Abstract
Red cell alloimmunization may induce severe hemolytic side effects. Identification of risk-modifying conditions will help tailor preventative strategies. This study aims to quantify the associations of hematologic malignancies and solid cancers with red cell alloimmunization in patients receiving red cell transfusions. We performed a nested multicenter case-control study in a source population of 24,063 patients receiving their first and subsequent red cell transfusions during an 8-year follow-up period. Cases (n=505), defined as patients developing a first transfusion-induced red cell alloantibody, were each compared with 2 non-alloimmunized controls (n=1010) who received a similar number of red cell units. Using multivariate logistic regression analyses, we evaluated the association of various malignancies and treatment regimens with alloimmunization during a delineated 5-week risk period. The incidence of alloimmunization among patients with acute (myeloid or lymphoid) leukemia and mature (B- or T-cell) lymphoma was significantly reduced compared to patients without these malignancies: adjusted relative risks (RR) with 95% confidence interval (CI) 0.36 (range 0.19–0.68) and 0.30 (range 0.12–0.81). Associations were primarily explained by immunosuppressive treatments [RR for (any type of) chemotherapy combined with immunotherapy 0.27 (95%CI: 0.09–0.83)]. Alloimmunization risks were similarly diminished in allogeneic or autologous stem cell transplanted patients (RR 0.34, 95%CI: 0.16–0.74), at least during the six months post transplant. Alloimmunization risks of patients with other hematologic diseases or solid cancers, and their associated treatment regimens were similar to risks in the general transfused population. Our findings suggest that, in contrast to malignancies in general, hemato-oncological patients treated with dose-intensive regimens have strongly diminished risk of red cell alloimmunization.
Introduction
Transfusion of red cells exposes recipients to non-self antigens and, consequently, may induce alloantibody formation. Although prior alloimmunization requires the exclusive administration of donor blood that is negative for the cognate antigen, accidental re-exposure may induce severe hemolytic transfusion reactions.1,2 Prevention of alloimmunization and its consequences is promoted by transfusion of ABO/RhD compatible units to all red cell recipients. In addition, matching beyond those antigens is recommended for certain patients considered to be at high risk of alloimmunization due to repeated exposure, since the number of transfusions is strongly associated with the likelihood of alloimmunization.3–5 As such, in several high-income countries, patients with hemoglobinopathies and with myelodysplastic syndrome (MDS), who often face regular transfusions over long periods of time, receive red cell units matched for the most immunogenic and clinically relevant antigens C, c, E, e, and K.3,4
The ability of the recipient’s immune system to evoke a humoral alloimmune response upon red cell alloantigen exposure is likely modulated by his or her clinical condition.6–8 In this regard, while oncological patients were suggested to have a similar alloimmunization risk to the general transfused population,9–11 some studies reported high incidences of alloimmunization among MDS patients.12,13 Importantly, apart from the study by Sanz et al.,13 these reports did not take into account the cumulative red cell exposure, which in the oncological patient population is often considerable and a main determinant of alloimmunization.5 Therefore, the possible influence of disease-specific features remains to be clarified. In addition, cancer types differ from one another in their intrinsic immunobiological characteristics as well as in the immunosuppressive nature of their treatments. Therefore, alloimmunization rates observed in a heterogeneous oncological patient population cannot be extrapolated to specific diseases.
Here we report the results of a nested case-control study quantifying the associations of various hematologic malignancies and solid cancers with the risk of red cell alloimmunization in a cohort of red cell transfusion recipients.
Methods
Study design and setting
We performed a nested case-control study within a mainly Caucasian source population of patients receiving their first and subsequent red cell transfusion between 2005 and 2013 at one of six Dutch hospitals. All six hospitals treat patients diagnosed with oncological pathologies; treatment includes standard remission-induction chemotherapy for acute leukemia patients. Allogeneic hematopoietic stem cell transplantation (HSCT) is performed at three and autologous HSCT at four of these centers.
Details of the source population, including eligibility criteria, study period per hospital, and the methods adopted have been published previously5,14,15 (see the Online Supplementary Appendix for details).
Briefly, cases were all patients who developed a first transfusion-induced alloantibody against c, C, e, E, K, Cw, Fya, Fyb, Jka, Jkb, Lua, Lub, M, N, S, or s. For all cases, we assumed the last antigen mismatched transfusion preceding the first positive screen (the ‘Nth’ transfusion) to have been likely to elicit alloimmunization and defined this as the implicated transfusion. If, due to incomplete donor typing, this last mismatched transfusion could not be identified, the last non-tested unit preceding the first positive screen was considered as the implicated transfusion. For each case, we then randomly sampled 2 non-alloimmunized controls on the pre-condition that these patients received at least N or more transfusions at the same hospital, hereby following an ‘incidence-density sampling strategy’.16 After marking the Nth transfusion in the 2 matched controls, we subsequently constructed a so-called ‘alloimmunization risk period’ in both the case and the 2 controls, which stretches from 30 days before to seven days after this Nth (implicated) transfusion (Figure 1).15 Next, hospital electronic laboratory information systems and patient medical charts were consulted to record the presence of various clinical conditions during this period.
Figure 1.
