Abstract
Allogeneic hematopoietic cell transplantation is widely used to treat adults with high-risk acute lymphoblastic leukemia. The aim of this study was to analyze whether the results changed over time and to identify prognostic factors. Adult patients treated between 1993 and 2012 with myeloablative allogeneic hematopoietic cell transplantation from HLA matched sibling (n=2681) or unrelated (n=2178) donors in first complete remission were included. For transplantations from sibling donors performed between 2008 and 2012, 2-year probabilities of overall survival were: 76% (18–25 years old), 69% (26–35 and 36–45 years old) and 60% (46–55 years old). Among recipients of transplantations from unrelated donors, the respective survival rates were 66%, 70%, 61%, and 62%. In comparison with the 1993–2007 period, significant improvements were observed for all age groups except for the 26–35-year old patients. In a multivariate model, transplantations performed between 2008 and 2012, when compared to 1993–2007, were associated with significantly reduced risks of non-relapse mortality (Hazard Ratio 0.77, P=0.00006), relapse (Hazard Ratio 0.85, P=0.007), treatment failure (Hazard Ratio 0.81, P<0.00001), and overall mortality (Hazard Ratio 0.79, P<0.00001). In the analysis restricted to transplantations performed between 2008 and 2012, the use of total body irradiation-based conditioning was associated with reduced risk of relapse (Hazard Ratio 0.48, P=0.004) and treatment failure (Hazard Ratio 0.63, P=0.02). We conclude that results of allogeneic hematopoietic cell transplantation for adults with acute lymphoblastic leukemia improved significantly over time. Total body irradiation should be considered as the preferable type of myeloablative conditioning.
Introduction
Allogeneic hematopoietic cell transplantation (alloHCT) is widely used for the treatment of adult patients with acute lymphoblastic leukemia (ALL) in first complete remission (CR1) with high risk of relapse. It allows for administration of high doses of total body irradiation (TBI) or myeloablative chemotherapy, which may contribute to eradication of residual disease. The effect may be further strengthened by graft-versus-leukemia reaction driven by lymphocytes of donor origin.1 Unfortunately, alloHCT is also associated with high risk of early and late complications, including infections, graft-versus-host disease (GvHD) and secondary malignancies, which result in significant mortality and morbidity. Hence, the balance between potential advantages and disadvantages should be carefully considered in all clinical situations.2
Over the past two decades, advances have been made in the care of patients undergoing transplantation. Results of several studies indicated reduced risk of non-relapse mortality (NRM) after alloHCT, observed over the period 1990–2007, which could be a consequence of improved supportive care and more accurate donor selection.3–5 Those studies, however, included heterogenous populations, most frequently patients with acute myeloid leukemia. Trends in outcome of alloHCT for patients with ALL are less well documented. In particular, no large scale analyses are available for patients treated after 2007. In addition, prognostic factors for alloHCT performed in recent years are still not known. Such data are essential for proper patient selection and optimization of the transplantation procedure.
The goal of this study was to analyze the results of myeloablative alloHCT for patients with ALL in various age groups and according to donor type, as well as to evaluate whether results changed over time during the 20-year period (1993–2012). In addition, we performed an analysis of prognostic factors for transplantations performed in more recent years (2008–2012).
Methods
Study design and data collection
This was a retrospective, multicenter analysis. Data were provided by the registry of the Acute Leukemia Working Party (ALWP) of the European Society for Blood and Marrow Transplantation (EBMT). The EBMT is a non-profit, scientific society representing more than 600 transplant centers, mainly in Europe. Data are entered, managed, and maintained in a central database; each EBMT center is represented in this database. The validation and quality control program includes verification of the computer printout of the entered data, cross-checking with the national registries, and on-site visits of selected teams.
The study was approved by the ALWP of the EBMT. Patients provided informed consent authorizing the use of their personal information for research purposes.
Criteria of selection
Inclusion criteria were: 1) patients with ALL in CR1; 2) age 18–55 years; 3) alloHCT from either matched sibling donor (MSD-HCT) or unrelated donor (URD-HCT) performed between 1993 and 2012 in centers reporting to the EBMT; 4) T-replete bone marrow or peripheral blood used as a source of stem cells (cord blood transplantations were excluded); 5) myeloablative conditioning, i.e. regimen based on busulfan administered at the total dose of 8 mg/kg or more or total body irradiation (TBI) applied at 6 Gy or more.
For the analysis of prognostic factors, only transplantations performed between 2008 and 2012 were considered. The population was restricted to subjects with available data regarding initial disease characteristics (cytogenetics, immune subtype, white blood cell count).
Patients, donors, and HSCT procedure
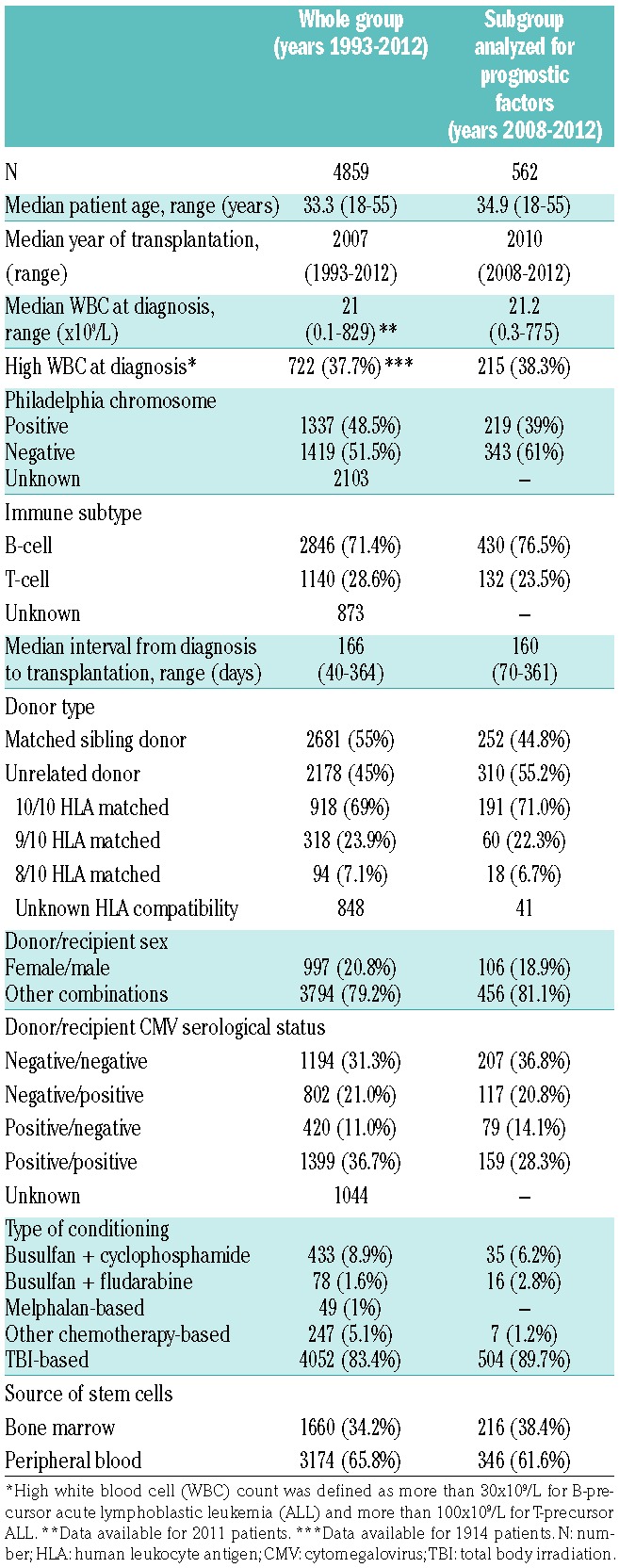
Altogether data on 4859 patients treated in 203 transplant centers were included in the analysis. Median age was 33.3 years (range 18–55 years). Data on cytogenetics were available for 2577 patients among whom 1242 (50.1%) had Philadelphia (Ph)-positive ALL. Transplantations were performed from HLA identical sibling in 2681 patients (55%) and from unrelated volunteer in 2178 cases (45%). Peripheral blood was used as a source of stem cells in 3174 (65.8%) cases.
