Abstract
We here report on a 20-year-old female patient with EDS due to a homozygous CHST14 single nucleotide deletion resulting in D4ST-1 deficiency, accompanied by muscle hypoplasia and muscle weakness. Findings of muscle ultrasound, electromyography, and muscle biopsy pointed to a myopathy, similarly as in other EDS types. This myopathy probably contributes to the gross motor developmental delay in this type of EDS.
Keywords: Ehlers-Danlos syndrome, myopathy, neuromuscular features, muscle biopsy, delayed motor development, connective tissue, CHST14, musculocontractural Ehlers-Danlos syndrome, adducted thumb-clubfoot syndrome
INTRODUCTION
Recently, a new type of Ehlers-Danlos syndrome (EDS) caused by recessive CHST14 mutations, dermatan-4-O-sulfotransferase I (D4ST-1 deficiency) has been described [Dundar et al., 2009; Malfait et al., 2010; Miyake et al., 2010]. Interestingly, D4ST-1 deficiency was independently found in an arthrogryposis syndrome “Adducted Thumb-Clubfoot Syndrome” (ATCS) [Dundar et al., 2009]; in a specific form of EDS tentatively named as EDS, Kosho Type (EDSKT) [Kosho et al., 2005, 2010; Miyake et al., 2010]; and in a subset of kyphoscoliosis type EDS without evidence of lysyl hydroxylase deficiency (EDS type VIB or musculocontractural (MC) EDS) [Malfait et al., 2010]. Malfait et al. [2010] proposed that these three clinical conditions should be considered as a single entity, categorized into a form of EDS [Malfait et al., 2010]. Subsequently, Janecke et al. [2011] claimed that the disorder should be named not as a form of EDS but as ATCS (“dermatan sulfate-deficient ATCS, representing another disorder of congenital glycosylation”). Very recently, Shimizu et al. [2011] have presented two additional case reports as well as a comprehensive review of all 20 reported patients, concluding the disorder to be categorized into a form of EDS both from clinical and etiological standpoints.
The clinical features of this new EDS type are either related to progressive multisystem fragility secondary to impaired assembly of collagen fibrils (joint dislocations and deformities, skin hyperextensibility, bruisability, and fragility; recurrent large subcutaneous hematomas, and other cardiac valvular, respiratory, gastrointestinal, and ophthalmological complications), or classified as malformations resulting from inborn errors of development (distinct craniofacial features, multiple congenital contractures, and congenital defects in cardiovascular, gastrointestinal, renal, ocular, and central nervous systems) [Kosho et al., 2010; Malfait et al., 2010; Shimizu et al., 2011]. Gross motor developmental delay with muscle hypotonia is considered to be a common feature in the disorder [Shimizu et al., 2011], but only one early case report suggested an underlying myopathic process in ATCS based on limited neuromuscular examinations [Dundar et al., 1997].
We here report on a patient with this novel type of EDS caused by a homozygous CHST14 single nucleotide deletion with muscle hypoplasia and weakness in whom findings of muscle ultrasound, electromyography, and muscle biopsy pointed to a myopathy.
CLINICAL REPORT
A 20-year-old patient with Ehlers-Danlos syndrome was invited to participate in a study on neuromuscular features in EDS through our outpatient department [Voermans et al., 2009c]. She had taken part in a questionnaire study on fatigue and pain in EDS [Voermans et al., 2010a,b], in which she had indicated that she had the vascular type of EDS. At the day of the study protocol, however, it was noted that she lacked the typical clinical features of the vascular type. She was therefore not included in the study and we referred her to our clinical geneticist. She agreed to participate in the study protocol anyhow [Voermans et al., 2009c], since she had several neuromuscular complaints. Three years later, after recognizing the similarity in her phenotype with that in the publications of Kosho et al. [2010] and Miyake et al. [2010], mutation analysis was performed by sequencing the entire coding region of CHST14 and a homozygous 1 bp deletion (c.124delG, p.Val48X) was detected.