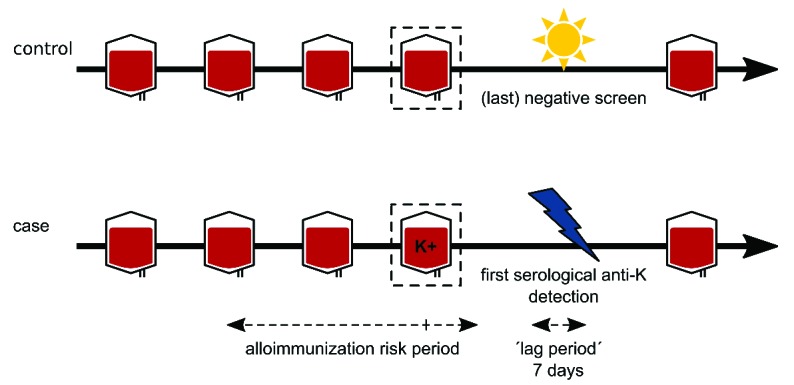
Illustration of the alloimmunization risk period. For each case, the last antigen mismatched transfusion preceding the first serological detection of an alloantibody was defined as the ‘implicated (Nth) transfusion’ since this transfusion most likely triggered alloimmunization. Alloimmunizations within seven days of the first antigen mismatched transfusion were not taken into consideration as these most likely represented boosting rather than primary alloimmunizations. An alloimmunization risk period was then constructed starting 30 days before and finishing seven days after the defined implicated transfusion. Subsequently, for each case, 2 controls who received at least the same number of red cell units were randomly selected and a similar alloimmunization risk period was constructed around the Nth transfusion. In this example, as the fourth red cell unit most likely elicited red cell alloimmunization, the alloimmunization risk period in both the case and control was constructed around the fourth transfusion. Figure adapted from: Evers et al.15
The study protocol was approved by the Ethical Review Board in Leiden and by the board of each participating center.
Malignancies and their treatments
We used internationally approved response criteria to define the remission state of various hematologic malignancies.17–21 Malignancies in complete remission during the alloimmunization risk period were considered as absent. The presence of minimal residual disease was not taken into account. All medication under subcategory L01 in the World Health Organization’s Anatomic Therapeutic Chemical (ATC) classification index22 was defined as chemotherapy, with the exception of agents in the pharmacological subgroup L01XC, as these involve monoclonal antibodies. Within subgroups L01XC and L04AA, we defined rituximab, alemtuzumab, and rabbit- or horse-derived anti-thymocyte globulin (ATG) as anti-lymphocyte immunotherapy.
Statistical analysis
Multiple imputation was used to account for missing data. Potential confounders were identified on the basis of their association with the assessed determinant among the source population (i.e. the non-alloimmunized controls).
Using multivariate logistic regression analyses conditioning on the matched variables and on the identified potential confounders, we evaluated the associations of various hematologic malignancies and solid cancers, treatment modalities, and degree of leukopenia with the development of red cell alloimmunization.
As we used an incidence-density sampling procedure to select controls,16 all odds ratios are presented as relative risks (RRs).23,24
Further details on the statistical analytical methods adopted are provided in the Online Supplementary Appendix.
Results
Among 54,347 newly-transfused patients, 24,063 met all study criteria. The majority of excluded patients were ineligible due to the absence of an antibody screen following a single transfusion episode (n=25,037).
First-formed red cell alloantibodies were identified in 505 patients (2.1%) (Online Supplementary Table S1). Thirty-seven of those patients (7.3%), including 21 of 32 (65.6%) who formed anti-Lua, only received units for which testing of the cognate antigen had not been performed; we assumed the last non-tested unit preceding the first positive screen to have elicited alloimmunization.
General and clinical characteristics of the 505 alloimmunized patients and their 1010 matched control subjects are presented in Online Supplementary Tables S1 and S2.
Malignancies present during the alloimmunization risk period
A total of 606 patients (40.0%) had at least one type of malignancy (270 had a hematologic malignancy and 338 a solid tumor; 2 patients presented with both types of malignancies). Online Supplementary Table S3 presents types and subtypes of malignancies.
The presence of a malignancy could not be confirmed for 12 patients: 4 patients with a clinical condition suspected for a malignancy that was not further evaluated, 4 patients with a suspected malignancy in whom a malignancy was later confirmed, and 4 patients receiving treatment for a solid tumor for whom the remission status at the time of the risk period was unclear. These 12 patients were not included in the corresponding analyses.
Online Supplementary Tables S4 and S5 show identified confounders for each type of malignancy. Control patients with acute leukemia and lymphoma, as compared to control patients without these diseases, were younger and had less comorbidity (including renal insufficiency and presence of other malignancies). They more frequently received chemotherapy and immunosuppressant medication and more frequently had decreased leukocyte counts.
Maximum frequency of missing data per identified confounder was 2.7% (Online Supplementary Table S6).
