Abstract
The myeloproliferative neoplasms, including polycythemia vera, essential thrombocythemia and myelofibrosis, are distinguished by their debilitating symptom profiles, life-threatening complications and profound impact on quality of life. The role gender plays in the symptomatology of myeloproliferative neoplasms remains under-investigated. In this study we evaluated how gender relates to patients’ characteristics, disease complications and overall symptom expression. A total of 2,006 patients (polycythemia vera=711, essential thrombocythemia=830, myelofibrosis=460, unknown=5) were prospectively evaluated, with patients completing the Myeloproliferative Neoplasm-Symptom Assessment Form and Brief Fatigue Inventory Patient Reported Outcome tools. Information on the individual patients’ characteristics, disease complications and laboratory data was collected. Consistent with known literature, most female patients were more likely to have essential thrombocythemia (48.6% versus 33.0%; P<0.001) and most male patients were more likely to have polycythemia vera (41.8% versus 30.3%; P<0.001). The rate of thrombocytopenia was higher among males than females (13.9% versus 8.2%; P<0.001) and males also had greater red-blood cell transfusion requirements (7.3% versus 4.9%; P=0.02) with shorter mean disease duration (6.4 versus 7.2 years, P=0.03). Despite there being no statistical differences in risk scores, receipt of most therapies or prior complications (hemorrhage, thrombosis), females had more severe and more frequent symptoms for most individual symptoms, along with overall total symptom score (22.8 versus 20.3; P<0.001). Females had particularly high scores for abdominal-related symptoms (abdominal pain/discomfort) and microvascular symptoms (headache, fatigue, insomnia, concentration difficulties, dizziness; all P<0.01). Despite complaining of more severe symptom burden, females had similar quality of life scores to those of males. The results of this study suggest that gender contributes to the heterogeneity of myeloproliferative neoplasms by influencing phenotypic profiles and symptom expression.
Introduction
The myeloproliferative neoplasms (MPN) have a reputation for molecular complexity, clinical heterogeneity and profound impact on duration and quality of life. Polycythemia vera (PV), essential thrombocythemia (ET) and myelofibrosis (MF) are debilitating MPN associated with arterial and venous thrombosis, cytopenias, marked splenomegaly, persistent constitutional symptoms and a predilection for transformation into acute myelogenous leukemia or MF (in ET and PV).
There is emerging interest in understanding how gender affects the development of MPN as well as the manifestations and progression of the disease. As exemplified by the higher prevalence of females with ET and males with PV, it has long been recognized that males and females may be affected differently. However, recent literature supports the potential for gender to influence genotypic expression and, potentially, clonal expansion. For example, an investigation of gene expression in circulating CD34+ cells from 19 JAK2V617F-positive PV patients found that fewer genes were differentially expressed in females (235 genes) than in males (571 genes), but that more than three times as many molecular pathways were activated in females.1 Females also have dramatically lower JAK2V617F allele burdens.2,3 Furthermore, it has been shown that there are female-dominant MPN clusters (both PV and ET) typified by a high prevalence of laboratory abnormalities and sexuality-related complaints.4
Despite these new insights, little is known about how gender relates to symptom profiles. The timely development of MPN-specific Patient Reported Outcome (PRO) tools has allowed us to objectively quantify MPN symptom burden and evaluate the impact of this disease on quality of life. The Myelofibrosis Symptom Assessment Form (MF-SAF), Myeloproliferative Neoplasm Symptom Assessment Form (MPN-SAF) and MPN-10 have been applied in both clinical and trial settings, yielding significant insights into how observed clinical and symptomatic heterogeneity may, in fact, follow predictable patterns and/or harbor otherwise unrecognized associations. In this study, we examine associations between gender and patients’ symptomatology, along with disease features, laboratory abnormalities and overall quality of life.
Methods
Survey development and collection
This study was approved by the Mayo Clinic Institutional Review Board. Data were collected from an international cohort of patients with MPN including ET, PV and MF. All patients were recruited using the methods described previously during the validation of the MPN-SAF.5 The development and validation of the MPN-SAF PRO are described in the Online Supplementary Appendix. The language translation process is also detailed in the Online Supplementary Appendix and was based on standard PRO translation methods.6 In addition to the MPN-SAF, subjects also completed the Brief Fatigue Inventory (BFI).7 Data were collected in various languages: English, Dutch, Italian, French, German, Chinese, Swedish and Spanish. Gender was recorded based on patients’ self-reporting under the question of ‘sex’ with respondent options of ‘male’ or ‘female’. Evaluation of cultural/regional variations in symptom expression involved comparisons of Chinese patients with ‘Western’ patients, who were predominantly Caucasian individuals from western Europe and the USA.
Symptom evaluation
Symptoms listed in the MPN-SAF included the patient’s perceptions of common MPN-related symptoms and overall quality of life on a scale from 0 (absent) to 10 (worst imaginable). The symptoms assessed included items related to sadness, quality of life, inactivity, concentration problems, abdominal pain/discomfort, dizziness, insomnia, night sweats, worst fatigue, early satiety, bone pain, numbness, cough, itching, headache, fever and weight loss. Total symptom score was computed based on ten symptom items. For individuals completing at least six of the ten MPN-SAF total symptom score items, the survey was scored by multiplying the average score across items by ten to obtain a scaled score from 0 to 100.