The patient had been diagnosed with a probable diagnosis of the vascular type of EDS at age 10 in the Netherlands; this was based upon extensive bruising, very severe hematomas after mild trauma, and generalized joint hypermobility with recurrent luxations. Her parents, brother, and sister were unaffected. Furthermore, subsequent electrophoresis of the collagen from the skin biopsy showed no abnormalities, and COL3A1 mutation analysis had not been performed. At the age of 14, her case was investigated in the USA, and the diagnosis of the vascular type of EDS was questioned. The differential diagnosis then included the kyphoscoliotic type of EDS, or “EDS with soft-tissue contractures” as described by Hamada et al. [1992], but no ancillary investigations were performed at that time. She was lost to follow-up, because she moved back with her family to the Netherlands.
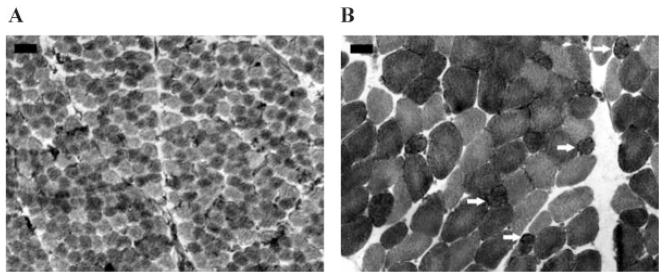
The mother had reported less intrauterine movements than in the previous pregnancy. The patient was born at 37 weeks gestational age via normal spontaneous vaginal delivery. Her birth weight was 3,000 g, however birth length was 60 cm (>97th centile). Immediately after birth, an unusual appearance with contracted thumbs and curved out, flexed clubfoot were noticed. Motor milestones were delayed: sitting at 14 months of age, and walking at 3 years of age. Cognitive development was normal. Due to the motor development delay, a neuromuscular disorder was suspected and a muscle biopsy of the gracilis muscle was performed, which revealed no abnormalities (Fig. 4A). Since the age of two, she started presenting with easy bruising and once had a large scalp hematoma after she fell down. At the age of four, she had a spontaneous knee luxation, and 1 year later she had a shoulder luxation. She had not suffered from hemorrhages or ruptures of the internal organs. Due to severe myopia, she had to start wearing glasses at the age of four (−10 to −12 diopters). Glaucoma was diagnosed at the age of 11 years.
FIG. 4.
A: Normal biopsy of gracilis muscle at 1 year of age. The diameter of muscle biopsies is smaller in the neonatal period (A) than in adolescence (B). B: Vastus lateralis biopsy at 20 years of age with increased variation of muscle fiber diameters, type 1 predominance and type 1 fibers with incipient lobulation (arrows). A,B: NADH, the darkest fibers are type 1 fibers; Bar = 50 μm: the same magnifications are used for (A) and (B).
Further history revealed that she had remarkably decreased exercise tolerance since childhood. She could not keep up with her younger brother and older sister in swimming as a child. Performance in gymnastics in school was limited due to muscle weakness and easy fatigability. At the time of the investigations, she could walk approximately 1–2 km after which she was very fatigued. Walking upstairs was also limited due to proximal muscle weakness and easy fatigability. She had also marked muscle weakness in her hands and arms. Cycling was only possible using a tricycle or tandem.
Physical examination at the age of 20 revealed a Marfanoid habitus (length 175.9 cm (+1 SD); weight 50.9 kg (weight for length—2 SD)), drooping face, irregular lenses, microcornea, telecanthus, protruding ears, a high palate, and arachnodactyly. The Beighton score was 2/9 and the Bulbena score 3/10. She had mild scoliosis, widened atrophic scars, hypotonic dropping feet (Fig. 1), but no thorax deformities. Manual muscle strength testing revealed a sum score of 96/140 (28 muscles tested), with a mean MRC score of 3.4/5 (Table I) [Peterson-Kendall et al., 2005; Voermans et al., 2009c]. Findings of dynamometry confirmed the presence of this moderate muscle weakness: the force (N) measured in five muscles bilaterally was far below the 5th centile of the normal values in control subjects (Table I) [van der Ploeg et al., 1991; Voermans et al., 2009c]. There was generalized muscle hypoplasia, including the intrinsic hand muscles, with contractures of the thumb, fourth and fifth fingers (Fig. 1). Vibration sensation was mildly reduced in her feet, and joint position was normal. Deep tendon reflexes were reduced, and there was a mild generalized muscle hypotonia. Vignos lower extremity score was 2/10 (this measures lower extremity function; 1 = being able to walk and climb stairs without assistance; 10 = confined to bed) [Thompson and Vignos, 1959]; Brooke upper extremity score was 1/6 (this measures upper extremity function; 1 = patients being able to fully abduct their arms; 6 = no useful function of hands) [Brooke et al., 1981]; the Modified Rankin scale was 2/5 (this measures recovery of motor function after stroke; 0 = normal; 5 = severe disability; 6 = dead) [Rankin, 1957; van Swieten et al., 1988]; and the Rivermead Mobility Index was 13/15 (measures the ability to move its own body; 0 = severely dependent on others for mobility; 15 = normal) [Collen et al., 1991]; all indicating mild impairment.