The association between types of malignancies and red cell alloimmunization
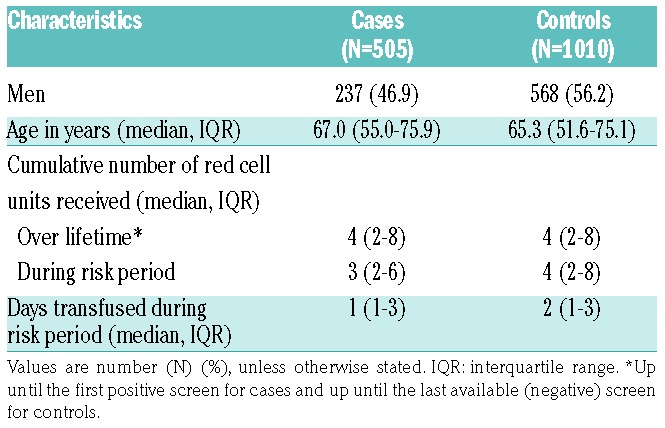
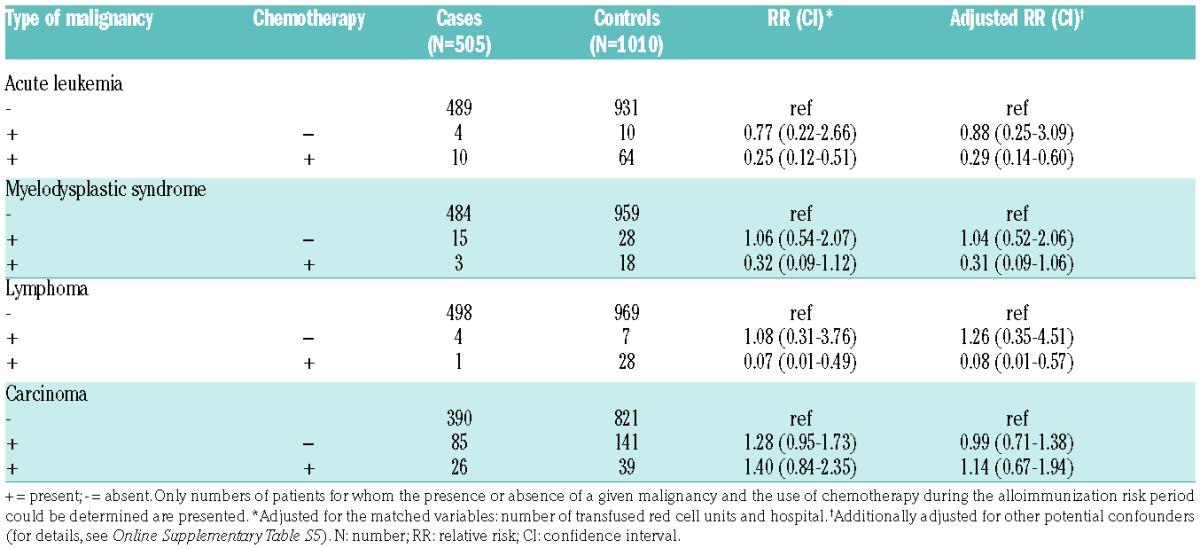
Table 2 presents the number of cases and controls according to various types of malignancies. Acute leukemia was present in 14 cases (2.8%) compared to 74 (7.3%) controls. There was a reduced incidence of red cell alloimmunization in patients with acute (myeloid or lymphoblastic) leukemia and in patients with mature (B- or T-cell) lymphoma [adjusted RR 0.36 (95%CI: 0.19–0.68) and 0.30 (95%CI: 0.12–0.81), respectively]. Conversely, patients with chronic lymphocytic leukemia (CLL) showed a modest, albeit statistically non-significant, increased risk [adjusted RR 1.20 (95%CI: 0.36–3.93)]. No association between the other types of malignancies and red cell alloimmunization was observed, including MDS and solid malignancies. Similarly, subtypes of solid tumors were not associated to red cell alloimmunization, although some RRs presented with wide 95% CIs (Online Supplementary Table S7). As extensive matching recommendations have only been introduced in the Netherlands since 2011,3 only one of 64 patients (1.6%) with MDS received CcEe- and K-matched units.
Table 2.
Association between various malignancies and red cell alloimmunization.
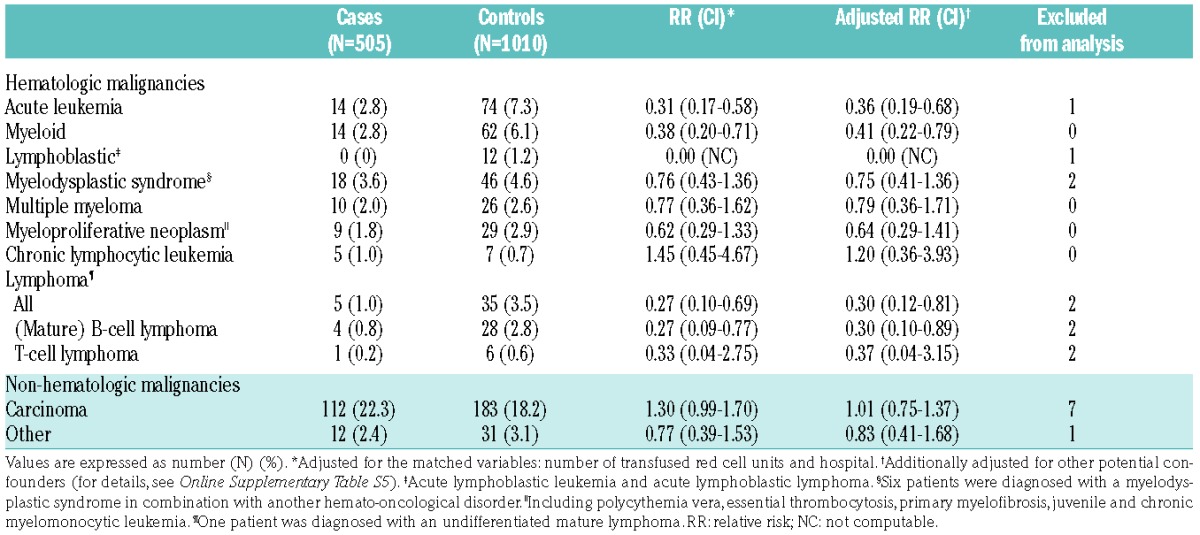
Table 1.
