Abstract
In addition to improving sexual function, testosterone has been reported to have beneficial effects in ameliorating lower urinary tract symptoms by increasing bladder capacity and compliance, while decreasing bladder pressure. However, the cellular mechanisms by which testosterone regulates detrusor smooth muscle (DSM) excitability have not been elucidated. Here, we used amphotericin-B perforated whole cell patch-clamp and single channel recordings on inside-out excised membrane patches to investigate the regulatory role of testosterone in guinea pig DSM excitability. Testosterone (100 nM) significantly increased the depolarization-induced whole cell outward currents in DSM cells. The selective pharmacological inhibition of the large-conductance voltage- and Ca2+-activated K+ (BK) channels with paxilline (1 μM) completely abolished this stimulatory effect of testosterone, suggesting a mechanism involving BK channels. At a holding potential of −20 mV, DSM cells exhibited transient BK currents (TBKCs). Testosterone (100 nM) significantly increased TBKC activity in DSM cells. In current-clamp mode, testosterone (100 nM) significantly hyperpolarized the DSM cell resting membrane potential and increased spontaneous transient hyperpolarizations. Testosterone (100 nM) rapidly increased the single BK channel open probability in inside-out excised membrane patches from DSM cells, clearly suggesting a direct BK channel activation via a nongenomic mechanism. Live-cell Ca2+ imaging showed that testosterone (100 nM) caused a decrease in global intracellular Ca2+ concentration, consistent with testosterone-induced membrane hyperpolarization. In conclusion, the data provide compelling mechanistic evidence that under physiological conditions, testosterone at nanomolar concentrations directly activates BK channels in DSM cells, independent from genomic testosterone receptors, and thus regulates DSM excitability.
Keywords: lower urinary tract symptoms, overactive bladder, testosterone
lower urinary tract symptoms (LUTS) and associated overactive bladder (OAB) are multifactorial in origin, and can range from urinary urgency, nocturia, and frequency, to slow stream, incomplete emptying, and urinary incontinence (2). Aging is considered one of the main factors for the development of urinary disorders in men and women (5). Studies suggest that LUTS are at least to some extent a function of reduced testosterone levels (12, 40), which in men declines gradually with age (9, 16). Testosterone replacement therapy in men improves the symptoms of many age-related diseases, including hypogonadism and erectile dysfunction (40, 41). However, the relationship between testosterone replacement therapy and LUTS is less clear (22). To effectively develop novel therapies for LUTS and associated OAB, we need to have a better understanding of the cellular mechanisms by which testosterone regulates the function of the detrusor smooth muscle (DSM), the main muscle component of the urinary bladder wall.
Contraction and relaxation of DSM are responsible for optimal storage and voiding of urine. Phasic and tonic DSM contractions are controlled by DSM action potentials and the resting membrane potential, which in turn are regulated by ion channels (29, 30). DSM cells express a variety of ion channels, including Ca2+ channels (11, 36), K+ channels (18, 29, 30), and TRP channels (21). Of all K+ channels, large-conductance voltage- and Ca2+-activated K+ (BK) channels are arguably the most important and physiologically relevant regulators of DSM excitability and contractility (15, 18, 20, 29, 30). Inhibition of these channels with selective blockers depolarizes the cell membrane potential, thus generating DSM phasic contractions (18, 29, 30). On the other hand, activation of the BK channels with selective BK channel openers hyperpolarizes the DSM cell membrane potential causing DSM relaxation (20, 29, 30). Modulation of BK channels, or the regulatory proteins controlling BK channel activity, could be a very effective approach for alternative pharmacological or genetic therapies for OAB (17, 20, 28–30). Because of their key role in DSM physiology, BK channels are regulated by a variety of signals, including intracellular Ca2+, voltage, protein kinases, phosphodiesterases, β-adrenergic and muscarinic receptors, and hormones (29). In addition, recent studies revealed that 17β-estradiol directly activates BK channels in DSM cells (19, 32). These studies provide compelling evidence that under physiological conditions estrogens control BK channel activity, and therefore DSM excitability (19, 32). However, the precise cellular mechanism by which testosterone regulates DSM cell excitability has not been investigated. In non-DSM cell types, testosterone modulates BK channels by both genomic (25, 26) and nongenomic (7, 8, 23, 34, 35) signaling pathways. Furthermore, a rapid, nongenomic, inhibitory effect of testosterone on the contractility of rat bladders has been reported (13). This inhibitory effect is independent of testosterone receptors, and the authors suggested that testosterone acts to modulate neuromuscular transmission of the bladder (13); however, the exact cellular mechanism of testosterone-induced bladder relaxation is not clear. To date, the regulatory role of testosterone on DSM excitability and BK channel activity at cellular level has not been investigated.
Here, we employed a combined electrophysiological approach including single BK channel recordings on inside-out excised membrane patches and the amphotericin-B perforated whole cell patch-clamp technique in combination with the selective BK channel inhibitor paxilline to elucidate the functional role of the BK channels as nongenomic targets of testosterone in guinea pig DSM cell excitability.
MATERIALS AND METHODS
DSM tissue collection and single cell isolation.
All experimental procedures were conducted in accordance with the animal use protocol #2186, reviewed, and approved by the Institutional Animal Care and Use Committee (IACUC) of the University of South Carolina. Fifty-five adult male Hartley-Albino guinea pigs (Charles River Laboratories, Raleigh, NC) of average weight 830.8 ± 55.0 g were euthanized by CO2 inhalation using a SMARTBOX automated CO2 delivery system (Euthanex, Palmer, PA) followed by thoracotomy. The urinary bladder was removed after a transverse incision superior to the bladder neck. Dissection of DSM tissues was performed as previously described (27, 32). DSM single cells were isolated from DSM tissues by enzymatic digestion using a combination of collagenase and papain as previously described (27, 32). Freshly isolated guinea pig DSM cells were used for patch-clamp and Ca2+ imaging experiments within 12 h of isolation. A suspension of DSM cells (0.3–0.5 ml) was placed in a glass bottom chamber to settle for at least 20–30 min before initiation of each patch-clamp recording.