Prognostic scoring
A prognostic score for ET was calculated using the International Prognostic Scoring for Essential Thrombocythemia (IPSET) system.8 This scoring system, which includes the variables of leukocyte count ≥11×109/L (1 point), age ≥60 years (2 points), and history of thrombosis (1 point), was used to stratify patients into different risk groups: low risk (0 points), intermediate risk (1–2 points) and high risk (3–4 points).
The prognostic score for survival of patients with PV was calculated using the Leukemia 2013 prognostic scoring model.9 This scoring system includes the variables of age ≥67 years(5 points), age 57–66 years (2 points), prior thrombosis (1 point) and leukocyte count ≥15×109/L (1 point) to stratify patients into low-risk (0 points), intermediate risk (1–2 points) and high-risk (≥3 points) groups.
The prognostic score for survival in patients with MF was calculated using the Dynamic International Prognostic Scoring System (DIPSS).10 This scoring model includes the variables of hemoglobin <10 g/dL (2 points), age ≥65 years (1 point), white blood cell count ≥25×109/L (1 point), the presence of constitutional symptoms (1 point) and ≥1% blasts (1 point) to stratify patients into low-risk (0 points), intermediate-1-risk (1–2 points), intermediate-2-risk (3–4 points) and high-risk (>4 points) groups.
Statistical analysis
All comparisons of patients’ symptoms were adjusted for type of MPN and age. Continuous variables were compared using analysis of variance and dichotomous data were compared using the chi-square test. Statistical significance was set at P<0.05. SAS version 9.3 (Cary, NC, USA) was used for the analyses.
Results
Patients’ demographics
A total of 2,006 patients (917 males, 1,089 females) with MPN completed the MPN-SAF and BFI (Table 1). MPN subtypes included PV (n=711), ET (n=830) and MF [n=460; primary MF (68.3%); post-ET MF (18%); post-PV MF (13.7%)]. Patients were of the expected age (mean 59.9 years; range, 15–94) for their disorders and consisted primarily of Chinese (27.1%) and French (23.0%) speakers. When separated by DIPSS risk categories, most MF patients were stratified as intermediate-1 risk (54.5%), followed by intermediate-2 risk (24.3%), low risk (18.3%) and high risk (3%). Most ET patients were stratified as intermediate risk (46.7%), followed by low risk (36.0%) and high risk (17.3%). For PV, most patients were in the high-risk category (49.5%), followed by intermediate-risk (29.7%) and low-risk (20.7%) categories. The mean hemoglobin concentration (13.4 g/dL, SD 3.17), white blood cell count (8.9×109/L, SD 7.15), and platelet count (429.5×109/L, SD 269.72) were evaluated, along with laboratory abnormalities including anemia (present in 8.5%), thrombocytopenia (present in 10.7%) and leukopenia (present in 10.0%). Prior thrombosis (21.2%) and prior hemorrhage (5.4%) were relatively uncommon and most patients (94.0%) did not require red blood cell transfusions.
Table 1.
MPN patients’ demographics by gender.
Clinical factors
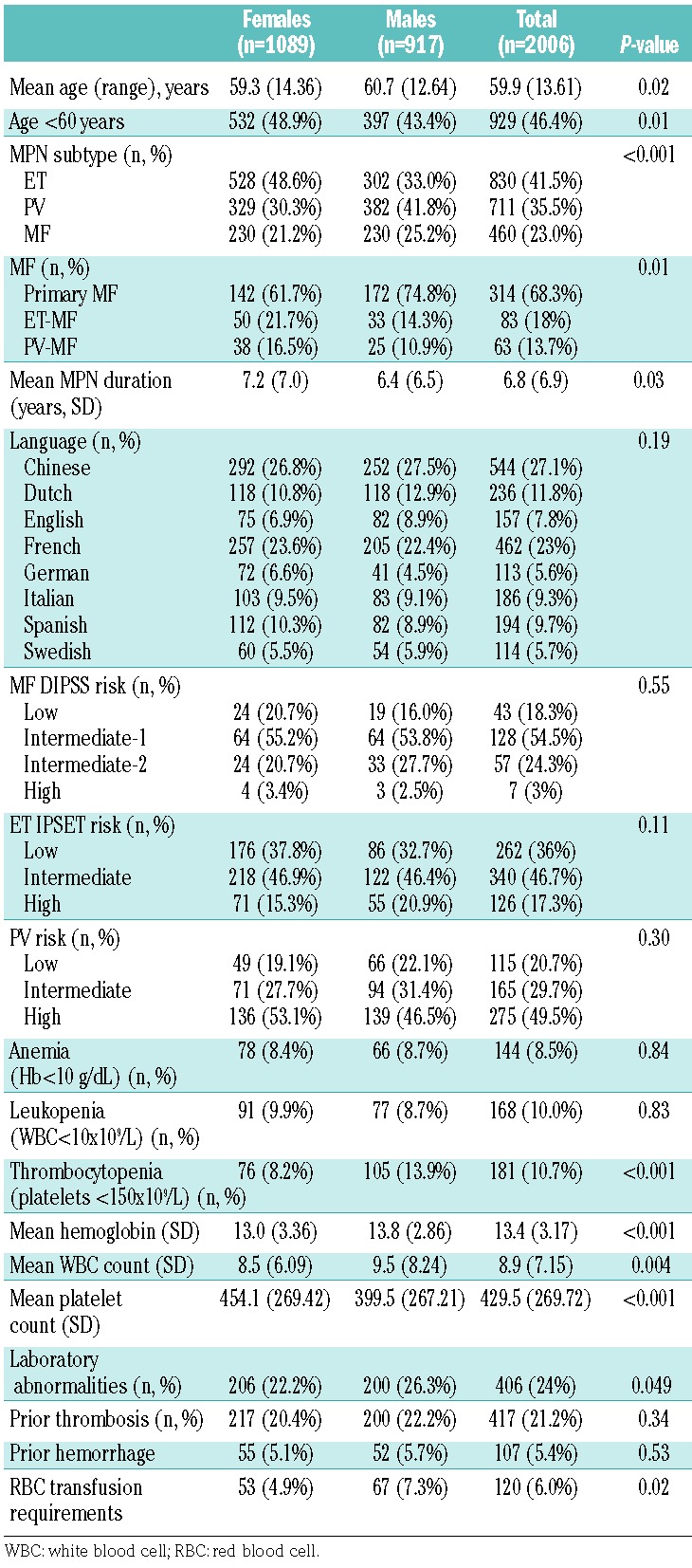