Patients’ characteristics during the alloimmunization risk period.
Effects were similar in all six hospitals (data not shown).
The association between treatment modalities and red cell alloimmunization
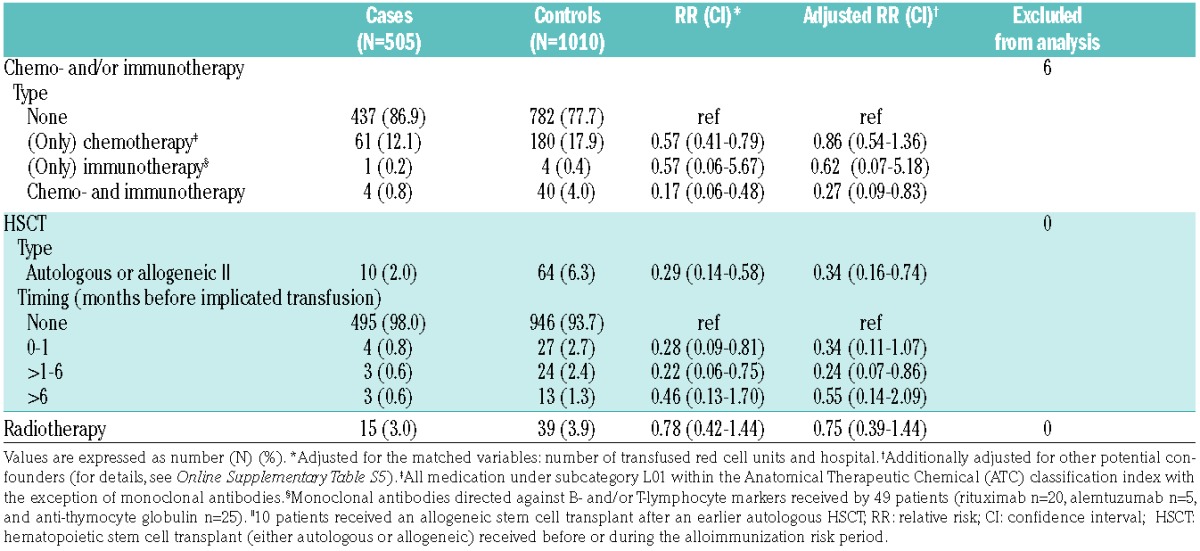
A total of 290 patients received chemo- and/or (anti-lymphocyte) immunotherapy during the implicated risk period. Use of any type of chemotherapy without immunotherapy was not associated with red cell alloimmunization. However, when regimens included lymphocyte-targeted monoclonal antibodies the adjusted RR was 0.27 (95%CI: 0.09–0.83) (Table 3). Twenty-five of the 49 patients (51%) treated with monoclonal antibodies received ATG (with or without alemtuzumab) for in vivo depletion of T cells in the context of an allogeneic HSCT (n=21), aplastic anemia (n=3), or combined pancreas-kidney organ transplant (n=1).
Table 3.
Treatment modalities and red cell alloimmunization risks.
Patients receiving chemotherapeutic agents for acute leukemia or lymphoma during the implicated risk period had substantially reduced alloimmunization incidences [RR 0.29 (95%CI: 0.14–0.60) and 0.08 (95%CI: 0.01–0.57), respectively]. This reduction in risk did not seem to be further influenced to any great extent by the time interval between the initial diagnosis and the period of risk (data not shown). In contrast, non-treated patients with these disorders demonstrated risks comparable to the remainder of the patient population (Table 4). Sixty-two of the 74 treated patients (84%) with acute leukemia received induction therapy during the alloimmunization risk period. Analogous to acute leukemia and mature lymphoma, the 22 patients who received treatment for MDS (including 13 patients receiving induction therapy and 7 receiving hypomethylating agents), demonstrated a trend towards reduced alloimmunization incidences [RR 0.31 (95%CI: 0.09–1.06)] (Table 4). Chemotherapy did not have any impact on risks in patients with other types of hematologic malignancies or carcinoma (Table 4 and Online Supplementary Table S8).
Table 4.
Chemotherapy and red cell alloimmunization risks.
A total of 54 patients received radiotherapy (of any dose and frequency), including 10 patients who received total body irradiation in the setting of an allogeneic HSCT. Radiotherapy was not associated with red cell alloimmunization (Table 3).
Respectively 51, 13, and 10 patients underwent an allogeneic HSCT, an autologous HSCT, or both before or during the risk period. In 51 patients, a reduced-intensity allogeneic HSCT conditioning regimen was followed (including 8 patients who received a double cord transplant), while 10 patients received a myeloablative conditioning regimen. Alloimmunization incidences were substantially decreased in these allogeneic or autologous stem cell transplant recipients [RR 0.34, (95%CI: 0.16–0.74)], at least during the first six months after transplant (Table 3). There was no difference in alloimmunization risk between recipients of an autologous or allogeneic HSCT (data not shown).