Patch-clamp electrophysiology.
To record voltage-step depolarization-induced whole cell BK currents, TBKCs, and resting membrane potential of freshly isolated guinea pig DSM cells, we applied the amphotericin-B perforated whole cell patch-clamp technique as previously described (27, 32). To determine the effects of testosterone on whole cell steady-state BK currents, DSM cells were voltage-clamped at a holding potential of −70 mV, and then voltage-step depolarizations were applied from −40 to +80 mV at 20-mV intervals for 200 ms. Membrane potential recordings were performed in current-clamp mode (I=0) of the patch-clamp technique. Single BK channel recordings were performed on inside-out excised membrane patches as previously described (27, 32). Single BK channel currents were measured at −60 mV with bath and pipette solutions containing symmetrical 140 mM KCl and ∼300 nM free [Ca2+] (see Solutions and drugs). These experiments were conducted using pCLAMP version 10.3 software (Molecular Devices, Sunnyvale, CA) with an Axopatch 200B amplifier (Digidata 1322A). Currents were filtered using an eight-pole Bessel filter (model 900CT/9L8L, Frequency Devices, Ottawa, IL). Borosilicate glass patch-clamp pipettes (Sutter Instruments, Novato, CA) were pulled using a Narishige glass micropipette puller (model PP-830, Narishige Group, Tokyo, Japan) and polished with a Microforge (model MF-830, Narishige Group). All patch-clamp experiments were conducted at room temperature (22–23°C).
Measurement of intracellular Ca2+ levels in DSM cells.
Ca2+ imaging experiments were performed as previously described (20). The DSM cell suspension (500 μl) was added to 27 mm Nunc glass base dishes (Thermo Scientific) for 30 min to allow the cells to adhere to the glass bottom. DSM cells were then incubated with fura 2-AM, a ratiometric fluorescent Ca2+ indicator, for 60 min in darkness. Then, DSM cells underwent three washes with a fresh bath solution. An OLYMPUS IX81 inverted microscope equipped with a ×40 oil objective and MetaFluor 7.7.2.0 software (Molecular Devices) was used to image cells. At 340- and 380-nm wavelengths of light, we excited the cells loaded with fura 2 for a period of 20 ms at 2-s intervals. Intracellular Ca2+ concentrations were quantified as the ratio (F340/F380) of emission intensities collected at 510 nm. Ca2+ imaging was conducted at room temperature (22–23°C).
Solutions and drugs.
Ca2+-free dissection solution contained (in mM) 80 monosodium glutamate, 55 NaCl, 6 KCl, 10 glucose, 10 HEPES, 2 MgCl2; NaOH was administered to attain pH 7.3. The extracellular solution for whole cell patch-clamp and Ca2+ imaging experiments had (in mM) 134 NaCl, 6 KCl, 1 MgCl2, 2 CaCl2, 10 glucose, and 10 HEPES, pH adjusted to 7.4 with NaOH. The patch-pipette solution contained (in mM) 110 potassium aspartate, 30 KCl, 10 NaCl, 1 MgCl2, 10 HEPES, 0.05 EGTA; NaOH was used to adjust the pH to 7.2. Symmetrical K+ solution used for single BK channel recordings contained (in mM) 140 KCl, 1.08 MgCl2, 5 EGTA, and 3.16 CaCl2, adjusted to pH 7.2 with NaOH. Stock amphotericin-B solution was freshly prepared in dimethyl sulfoxide (DMSO) daily and was added to the pipette solution (200–300 μg/ml) before the experiment. Testosterone and paxilline were purchased from Sigma (St. Louis, MO) and were dissolved in DMSO. The final concentration of DMSO in the bath solution did not exceed 0.01%.
Data analysis and statistics.
Single BK channel openings were analyzed over 5- to 10-min intervals before and after the addition of testosterone (100 nM). Single BK channel open probability of each excised patch (NPo) was calculated as previously described (27). The effects of testosterone on whole cell steady-state BK currents and the cell membrane potential were analyzed using Clampfit 10.3 software. The effects of testosterone on voltage-step depolarization-induced whole cell BK currents were analyzed by taking the average value of the last 50 ms of each pulse before and after the application of testosterone (100 nM). The effects of testosterone on the amplitude and frequency of TBKCs and spontaneous transient hyperpolarizations (STHs) were analyzed using Minianalysis software (Synaptosoft, Decatur, GA). The threshold of TBKCs was set at 9 pA. Data are presented as the means ± SE. In the summarized data, “n” indicates the number of DSM cells used and “N” represents the total number of guinea pigs. CorelDraw Graphics Suite X3 software (Corel, Mountain View, CA) and GraphPad Prism 4.03 software (GraphPad Software, La Jolla, CA) were used for statistical analysis and data illustration. Student's t-test was used for statistical analysis. Two-way ANOVA followed by Bonferroni posttest was used for analyzing current-voltage relationship of the whole cell currents. P values <0.05 were considered statistically significant.
RESULTS
Testosterone increases depolarization-induced whole cell steady-state outward BK currents in DSM cells.