When comparing clinical factors between genders, female patients were found to be slightly younger (59.3 versus 60.7 years, P=0.02) with more patients under the age of 60 at the time of data collection (48.9% versus 43.4%, P=0.01; Table 1). The prevalence of MPN subtypes also differed by gender (P=0.01) with more females having a diagnosis of ET (48.6%) than of PV (30.3%) and MF (21.2%) and more male patients having a history of PV (41.8%) than of ET (33.0%) and MF (25.2%). Gender distribution also differed by MPN subtype with primary MF being more prevalent in males (74.8% versus 61.7%; P=0.01) and post-ET MF being more common in females (21.7% versus 14.3%; P=0.01). Mean hemoglobin concentration (13.8 versus 13.0 g/dL, P<0.001) and white blood cell count (9.5 versus 8.5×109/L, P=0.004) were higher in males whereas females had a higher mean platelet count (454.1 versus 399.5×109/L, P<0.001). Thrombocytopenia was more common in males (13.9% versus 8.2%, P<0.001) whereas no differences were noted in the prevalence of anemia or leukopenia (P>0.05). Males were also more likely to have a history of red blood cell transfusion requirements (7.3% versus 4.9%, P=0.02). Risk scores, language prevalence, history of prior thrombosis or hemorrhage did not differ by gender (all P>0.05). Prior thrombosis was further stratified by gender and MPN type: no differences were noted in ET (males 23.5% versus females 19.3%, P=0.156), PV (males 26.7% versus females 29.8%, P=0.339) or MF (males 12.8% versus females 9.7%, P=0.298). Few differences were noted between genders when comparing prior therapies, with the exception of higher rates of phlebotomy/venesection and givinostat/vorinostat use in males (both P<0.05; Figure 1).
Figure 1.
Percentage of MPN patients who have received prior therapies (x axis) compared by gender.
Symptoms of myeloproliferative neoplasms analyzed by gender
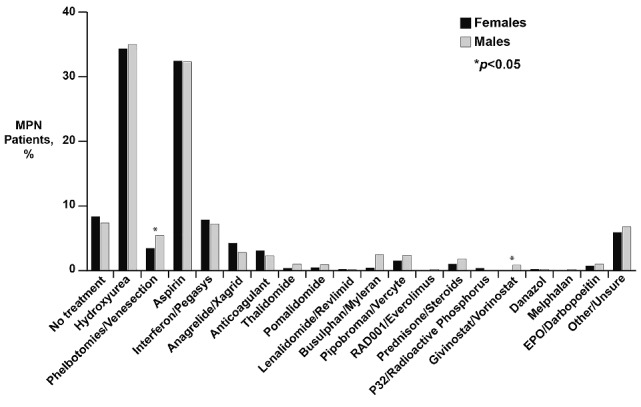
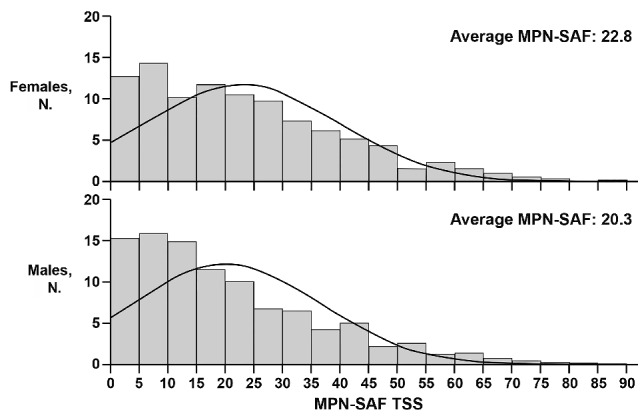
After adjusting for MPN subtype and age, the overall total symptom score was higher for females than males [22.8 (SD=17.0) versus 20.3 (SD=16.3), P<0.001; Figure 2]. Females also had higher scores for all individual MPN symptoms that met statistical significance (Figure 3). These included fatigue (4.5 versus 4.0, P<0.001), early satiety (2.6 versus 2.3, P=0.02), abdominal pain (1.6 versus 1.2, P=0.001), abdominal discomfort (2.1 versus 1.6, P<0.001), headache (2.2 versus 1.6, P<0.001), concentration difficulties (2.7 versus 2.3, P=0.01), dizziness (2.5 versus 2.0, P<0.001), numbness (2.6 versus 2.2, P=0.001), insomnia (3.4 versus 2.4, P<0.001), sadness (2.6 versus 2.3, P=0.01), night sweats (2.4 versus 2.0, P=0.002) and bone pain (2.3 versus 1.6, P<0.001). Items that did not show gender differences included inactivity, sexuality concerns, cough, pruritus, fever, weight loss and overall quality of life. Fatigue was the most severe symptom in both genders. The prevalence of symptoms differed between genders for many of the individual MPN items (Figure 4). With the exception of weight loss (males 37.4% versus females 31.7%, P=0.008), the prevalence of all symptoms that were statistically different between females and males were higher in the former. These symptoms included abdominal pain (46.0% versus 40.8%, P=0.02), abdominal discomfort (55.2% versus 50.7%, P=0.046), headache (58.1% versus 49.1%, P<0.001), dizziness (61.0% versus 56.6%, P=0.046), numbness (64.1% versus 58.1%, P=0.007), insomnia (70.5% versus 59.9%, P<0.001), night sweats (55.6% versus 49.8%, P=0.01) and bone pain (53.2% versus 43.4%, P<0.001).