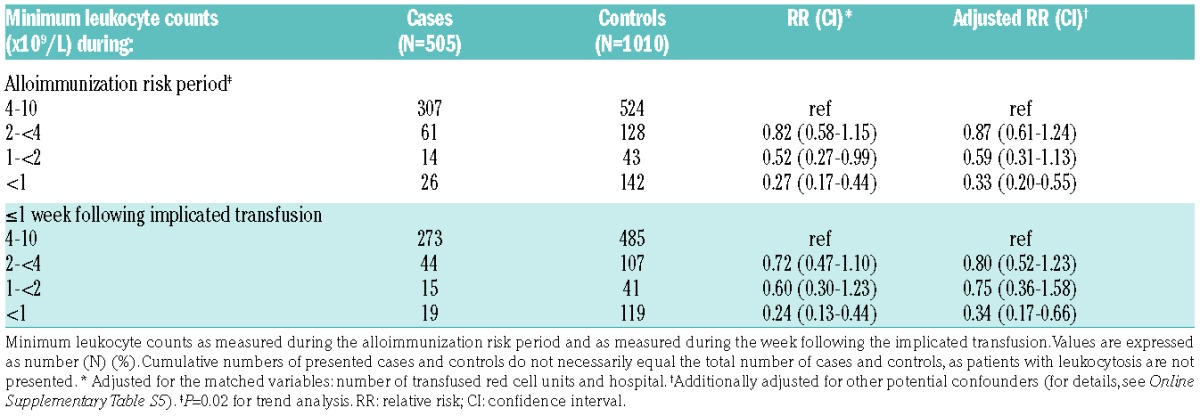
Finally, the degree of leukopenia was strongly associated with diminished red cell alloimmunization (Table 5). Here, patients with leukocyte counts of less than 1.0×109/L demonstrated an adjusted RR of 0.33 (95%CI: 0.20–0.55). Similar results were obtained when we restricted these analyses to leukocyte counts determined within the week following the implicated transfusion (Table 5). The degree of leukopenia was associated with the type of malignancy and whether or not the patient received chemotherapy. In this regard, minimum leukocyte counts of less than 1.0×109/L were observed respectively in 66.2%, 75.9%, and 13.8% of patients with acute leukemia, lymphoma, and carcinoma receiving chemotherapy during the risk period (P<0.0001 for carcinoma vs. acute leukemia and for carcinoma vs. lymphoma).
Table 5.
Leukopenia and red cell alloimmunization risks.
Discussion
In this nested case-control study, we evaluated whether patients diagnosed with hematologic malignancies and solid cancers differed from the general transfused patient population with regards to the risk of forming red cell alloantibodies. Patients treated for acute leukemia (of either myeloid or lymphoblastic origin) and patients with mature (B- or T-cell) lymphomas demonstrated a 3-fold decrease in the incidence of clinically relevant alloantibodies against red cell alloantigens. In contrast, the alloimmunization incidence among patients treated for other hematologic malignancies or solid tumors was similar to those among the non-malignant patient population.
Although earlier reports only observed similar or even increased red cell alloimmunization frequencies in the oncological patient population,9–11 these prevalence-based studies did not adjust for the substantial number of transfusions these patients usually receive. However, it is well known that the cumulative transfusion dose is an important determinant of alloimmunization.5 Consequently, the observed positive associations might have been due to the quite intensive red cell transfusion support that is generally needed in the treatment of certain malignancies rather than to disease-specific characteristics. So far, no studies have compared specific oncological diseases for alloimmunization risks.
Our findings suggest that especially the dose-intensive immunosuppressive therapy influences alloimmunization. This seems biologically plausible. Several classical cytotoxic agents frequently used in the treatment of acute leukemia and lymphoma, including cyclophosphamide, purine nucleoside analogs, and anthracyclines, are known to induce prolonged (mainly naïve) CD4+ T-cell and B-cell depletion.25–28 Moreover, chemotherapeutic regimens often include corticosteroids, a class of immunosuppressants which we earlier reported to protect against red cell alloimmunization.8 Significantly reduced incidence of red cell alloimmunization was also found in patients receiving anti-lymphocyte targeted agents (i.e. ATG, alemtuzumab, and rituximab). ATG is well known for its strong and prolonged T-cell depleting effects.29,30 In addition, ATG preparations contain antibodies against several B- and even plasma cell-specific markers.30,31 In agreement with this, eradication of B cells by rituximab has been shown to coincide with impaired primary as well as re-call vaccine responses.32–35 Finally, we observed profoundly lower alloimmunization rates in the setting of HSCT, either autologous or allogeneic, which appeared to be sustained at least during the first six months after transplant. Even though we cannot fully exclude the possibility that the 8 cases of alloimmunization following an allogeneic HSCT could have been elicited by donor-recipient red cell antigen mismatches (in addition to exposure via transfusion), these findings are consistent with previous studies reporting anti-D formation to be rare in RhD-negative HSCT recipients exposed to RhD.36–38 Reconstitution of adaptive immune cells generally takes up to 6–12 months following HSCT,39–44 depending on age-associated thymic functioning, type of stem cell harvest, and intensity of T-cell depletion strategies, while humoral immunity may continue to be deficient even after several years.45,46
Although treatment-induced immunosuppression seems to be the principle explanation for our observations, other non-measured factors associated with receiving treatment (e.g. co-morbidities and disease stage) might have interacted with disease-specific effects on the immune response. Therefore, we cannot exclude the possibility that part of the observed effects could be directly related to the diseases themselves, i.e. induction of an immunosuppressive but tumor tolerant state via host immune evasion mechanisms of malignant cells.47–50
Furthermore, as patients received a wide range of different chemotherapeutic regimens at varying times before the alloimmunization risk period, it is not possible to come to any firm conclusions as to whether or to what extent patients in complete remission of their treated malignancy should be considered to be significantly immunosuppressed. As such, our RRs might underestimate true effects and our results do not preclude the possibility that these patients have a diminished red cell alloimmunization risk.