The analysis of prognostic factors included a subgroup of 562 patients treated with either MSD-HCT (n=252, 44.8%) or URD-HCT (n=310, 55.2%), between 2008–2012. Ph-positive ALL was reported in 225 cases (40%).
Detailed patients’ and procedural characteristics are listed in Table 1.
Table 1.
Patients and donors: transplantation procedure.
Statistical analysis
Study end points were probabilities of NRM, relapse incidence (RI), leukemia-free survival (LFS), and overall survival (OS). The LFS was defined as time interval from alloHCT to either relapse or death in remission. Probabilities of OS and LFS were calculated using the Kaplan-Meier estimate. The RI and NRM were calculated using cumulative incidence curves in a competing risks setting, death in remission being treated as a competing event to relapse.6,7 Univariate analyses were made with the use of log-rank test for LFS and OS, while Gray test was used to compare RI and NRM. In a univariate analysis we compared results of alloHCT performed in three periods: 1993–2002 (10 years), 2003–2007 (5 years), and 2008–2012 (5 years). The first period was longer than subsequent ones to ensure a representative number of observations. Multivariate analyses were performed with the use of Cox proportional hazard model, adjusted for potential risk factors. The effect of year of alloHCT (2008–2012 vs. 1993–2007) was analyzed in a model adjusted for donor type and recipient age. The analysis of prognostic factors was restricted to years 2008–2012.
Median follow up for survivors was 38 months. All P-values are two-sided with type 1 error rate fixed at 0.05. Statistical analyses were performed with SPSS 22.0 (IBM Corp., Armonk, NY, USA) and R 3.1.1 software packages (R Development Core Team, Vienna, Austria).
Results
Outcome of MDS-HCT: changes over time according to recipient age
Results of alloHCT were analyzed separately for MSD-HCT and URD-HCT in four age groups: 18–25 years, 26–35 years, 36–45 years, and 46–55 years. Outcome of alloHCT performed in three study periods was compared. Detailed results are presented in Table 2.
Table 2.
Results of transplantation from matched sibling donors and unrelated donors in various different age groups and time periods.
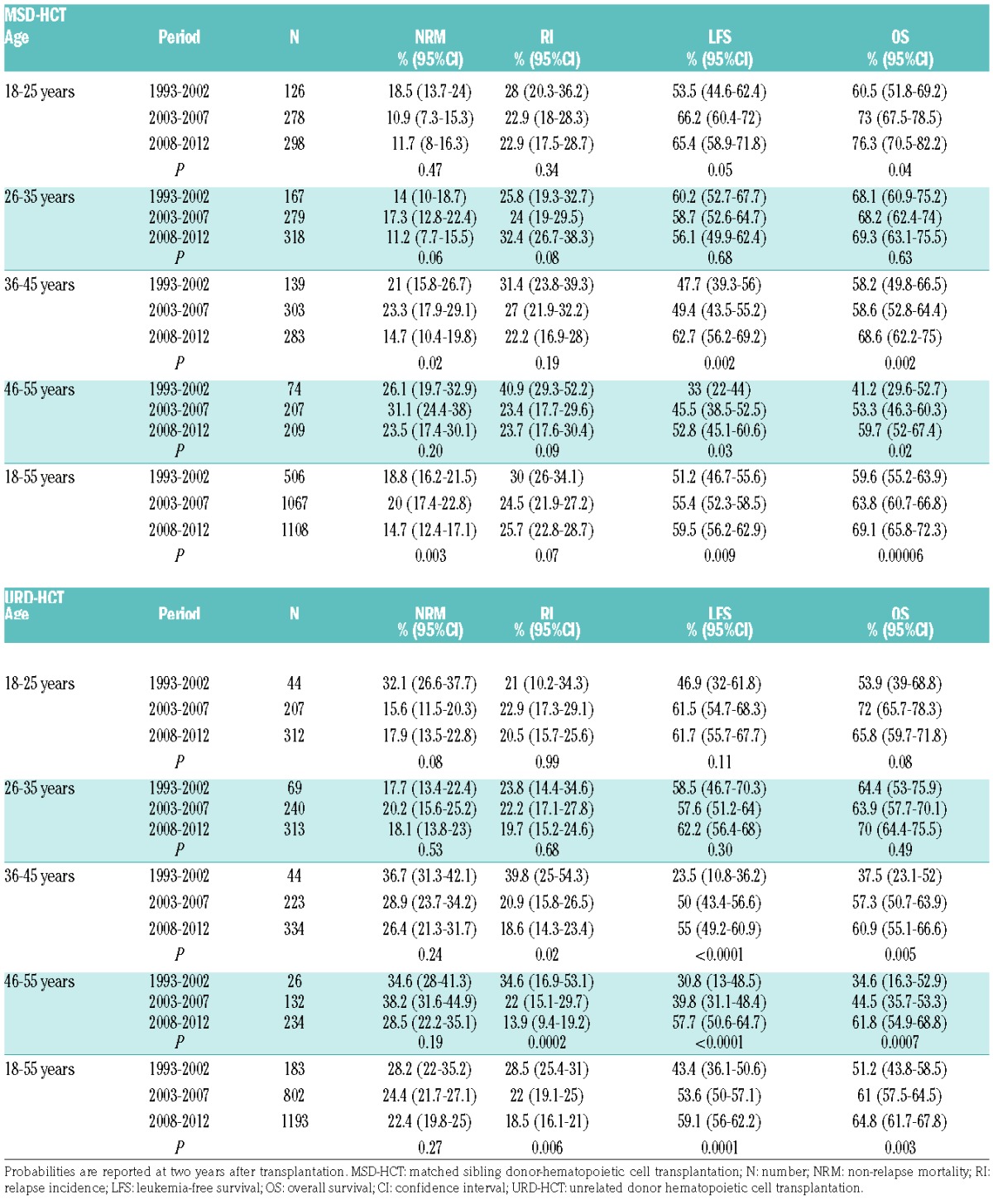
The cumulative incidence of NRM at two years after MSD-HCT decreased over time for all age groups; however, the differences reached statistical significance only among patients aged 36–45 years. In this age category, the incidence of NRM decreased from 21% and 23.3% in years 1993–2002 and 2003–2007, respectively, to 14.7% in the period 2008–2012 (P=0.02). No significant changes over time could be demonstrated for the incidence of relapse after MSD-HCT; however, there was significant improvement of LFS in all age groups except for patients aged 26–35 years. Among the youngest patients (18–25 years old) LFS rates increased from 53.5% between 1993–2002 to 66.2% between 2003 and 2007 and 65.4% between 2008 and 2012 (P=0.05). Respective LFS rates for patients aged 36–45 years were 47.7%, 58.7%, and 62.7% (P=0.002), while in the oldest group (46–55 years old) they equaled 33%, 45.5%, and 52.8% (P=0.03). In parallel, significant improvement could be demonstrated for the probabilities of OS. The 2-year OS rates for MSD-HCT performed between 2008–2012 in various age groups reached 76.3% (18–25 years), 69.3% (26–35 years), 68.6% (36–45 years), and 59.7% (46–55 years).