Figure 2.
Distribution of MPN-SAF scores according to age in females (top) and males (bottom) Evaluation of total number of patients in each gender (y axis) when compared by total MPN-SAF TSS value (x axis).
Figure 3.
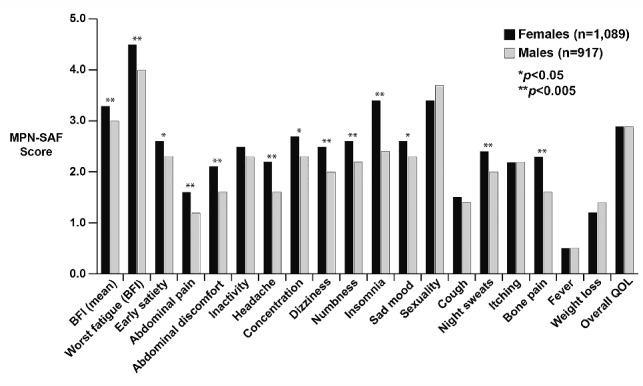
Comparison of scores for the individual items of the MPN-SAF between males and females.
Figure 4.
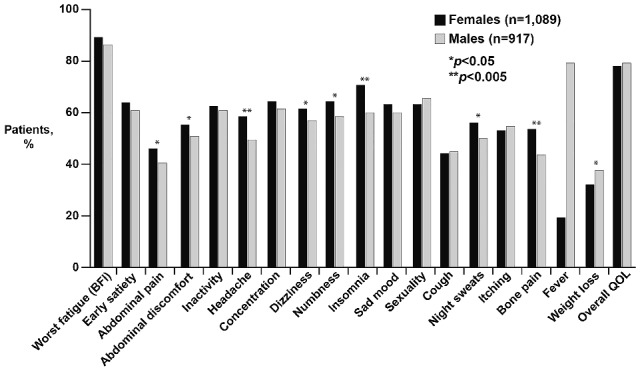
Comparison of the prevalence of MPN-SAF symptoms between males and females.
Symptoms of myeloproliferative neoplasms analyzed by region/culture
The influence of region/culture was also explored among male and female patients by comparing the Chinese cohort (n=544) with the Western cohort consisting of patients from Europe and the USA (n=1,462). Overall, female Chinese patients expressed more severe symptoms related to headaches (2.5 versus 2.0, P=0.01), dizziness (3.1 versus 2.2, P<0.0001), problems with sexuality (4.6 versus 2.9, P<0.0001), fever (0.6 versus 0.4, P=0.005) and weight loss (1.6 versus 1.1, P=0.001) with a higher total symptom score (22.2 versus 19.5, P=0.023) and worse overall quality of life (3.1 versus 2.8, P=0.048; Figure 5). Chinese females had higher scores for sexuality-related complaints (4.6/10) and insomnia (3.3/10). In contrast, Western females described worse fatigue (4.7 versus 4.0, P=0.0003) and abdominal pain (1.7 versus 1.1, P=0.0004). The highest scores for Western females were for fatigue (4.7/10) and insomnia (3.4/10).
Figure 5.
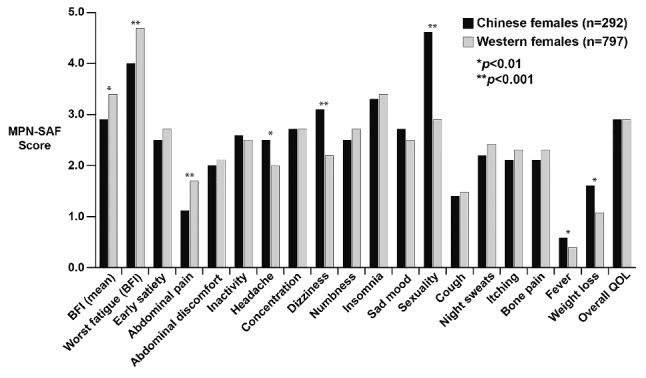
Comparison of scores for individual MPN-SAF items between Chinese and Western females.