In contrast to some other studies,12,13 our incidence-based analysis did not demonstrate an enhanced alloimmunization susceptibility with a diagnosis of MDS. However, and similar to intensively treated patients with acute leukemia and mature lymphoma, patients who received treatment for their MDS tended to show incidence of reduced alloimmunization. Consequently, the decision to transfuse extended donor-matched products to this patient population should not be based on the MDS diagnosis itself, but on other factors associated to an increased alloimmune response, e.g. a high transfusion burden.
Finally, the alloimmunization RR in patients with chronic lymphocytic leukemia (CLL) independent of their treatment seemed to be increased compared to lymphoma patients, although we acknowledge that the number of CLL patients in the current study is not sufficient to con firm such a hypothesis. However, CLL is characterized by profound immune disturbances including non-clonal formation of IgG auto-antibodies directed against blood cell antigens.51–53 Observations seem to suggest that the disease disturbs normal regulatory potential. Seemingly in contrast with these findings, antimicrobial vaccination responses are often compromised in CLL patients.54
Some final comments regarding our methods are appropriate. First, the use of an incidence-density sampling strategy guaranteed that controls were exposed to at least the same number of red cell units as their matched cases.16,55 Given this adjustment for cumulative number of red cell exposures, our RRs reflect relative risks independent of exposures. Our alloimmunization risk period was defined specifically to provide a comprehensive study of the influential effect of conditions present around the time of red cell exposure. As the immunosuppressive effects of various treatment regimens are slow to wear off, we preferred to use a relatively long period of risk to precede the implicated transfusion.
Second, our strategies do not fully guarantee the exclusion of all boosting events. Actual ‘lag periods’, i.e. the time needed before antibody levels become detectable after primary antigen encounter, are currently unknown and may even differ according to the antigen used. Regarding our chosen lag period of seven days, we cannot, therefore, fully exclude the possibility that our study included patients whose antibody titers became undetectable over time and who quickly demonstrated re-call responses upon re-exposure to the alloantigen. However, we had thought that a substantial amount of boosting reactions as primary alloimmunization events would have biased our RRs towards the null-effect. However, a sensitivity analysis, in which we excluded the 53 patients in whom alloantibodies were discovered during the second week after their first antigen-incompatible transfusion, showed no change in RRs (data not shown). We are confident, therefore, that any possible bias deriving from our choice of lag period is small.
Third, we observed no associations with red cell alloimmunization other than the above mentioned hematologic malignancies and specific types of solid malignancies, although the low numbers of some of these subgroups and the consequent wide CIs per RR prevent any firm conclusions to be made. A much larger study or a meta-analysis of similar studies is needed to assess whether these malignancies are indeed not associated to red cell alloimmunization. Also, due to the fact that remission evaluations available during the alloimmunization risk period were not always complete, we were unable to assess whether the disease stage itself is associated to cell alloimmunization. Finally, since patients treated with chemotherapy received a wide range of chemotherapeutic agents and combinations, as well as varying dose intensities, we were not able to quantify risks according to each single agent.
In conclusion, risks associated with red cell alloimmunization are significantly reduced in patients treated for acute leukemia and mature lymphomas, as well as in recipients of an autologous or allogeneic HSCT. These diminished immune responses most likely reflect the intensity of treatment-associated immunosuppression. In contrast, alloimmunization risks in patients with other hematologic diseases and in patients with solid cancers are similar to those in the general, non-oncological transfused patient population. These findings clearly indicate that, in addition to cumulative red cell exposure, disease-specific conditions should be taken into account when considering the risk of red cell alloimmunization in order to select those patients who would most benefit from extended matched red cell transfusions.
Supplementary Material
Acknowledgments
The authors would like to thank André Ringeling (UMC Utrecht, Utrecht), Ruud van Woensel (Catharina Hospital, Eindhoven), Leo van den Boogaard (Catharina Hospital, Eindhoven), Mai Lie Tjoa (VUMC, Amsterdam), Nel Som (VUMC, Amsterdam), Ton Wolfhagen (Jeroen Bosch Hospital, ‘s Hertogenbosch), Eugenie Gemen (Jeroen Bosch Hospital, ‘s Hertogenbosch), and Gerard Smouter (LabWest, The Hague) who all were very supportive regarding the data collection.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/102/1/52
References
- 1.TRIP Hemovigilance report 2014. Available at: https://www.tripnet.nl/pages/nl/documents/TRIP2014Hemovigilantie.pdf.
- 2.SHOT-report 2014. Available at: http://www.shotuk.org/shotreports/report-summary-supplement-2014.
- 3.CBO Blood Transfusion Guideline 2011. English version accessible at: http://www.sanquin.nl/repository/documenten/en/prod-en-dienst/287294/blood-transfusion-guideline.pdf.
- 4.Handbook of Transfusion Medicine, United Kingdom Blood Services, 5th edition, 2013. Available at: http://www.transfusionguide-lines.org.uk/transfusion-handbook.