Outcome of URD-HCT: changes over time according to recipient age
In a univariate analysis, NRM after URD-HCT did not change significantly over time; however, a tendency was observed in the youngest study group. For patients aged 18–25 years the 2-year NRM rates decreased from 32.1% between 1993 and 2002 to 15.6% between 2003 and 2007 and 17.9% between 2008 and 2012 (P=0.08) (Table 2). Significant reduction of the incidence of RI after URD-HCT could be demonstrated for older adults. For patients aged 36–45 years, the 2-year RI rates decreased from 39.8% between 1993 and 2002 to 20.9% between 2003 and 2007 and 18.6% between 2008 and 2012 (P=0.02). In the oldest study groups (46–55 years), respective RI rates were 34.6%, 22%, and 13.9% (P=0.0002).
Although the probabilities of LFS and OS increased over time in all age groups, significant differences could be demonstrated only for older adults. For patients aged 36–45 years, the 2-year LFS rates in subsequent study periods were 23.5%, 50%, and 55% (P<0.0001) while OS was 37.5%, 57.3% and 60.9%, respectively (P=0.005). Among those aged 46–55 years, the probabilities of LFS increased from 30.8% between 1993 and 2002 to 39.8% between 2003 and 2007 and 57.7% between 2008 and 2012 (P<0.0001) while OS rates were 34.6%, 44.5%, and 61.8%, respectively (P=0.0007).
Outcome of alloHCT: general trends
Univariate and multivariate analyses were performed for MSD-HCT and URD-HCT, including all age groups to see if outcome changed over time for a general population of adults aged 18–55 years (Tables 2 and 3).
Table 3.
Effect of the year of allogeneic HCT on outcome adjusted for recipient age and type of donor.
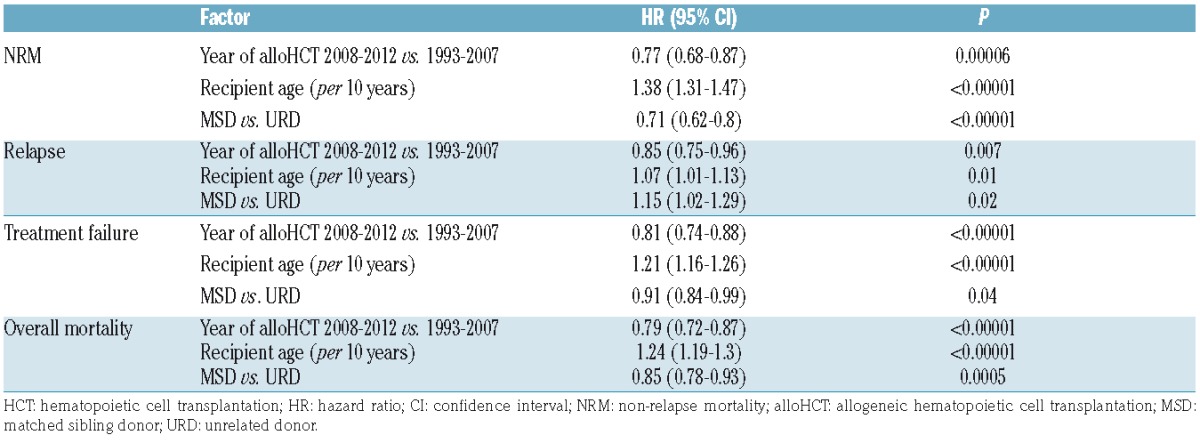
In a univariate model, improvement regarding LFS and OS rates could be demonstrated for both types of transplantations (Table 2 and Figures 1 and 2). As for NRM, a significant reduction could be demonstrated after MSD-HSCT (18.8%, 20%, and 14.7% in subsequent study periods; P=0.003) while the differences after URD-HCT were not statistically significant (Table 2). In contrast, significant reduction of RI rates was observed after URD-HCT (28.5%, 22%, and 18.5%, respectively, P=0.006), while not after MSD-HCT. In a multivariate analysis adjusted for recipient age and donor type significant effects of the treatment period were observed with regard to all study end points (Table 3). AlloHCT procedures performed between 2008–2012 were associated with reduced risk of NRM [Hazard Ratio (HR) 0.77, P=0.00006], relapse (HR 0.85, P=0.007), treatment failure (either relapse or NRM; HR 0.81, P<0.00001), and overall mortality (HR 0.79, P<0.00001).
