inflammation and functional iron deficiency regulate fibroblast growth factor 23 production. Kidney Int 89: 135–146, 2016. doi: 10.1038/ki.2015.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Durham BH, Joseph F, Bailey LM, Fraser WD. The association of circulating ferritin with serum concentrations of fibroblast growth factor-23 measured by three commercial assays. Ann Clin Biochem 44: 463–466, 2007. doi: 10.1258/000456307781646102. [DOI] [PubMed] [Google Scholar]
- 10.Farrow EG, Yu X, Summers LJ, Davis SI, Fleet JC, Allen MR, Robling AG, Stayrook KR, Jideonwo V, Magers MJ, Garringer HJ, Vidal R, Chan RJ, Goodwin CB, Hui SL, Peacock M, White KE. Iron deficiency drives an autosomal dominant hypophosphatemic rickets (ADHR) phenotype in fibroblast growth factor-23 (Fgf23) knock-in mice. Proc Natl Acad Sci USA 108: E1146–E1155, 2011. doi: 10.1073/pnas.1110905108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fliser D, Kollerits B, Neyer U, Ankerst DP, Lhotta K, Lingenhel A, Ritz E, Kronenberg F, MMKD Study Group, Kuen E, König P, Kraatz G, Mann JF, Müller GA, Köhler H, Riegler P. Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: the Mild to Moderate Kidney Disease (MMKD) Study. J Am Soc Nephrol 18: 2600–2608, 2007. doi: 10.1681/ASN.2006080936. [DOI] [PubMed] [Google Scholar]
- 12.Gutierrez O, Isakova T, Rhee E, Shah A, Holmes J, Collerone G, Jüppner H, Wolf M. Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol 16: 2205–2215, 2005. doi: 10.1681/ASN.2005010052. [DOI] [PubMed] [Google Scholar]
- 13.Gutiérrez OM, Januzzi JL, Isakova T, Laliberte K, Smith K, Collerone G, Sarwar A, Hoffmann U, Coglianese E, Christenson R, Wang TJ, deFilippi C, Wolf M. Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation 119: 2545–2552, 2009. doi: 10.1161/CIRCULATIONAHA.108.844506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hasegawa H, Nagano N, Urakawa I, Yamazaki Y, Iijima K, Fujita T, Yamashita T, Fukumoto S, Shimada T. Direct evidence for a causative role of FGF23 in the abnormal renal phosphate handling and vitamin D metabolism in rats with early-stage chronic kidney disease. Kidney Int 78: 975–980, 2010. doi: 10.1038/ki.2010.313. [DOI] [PubMed] [Google Scholar]
- 15.Hsu CY, McCulloch CE, Curhan GC. Epidemiology of anemia associated with chronic renal insufficiency among adults in the United States: results from the Third National Health and Nutrition Examination Survey. J Am Soc Nephrol 13: 504–510, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Imel EA, Peacock M, Gray AK, Padgett LR, Hui SL, Econs MJ. Iron modifies plasma FGF23 differently in autosomal dominant hypophosphatemic rickets and healthy humans. J Clin Endocrinol Metab 96: 3541–3549, 2011. doi: 10.1210/jc.2011-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Isakova T, Wahl P, Vargas GS, Gutiérrez OM, Scialla J, Xie H, Appleby D, Nessel L, Bellovich K, Chen J, Hamm L, Gadegbeku C, Horwitz E, Townsend RR, Anderson CA, Lash JP, Hsu CY, Leonard MB, Wolf M. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int 79: 1370–1378, 2011. doi: 10.1038/ki.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Isakova T, Xie H, Yang W, Xie D, Anderson AH, Scialla J, Wahl P, Gutiérrez OM, Steigerwalt S, He J, Schwartz S, Lo J, Ojo A, Sondheimer J, Hsu CY, Lash J, Leonard M, Kusek JW, Feldman HI, Wolf M; Chronic Renal Insufficiency Cohort (CRIC) Study Group . Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA 305: 2432–2439, 2011. doi: 10.1001/jama.2011.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jia T, Olauson H, Lindberg K, Amin R, Edvardsson K, Lindholm B, Andersson G, Wernerson A, Sabbagh Y, Schiavi S, Larsson TE. A novel model of adenine-induced tubulointerstitial nephropathy in mice. BMC Nephrol 14: 116, 2013. doi: 10.1186/1471-2369-14-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim A, Fung E, Parikh SG, Valore EV, Gabayan V, Nemeth E, Ganz T. A mouse model of anemia of inflammation: complex pathogenesis with partial dependence on hepcidin. Blood 123: 1129–1136, 2014. doi: 10.1182/blood-2013-08-521419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kovesdy CP, Trivedi BK, Kalantar-Zadeh K, Anderson JE. Association of anemia with outcomes in men with moderate and severe chronic kidney disease. Kidney Int 69: 560–564, 2006. doi: 10.1038/sj.ki.5000105. [DOI] [PubMed] [Google Scholar]