Like Chinese females, Chinese males also expressed more severe symptoms related to headaches (1.9 versus 1.4, P=0.001), dizziness (2.6 versus 1.8, P<0.0001), sexuality problems (4.5 versus 3.4, P=0.0001), fever (0.7 versus 0.4, P=0.0002) and weight loss (2.2 versus 1.1, P<0.0001) (Figure 6) than their Western counterparts. The highest scores for Chinese males were for sexuality concerns (4.5/10) and fatigue (3.9/10). The same pattern was seen for Western males, with the highest scores being for fatigue (4.1/10) and sexuality concerns (3.4/10).
Figure 6.
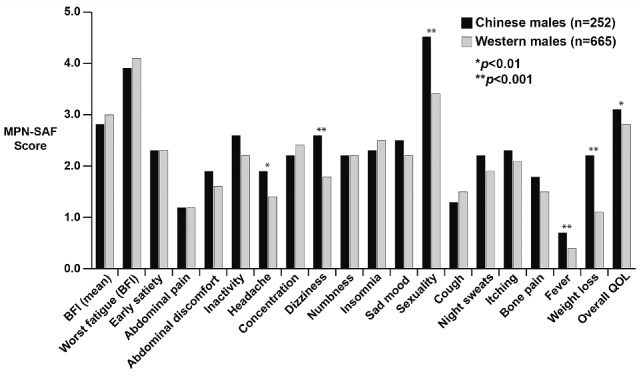
Comparison of scores for individual MPN-SAF items between Chinese and Western males.
Discussion
The diversity of the various types of MPN has made full characterization of their symptom profiles challenging. PV, ET and MF may concurrently shorten survival and impair quality of life. For decades, gender differences in MPN have been observed and documented but remained of low investigational priority given the paucity of exploratory tools. Objective examination of symptom heterogeneity has now emerged as a possibility following the development of MPN-specific PRO tools (MF-SAF, MPN-SAF and MPN-10), enhanced precision of risk scoring algorithms and advances in genomic sequencing.5,11,12 Applying many of these novel instruments, this study represents the first large-scale investigation into the correlates between gender, clinical features and patients’ symptoms.
This investigation yielded a number of important findings. The first is the observation that female patients were more likely to have ET (48.6%) whereas males were more likely to have PV (30.3%). This is consistent with previous findings, with published data historically supporting a prevalence of females among ET patients and a prevalence of males among patients with PV.13–17 Gender discrepancies within hematologic malignancies are not unique to MPN. Similar discordances in gender prevalence have been demonstrated in other disorders such as acute lymphoblastic leukemia, chronic lymphocytic leukemia and multiple myeloma.18,19 Although the etiological cause of this discordance remains unclear, sex chromosome complement/aberrations/aneuploidy, an influence of sex hormones, immune-competence, and gene expression may all be potential contributors.20–23 Investigation of these factors was beyond the scope of this study but they would be worth exploring in future studies.
This study also found that males and females have similar rates of thrombosis. Previous investigations showed that thrombotic risk typically differs by sex among the MPN subtypes.2,24–26 Within the ECLAP study, female PV patients were more likely than males to suffer thrombotic complications (11% versus 8%), particularly within the splanchnic system.27 Similarly, multivariable analysis of data from a recent international collaborative study of 891 ET patients identified that only male gender was predictive of venous thrombosis.28 Gender also appears to influence the location of vascular events. A recent investigation identified that women were more likely to experience macrothrombosis within the abdominal venous system (hepatic, portal, mesenteric or splenic veins) whereas males were more likely to experience events in the deep venous system, including limb thrombosis and pulmonary emboli.16
Although the influence of gender on the pathogenesis of thrombosis remains unclear, mounting evidence suggests that both the type and ratio of circulating sex hormones plays an important role in the thrombotic cascade. In an investigation involving exogenous sex-steroid administration, ET patients exposed to hormone replacement therapy (estrogen only) had similar rates of arterial and venous thrombosis when compared to ET patients not on therapy.29 Importantly, this finding conflicts with studies of healthy populations in which females taking hormone replacement therapy have been observed to be at greater thrombotic risk. However, ET patients utilizing oral contraceptive therapy (estrogen and progesterone combined) had increased rates of venous thrombosis, and specifically, a 5-fold increased risk of splanchnic venous thrombosis (15% versus 3%). From a hormonal standpoint, it remains unclear why male ET patients seem to face a higher risk of thrombosis than females, but this serves to show that the pathogenesis is likely multifactorial. We have no explanation for why males and females in our specific study population had similar rates of thrombosis, independently of MPN subtype. It should be noted that neither the location nor the type of thrombosis (arterial versus venous) was recorded in this study, and we suspect this information might have shed some light on our discrepant finding.
We found it interesting that despite not differing by total number of thrombotic events, our female population still described more abdominal pain. Given that this study utilized reported events only (and did not prospectively investigate for thrombosis), it is possible that some female patients had unrecognized macrothrombosis in the abdominal cavity, accounting for this symptom. Alternatively, the discrepancy may be related to differences in spleen size, which were not investigated in this study, or to differences in symptom expression, which are discussed below.