Figure 1.
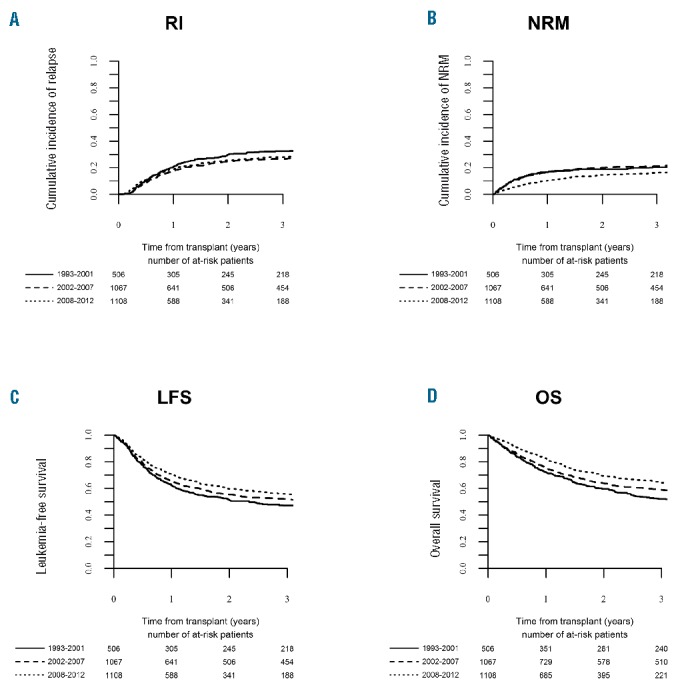
Outcome of matched sibling donor – hematopoietic cell transplantation for adults with acute lymphoblastic leukemia (ALL) in first complete remission (CR1). Changes over time in the period 1993–2012. (A) Relapse incidence (RI), (B) non-relapse mortality (NRM), (C) leukemia-free survival (LFS), (D) overall survival (OS).
Figure 2.
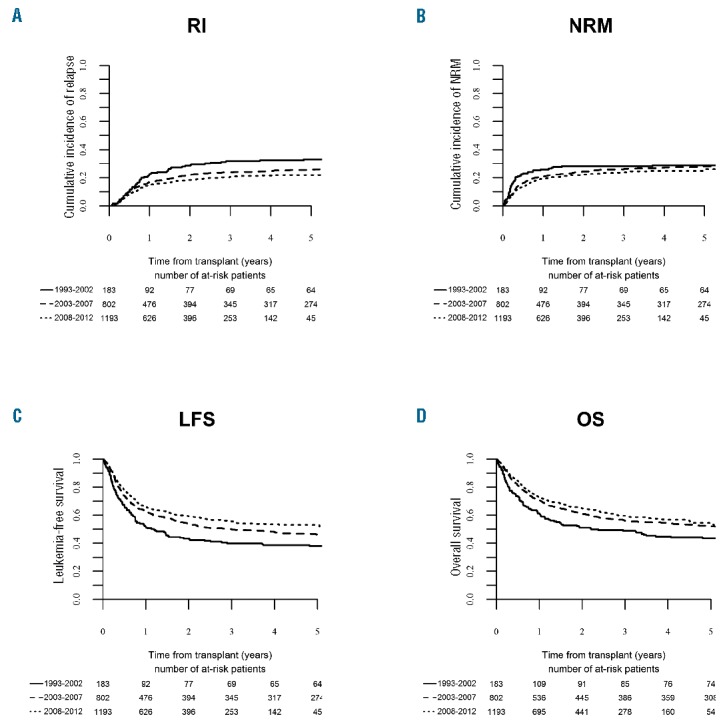
Outcome of unrelated donor – hematopoietic cell transplantation for adults with acute lymphoblastic leukemia (ALL) in first complete remission (CR1). Changes over time in the period 1993–2012. (A) Relapse incidence (RI), (B) non-relapse mortality (NRM), (C) leukemia-free survival (LFS), (D) overall survival (OS).
Additional analyses were performed for patients with known Ph-status. Survival improvement was observed for patients with Ph-positive ALL and Ph-negative ALL (Online Supplementary Tables S1 and S2).
Prognostic factors for alloHCT performed between 2008–2012
Results of univariate and multivariate analyses restricted to transplantations performed in the most recent era are reported in Tables 4 and 5.
Table 4.
Univariate analysis of factors affecting outcome after allogeneic HCT performed between 2008–2012.
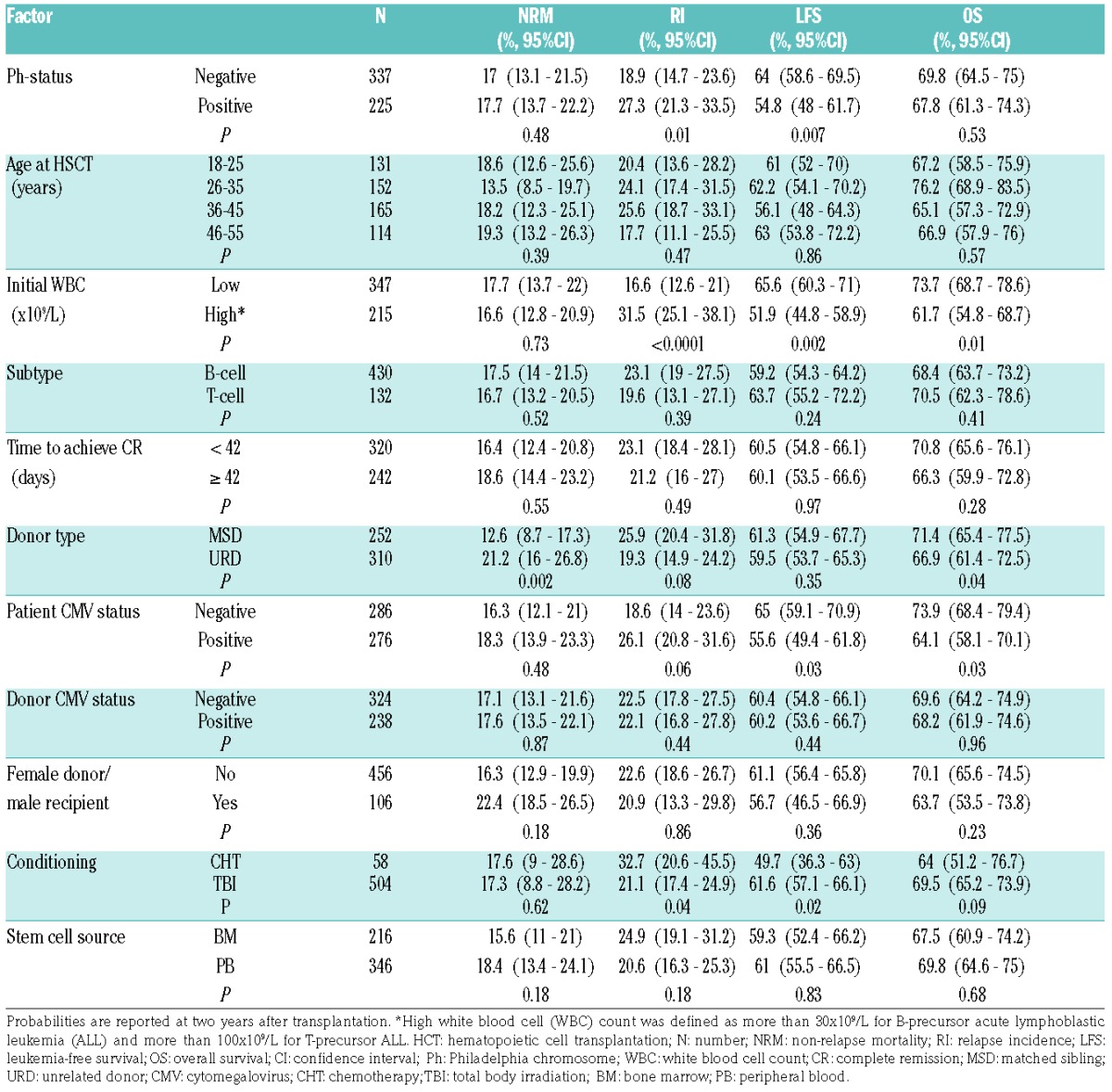
Table 5.