- 22.Larsson T, Nisbeth U, Ljunggren O, Jüppner H, Jonsson KB. Circulating concentration of FGF-23 increases as renal function declines in patients with chronic kidney disease, but does not change in response to variation in phosphate intake in healthy volunteers. Kidney Int 64: 2272–2279, 2003. doi: 10.1046/j.1523-1755.2003.00328.x. [DOI] [PubMed] [Google Scholar]
- 23.Lavi-Moshayoff V, Wasserman G, Meir T, Silver J, Naveh-Many T. PTH increases FGF23 gene expression and mediates the high-FGF23 levels of experimental kidney failure: a bone parathyroid feedback loop. Am J Physiol Renal Physiol 299: F882–F889, 2010. doi: 10.1152/ajprenal.00360.2010. [DOI] [PubMed] [Google Scholar]
- 24.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25: 402–408, 2001. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 25.López I, Rodríguez-Ortiz ME, Almadén Y, Guerrero F, de Oca AM, Pineda C, Shalhoub V, Rodríguez M, Aguilera-Tejero E. Direct and indirect effects of parathyroid hormone on circulating levels of fibroblast growth factor 23 in vivo. Kidney Int 80: 475–482, 2011. doi: 10.1038/ki.2011.107. [DOI] [PubMed] [Google Scholar]
- 26.Martin A, David V, Quarles LD. Regulation and function of the FGF23/klotho endocrine pathways. Physiol Rev 92: 131–155, 2012. doi: 10.1152/physrev.00002.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McMahon S, Grondin F, McDonald PP, Richard DE, Dubois CM. Hypoxia-enhanced expression of the proprotein convertase furin is mediated by hypoxia-inducible factor-1: impact on the bioactivation of proproteins. J Biol Chem 280: 6561–6569, 2005. doi: 10.1074/jbc.M413248200. [DOI] [PubMed] [Google Scholar]
- 28.Mohanram A, Zhang Z, Shahinfar S, Keane WF, Brenner BM, Toto RD. Anemia and end-stage renal disease in patients with type 2 diabetes and nephropathy. Kidney Int 66: 1131–1138, 2004. doi: 10.1111/j.1523-1755.2004.00863.x. [DOI] [PubMed] [Google Scholar]
- 29.Pereira RC, Juppner H, Azucena-Serrano CE, Yadin O, Salusky IB, Wesseling-Perry K. Patterns of FGF-23, DMP1, and MEPE expression in patients with chronic kidney disease. Bone 45: 1161–1168, 2009. doi: 10.1016/j.bone.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Portale AA, Wolf M, Jüppner H, Messinger S, Kumar J, Wesseling-Perry K, Schwartz GJ, Furth SL, Warady BA, Salusky IB. Disordered FGF23 and mineral metabolism in children with CKD. Clin J Am Soc Nephrol 9: 344–353, 2014. doi: 10.2215/CJN.05840513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Portale AA, Wolf MS, Messinger S, Perwad F, Jüppner H, Warady BA, Furth SL, Salusky IB. Fibroblast growth factor 23 and risk of CKD progression in children. Clin J Am Soc Nephrol CJN.02110216, 2016 10.2215/CJN.02110216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saito H, Maeda A, Ohtomo S, Hirata M, Kusano K, Kato S, Ogata E, Segawa H, Miyamoto K, Fukushima N. Circulating FGF-23 is regulated by 1alpha,25-dihydroxyvitamin D3 and phosphorus in vivo. J Biol Chem 280: 2543–2549, 2005. doi: 10.1074/jbc.M408903200. [DOI] [PubMed] [Google Scholar]