FIG. 1.
Hypotonic, dropping feet, facial features, flexion contractures of thumb (right >left), fourth and fifth finger, and hypoplasia of intrinsic hand muscles.
TABLE I.
Results of Manual Muscle Testing (MMT) and Dynamometry [van der Ploeg et al., 1991; Peterson-Kendall et al., 2005]
| Movement tested | MMT muscle force (0–5)
|
|||
|---|---|---|---|---|
| Right | Left | |||
| Shoulder anteflexion | 3 | 3 | ||
| Shoulder abduction | 4 | 4 | ||
| Elbow flexion | 4 | 4 | ||
| Elbow extension | 3 | 3 | ||
| Wrist flexion | 4 | 4 | ||
| Wrist extension | 3 | 3 | ||
| Hip flexion | 3 | 3 | ||
| Hip extension | 4 | 4 | ||
| Hip abduction | 4 | 4 | ||
| Knee flexion | 4 | 3 | ||
| Knee extension | 3 | 3 | ||
| Foot dorsal flexion | 3 | 3 | ||
| Foot plantar flexion | 3 | 3 | ||
| Neck flexion | 4 | |||
| Trunk flexion | 3 | |||
| Dynamometry muscle force (N)
|
||||
| Right | Left | p5 of normal female subjects | ||
| Shoulder abduction | 32 | 37 | 72 | |
| Flexion elbow | 53 | 33 | 127 | |
| Extension elbow | 33 | 26 | 68 | |
| Grip strength | 27 | 14 | 58 | |
| Knee extension | 27 | 27 | >160 | |
The muscle force was measured three times, and the outcomes were averaged.
The measurement of knee extension was painful at the shin and therefore probably reflecting submaximal force.
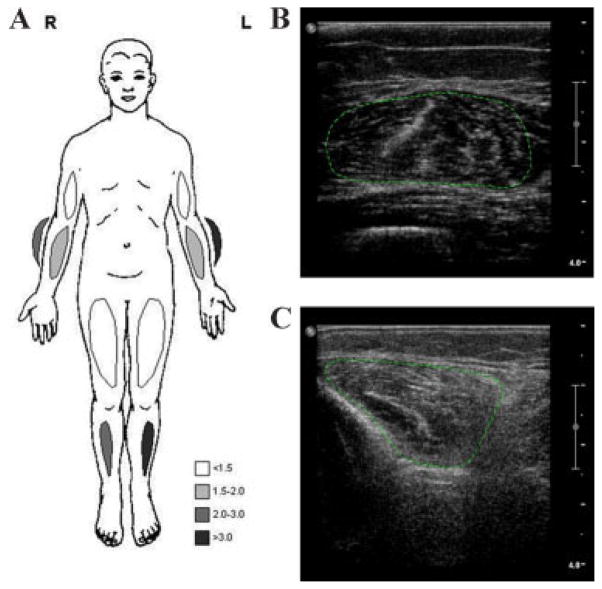
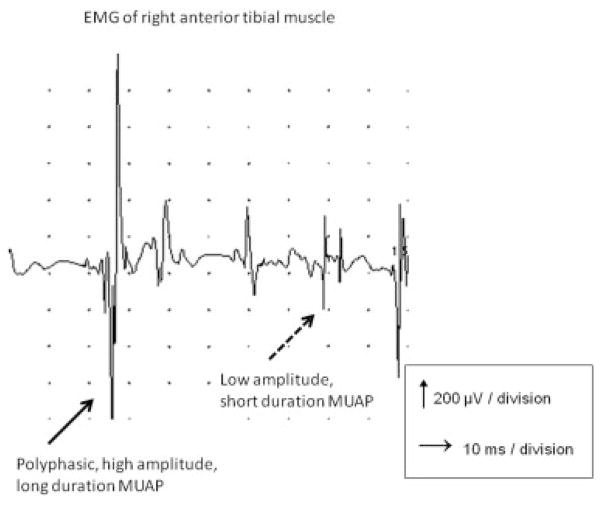
The lysyl/hydroxylysyl pyridinoline ratio was normal, and no PLOD1 mutation was detected. Bone densitometry revealed osteopenia (lumbar spine T −2.37; femoral neck −2.48), for which calcium supplementation and vitamin D3 were prescribed. Cardiological evaluation, including electrocardiography and cardiac ultrasound was normal. Creatine kinase was mildly elevated (CK 277 U/l; N <160 U/l). We performed a quantitative muscle ultrasound, which is a reliable tool for assessing structural abnormalities in neuromuscular disorders that shows increasing echogenecity of muscle with progression of structural abnormalities such as fibrosis and fatty degeneration. Several prospective studies have reported high sensitivities and specificities in the detection of neuromuscular disorders [Pillen et al., 2008]. The quantitative muscle ultrasound in this patient revealed an increased echo intensity (Z-score ≥2) in the forearm extensors and of the anterior tibial muscles. There was marked atrophy (Z-score ≤−2) of the forearm flexors, forearm extensors and quadriceps muscles bilaterally (Fig. 2) [Pillen et al., 2008; Arts et al., 2009]. Nerve conduction studies showed low compound muscle action potential amplitudes in distal muscles and H-reflexes of the soleus muscle were absent. The sensory nerve action potential of the sural nerve was absent on the right side (probably due to previous operation) and was normal on the left side. Needle electromyography showed an abnormal and mixed pattern of shorter duration, low amplitude, polyphasic motor units, and polyphasic motor units with longer duration and higher amplitudes, reflecting an increase in fiber size diameter (Fig. 3). Together, these findings suggested mainly myopathic involvement, which could also explain the lower CMAP amplitudes and findings on needle examination. A needle biopsy of the vastus lateralis muscle showed fiber type 1 predominance (70%, N 20–46%) without fiber type grouping, increased variation of fiber diameter of both type 1 and type 2 fibers, and some type 1 fibers coming close to lobulated fibers, compatible with a mild myopathy (Fig. 4B).
FIG. 2.
Results of quantitative muscle ultrasound. Measurements were performed with a Philips IU 22 ultrasound system using a 17–5 MHz broadband linear probe (framerate 20 Hz, gain 70%, compression 55, greymap 2). Regions of interest for quantitative grey scale histogram analysis are encircled (green dotted lines). A: Z-scores of the echo intensity of the quantative muscle ultrasound. B: Muscle ultrasound of the right rectus femoris muscle: normal echo intensity (Z-score 0.1). C: Muscle ultrasound of the right anterior tibial muscle: increased echo intensity (Z-score 2.8).
FIG. 3.
Electromyography of the right anterior tibial muscle showed an abnormal, mixed pattern of both shorter duration, low amplitude, polyphasic motor units, and polyphasic motor units with longer duration and higher amplitudes, reflecting an increase in fiber size diameter. These findings imply mainly myopathic involvement.
DISCUSSION
We here report on a 20-year-old female patient with EDS due to a homozygous CHST14 single nucleotide deletion resulting in D4ST-1 deficiency, accompanied by muscle hypoplasia and muscle weakness. Findings of muscle ultrasound, electromyography, and muscle biopsy pointed to a myopathy, similarly as in other EDS types.