Multivariate analysis of factors affecting outcome after allogeneic HCT performed between 2008–2012.
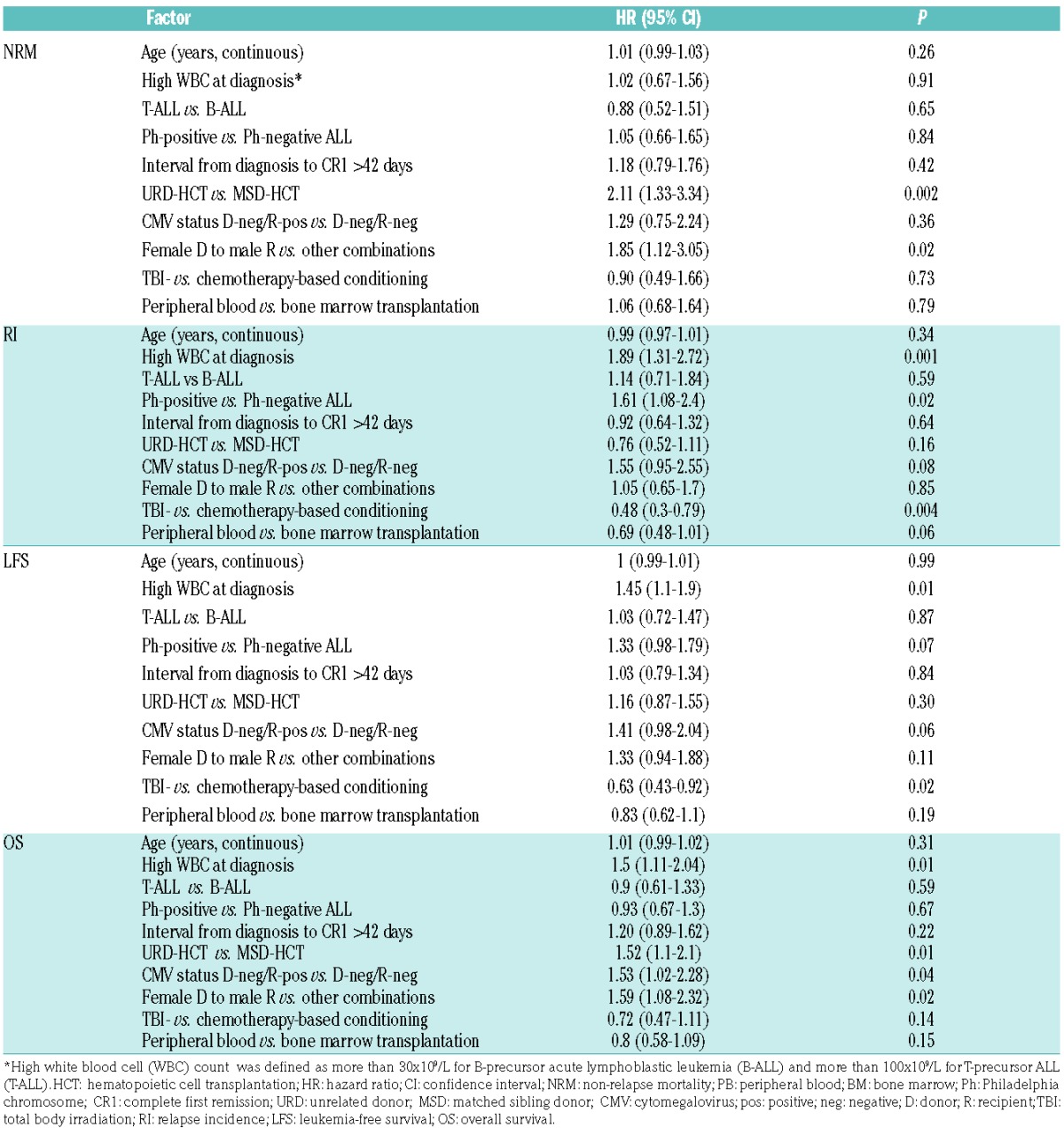
Among disease-related factors, increased risk of relapse was observed for patients with high leukocyte count at diagnosis (HR 1.89, P=0.001) and the presence of Philadelphia chromosome (HR 1.61, P=0.02). High initial leukocyte count was also associated with increased risk of treatment failure (HR 1.45, P=0.01) and overall mortality (HR 1.5, P=0.01). The effect of Ph-status on LFS was significant in a univariate analysis (P=0.007) (Online Supplementary Figure S1), but not in a multivariate model. Neither immune subgroups (B- vs. T-ALL) nor time to achieve CR were associated with outcome.