The observation that males and females reported different symptom burdens remains a major finding. Overwhelmingly, females described symptoms with greater frequency and severity than males. In particular, abdominal complaints (abdominal pain, discomfort) and microvascular symptoms (headache, fatigue, insomnia, concentration difficulties, dizziness) dominated the female symptom burden. Factors that might have accounted for higher symptom scores (such as anemia, high-risk disease status or increased counts of hemorrhagic/thrombotic complications) were not observed to occur at higher rates in females. In fact, males were more likely to have increased transfusion requirements (despite describing less fatigue) and thrombocytopenia. The underlying cause of our observations is, therefore, uncertain. It is well recognized that the prevalence of abdominal pain is higher among females.30 Irritable bowel syndrome, a chronic constellation of abdominal symptoms including pain, discomfort and alterations in bowel habits, has been reported to occur in a female-to-male ratio of 3:1 and remains a common source of abdominal complaints in younger populations.31 However, the prevalence of irritable bowel syndrome declines in individuals over 60 years old and given the average age of MPN females, this syndrome is unlikely to serve as a primary symptom driver. It is plausible that in addition to having a higher risk of macrovascular events, females also incur more microvascular events. Microthrombosis contributes to microvascular symptoms (lightheadedness, dizziness, vertigo, concentration problems, numbness/tingling and sexual dysfunction) by compromising endothelial function and inducing local hypoxia.32 In this study, females clearly described more frequent and more severe microvascular symptoms than did males. Mechanisms that may account for different risks of microvascular dysfunction are worthy of further exploration and may parallel those driving macrothrombosis. Underreporting of microvascular symptoms by males is also a potential explanation of our observations and is discussed further below. Congruent with previous investigations, males and females described similar degrees of sexual dysfunction and fatigue remained the most symptomatic facet of the disease burden.
Patients’ ethnicity and culture also appear to contribute to symptom burden. Variations in symptom expression were noted when Western and Eastern patients were compared. Independent of gender, Chinese patients described more microvascular symptoms (headaches, dizziness) and more concerns related to sexuality. In contrast, fatigue was the most prominent symptom among Western male and female patients. Variations between MPN in Eastern and Western patients have been highlighted by the presence of fundamental biological and clinical differences, which are being increasingly discussed in the literature.33,34 For example, Eastern patients with MF are more likely to be younger and less likely to struggle with constitutional symptoms or splenomegaly. Survival differences between the two cohorts has also been observed, with median survival being slightly longer in patients of Chinese ethnicity. Given the subjectivity inherent to symptom reporting, it remains unclear whether the differences in MPN-SAF scores between races are related to norms of cultural expression (especially willingness to verbalize problems related to sexuality) or the natural outworking of true genotypic and phenotypic differences between races.
The possibility that our observations are related to reporting discrepancies is also an important matter of discussion. Published data show that females tend to describe more numerous and more intense symptoms than males, independent of location or organ system involved. In a study of 13,538 non-patient community residents, participants evaluated the lifetime prevalence of non-menstrual complaints and 20 of the 22 most common symptoms were reported more frequently by females.35 Similarly, experimental studies involving induction of pain have shown that females have a lower threshold of pain tolerance and report more symptoms than males.36 These findings may be driven by biological differences in somatic and visceral sensation, sex-influenced descriptiveness in symptom labeling and reporting, social acceptance of symptom revelation, sex-related differences in the prevalence of depression and anxiety and gender biases inherent to the research process. Some studies have suggested that females engender greater bodily vigilance, potentially as an innate mechanism to optimize fertility.37,38 Other studies advocate that social cues have impressed upon males the importance of limiting expression of discomfort/illness, maintaining a stoic appearance and underemphasizing complaints.39
Independently of the foregoing, we find it intriguing that female MPN patients had the same quality of life scores as males, despite having more frequent and severe symptomatology. The literature supports health-related quality of life as being typically rated lower among females. This has traditionally been attributed to the higher prevalence of disability and chronic conditions in this population.40 However, in this study MPN-related comorbidities were similar between the two sexes. It is plausible that MPN females have socially adapted to compensate for their intensified symptom burden. Alternatively, female patients may simply be more disposed to voice their complaints. We also note that females described greater symptom burdens but their risk scores were similar to those of the males. This information corroborates the previous finding of the MPN Symptom Burden study that MPN symptoms are not surrogates for disease severity.4
It is important to recognize that there are a number of limitations to this exploratory investigation. The first is that the term ‘gender’ is being used synonymously with genotypically-defined ‘sex’. As stated, the surveys allowed patients to self-report their sex as either ‘male’ or ‘female’. Although it may be assumed that the recorded choice referred to genotypic makeup, it is possible that some patients recorded their ‘gender identity’ instead, which may not be synonymous with chromosomal makeup. Should this have occurred, we believe that the number of cases would have been small and likely consistent with the prevalence of discordant associations in the community. We also lack information on the exact location of events (peripheral versus central) and note that males had greater transfusion requirements than females despite similar rates of anemia. We suspect that this is related to the averaging of pre- and post-transfusion hemoglobin controls in males, resulting in falsely high hemoglobin levels. It is worth noting that the majority of patients within this population of MPN patients were classified as low to intermediate risk, which potentially skews symptom burden towards lower values. Although an evaluation of differences in symptom burden between genders separated by risk category was beyond the scope of this study, future investigations could further explore this issue to determine whether symptom progression differs between the sexes. It is important to note that there are inherent flaws in using a ‘self-reporting’ format. However, we believe that the use of validated MPN-specific PRO tools greatly improves the cogency of the results. In addition, members of the clinical team were primarily responsible for all data collection not related to symptom expression, conceivably limiting errors in the recording process. It is regrettable that underlying mutations could not be analyzed, as doing so could have offered yielded interesting information.