This myopathy probably contributes to the gross motor developmental delay in this type of EDS, which has only been mentioned shortly in the extensive clinical description of the six patients by Kosho et al. [2010], and is discussed in little more detail in the review on 20 patients by Shimizu et al. [2011]. This delayed gross motor development occurred in patients previously diagnosed as EDSKT, ATCS, and MCEDS. The reduced compound muscle action potentials on electromyography in a patient with ATCS pointed to a myopathic process; however this was deemed unlikely since the neonatal muscle biopsy did not show any myopathic changes [Dundar et al., 1997]. The myopathy probably also contributed to the mild functional impairments in the patient presented here, and should be taken into account in rehabilitation programs. Furthermore, the fact that this patient had also undergone a muscle biopsy in infancy indicates that this type of EDS should be part of the differential diagnosis of neonatal hypotonia, similar as the kyphoscoliotic type of EDS [Yis et al., 2008; Voermans et al., 2009b].
In fact, mild-to-moderate myopathy and polyneuropathy are common in various types of EDS and point to the possible role of the extracellular matrix in muscle and peripheral nerve function [Voermans et al., 2009b,c]. In a study of 40 EDS patients of various types, muscle weakness, myalgia, and easy fatigability were reported by the majority of patients. Mild to moderate muscle weakness (85%) and mild reduction of vibration sense (60%) were common. Nerve conduction studies revealed axonal polyneuropathy in five patients (13%), needle electromyography showed predominantly myopathic features in nine patients (26%), and a mixed neurogenic-myopathic pattern in most (60%). Muscle ultrasound revealed increased echo intensity (48%) and atrophy (50%). Mild myopathic features were seen on muscle biopsy of five patients (28%). Furthermore, we have reported on a 16-year-old patient with the kyphoscoliotic type of EDS with generalized muscle weakness and hypotonia. Electromyography, muscle ultrasound, MRI, and muscle biopsy showed myopathic changes, and nerve conduction studies revealed a mild axonal polyneuropathy, while laboratory investigations had excluded the common causes of axonal polyneuropathy.
CHST14 is involved in collagen formation: the gene encodes dermatan 4-O-sulfotransferase 1 (D4ST1), which transfers active sulfate from 3-phosphoadenosine 5-phosphosulfate to position 4 of the N-acetyl-D-galactosamine (GalNAc) residues of dermatan sulfate. The loss of the decorin dermatan sulfate proteoglycan may preclude proper collagen bundle formation and maintenance of collagen bundles also in muscle connective tissue (endo-, peri-, and epimysium). In the dermis, this results in more fine and less normal collagen fibrils and increased dispersion of collagen fibrils [Miyake et al., 2010]. Since decorin is also expressed in the muscle connective tissue, collagen fiber size, and distribution in endo-, peri- and epimysium might be altered in a similar way, in parallel with observations in other EDS types [Voermans et al., 2009c]. Muscle electronmicroscopy in a patient with the classical type of EDS showed shorter and less collagen fibrils between muscle fibers [Voermans et al., 2009c]. In the TNX-deficient type EDS these structural extracellular matrix changes results in altered contractile properties of the muscle connective tissue with subsequent changes in the myofascial force transmission within and between the myotendinous complexes [Huijing, 2009]. This leads to reduced muscle function since TNX-deficient muscles act more independently and probably less effectively [Voermans et al., 2007a; Huijing et al., 2010].
The combination of joint hypermobility and congenital (nonprogressive) contractures in this new type of EDS appears quite specific. Joint hypermobility is a typical feature of inherited connective tissue disorders, whereas contractures (e.g., many distal arthrogryposes) can occur in congenital myopathies. However, clinical features of these two groups of disorders overlap [Kirschner et al., 2005; Voermans et al., 2008]: joint hypermobility may also occur in congenital myopathies (e.g., Bethlem myopathy, Ullrich congenital myopathy, and central core disease) [Voermans et al., 2009a], and proximal contractures occur in inherited connective tissue disorders, although most often later in the course of the disease (e.g., Marfan syndrome). Most likely, these two groups of disorders are part of a continuum of extracellular matrix disorders which affect muscles to a variable degree [Voermans et al., 2007b, 2008]. The D4ST1 deficient type EDS fits well in this continuum, similarly as the kyphoscoliotic type EDS. Of note, genitopatellar syndrome is another condition sharing congenital joint hypermobility and arthrogryposis [Cormier-Daire et al., 2000; Reardon, 2002]. In conclusion, this new D4ST1 deficient type EDS due to mutations in CHST14 (former EDS type VIB; EDSKT, MCEDS, or ACTD) [Dundar et al., 2009; Kosho et al., 2010; Miyake et al., 2010] can be accompanied by a myopathy, similarly as in other EDS types Voermans et al.,c].