The risk of NRM was increased for URD-HCT compared to MSD-HCT (HR 2.11, P=0.002) (Online Supplementary Figure S1), and in the case of female donor/male recipient sex combination (HR 1.85, P=0.02). Both these factors were also associated with increased risk of the overall mortality (HR 1.52, P=0.01 and HR 1.59, P=0.02, respectively). In addition, the risk of mortality was increased for patients with CMV-positive serological status receiving transplantation from CMV-negative donors (HR 1.53, P=0.04).
Among procedure-related variables, decreased risk of relapse was demonstrated for transplantations preceded by total body irradiation (TBI)-based preparative regimens (HR=0.48, P=0.001). The use of TBI was also associated with reduced risk of treatment failure (HR=0.63, P=0.02) (Figure 3). The choice of stem cell source did not significantly affect outcome; however, there was a trend to reduced risk of relapse after peripheral blood compared to bone marrow transplantations (P=0.06).
Figure 3.
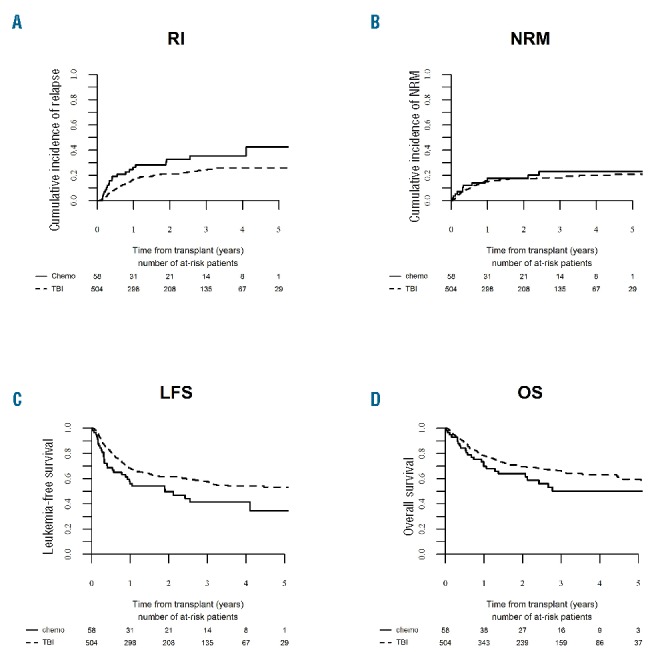
Outcome of allogeneic hematopoietic stem cell transplantation (alloHSCT) performed in the period 2008–2012 according to the type of conditioning (total body irradiation-based vs. chemotherapy-based). (A) Relapse incidence (RI), (B) non-relapse mortality (NRM), (C) leukemia-free survival (LFS), (D) overall survival (OS).
Data on minimal residual disease (MRD) status before transplantation were available for 716 patients, including 502 patients with Ph-positive ALL (MRD-positive, n=314; MRD-negative, n=188) and 214 subjects with Ph-negative disease (MRD-positive, n=162; MRD-negative, n=52) treated between 2008–2012. Methods for MRD assessment were not specified. In a univariate analysis, among patients with Ph-negative ALL, the positive MRD status was associated with increased incidence of relapse (36.5% vs. 17.6%; P=0.0005) and decreased probability of LFS (54.3% vs. 65.9%; P=0.04), while no significant effect was observed with regard to OS and NRM. For patients with Ph-positive ALL, the MRD status did not significantly affect outcome.
Discussion
AlloHCT is used as part of consolidation treatment of adults with ALL with intention to reduce the risk of relapse. However, this benefit may be counterbalanced by NRM. Results of the UK ALL XII/ECOG 2993 trial revealed that, among standard-risk patients with a sibling donor, the cumulative incidence of NRM was 19.5%, compared with 35.8% in the high-risk group.8 Consequently, survival advantage could be demonstrated for standard- but not high-risk ALL. In the HOVON studies, NRM among patients with a sibling donor was lower: 16% for standard-risk and 15% for high-risk ALL.2 Collectively, it has been concluded that patients with an over 50% risk of relapse and a less than 20%–25% risk of NRM are most likely to benefit from alloHCT performed in CR1. Therefore, assessing the risk of transplantation-related mortality is imperative in the decision-making process and there is a need for estimates related to procedures performed in the most recent era.2
In the current study, we focused on alloHCT performed in the period of 2008–2012, which reflected current clinical practice with regard to supportive care, HLA typing and other factors potentially influencing outcome. Results were compared with preceding periods. We were able to demonstrate a significant improvement in OS and LFS over time, which for the major part was related to reduced risk of NRM, and to a lesser extent the risk of relapse. For the entire population of adults between 18–55 years old treated between 2008 and 2012, the cumulative incidence of NRM at two years was 14.7% after MSD-HCT and 22.4% after URD-HCT.
The improvement over time has already been reported by Gooley et al. who compared results of transplantations performed between 2003 and 2007 with those performed between 1993 and 1997. In a heterogeneous population including 13% patients with ALL in various disease phases, the authors demonstrated reduction of the NRM rates from 41% to 26%.4 Hahn et al. reported general survival improvement for patients with hematologic malignancies observed over the period 1994–2005; however, the analysis included children, and data on NRM were not reported.5 In the previous analysis of the ALWP of the EBMT, we demonstrated that, for patients with acute leukemia treated with MSD-HCT in Eastern European countries, the rates of NRM decreased from 22% between 1990 and 2002 to 15% in the period 2002 and 2005.3 Two studies were restricted to patients with ALL, transplanted in both CR1 and CR2. In a single center experience from the University of Minnesota, USA, alloHCT performed between 2000 and 2005 were associated with a significant reduction of NRM compared to preceding periods (1990–1994 and 1995–1999).9 In a study by Wood et al., reduction of NRM from 43% between 1990 and 1995 to 31% between 2002 and 2007 was reported among adolescents and young adults, i.e. patients 18–40 years old.10
In the current analysis, the estimated rates of NRM were lower compared to most previously reported results. This may reflect further improvement which occurred in the most recent time period. As suggested by Gooley et al., reduction of NRM may be dependent on improving supportive care, but also on the reduction of the incidence of graft-versus-host disease.4 The latter could be a consequence of more accurate HLA typing, and in particular, the introduction of high resolution techniques in the process of unrelated donor search.11 On the other hand, it may be speculated that decreased NRM may be a consequence of more appropriate patient selection and that toxicity of conventional-dose chemotherapy decreased in parallel, positively affecting biological status of the transplant recipients. Results of our study indicate survival improvement after MSD-HCT for all age groups except for patients 26–35 years old. A trend towards reduction of NRM in this age category was counterbalanced by a tendency to increased RI. It may be speculated that selection criteria for alloHCT changed over time, and patients with higher risk of relapse were referred for transplantation in recent years. Among recipients of URD-HCT, the most prominent improvement could be shown in older age groups (36–55 years old), which, however, was mainly attributed to reduced RI.