- 5.Evers D, Middelburg RA, de Haas M, et al. Red-blood-cell alloimmunisation in relation to antigens’ exposure and their immunogenicity: a cohort study. Lancet Haematol. 2016;3(6):e284–292. [DOI] [PubMed] [Google Scholar]
- 6.Bauer MP, Wiersum-Osselton J, Schipperus M, Vandenbroucke JP, Briet E. Clinical predictors of alloimmunization after red blood cell transfusion. Transfusion. 2007; 47(11):2066–2071. [DOI] [PubMed] [Google Scholar]
- 7.Fasano RM, Booth GS, Miles M, et al. Red blood cell alloimmunization is influenced by recipient inflammatory state at time of transfusion in patients with sickle cell disease. Br J Haematol. 2015; 168(2):291–300. [DOI] [PubMed] [Google Scholar]
- 8.Zalpuri S, Evers D, Zwaginga JJ, et al. Immunosuppressants and alloimmunization against red blood cell transfusions. Transfusion. 2014;54(8):1981–1987. [DOI] [PubMed] [Google Scholar]
- 9.Arriaga F, Bonanad S, Larrea L, et al. Immunohematologic study in 112 patients with myelodysplastic syndromes: 10-year analysis. Sangre (Barc). 1995; 40(3):177–180. [PubMed] [Google Scholar]
- 10.Schonewille H, Haak HL, van Zijl AM. Alloimmunization after blood transfusion in patients with hematologic and oncologic diseases. Transfusion. 1999;39(7):763–771. [DOI] [PubMed] [Google Scholar]
- 11.Stiegler G, Sperr W, Lorber C, Fabrizii V, Hocker P, Panzer S. Red cell antibodies in frequently transfused patients with myelodysplastic syndrome. Ann Hematol. 2001;80(6):330–333. [DOI] [PubMed] [Google Scholar]
- 12.Novaretti MC, Sopelete CR, Velloso ER, Rosa MF, Dorlhiac-Llacer PE, Chamone DA. Immunohematological findings in myelodysplastic syndrome. Acta Haematol. 2001;105(1):1–6. [DOI] [PubMed] [Google Scholar]
- 13.Sanz C, Nomdedeu M, Belkaid M, Martinez I, Nomdedeu B, Pereira A. Red blood cell alloimmunization in transfused patients with myelodysplastic syndrome or chronic myelomonocytic leukemia. Transfusion. 2013;53(4):710–715. [DOI] [PubMed] [Google Scholar]
- 14.Zalpuri S, Zwaginga JJ, van der Bom JG. Risk Factors for Alloimmunisation after red blood Cell Transfusions (R-FACT): a case cohort study. BMJ Open. 2012;2:e001150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evers D, van der Bom JG, Tijmensen J, et al. Red cell alloimmunisation in patients with different types of infections. Br J Haematol. 2016. August 18 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rothman Kenneth J. Modern Epidemiology. Philadelphia, Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 17.Alvarnas JC, Brown PA, Aoun P, et al. Acute lymphoblastic leukemia. J Natl Compr Canc Netw. 2012;10(7):858–914. [DOI] [PubMed] [Google Scholar]
- 18.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21(24):4642–4649. [DOI] [PubMed] [Google Scholar]
- 19.Cheson BD, Greenberg PL, Bennett JM, et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood. 2006;108(2):419–425. [DOI] [PubMed] [Google Scholar]
- 20.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25(5):579–586. [DOI] [PubMed] [Google Scholar]
- 21.Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008; 111(12):5446–5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.WHO Collaborating Centre for Drug Statistics Methodology, Anatomic Therapeutic Chemical index 2016. Available at: http://www.whocc.no/atc_ddd_index.
- 23.Knol MJ, Vandenbroucke JP, Scott P, Egger M. What do case-control studies estimate? Survey of methods and assumptions in published case-control research. Am J Epidemiol. 2008;168(9):1073–1081. [DOI] [PubMed] [Google Scholar]
- 24.Miettinen O. Estimability and estimation in case-referent studies. Am J Epidemiol. 1976;103(2):226–235. [DOI] [PubMed] [Google Scholar]
- 25.Ferraro C, Quemeneur L, Prigent AF, Taverne C, Revillard JP, Bonnefoy-Berard N. Anthracyclines trigger apoptosis of both G0–G1 and cycling peripheral blood lymphocytes and induce massive deletion of mature T and B cells. Cancer Res. 2000; 60(7):1901–1907. [PubMed] [Google Scholar]
- 26.Gafter-Gvili A, Polliack A. Bendamustine associated immune suppression and infections during therapy of hematological malignancies. Leuk Lymphoma. 2016; 57(3):512–519-8. [DOI] [PubMed] [Google Scholar]
- 27.Mackall CL, Fleisher TA, Brown MR, et al. Lymphocyte depletion during treatment with intensive chemotherapy for cancer. Blood. 1994;84(7):2221–2228. [PubMed] [Google Scholar]
- 28.Tolaney SM, Najita J, Winer EP, Burstein HJ. Lymphopenia associated with adjuvant anthracycline/taxane regimens. Clin Breast Cancer. 2008;8(4):352–356. [DOI] [PubMed] [Google Scholar]
- 29.Remberger M, Sundberg B. Rabbit-immunoglobulin G levels in patients receiving thymoglobulin as part of conditioning before unrelated donor stem cell transplantation. Haematologica. 2005; 90(7):931–938. [PubMed] [Google Scholar]
- 30.Mohty M. Mechanisms of action of antithymocyte globulin: T-cell depletion and beyond. Leukemia. 2007;21(7):1387–1394. [DOI] [PubMed] [Google Scholar]
- 31.Zand MS, Vo T, Huggins J, et al. Polyclonal rabbit antithymocyte globulin triggers B-cell and plasma cell apoptosis by multiple pathways. Transplantation. 2005; 79(11):1507–1515. [DOI] [PubMed] [Google Scholar]