Acknowledgments
Grant sponsor: Prinses Beatrix Fonds.
N.C. Voermans was supported by a clinical Neuromuscular fellowship grant by the Prinses Beatrix Fonds. We are thankful to Dr. Miyake, Department of Human Genetics, Yokohama City University Graduate School of Medicine, Japan for performing the CHST14 mutation analysis in this patient.
References
- Arts IM, Pillen S, Schelhaas HJ, Overeem S, Zwarts MJ. Normal values for quantitative muscle ultrasonography in adults. Muscle Nerve. 2010;41:32–41. doi: 10.1002/mus.21458. [DOI] [PubMed] [Google Scholar]
- Brooke MH, Griggs RC, Mendell JR, Fenichel GM, Shumate JB, Pellegrino RJ. Clinical trial in Duchenne dystrophy. I. The design of the protocol. Muscle Nerve. 1981;4:186–197. doi: 10.1002/mus.880040304. [DOI] [PubMed] [Google Scholar]
- Collen FM, Wade DT, Robb GF, Bradshaw CM. The Rivermead Mobility Index: A further development of the Rivermead Motor Assessment. Int Disabil Stud. 1991;13:50–54. doi: 10.3109/03790799109166684. [DOI] [PubMed] [Google Scholar]
- Cormier-Daire V, Chauvet ML, Lyonnet S, Briard ML, Munnich A, Le MM. Genitopatellar syndrome: A new condition comprising absent patellae, scrotal hypoplasia, renal anomalies, facial dysmorphism, and mental retardation. J Med Genet. 2000;37:520–524. doi: 10.1136/jmg.37.7.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dundar M, Demiryilmaz F, Demiryilmaz I, Kumandas S, Erkilic K, Kendirci M, Tuncel M, Ozyazgan I, Tolmie JL. An autosomal recessive adducted thumb-club foot syndrome observed in Turkish cousins. Clin Genet. 1997;51:61–64. doi: 10.1111/j.1399-0004.1997.tb02417.x. [DOI] [PubMed] [Google Scholar]
- Dundar M, Muller T, Zhang Q, Pan J, Steinmann B, Vodopiutz J, Gruber R, Sonoda T, Krabichler B, Utermann G, Baenziger JU, Zhang L, Janecke AR. Loss of dermatan-4-sulfotransferase 1 function results in adducted thumb-clubfoot syndrome. Am J Hum Genet. 2009;85:873–882. doi: 10.1016/j.ajhg.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada S, Hiroshima K, Oshita S, Doi T, Ono K. Ehlers-Danlos syndrome with soft-tissue contractures. J Bone Joint Surg Br. 1992;74:902–905. doi: 10.1302/0301-620X.74B6.1447255. [DOI] [PubMed] [Google Scholar]
- Huijing PA. Epimuscular myofascial force transmission: A historical review and implications for new research. International Society of Biomechanics Muybridge Award Lecture, Taipei 2007. J Biomech. 2009;42:9–21. doi: 10.1016/j.jbiomech.2008.09.027. [DOI] [PubMed] [Google Scholar]
- Huijing PA, Voermans NC, Baan GC, Buse TE, van Engelen BG, de Haan A. Muscle characteristics and altered myofascial force transmission in tenascin-X-deficient mice, a mouse model of Ehlers-Danlos syndrome. J Appl Physiol. 2010;109:986–995. doi: 10.1152/japplphysiol.00723.2009. [DOI] [PubMed] [Google Scholar]
- Janecke AR, Baenziger JU, Muller T, Dundar M. Loss of dermatan-4-sulfotransferase 1 (D4ST1/CHST14) function represents the first dermatan sulfate biosynthesis defect, “dermatan sulfate-deficient adducted thumb-clubfoot syndrome”. Hum Mutat. 2011;32:484–485. doi: 10.1002/humu.21440. [DOI] [PubMed] [Google Scholar]