Identification of prognostic factors in the most recent time period was a secondary goal of our study. Among donor/recipient-related variables, the use of unrelated donor and female donor to male recipient sex combination had the strongest impact, negatively affecting both the risk of NRM and survival. Several previous reports had suggested that results of MSD-HSCT and URD-HCT for patients with ALL may be comparable. In an analysis by Tomblyn et al., the use of well-matched or partially matched unrelated donor was not associated with inferior outcome compared to MSD.9 In a Japanese study, the OS rates after URD-HCT and MSD-HCT for patients in CR1 were superimposable; however, HLA disparities were associated with increased risk of NRM.12 In the present analysis, 29% of URD-HCT were performed across single or double HLA mismatch. However, in a univariate analysis this factor did not influence significantly any of the study outcomes (data not shown). Female donor to male recipient sex combination is a well-recognized risk factor associated with increased risk of graft-versus-host disease and NRM.13 Results of our study confirm that, whenever possible, this combination should be avoided.
The definition of high risk in adult ALL varies among countries and study groups; however, most of the stratification systems include high initial leukocyte count. This factor influences the overall outcome but its impact on results of alloHCT has not been well recognized. In our study, high WBC at diagnosis was a strong predictor of risk of relapse, treatment failure and survival. Our findings suggest the need for additional intervention in this patient population, e.g. therapy oriented to eradicate MRD prior to alloHSCT, intensification of the conditioning regimen, and close monitoring of MRD after alloHSCT followed by pre-emptive donor lymphocyte infusions.
General outcome of Ph-positive ALL improved with the introduction of tyrosine kinase inhibitors (TKIs).14 Results of a recent analysis by the ALWP of the EBMT indicate that using TKIs both pre- and post-transplant is associated with reduced risk of relapse and improved survival.15 Both pre-emptive or prophylactic use of imatinib after alloHCT may be considered.16 In the current analysis, the presence of t(9;22) was associated with increased risk of relapse but not overall mortality. As our study population was quite young, longer follow up may be needed in order to obtain a final evaluation of the effect on OS. On the other hand, it may be speculated that some patients with Ph-positive ALL who relapse after alloHCT may still be salvaged, possibly with the use of 2nd- or 3rd-generation TKIs. Furthermore, the probabilities of LFS for Ph-positive ALL improved markedly between 2008 and 2012 compared to preceding periods as a consequence of reduced incidence of both relapse and NRM (Online Supplementary Table S1 and Online Supplementary Figure S3). It could be speculated that the introduction of TKIs not only increased the treatment efficacy but also, by allowing for a reduction in chemotherapy intensity, contributed to a reduction in the overall toxicity.
Among procedure-related factors, the use of TBI-based conditioning was the strongest predictor of relapse and was associated with an over 50% reduction of the risk of this event. Although comparison of TBI with myeloblative chemotherapy in a setting of ALL has never been a subject of a prospective trial, some retrospective analyses confirmed the advantage of TBI.17,18 Results of the current study strongly support this statement. New, irradiation-free regimens based on the use of thiotepa are under development; however, their utility requires further evaluation.19 Our study is the largest to focus on alloHCT performed in a very large cohort of adults with ALL in CR1. However, it does have several limitations related to its retrospective nature. We were unable to analyze the reasons of NRM after transplantation. In addition, some important variables related to the disease characteristics were unavailable. Among karyotype features we focused on the Ph-status, while the effect of other known high-risk abberrations, e.g. t(4;11) and molecular markers, could not be evaluated. Furthermore, for the majority of patients, we were unable to collect MRD data, which is a well-recognized risk factor in a setting of ALL.20,21 Finally, selection procedures for patients to go forward to alloHCT could vary among centers and countries. The effect of center experience and national socio-economic status could have caused an additional bias.22
The role of alloHCT in first-line treatment of adults with ALL is a matter of debate in view of improving results of conventional-dose chemotherapy.23 In this study, we demonstrate that results of alloHCT improved in parallel, due to the reduced risk of both NRM and relapse. The improvement is observed in most age categories after both MSD-HCT and URD-HCT. Therefore, we conclude that current estimates of NRM justify the use of alloHCT as consolidation in patients with a high risk of relapse. TBI-based regimens should still be considered the preferable type of conditioning for patients with ALL in CR1.
Supplementary Material
Acknowledgments
The authors would like to thank all EBMT centers reporting their data for the purpose of this analysis. Fifty centers with the highest number of patients, are listed below:
Karolinska University Hospital, Stockholm, Sweden; CHU Bordeaux, Hôpital Haut-leveque, Pessac, France; University of Freiburg, Freiburg, Germany; University Hospital Eppendorf, Hamburg, Germany; University Hospital Leipzig, Leipzig, Germany; National University Hospital, Rigshospitalet, Copenhagen, Denmark; University Hospital Birmingham NHSTrust, Queen Elizabeth Medical Centre, Edgbaston, Birmingham, United Kingdom; University of Münster, Münster, Germany; Universitaetsklinikum Dresden, Dresden, Germany; Royal Marsden Hospital, London, United Kingdom; Medizinische Universitaet Wien, Vienna, Austria; Hannover Medical School, Hannover, Germany; Universität Tübingen, Tübingen, Germany; CHU Nantes, Nantes, France; University Hospital, Basel, Switzerland; Deutsche Klinik für Diagnostik, KMT Zentrum, Wiesbaden, Germany; West of Scotland Cancer Centre, Gartnaval General Hospital, Glasgow, United Kingdom; Ospedale di Careggi, Firenze, Italy; Azienda Ospedaliera Papa Giovanni XXIII, Bergamo, Italy; University Hospital, Bratislava, Slovakia; Institute of Hematology and Blood Transfusion, Prague, Czech Republic; A.O.U Citta della Salute e della Scienza di Torino, Torino, Italy; Imperial College, Hammersmith