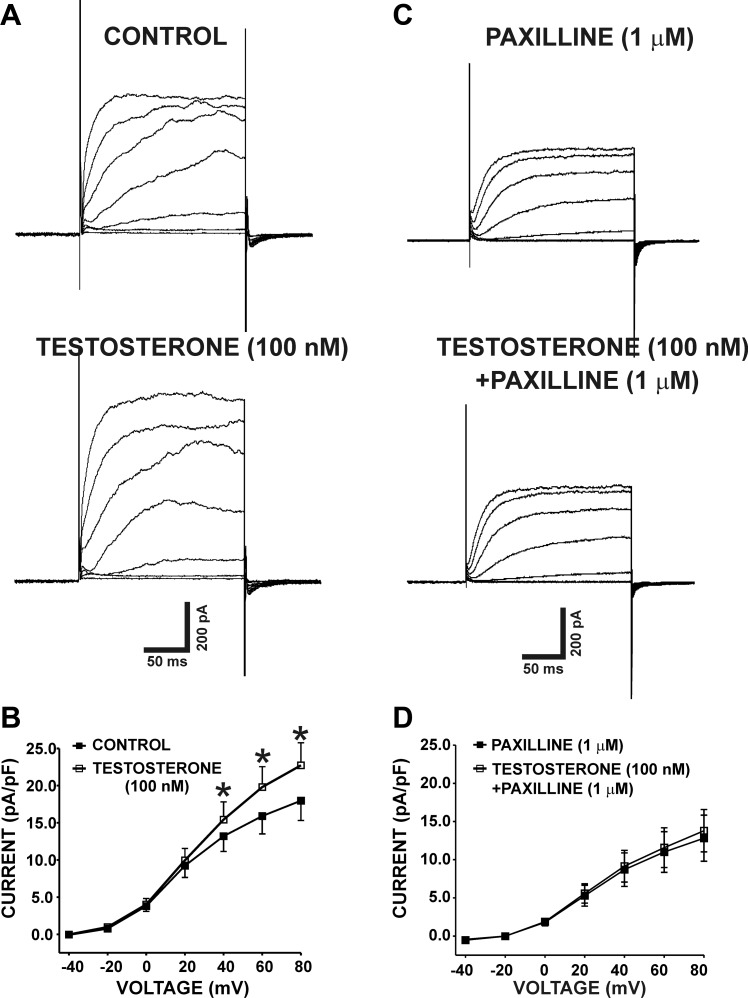
We studied the effects of testosterone on depolarization-induced whole cell steady-state outward currents on freshly isolated guinea pig DSM cells. DSM cells used in the present study had an average capacitance of 24.8 ± 1.4 pF (n = 63, N = 34). Whole cell outward currents were increased gradually in response to voltage-step depolarization from −40 to +80 mV as shown in Fig. 1A. As illustrated in Fig. 1B, testosterone (100 nM) significantly increased the whole cell current density. At the highest recording voltage of +80 mV, the whole cell outward currents were 17.9 ± 2.7 and 22.7 ± 3.2 pA/pF in the absence and in the presence of testosterone (100 nM), respectively (n = 15, N = 6; P < 0.05; Fig. 1, A–B). The effects of testosterone on whole cell outward K+ currents were completely abolished by the selective BK channel inhibitor paxilline (1 μM). At +80 mV, the current amplitudes were 12.8 ± 3.0 and 13.8 ± 2.8 pA/pF in the presence of 1 μM paxilline alone and in the presence of both paxilline (1 μM) and testosterone (100 nM), respectively (n = 7, N = 7; P > 0.05; Fig. 1, C–D). The lack of testosterone-induced stimulatory effects on whole cell outward currents in the presence of paxilline suggests that the potentiating effects of testosterone were due to BK channel activation.
Fig. 1.
Testosterone increases the depolarization-induced whole cell outward currents in freshly isolated detrusor smooth muscle (DSM) cells. A: representative original recordings illustrating the depolarization-induced whole cell outward currents in the absence (control) and in the presence of 100 nM testosterone. B: current-voltage relationship curve summarizes the stimulatory effects of 100 nM testosterone on the whole cell outward currents (n = 15, N = 6; *P < 0.05). C: representative original recordings illustrating the depolarization-induced whole cell outward currents in the presence of 1 μM paxilline alone and in the presence of both 1 μM paxilline and 100 nM testosterone. D: current-voltage relationship curve summarizes the lack of stimulatory effects of 100 nM testosterone on the whole cell outward currents in the presence of 1 μM paxilline (n = 7, N = 7; P > 0.05). The original recordings represented in A and C are from 2 separate DSM cells.
Testosterone increases TBKC activity in DSM cells.
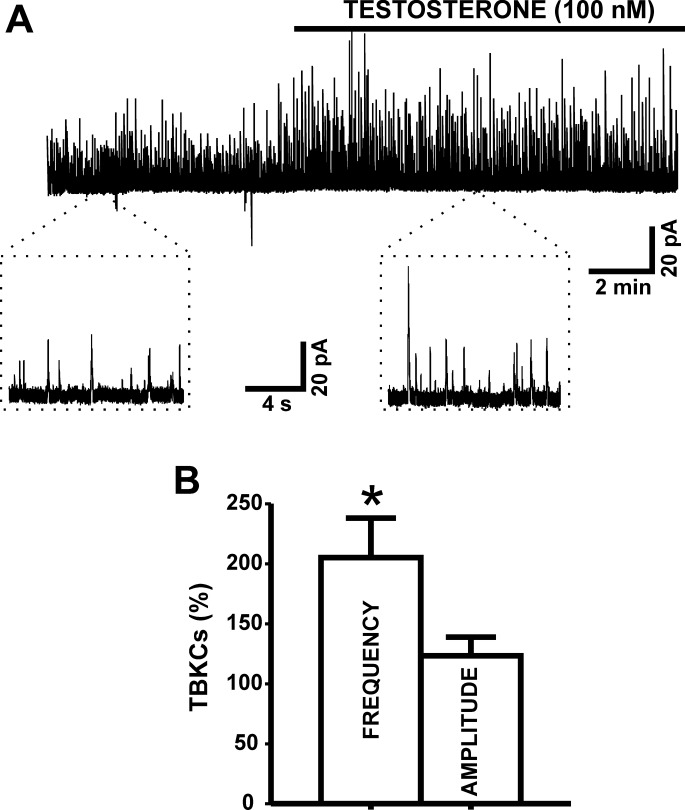
DSM cells generate TBKCs, caused by localized Ca2+ release events from the sarcoplasmic reticulum known as Ca2+ sparks. At a holding potential of −20 mV, testosterone (100 nM) increased the frequency of TBKCs by 105.1 ± 32.9% (n = 9, N = 9; P < 0.05; Fig. 2). The results reveal that activation of BK channels with testosterone increases TBKC frequency in DSM cells.