The concomitant development of innovative technologies and novel symptom assessment tools has revolutionized the treatment landscape for MPN. Few fields of study can boast of a faster or more cooperative manner via which pioneering research has translated into improved outcomes for patients. In this study, we have determined that gender integrally relates to disease features and symptom burden. These results further underscore the importance of considering each gender individually as treatment regimens are designed. Understanding that males may be less likely to voice their MPN symptoms should influence clinicians to explore potentially under-expressed complaints. Similarly, acknowledging that females may have greater symptom burdens should motivate healthcare providers to consider novel therapies and explore trial options. This exploratory study indicates the importance of including gender as a contributor to heterogeneity and as an object of investigation in future studies.
Supplementary Material
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/102/1/85
References
- 1.Spivak JL, Considine M, Williams DM, et al. Two clinical phenotypes in polycythemia vera. N Engl J Med. 2014;371(9):808–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stein BL, Williams DM, Wang NY, et al. Sex differences in the JAK2 V617F allele burden in chronic myeloproliferative disorders. Haematologica. 2010;95(7):1090–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Passamonti F, Randi ML, Rumi E, et al. Increased risk of pregnancy complications in patients with essential thrombocythemia carrying the JAK2 (617V>F) mutation. Blood. 2007;110(2):485–489. [DOI] [PubMed] [Google Scholar]
- 4.Geyer HL, Scherber RM, Dueck AC, et al. Distinct clustering of symptomatic burden among myeloproliferative neoplasm patients: retrospective assessment in 1470 patients. Blood. 2014;123(24):3803–3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scherber R, Dueck AC, Johansson P, et al. The Myeloproliferative Neoplasm Symptom Assessment Form (MPN-SAF): international prospective validation and reliability trial in 402 patients. Blood. 2011;118(2):401–408. [DOI] [PubMed] [Google Scholar]
- 6.Wild D, Grove A, Martin M, et al. Principles of good practice for the translation and cultural adaptation process for patient-reported outcomes (PRO) measures: report of the ISPOR Task Force for Translation and Cultural Adaptation. Value Health. 2005;8(2):94–104. [DOI] [PubMed] [Google Scholar]
- 7.Mendoza TR, Wang XS, Cleeland CS, et al. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer. 1999;85(5):1186–1196. [DOI] [PubMed] [Google Scholar]
- 8.Passamonti F, Thiele J, Girodon F, et al. A prognostic model to predict survival in 867 World Health Organization-defined essential thrombocythemia at diagnosis: a study by the International Working Group on Myelofibrosis Research and Treatment. Blood. 2012;120(6):1197–1201. [DOI] [PubMed] [Google Scholar]
- 9.Tefferi A, Rumi E, Finazzi G, et al. Survival and prognosis among 1545 patients with contemporary polycythemia vera: an international study. Leukemia. 2013;27(9):1874–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Passamonti F, Cervantes F, Vannucchi AM, et al. A dynamic prognostic model to predict survival in primary myelofibrosis: a study by the IWG-MRT (International Working Group for Myeloproliferative Neoplasms Research and Treatment). Blood. 2010;115(9):1703–1708. [DOI] [PubMed] [Google Scholar]
- 11.Mesa RA, Schwager S, Radia D, et al. The Myelofibrosis Symptom Assessment Form (MFSAF): an evidence-based brief inventory to measure quality of life and symptomatic response to treatment in myelofibrosis. Leuk Res. 2009;33(9):1199–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Emanuel RM, Dueck AC, Geyer HL, et al. Myeloproliferative neoplasm (MPN) symptom assessment form total symptom score: prospective international assessment of an abbreviated symptom burden scoring system among patients with MPNs. J Clin Oncol. 2012;30(33):4098–4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Passamonti F, Rumi E, Pungolino E, et al. Life expectancy and prognostic factors for survival in patients with polycythemia vera and essential thrombocythemia. Am J Med. 2004;117(10):755–761. [DOI] [PubMed] [Google Scholar]
- 14.Bellucci S, Janvier M, Tobelem G, et al. Essential thrombocythemias. Clinical evolutionary and biological data. Cancer. 1986;58(11):2440–2447. [DOI] [PubMed] [Google Scholar]
- 15.Fenaux P, Simon M, Caulier MT, Lai JL, Goudemand J, Bauters F. Clinical course of essential thrombocythemia in 147 cases. Cancer. 1990;66(3):549–556. [DOI] [PubMed] [Google Scholar]