- Kirschner J, Hausser I, Zou Y, Schreiber G, Christen HJ, Brown SC, Anton-Lamprecht I, Muntoni F, Hanefeld F, Bonnemann CG. Ullrich congenital muscular dystrophy: Connective tissue abnormalities in the skin support overlap with Ehlers-Danlos syndromes. Am J Med Genet A. 2005;132A:296–301. doi: 10.1002/ajmg.a.30443. [DOI] [PubMed] [Google Scholar]
- Kosho T, Takahashi J, Ohashi H, Nishimura G, Kato H, Fukushima Y. Ehlers-Danlos syndrome type VIB with characteristic facies, decreased curvatures of the spinal column, and joint contractures in two unrelated girls. Am J Med Genet A. 2005;138A:282–287. doi: 10.1002/ajmg.a.30965. [DOI] [PubMed] [Google Scholar]
- Kosho T, Miyake N, Hatamochi A, Takahashi J, Kato H, Miyahara T, Igawa Y, Yasui H, Ishida T, Ono K, Kosuda T, Inoue A, Kohyama M, Hattori T, Ohashi H, Nishimura G, Kawamura R, Wakui K, Fukushima Y, Matsumoto N. A new Ehlers-Danlos syndrome with craniofacial characteristics, multiple congenital contractures, progressive joint and skin laxity, and multisystem fragility-related manifestations. Am J Med Genet A. 2010;152A:1333–1346. doi: 10.1002/ajmg.a.33498. [DOI] [PubMed] [Google Scholar]
- Malfait F, Syx D, Vlummens P, Symoens S, Nampoothiri S, Hermanns-Le T, Van LL, De PA. Musculocontractural Ehlers-Danlos syndrome (former EDS type VIB) and adducted thumb clubfoot syndrome (ATCS) represent a single clinical entity caused by mutations in the dermatan-4-sulfotransferase 1 encoding CHST14 gene. Hum Mutat. 2010;31:1233–1239. doi: 10.1002/humu.21355. [DOI] [PubMed] [Google Scholar]
- Miyake N, Kosho T, Mizumoto S, Furuichi T, Hatamochi A, Nagashima Y, Arai E, Takahashi K, Kawamura R, Wakui K, Takahashi J, Kato H, Yasui H, Ishida T, Ohashi H, Nishimura G, Shiina M, Saitsu H, Tsurusaki Y, Doi H, Fukushima Y, Ikegawa S, Yamada S, Sugahara K, Matsumoto N. Loss-of-function mutations of CHST14 in a new type of Ehlers-Danlos syndrome. Hum Mutat. 2010;31:966–974. doi: 10.1002/humu.21300. [DOI] [PubMed] [Google Scholar]
- Peterson-Kendall F, Kendall-McCreary E, Geise-Provance P, McIntyre-Rodgers M, Romani WA. Muscles testing and function with posture and pain. Baltimore, MD: Lipincott Williams & Wilkins; 2005. [Google Scholar]
- Pillen S, Arts IM, Zwarts MJ. Muscle ultrasound in neuromuscular disorders. Muscle Nerve. 2008;37:679–693. doi: 10.1002/mus.21015. [DOI] [PubMed] [Google Scholar]
- Rankin J. Cerebral vascular accidents in patients over the age of 60. III. Diagnosis and treatment. Scott Med J. 1957;2:254–268. [PubMed] [Google Scholar]
- Reardon W. Genitopatellar syndrome: A recognizable phenotype. Am J Med Genet. 2002;111:313–315. doi: 10.1002/ajmg.10590. [DOI] [PubMed] [Google Scholar]
- Shimizu K, Okamoto N, Miyake N, Taira K, Sato Y, Matsuda K, Akimaru N, Ohashi H, Wakui K, Fukushima Y, Matsumoto N, Kosho T. Delineation of dermatan 4-O-sulfotransferase 1 deficient Ehlers-Danlos syndrome: Observation of two additional patients and comprehensive review of 20 reported patients. Am J Med Genet A. 2011;155A:1949–1958. doi: 10.1002/ajmg.a.34115. [DOI] [PubMed] [Google Scholar]