Fig. 2.
Testosterone increases transient BK currents (TBKCs) in DSM cells. A: representative original recording illustrating the stimulatory effect of 100 nM testosterone on the TBKC activity in an isolated DSM cell. A portion of the recording before (control) and after testosterone application is shown on an expanded time scale. B: summary data illustrating the stimulatory effects of 100 nM testosterone on TBKC frequency (n = 9, N = 9; *P < 0.05). TBKCs were recorded at a holding potential of −20 mV. The data were normalized to control values (before testosterone addition) taken as 100% and were presented in percentages (%).
Testosterone increases single BK channel open probability in excised membrane patches of DSM cells.
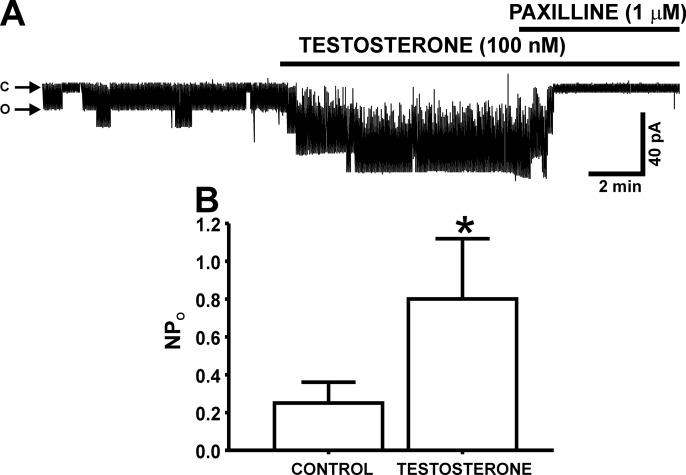
To further investigate the mechanism by which testosterone activates BK channels in DSM cells, we conducted experiments on single BK channel activity by using the inside-out excised patch configuration of the patch-clamp technique. Testosterone (100 nM) significantly increased the single BK channel open probability (NPo) from 0.25 ± 0.1 to 0.80 ± 0.3 (n = 13, N = 11; P < 0.05; Fig. 3). As illustrated in Fig. 3, the selective BK channel inhibitor paxilline (1 μM) completely abolished the BK channel activity, suggesting a functional involvement of BK channels.
Fig. 3.
Testosterone increases the single BK channel open probability (NPo) in inside-out excised membrane patches from DSM cells. A: representative original recording from an excised membrane patch of a DSM cell illustrating the stimulatory effect of 100 nM testosterone on NPo in inside-out configuration. Posttreatment of DSM cell membrane patches with 1 μM paxilline completely abolishes the single BK channel activity. “C” and “O” represent the closing and opening states of BK channels, respectively. B: summary data illustrating the stimulatory effects of 100 nM testosterone on NPo observed in inside-out excised patches (n = 13, N = 11; *P < 0.05).
BK channel activation with testosterone hyperpolarizes the resting membrane potential and increases the frequency of spontaneous transient hyperpolarizations in DSM cells.
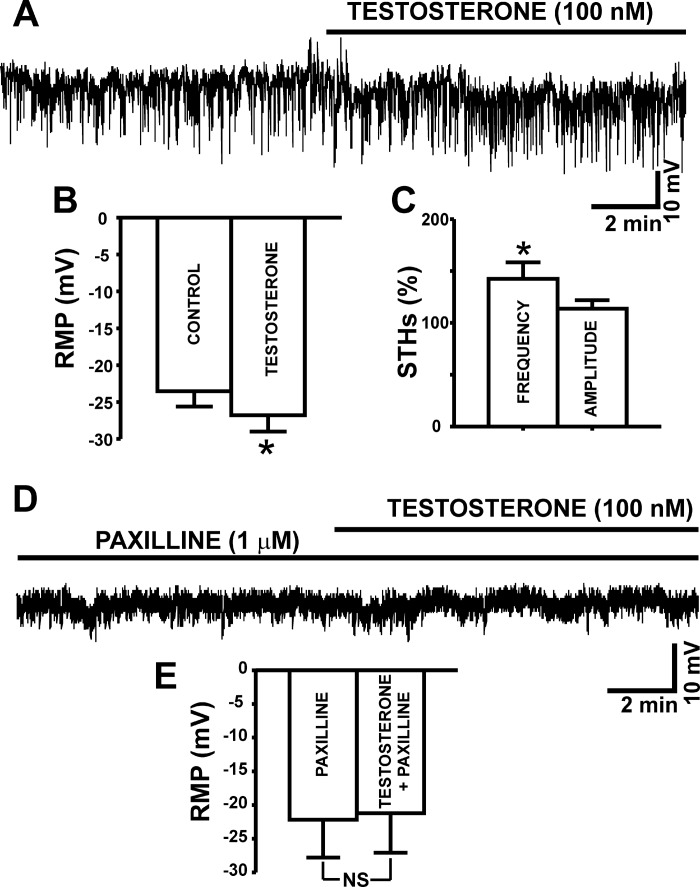
Next, we aimed to investigate the BK channel-dependent regulation of the DSM cell resting membrane potential by testosterone. The current-clamp experiments showed that testosterone (100 nM) significantly hyperpolarized the DSM cell resting membrane potential from a control value of −23.5 ± 2.0 to −26.8 ± 2.2 mV (n = 27, N = 21; P < 0.05; Fig. 4, A–B). Testosterone (100 nM) increased the frequency of spontaneous transient hyperpolarizations (STHs) by 42.5 ± 16.0% (n = 9, N = 8; P < 0.05; Fig. 4C). As shown in Fig. 4D, testosterone had no effects on DSM cell resting membrane potential when administered in the presence of the BK channel inhibitor paxilline (1 μM). The cell resting membrane potential was −21.2 ± 5.8 mV under control (1 μM paxilline only) condition, and −22.2 ± 5.6 mV in the presence of both 100 nM testosterone and 1 μM paxilline (n = 6, N = 4; P > 0.05; Fig. 4E). These data support the concept that testosterone regulates DSM cell resting membrane potential through a direct modulation of BK channel activity.