- 16.Stein BL, Rademaker A, Spivak JL, Moliterno AR. Gender and vascular complications in the JAK2 V617F-positive myeloproliferative neoplasms. Thrombosis. 2011;2011:874146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ania BJ, Suman VJ, Sobell JL, Codd MB, Silverstein MN, Melton LJ., 3rd Trends in the incidence of polycythemia vera among Olmsted County, Minnesota residents, 1935–1989. Am J Hematol. 1994;47(2):89–93. [DOI] [PubMed] [Google Scholar]
- 18.Ward E, DeSantis C, Robbins A, Kohler B, Jemal A. Childhood and adolescent cancer statistics, 2014. CA Cancer J Clin. 2014;64(2):83–103. [DOI] [PubMed] [Google Scholar]
- 19.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. [DOI] [PubMed] [Google Scholar]
- 20.Ellegren H, Parsch J. The evolution of sex-biased genes and sex-biased gene expression. Nat Rev Genet. 2007;8(9):689–698. [DOI] [PubMed] [Google Scholar]
- 21.Rinn JL, Snyder M. Sexual dimorphism in mammalian gene expression. Trends Genet. 2005;21(5):298–305. [DOI] [PubMed] [Google Scholar]
- 22.Ober C, Loisel DA, Gilad Y. Sex-specific genetic architecture of human disease. Nat Rev Genet. 2008;9(12):911–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bouman A, Heineman MJ, Faas MM. Sex hormones and the immune response in humans. Hum Reprod Update. 2005;11(4): 411–423. [DOI] [PubMed] [Google Scholar]
- 24.Kittur J, Knudson RA, Lasho TL, et al. Clinical correlates of JAK2V617F allele burden in essential thrombocythemia. Cancer. 2007;109(11):2279–2284. [DOI] [PubMed] [Google Scholar]
- 25.Larsen TS, Pallisgaard N, Moller MB, Hasselbalch HC. The JAK2 V617F allele burden in essential thrombocythemia, polycythemia vera and primary myelofibrosis–impact on disease phenotype. Eur J Haematol. 2007;79(6):508–515. [DOI] [PubMed] [Google Scholar]
- 26.Girodon F, Schaeffer C, Cleyrat C, et al. Frequent reduction or absence of detection of the JAK2-mutated clone in JAK2V617F-positive patients within the first years of hydroxyurea therapy. Haematologica. 2008;93(11):1723–1727. [DOI] [PubMed] [Google Scholar]
- 27.Landolfi R, Marchioli R. European Collaboration on Low-dose Aspirin in Polycythemia Vera (ECLAP): a randomized trial. Semin Thromb Hemost. 1997;23(5): 473–478. [DOI] [PubMed] [Google Scholar]
- 28.Carobbio A, Thiele J, Passamonti F, et al. Risk factors for arterial and venous thrombosis in WHO-defined essential thrombocythemia: an international study of 891 patients. Blood. 2011;117(22):5857–5859. [DOI] [PubMed] [Google Scholar]
- 29.Gangat N, Wolanskyj AP, Schwager SM, Mesa RA, Tefferi A. Estrogen-based hormone therapy and thrombosis risk in women with essential thrombocythemia. Cancer. 2006;106(11):2406–2411. [DOI] [PubMed] [Google Scholar]
- 30.Unruh AM. Gender variations in clinical pain experience. Pain. 1996;65(2–3):123–167. [DOI] [PubMed] [Google Scholar]
- 31.Sandler RS. Epidemiology of irritable bowel syndrome in the United States. Gastroenterology. 1990;99(2):409–415. [DOI] [PubMed] [Google Scholar]
- 32.Papma JM, den Heijer T, de Koning I, et al. The influence of cerebral small vessel disease on default mode network deactivation in mild cognitive impairment. Neuroimage Clin. 2012;2:33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu Z, Gale RP, Zhang Y, et al. Unique features of primary myelofibrosis in Chinese. Blood. 2012;119(11):2469–2473. [DOI] [PubMed] [Google Scholar]
- 34.Gill H, Leung AY, Chan CC, et al. Clinicopathologic features and prognostic indicators in Chinese patients with myelofibrosis. Hematology. 2016;21(1):10–18. [DOI] [PubMed] [Google Scholar]
- 35.Kroenke K, Price RK. Symptoms in the community. Prevalence, classification, and psychiatric comorbidity. Arch Intern Med. 1993;153(21):2474–2480. [PubMed] [Google Scholar]
- 36.Riley JL, 3rd, Robinson ME, Wise EA, Myers CD, Fillingim RB. Sex differences in the perception of noxious experimental stimuli: a meta-analysis. Pain. 1998;74(2–3):181–187. [DOI] [PubMed] [Google Scholar]
- 37.Lieban RW. Gender and symptom sensitivity: report on a Philippine study. Am J Orthopsychiatry. 1985;55(3):446–450. [DOI] [PubMed] [Google Scholar]
- 38.Warner CD. Somatic awareness and coronary artery disease in women with chest pain. Heart Lung. 1995;24(6):436–443. [DOI] [PubMed] [Google Scholar]
- 39.Mechanic D. Social psychologic factors affecting the presentation of bodily complaints. N Engl J Med. 1972;286(21):1132–1139. [DOI] [PubMed] [Google Scholar]
- 40.Orfila F, Ferrer M, Lamarca R, Tebe C, Domingo-Salvany A, Alonso J. Gender differences in health-related quality of life among the elderly: the role of objective functional capacity and chronic conditions. Soc Sci Med. 2006;63(9):2367–2380. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