- 32.Bedognetti D, Zoppoli G, Massucco C, et al. Impaired response to influenza vaccine associated with persistent memory B cell depletion in non-Hodgkin’s lymphoma patients treated with rituximab-containing regimens. J Immunol. 2011;186(10):6044–6055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cha Z, Li C, Zang Y, et al. Adaptive B cell responses in rituximab-treated diffuse large B cell lymphoma patients during complete remission. Tumour Biol. 2015;37(1):829–835. [DOI] [PubMed] [Google Scholar]
- 34.van der Kolk LE, Baars JW, Prins MH, van Oers MH. Rituximab treatment results in impaired secondary humoral immune responsiveness. Blood. 2002;100(6):2257–2259. [PubMed] [Google Scholar]
- 35.Yri OE, Torfoss D, Hungnes O, et al. Rituximab blocks protective serologic response to influenza A (H1N1) 2009 vaccination in lymphoma patients during or within 6 months after treatment. Blood. 2011;118(26):6769–6771. [DOI] [PubMed] [Google Scholar]
- 36.Asfour M, Narvios A, Lichtiger B. Transfusion of RhD-incompatible blood components in RhD-negative blood marrow transplant recipients. MedGenMed. 2004; 6(3):22. [PMC free article] [PubMed] [Google Scholar]
- 37.Cid J, Lozano M, Fernandez-Aviles F, et al. Anti-D alloimmunization after D-mismatched allogeneic hematopoietic stem cell transplantation in patients with hematologic diseases. Transfusion. 2006;46(2):169–173. [DOI] [PubMed] [Google Scholar]
- 38.Mijovic A. Alloimmunization to RhD antigen in RhD-incompatible haemopoietic cell transplants with non-myeloablative conditioning. Vox Sang. 2002;83(4):358–362. [DOI] [PubMed] [Google Scholar]
- 39.Booth C, Lawson S, Veys P. The current role of T cell depletion in paediatric stem cell transplantation. Br J Haematol. 2013; 162(2):177–190. [DOI] [PubMed] [Google Scholar]
- 40.Mackall CL, Fleisher TA, Brown MR, et al. Age, thymopoiesis, and CD4+ T-lymphocyte regeneration after intensive chemotherapy. N Engl J Med. 1995; 332(3):143–149. [DOI] [PubMed] [Google Scholar]
- 41.Mackall CL, Stein D, Fleisher TA, et al. Prolonged CD4 depletion after sequential autologous peripheral blood progenitor cell infusions in children and young adults. Blood. 2000;96(2):754–762. [PubMed] [Google Scholar]
- 42.Oshrine BR, Li Y, Teachey DT, Heimall J, Barrett DM, Bunin N. Immunologic recovery in children after alternative donor allogeneic transplantation for hematologic malignancies: comparison of recipients of partially T cell-depleted peripheral blood stem cells and umbilical cord blood. Biol Blood Marrow Transplant. 2013;19(11): 1581–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Storek J, Witherspoon RP, Storb R. T cell reconstitution after bone marrow transplantation into adult patients does not resemble T cell development in early life. Bone Marrow Transplant. 1995;16(3):413–425. [PubMed] [Google Scholar]
- 44.Storek J, Dawson MA, Storer B, et al. Immune reconstitution after allogeneic marrow transplantation compared with blood stem cell transplantation. Blood. 2001;97(11):3380–3389. [DOI] [PubMed] [Google Scholar]
- 45.Korholz D, Kunst D, Hempel L, et al. Humoral immunodeficiency in patients after bone marrow transplantation. Bone Marrow Transplant. 1996;18(6):1123–1130. [PubMed] [Google Scholar]
- 46.Storek J, Witherspoon RP, Storb R. Reconstitution of membrane IgD− (mIgD−) B cells after marrow transplantation lags behind the reconstitution of mIgD+ B cells. Blood. 1997;89(1):350–351. [PubMed] [Google Scholar]
- 47.Chow MT, Moller A, Smyth MJ. Inflammation and immune surveillance in cancer. Semin Cancer Biol. 2012;22(1):23–32. [DOI] [PubMed] [Google Scholar]
- 48.Meirow Y, Kanterman J, Baniyash M. Paving the Road to Tumor Development and Spreading: Myeloid-Derived Suppressor Cells are Ruling the Fate. Front Immunol. 2015;6:523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Upadhyay R, Hammerich L, Peng P, Brown B, Merad M, Brody JD. Lymphoma: immune evasion strategies. Cancers. 2015; 7(2):736–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6(4):295–307. [DOI] [PubMed] [Google Scholar]
- 51.Forconi F, Moss P. Perturbation of the normal immune system in patients with CLL. Blood. 2015;126(5):573–581. [DOI] [PubMed] [Google Scholar]
- 52.Hodgson K, Ferrer G, Pereira A, Moreno C, Montserrat E. Autoimmune cytopenia in chronic lymphocytic leukaemia: diagnosis and treatment. Br J Haematol. 2011; 154(1):14–22. [DOI] [PubMed] [Google Scholar]
- 53.Visco C, Barcellini W, Maura F, Neri A, Cortelezzi A, Rodeghiero F. Autoimmune cytopenias in chronic lymphocytic leukemia. Am J Hematol. 2014; 89(11):1055–1062. [DOI] [PubMed] [Google Scholar]
- 54.Sinisalo M, Aittoniemi J, Kayhty H, Vilpo J. Vaccination against infections in chronic lymphocytic leukemia. Leuk Lymphoma. 2003;44(4):649–652. [DOI] [PubMed] [Google Scholar]
- 55.Middelburg RA, Wiersum-Osselton JC, van de Watering LM, van der Bom JG. Observational etiologic research: part 3-case-control studies: it’s all about the source population. Transfusion. 2014; 54(1):12–16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