Fig. 4.
Testosterone hyperpolarizes the resting membrane potential (RMP) and increases spontaneous transient hyperpolarizations (STHs) in DSM cells. A: representative trace of an RMP recording in current-clamp mode illustrating the hyperpolarizing effects of 100 nM testosterone in an isolated DSM cell. B: summary data illustrating the hyperpolarizing effects of testosterone on DSM cell RMP (n = 27, N = 21; *P < 0.05). C: summary data illustrating the effect of testosterone on STH amplitude and frequency (n = 9, N = 8; *P < 0.05). D: representative RMP recording in current-clamp mode demonstrating that when the BK channels are blocked with 1 μM paxilline, testosterone (100 nM) did not cause a membrane hyperpolarization in DSM cells. E: summary data illustrating that 100 nM testosterone had no effect on the DSM cell RMP in the presence of 1 μM paxilline (n = 6, N = 4; P > 0.05). NS, nonsignificant.
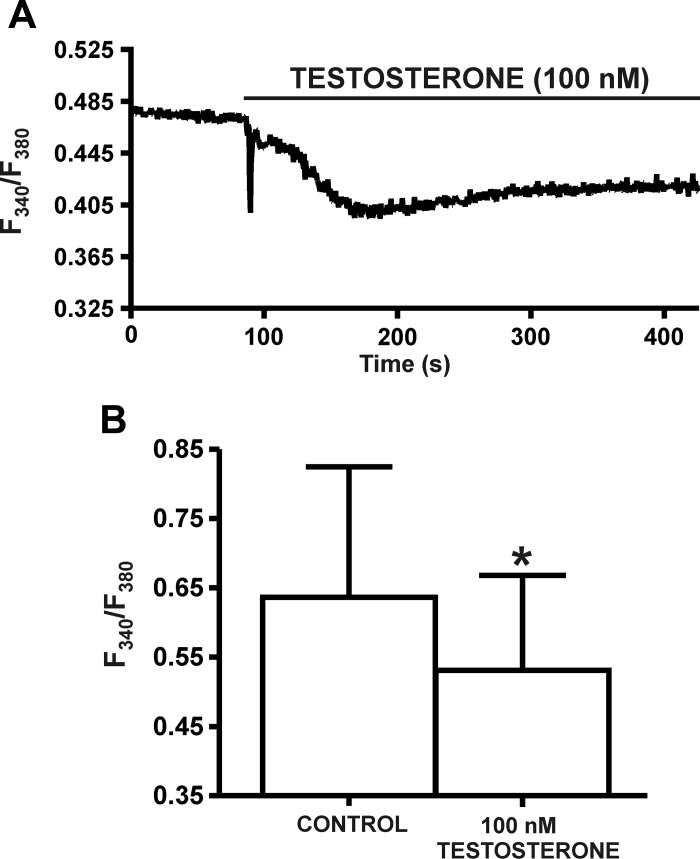
Testosterone (100 nM) reduces the intracellular Ca2+ levels in DSM cells.
To study the effects of testosterone on intracellular Ca2+ levels, live-cell Ca2+ imaging was conducted using the ratiometric fluorescence Ca2+ indicator fura 2-AM. Under control conditions, the mean value for emitted intensity (F340/F380) before testosterone (100 nM) application was 0.64 ± 0.19 (n = 8, N = 5). Testosterone (100 nM) caused a statistically significant decrease in the F340/F380 ratio to 0.53 ± 0.14 of the control, respectively (n = 8, N = 5; P < 0.05; Fig. 5), effects consistent with testosterone (100 nM)-induced DSM membrane hyperpolarization (Fig. 4).
Fig. 5.
Testosterone reduces the intracellular Ca2+ levels in DSM cells. A: original recording showing a decrease in the global intracellular Ca2+ concentration by 100 nM testosterone in a DSM cell. B: summary data demonstrating the significant decrease in global intracellular Ca2+ concentration by 100 nM testosterone (n = 8, N = 5; *P < 0.05). Data are reported as the ratio of fura-2 AM fluorescent emission at 510 nm with excitation at 340 and 380 nm.
DISCUSSION
This study represents an important step in revealing a novel cellular mechanism by which testosterone regulates urinary bladder function. The data support the concept that testosterone at nanomolar concentrations decreases DSM cell excitability by directly activating BK channels via a nongenomic mechanism.
To a certain extent, LUTS and related OAB are a function of reduced testosterone levels in aging men (12, 40). Although the exact relationship between testosterone and LUTS is not well-established, studies in rats showed that testosterone replacement therapy improves bladder capacity, reverses the parameters of DSM contractility, and improves smooth muscle/collagen ratio (1, 37, 38). Furthermore, it has been reported that testosterone relaxes the pig urinary bladder neck (10), and rapidly inhibits contractility in rat and shrew DSM preparations (13). In rat DSM, the inhibition of testosterone receptors with flutamide does not abolish testosterone ability to relax DSM, suggesting a nongenomic mechanism (13). To explain these rapid nongenomic effects, the authors hypothesized that testosterone could inhibit neuromuscular transmission, or affect molecular machinery governing contractile activity and Ca2+ homeostasis (13). However, the exact molecular and cellular mechanisms are unclear. It is not known how testosterone affects DSM excitability at the cellular level.