- Thompson RA, Vignos PJ., Jr Serum aldolase in muscle disease. AMA Arch Intern Med. 1959;103:551–564. doi: 10.1001/archinte.1959.00270040037004. [DOI] [PubMed] [Google Scholar]
- van der Ploeg RJ, Fidler V, Oosterhuis HJ. Hand-held myometry: Reference values. J Neurol Neurosurg Psychiatry. 1991;54:244–247. doi: 10.1136/jnnp.54.3.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19:604–607. doi: 10.1161/01.str.19.5.604. [DOI] [PubMed] [Google Scholar]
- Voermans NC, Altenburg TM, Hamel BC, de Haan A, van Engelen BG. Reduced quantitative muscle function in tenascin-X deficient Ehlers-Danlos patients. Neuromuscul Disord. 2007a;17:597–602. doi: 10.1016/j.nmd.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Voermans NC, Jenniskens GJ, Hamel BC, Schalkwijk J, Guicheney P, van Engelen BG. Ehlers-Danlos syndrome due to tenascin-X deficiency: Muscle weakness and contractures support overlap with collagen VI myopathies. Am J Med Genet A. 2007b;143A:2215–2219. doi: 10.1002/ajmg.a.31899. [DOI] [PubMed] [Google Scholar]
- Voermans NC, Bonnemann CG, Huijing PA, Hamel BC, van Kuppevelt TH, de Haan A, Schalkwijk J, van Engelen BG, Jenniskens GJ. Clinical and molecular overlap between myopathies and inherited connective tissue diseases. Neuromuscul Disord. 2008;18:843–856. doi: 10.1016/j.nmd.2008.05.017. [DOI] [PubMed] [Google Scholar]
- Voermans NC, Bonnemann CG, Hamel BC, Jungbluth H, van Engelen BG. Joint hypermobility as a distinctive feature in the differential diagnosis of myopathies. J Neurol. 2009a;256:13–27. doi: 10.1007/s00415-009-0105-1. [DOI] [PubMed] [Google Scholar]
- Voermans NC, Bonnemann CG, Lammens M, van Engelen BG, Hamel BC. Myopathy and polyneuropathy in an adolescent with the kyphoscoliotic type of Ehlers-Danlos syndrome. Am J Med Genet A. 2009b;149A:2311–2316. doi: 10.1002/ajmg.a.32997. [DOI] [PubMed] [Google Scholar]
- Voermans NC, van Alfen N, Pillen S, Lammens M, Schalkwijk J, Zwarts MJ, van Rooij I, Hamel BC, van Engelen BG. Neuromuscular involvement in various types of Ehlers-Danlos syndrome. Ann Neurol. 2009c;65:687–697. doi: 10.1002/ana.21643. [DOI] [PubMed] [Google Scholar]
- Voermans NC, Knoop H, Bleijenberg G, van Engelen BG. Pain in Ehlers-Danlos syndrome is common, severe, and associated with functional impairment. J Pain Symptom Manage. 2010a;40:370–378. doi: 10.1016/j.jpainsymman.2009.12.026. [DOI] [PubMed] [Google Scholar]
- Voermans NC, Knoop H, van de Kamp N, Hamel BC, Bleijenberg G, van Engelen BG. Fatigue is a frequent and clinically relevant problem in Ehlers-Danlos syndrome. Semin Arthritis Rheum. 2010b;40:267–274. doi: 10.1016/j.semarthrit.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Yis U, Dirik E, Chambaz C, Steinmann B, Giunta C. Differential diagnosis of muscular hypotonia in infants: the kyphoscoliotic type of Ehlers-Danlos syndrome (EDS VI) Neuromuscul Disord. 2008;18:210–214. doi: 10.1016/j.nmd.2007.11.006. [DOI] [PubMed] [Google Scholar]