- 33.Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, Fujita T, Fukumoto S, Tomizuka K, Yamashita T. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest 113: 561–568, 2004. doi: 10.1172/JCI200419081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shimada T, Urakawa I, Isakova T, Yamazaki Y, Epstein M, Wesseling-Perry K, Wolf M, Salusky IB, Jüppner H. Circulating fibroblast growth factor 23 in patients with end-stage renal disease treated by peritoneal dialysis is intact and biologically active. J Clin Endocrinol Metab 95: 578–585, 2010. doi: 10.1210/jc.2009-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shimada T, Yamazaki Y, Takahashi M, Hasegawa H, Urakawa I, Oshima T, Ono K, Kakitani M, Tomizuka K, Fujita T, Fukumoto S, Yamashita T. Vitamin D receptor-independent FGF23 actions in regulating phosphate and vitamin D metabolism. Am J Physiol Renal Physiol 289: F1088–F1095, 2005. doi: 10.1152/ajprenal.00474.2004. [DOI] [PubMed] [Google Scholar]
- 36.Silvestri L, Pagani A, Camaschella C. Furin-mediated release of soluble hemojuvelin: a new link between hypoxia and iron homeostasis. Blood 111: 924–931, 2008. doi: 10.1182/blood-2007-07-100677. [DOI] [PubMed] [Google Scholar]
- 37.Singh S, Grabner A, Yanucil C, Schramm K, Czaya B, Krick S, Czaja MJ, Bartz R, Abraham R, Di Marco GS, Brand M, Wolf M, Faul C. Fibroblast growth factor 23 directly targets hepatocytes to promote inflammation in chronic kidney disease. Kidney Int 90: 985–996, 2016. doi: 10.1016/j.kint.2016.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith ER, Cai MM, McMahon LP, Holt SG. Biological variability of plasma intact and C-terminal FGF23 measurements. J Clin Endocrinol Metab 97: 3357–3365, 2012. doi: 10.1210/jc.2012-1811. [DOI] [PubMed] [Google Scholar]
- 39.Tagliabracci VS, Engel JL, Wiley SE, Xiao J, Gonzalez DJ, Nidumanda Appaiah H, Koller A, Nizet V, White KE, Dixon JE. Dynamic regulation of FGF23 by Fam20C phosphorylation, GalNAc-T3 glycosylation, and furin proteolysis. Proc Natl Acad Sci USA 111: 5520–5525, 2014. doi: 10.1073/pnas.1402218111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tamura M, Aizawa R, Hori M, Ozaki H. Progressive renal dysfunction and macrophage infiltration in interstitial fibrosis in an adenine-induced tubulointerstitial nephritis mouse model. Histochem Cell Biol 131: 483–490, 2009. doi: 10.1007/s00418-009-0557-5. [DOI] [PubMed] [Google Scholar]
- 41.Tanaka T, Doi K, Maeda-Mamiya R, Negishi K, Portilla D, Sugaya T, Fujita T, Noiri E. Urinary L-type fatty acid-binding protein can reflect renal tubulointerstitial injury. Am J Pathol 174: 1203–1211, 2009. doi: 10.2353/ajpath.2009.080511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thorp ML, Johnson ES, Yang X, Petrik AF, Platt R, Smith DH. Effect of anaemia on mortality, cardiovascular hospitalizations and end-stage renal disease among patients with chronic kidney disease. Nephrology (Carlton) 14: 240–246, 2009. doi: 10.1111/j.1440-1797.2008.01065.x. [DOI] [PubMed] [Google Scholar]
- 43.Warady BA, Abraham AG, Schwartz GJ, Wong CS, Muñoz A, Betoko A, Mitsnefes M, Kaskel F, Greenbaum LA, Mak RH, Flynn J, Moxey-Mims MM, Furth S. Predictors of rapid progression of glomerular and nonglomerular kidney disease in children and adolescents: the chronic kidney disease in children (CKiD) cohort. Am J Kidney Dis 65: 878–888, 2015. doi: 10.1053/j.ajkd.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wesseling-Perry K, Pereira RC, Sahney S, Gales B, Wang HJ, Elashoff R, Jüppner H, Salusky IB. Calcitriol and doxercalciferol are equivalent in controlling bone turnover, suppressing parathyroid hormone, and increasing fibroblast growth factor-23 in secondary hyperparathyroidism. Kidney Int 79: 112–119, 2011. doi: 10.1038/ki.2010.352. [DOI] [PubMed] [Google Scholar]
- 45.Wolf M, Koch TA, Bregman DB. Effects of iron deficiency anemia and its treatment on fibroblast growth factor 23 and phosphate homeostasis in women. J Bone Miner Res 28: 1793–1803, 2013. doi: 10.1002/jbmr.1923. [DOI] [PubMed] [Google Scholar]
- 46.Zaritsky J, Young B, Wang HJ, Westerman M, Olbina G, Nemeth E, Ganz T, Rivera S, Nissenson AR, Salusky IB. Hepcidin--a potential novel biomarker for iron status in chronic kidney disease. Clin J Am Soc Nephrol 4: 1051–1056, 2009. doi: 10.2215/CJN.05931108. [DOI] [PMC free article] [PubMed] [Google Scholar]