DSM contractility is a function of DSM cell excitability, which in turn is regulated by the coordinated activity of multiple ion channels expressed in the DSM cell membrane (29, 30). In this process, BK channels play a key role, as evidenced by selective BK channel pharmacological modulators, which can dramatically affect the action potentials, intracellular Ca2+ levels, and DSM contractions (18, 20, 29, 30). In non-DSM smooth muscle, testosterone can activate BK channels, and thus significantly decrease cellular excitability (3, 7, 8, 14, 33–35). Here, for the first time, we demonstrated that testosterone at a nanomolar concentration (100 nM) increased the whole cell BK current in freshly isolated guinea pig DSM cells (Fig. 1), suggesting a regulatory role of testosterone in DSM cell excitability. Furthermore, testosterone increases TBKCs (Fig. 2), which are a function of transient elevation of BK channel activity in DSM (18). In addition, the current-clamp data showed that testosterone significantly hyperpolarizes DSM cell resting membrane potential and activates STHs (Fig. 4). In DSM cells, similarly to TBKCs, STHs are due to transient and localized increases of BK channel activity (18). Moreover, the hyperpolarizing effect of testosterone on DSM cell resting membrane potential (Fig. 4) is consistent with the effects observed after BK channel activation with a selective BK channel opener (20). More importantly, the testosterone-induced hyperpolarization of DSM cells was completely abolished by the selective BK channel inhibitor paxilline (Fig. 4), further suggesting the functional role of BK channels. Collectively, our perforated patch-clamp data support the concept that under physiological conditions testosterone activates BK channels, hyperpolarizes the resting membrane potential, and decreases DSM cell excitability. In turn, this leads to a reduction in the global intracellular Ca2+ concentration (Fig. 5), which is consistent with testosterone-induced DSM membrane hyperpolarization and DSM relaxation.
Testosterone can activate BK channels indirectly, via intracellular signaling mechanisms, such as cGMP-dependent protein kinase pathway (7), or directly by binding to the regulatory BK β-subunit, thus providing rapid activation of the channel (23). It has been shown that DSM cells express BK β1- and β4-subunits (4, 18, 31), both of which can bind steroid hormones, such as testosterone and estrogen, and thus promote BK channel activation (23). While the BK β1-subunit is smooth muscle-specific, and therefore an important regulator of DSM excitability and contractility (31), the functional role of the BK β4-subunit in DSM is less clear (4). Our excised patch, single channel experiments demonstrated that testosterone significantly increases single BK channel open probability in DSM cell excised patches by more than 300% (Fig. 3). These results suggest that the underlying cellular mechanism of testosterone-induced BK channel activation is most likely independent of the genomic testosterone receptors, or second messenger signaling. These data are consistent with the hypothesis for direct modulation of the BK β-subunit by testosterone in DSM cells.
The testosterone-induced elevation of single BK channel open probability in excised membrane patches (Fig. 3) can explain the observation that testosterone increases the frequency of TBKCs in DSM cells, without having any significant effects on the average TBKC amplitude (Fig. 2). The increase in TBKC frequency (Fig. 2) could be attributed to an increase in BK channel NPo by testosterone, which can thus increase the number of single BK channel opening events at −20 mV. Therefore, testosterone increased the number of small BK channel opening events reaching the TBKC threshold of 9 pA, (see materials and methods) as a result of direct modulation of BK channel NPo while having no potentiating effects on the average amplitude of TBKCs in DSM cells.
Studies with HEK239 cells showed that steroid hormones can bind to BK β-subunits, and thus differentially regulate BK channels (23). Recently, we demonstrated a rapid nongenomic stimulatory effect of 17β-estradiol on BK channels in DSM cells (32), consistent with findings in non-DSM cell types (6, 24, 39). Similarly to testosterone (this study), 17β-estradiol (100 nM) increases the whole cell and single channel BK currents, TBKCs, and hyperpolarizes DSM cell resting membrane potential in guinea pig DSM cells (32). Since variation in steroid sensitivity to different BK channel β-subunits has been reported (23), we decided to examine the concept that testosterone and 17β-estradiol differentially regulate BK channels in guinea pig DSM cells. In Table 1, we compare the relative effects of testosterone (100 nM) and 17β-estradiol (100 nM) on whole cell BK currents, TBKCs, single BK channel currents, and the resting membrane potential in DSM cells. The analysis reveals no statistically significant differences of the relative effects of testosterone and 17β-estradiol in any of the investigated parameters (Table 1). However, the hypothesis that testosterone and 17β-estradiol differentially regulate BK channels in DSM requires further detailed investigations.
Table 1.
Comparison of the effects of 17β-estradiol (100 nM) and testosterone (100 nM)
| Whole Cell Current at +40 mV, % | TBKC Frequency, % | Single BK channel NPO, % | Membrane Potential, % | |
|---|---|---|---|---|
| 17β-Estradiol (100 nM) effect | 32.9 ± 11.7 n = 11/N = 10 | 35.5 ± 11.1 n = 9/N = 9 | 464.6 ± 259.7 n = 13/N = 11 | 26.0 ± 14.4 n = 12/N = 11 |
| Testosterone (100 nM) effect | 20.0 ± 7.3 n = 15/N = 6 | 105.1 ± 32.9 n = 9/N = 9 | 307.1 ± 107.7 n = 13/N = 11 | 15.9 ± 4.5 n = 23/N = 21 |
| Unpaired Student's t-test | P > 0.05 | P > 0.05 | P > 0.05 | P > 0.05 |
Data are presented as means ± SE in percentages. Comparison of the effects of 17β-estradiol (100 nM) and testosterone (100 nM) on the whole cell currents, TBKC frequency, single BK channel NPO, and resting membrane potential in guinea pig detrusor smooth muscle cells. 17β-Estradiol data are taken from Provence et al., 2015 (32).
In conclusion, our findings provide a novel understanding of the regulatory roles of testosterone on DSM cell excitability. The demonstrated stimulatory effects of testosterone on BK channel activity could at least in part contribute to the rapid, nongenomic effects of this sex hormone on bladder contractility (13). As the role of testosterone in bladder physiology and pathophysiology is not fully understood, the current study provides a foundational basis for future investigations in human DSM. The nongenomic effects of testosterone on BK channel activity should be taken into account in the development of novel hormonal therapies for treatment of age-related bladder disorders, such as LUTS and associated OAB.
GRANTS
This study was supported by National Institutes of Health Grants R01 DK106964 and R01 DK084284 to G. V. Petkov. A. Provence was a recipient of F31 DK104528 fellowship under Dr. Petkov's mentorship.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
K.L.H. and G.V.P. conception and design of research; K.L.H., S.P.P., A.P., and G.V.P. performed experiments; K.L.H., A.P., and G.V.P. analyzed data; K.L.H., A.P., and G.V.P. interpreted results of experiments; K.L.H., A.P., and G.V.P. prepared figures; K.L.H., A.P., and G.V.P. drafted manuscript; K.L.H., S.P.P., A.P., and G.V.P. edited and revised manuscript; K.L.H., S.P.P., A.P., and G.V.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Damiano Angoli for help with the patch-clamp experiments.
REFERENCES
- 1.Abdel-Hamid AAM, Ali EMT. Effect of testosterone therapy on the urinary bladder in experimental hypogonadism of rats. J Mol Histol 46: 263–272, 2015. [DOI] [PubMed] [Google Scholar]
- 2.Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, Van Kerrebroeck P, Victor A, Wein A. The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology 61: 37–49, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Cairrao E, Alvarez E, Santos-Silva AJ, Verde I. Potassium channels are involved in testosterone-induced vasorelaxation of human umbilical artery. Naunyn Schmiedebergs Arch Pharmacol 376: 375–383, 2008. [DOI] [PubMed] [Google Scholar]
- 4.Chen M, Petkov GV. Identification of large conductance calcium activated potassium channel accessory beta4 subunit in rat and mouse bladder smooth muscle. J Urol 182: 374–381, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coyne KS, Kaplan SA, Chapple CR, Sexton CC, Kopp ZS, Bush EN, Aiyer LP. Risk factors and comorbid conditions associated with lower urinary tract symptoms: EpiLUTS. BJU Int 103, Suppl 3: 24–32, 2009. [DOI] [PubMed] [Google Scholar]
- 6.De Wet H, Allen M, Holmes C, Stobbart M, Lippiat JD, Callaghan R. Modulation of the BK channel by estrogens: examination at single channel level. Mol Membr Biol 23: 420–429, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Deenadayalu V, Puttabyatappa Y, Liu AT, Stallone JN, White RE. Testosterone-induced relaxation of coronary arteries: activation of BKCa channels via the cGMP-dependent protein kinase. Am J Physiol Heart Circ Physiol 302: H115–H123, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deenadayalu VP, White RE, Stallone JN, Gao X, Garcia AJ. Testosterone relaxes coronary arteries by opening the large-conductance, calcium-activated potassium channel. Am J Physiol Heart Circ Physiol 281: H1720–H1727, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Feldman HA, Longcope C, Derby CA, Johannes CB, Araujo AB, Coviello AD, Bremner WJ, McKinlay JB. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab 87: 589–598, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Fernandes VS, Barahona MV, Recio P, Martinez-Saenz A, Ribeiro ASF, Contreras C, Martinez AC, Bustamante S, Carballido J, Garcia-Sacristan A, Prieto D, Hernandez M. Mechanisms involved in testosterone-induced relaxation to the pig urinary bladder neck. Steroids 77: 394–402, 2012. [DOI] [PubMed] [Google Scholar]
- 11.Fry CH, Jabr RI. T-type Ca2+ channels and the urinary and male genital tracts. Pflügers Arch 466: 781–789, 2014. [DOI] [PubMed] [Google Scholar]
- 12.Haider A, Gooren LJ, Padungtod P, Saad F. Concurrent improvement of the metabolic syndrome and lower urinary tract symptoms upon normalisation of plasma testosterone levels in hypogonadal elderly men. Andrologia 41: 7–13, 2009. [DOI] [PubMed] [Google Scholar]
- 13.Hall R, Andrews PLR, Hoyle CHV. Effects of testosterone on neuromuscular transmission in rat isolated urinary bladder. Eur J Pharmacol 449: 301–309, 2002. [DOI] [PubMed] [Google Scholar]
- 14.Han DH, Chae MR, Jung JH, So I, Park JK, Lee SW. Effect of testosterone on potassium channel opening in human corporal smooth muscle cells. J Sex Med 5: 822–832, 2008. [DOI] [PubMed] [Google Scholar]
- 15.Hanna-Mitchell AT, Robinson D, Cardozo L, Everaert K, Petkov GV. Do we need to know more about the effects of hormones on lower urinary tract dysfunction? ICI-RS 2014. Neurourol Urodyn 35: 299–303, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore longitudinal study of aging. J Clin Endocrinol Metab 86: 724–731, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Hristov KL, Afeli SAY, Parajuli SP, Cheng QP, Rovner ES, Petkov GV. Neurogenic detrusor overactivity is associated with decreased expression and function of the large conductance voltage- and Ca2+-activated K+ channels. PLos One 8: e68052, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hristov KL, Chen MY, Kellett WF, Rovner ES, Petkov GV. Large-conductance voltage- and Ca2+-activated K+ channels regulate human detrusor smooth muscle function. Am J Physiol Cell Physiol 301: C903–C912, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hristov KL, Parajuli SP, Provence A, Rovner ES, Petkov GV. 17β-Estradiol direct activation of large conductance voltage- and Ca2+-activated K+ channels: novel regulatory mechanism in human detrusor smooth muscle. J Urol 195: e378, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hristov KL, Parajuli SP, Soder RP, Cheng QP, Rovner ES, Petkov GV. Suppression of human detrusor smooth muscle excitability and contractility via pharmacological activation of large conductance Ca2+-activated K+ channels. Am J Physiol Cell Physiol 302: C1632–C1641, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hristov KL, Smith AC, Parajuli SP, Malysz J, Rovner ES, Petkov GV. Novel regulatory mechanism in human urinary bladder: Central role of transient receptor potential melastatin 4 channels in detrusor smooth muscle function. Am J Physiol Cell Physiol 310: C600–C611, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kathrins M, Doersch K, Nimeh T, Canto A, Niederberger C, Seftel A. The relationship between testosterone-replacement therapy and lower urinary tract symptoms: a systematic review. Urology 88: 22–32, 2016. [DOI] [PubMed] [Google Scholar]
- 23.King JT, Lovell PV, Rishniw M, Kotlikoff MI, Zeeman ML, McCobb DP. Beta2 and beta4 subunits of BK channels confer differential sensitivity to acute modulation by steroid hormones. J Neurophysiol 95: 2878–2888, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Kow LM, Pfaff DW. Rapid estrogen actions on ion channels: a survey in search for mechanisms. Steroids 111: 46–53, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lai GJ, McCobb DP. Regulation of alternative splicing of Slo K+ channels in adrenal and pituitary during the stress-hyporesponsive period of rat development. Endocrinology 147: 3961–3967, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Mahmoud SF, McCobb DP. Regulation of Slo potassium channel alternative splicing in the pituitary by gonadal testosterone. J Neuroendocrinol 16: 237–243, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Malysz J, Afeli SA, Provence A, Petkov GV. Ethanol-mediated relaxation of guinea pig urinary bladder smooth muscle: involvement of BK and L-type Ca2+ channels. Am J Physiol Cell Physiol 306: C45–C58, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Melman A, Bar-Chama N, McCullough A, Davies K, Christ G. Plasmid-based gene transfer for treatment of erectile dysfunction and overactive bladder: results of a phase I trial. Israel Med Assoc J 9: 143–146, 2007. [PubMed] [Google Scholar]
- 29.Petkov GV. Central role of the BK channel in urinary bladder smooth muscle physiology and pathophysiology. Am J Physiol Regul Integr Comp Physiol 307: R571–R584, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petkov GV. Role of potassium ion channels in detrusor smooth muscle function and dysfunction. Nat Rev Urol 9: 30–40, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petkov GV, Bonev AD, Heppner TJ, Brenner R, Aldrich RW, Nelson MT. Beta1-subunit of the Ca2+-activated K+ channel regulates contractile activity of mouse urinary bladder smooth muscle. J Physiol 537: 443–452, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Provence A, Hristov KL, Parajuli SP, Petkov GV. Regulation of guinea pig detrusor smooth muscle excitability by 17beta-estradiol: the role of the large conductance voltage- and Ca2+-activated K+ channels. PLos One 10: e0141950, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saldanha PA, Cairrao E, Maia CJ, Verde I. Long- and short-term effects of androgens in human umbilical artery smooth muscle. Clin Exp Pharmacol Physiol 40: 181–189, 2013. [DOI] [PubMed] [Google Scholar]
- 34.Seyrek M, Irkilata HC, Vural IM, Yildirim I, Basal S, Yildiz O, Dayanc M. Testosterone relaxes human internal spermatic vein through potassium channel opening action. Urology 78: 233 e231–235, 2011. [DOI] [PubMed] [Google Scholar]
- 35.Seyrek M, Yildiz O, Ulusoy HB, Yildirim V. Testosterone relaxes isolated human radial artery by potassium channel opening action. J Pharm Sci 103: 309–316, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Sui GP, Wu C, Fry CH. A description of Ca2+ channels in human detrusor smooth muscle. BJU Int 92: 476–482, 2003. [DOI] [PubMed] [Google Scholar]
- 37.Tanidir Y, Ercan F, Tarcan T. Exogenous testosterone and estrogen affect bladder tissue contractility and histomorphology differently in rat ovariectomy model. J Sex Med 8: 1626–1637, 2011. [DOI] [PubMed] [Google Scholar]
- 38.Tek M, Balli E, Cimen B, Efesoy O, Oguz I, Cayan S. The effect of testosterone replacement therapy on bladder functions and histology in orchiectomized mature male rats. Urology 75: 886–890, 2010. [DOI] [PubMed] [Google Scholar]
- 39.Valverde MA, Rojas P, Amigo J, Cosmelli D, Orio P, Bahamonde MI, Mann GE, Vergara C, Latorre R. Acute activation of Maxi-K channels (hSlo) by estradiol binding to the beta subunit. Science 285: 1929–1931, 1999. [DOI] [PubMed] [Google Scholar]
- 40.Yassin AA, El-Sakka AI, Saad F, Gooren LJ. Lower urinary-tract symptoms and testosterone in elderly men. World J Urol 26: 359–364, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yassin DJ, Doros G, Hammerer PG, Yassin AA. Long-term testosterone treatment in elderly men with hypogonadism and erectile dysfunction reduces obesity parameters and improves metabolic syndrome and health-related quality of life. J Sex Med 11: 1567–1576, 2014. [DOI] [PubMed] [Google Scholar]