Hospital, London, United Kingdom; Inst. Português de Oncologia do Porto, Porto, Portugal; University of Heidelberg, Heidelberg, Germany; Northern Centre for Bone Marrow Transplantation, Newcastle-Upon-Tyne, United Kingdom; University Hospital, Zürich, Switzerland; George Papanicolaou General Hospital, Thessaloniki, Greece; Nottingham City Hospital, Nottingham, United Kingdom; Institut Universitaire du Cancer Toulouse, Toulouse, France; University Medical Center Mainz, Mainz, Germany; University Hospital Center Rebro, Zagreb, Croatia; Klinikum Grosshadern, Munich, Germany; Hopital Saint Antoine, Paris, France; Bologna University, S. Orsola-Malpighi Hospital, Institute of Hematology & Medical, Oncology L & A Seràgnoli, Bologna, Italy; Rambam Medical Center, Haifa, Israel; Centre Hospitalier Universitaire de Rennes, Rennes, France; Hopital d’Enfants, Vandoeuvre_Les_Nancy, France; Charles University Hospital, Pilsen, Czech Republic; Cliniques Universitaires St. Luc, Brussels, Belgium; St. István and St. László Hospital, Semmelweis University St. Laszlo, Budapest, Hungary; Nouvel Hopital Civil, Strasbourg, France; Heinrich Heine Universität, Düsseldorf, Germany; Christie NHS Trust Hospital, Manchester, United Kingdom; Chaim Sheba Medical Center, Chaim Sheba Medical Center, Tel-Hashomer, Israel; CHU Lapeyronie, Montpellier, France; Gustave Roussy, Institut de Cancérologie, Villejuif, France; Ospedale San Martino, Genova, Italy; University Hospital Schleswig-Holstein Kiel Campus, Kiel, Germany; Ankara University Faculty of Medicine, Ankara, Turkey; Centre de Recherche en Cancérologie de Marseille, Institut Paoli Calmettes, Marseille, France; Ospedale di Niguarda Cà Granda, Milano, Italy; Centre Pierre et Marie Curie, Alger, Algeria.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/102/1/139
References
- 1.Weiden PL, Flournoy N, Thomas ED, et al. Antileukemic effect of graft-versus-host disease in human recipients of allogeneic-marrow grafts. N Engl J Med. 1979; 300(19):1068–1073. [DOI] [PubMed] [Google Scholar]
- 2.Cornelissen JJ, van der Holt B, Verhoef GE, et al. Myeloablative allogeneic versus autologous stem cell transplantation in adult patients with acute lymphoblastic leukemia in first remission: a prospective sibling donor versus no-donor comparison. Blood 2009;113(6):1375–1382. [DOI] [PubMed] [Google Scholar]
- 3.Giebel S, Labopin M, Holowiecki J, et al. Outcome of HLA-matched related allogeneic hematopoietic stem cell transplantation for patients with acute leukemia in first complete remission treated in Eastern European centers. Better results in recent years. Ann Hematol. 2009;88(10):1005–1013. [DOI] [PubMed] [Google Scholar]
- 4.Gooley TA, Chien JW, Pergam SA, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010;363(22):2091–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hahn T, McCarthy PL, Jr, Hassebroek A, et al. Significant improvement in survival after allogeneic hematopoietic cell transplantation during a period of significantly increased use, older recipient age, and use of unrelated donors. J Clin Oncol. 2013; 31(19):2437–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18(6):695–706. [DOI] [PubMed] [Google Scholar]
- 7.Fine JP, Gray RJ. A proportional hazards model for subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. [Google Scholar]
- 8.Goldstone AH, Richards SM, Lazarus HM, et al. In adults with standard-risk acute lymphoblastic leukemia, the greatest benefit is achieved from a matched sibling allogeneic transplantation in first complete remission, and an autologous transplantation is less effective than conventional consolidation/maintenance chemotherapy in all patients: final results of the International ALL Trial (MRC UKALL XII/ECOG E2993). Blood 2008;111(4):1827–1833. [DOI] [PubMed] [Google Scholar]
- 9.Tomblyn MB, Arora M, Baker KS, et al. Myeloablative hematopoietic cell transplantation for acute lymphoblastic leukemia: analysis of graft sources and long-term outcome. J Clin Oncol. 2009; 27(22):3634–3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wood WA, Lee SJ, Brazauskas R, et al. Survival improvements in adolescents and young adults after myeloablative allogeneic transplantation for acute lymphoblastic leukemia. Biol Blood Marrow Transplant. 2014;20(6):829–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giebel S, Giorgiani G, Martinetti M, et al. Low incidence of severe acute graft-versus-host disease in children given haematopoietic stem cell transplantation from unrelated donors prospectively matched for HLA class I and II alleles with high-resolution molecular typing. Bone Marrow Transplant. 2003;31(11):987–993. [DOI] [PubMed] [Google Scholar]
- 12.Nishiwaki S, Miyamura K, Ohashi K, et al. Impact of a donor source on adult Philadelphia chromosome-negative acute lymphoblastic leukemia: a retrospective analysis from the Adult Acute Lymphoblastic Leukemia Working Group of the Japan Society for Hematopoietic Cell Transplantation. Ann Oncol. 2013; 24(6):1594–1602. [DOI] [PubMed] [Google Scholar]
- 13.Gratwohl A, Hermans J, Goldman JM, et al. Risk assessment for patients with chronic myeloid leukaemia before allogeneic blood or marrow transplantation. Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Lancet 1998; 352(9134): 1087–1092. [DOI] [PubMed] [Google Scholar]
- 14.de Labarthe A, Rousselot P, Huguet-Rigal F, et al. Imatinib combined with induction or consolidation chemotherapy in patients with de novo Philadelphia chromosome-positive acute lymphoblastic leukemia: results of the GRAAPH-2003 study. Blood 2007;109(4):1408–1413. [DOI] [PubMed] [Google Scholar]
- 15.Brissot E, Labopin M, Beckers MM, et al. Tyrosine kinase inhibitors improve long-term outcome of allogeneic hematopoietic stem cell transplantation for adult patients with Philadelphia chromosome positive acute lymphoblastic leukemia. Haematologica 2015;100(3):392–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pfeifer H, Wassmann B, Bethge W, et al. Randomized comparison of prophylactic and minimal residual disease-triggered imatinib after allogeneic stem cell transplantation for BCR-ABL1-positive acute lymphoblastic leukemia. Leukemia 2013; 27(6):1254–1262. [DOI] [PubMed] [Google Scholar]
- 17.Davies SM, Ramsay NK, Klein JP, et al. Comparison of preparative regimens in transplants for children with acute lymphoblastic leukemia. J Clin Oncol. 2000; 18(2):340–347. [DOI] [PubMed] [Google Scholar]
- 18.Eroglu C, Pala C, Kaynar L, et al. Comparison of total body irradiation plus cyclophosphamide with busulfan plus cyclophosphamide as conditioning regimens in patients with acute lymphoblastic leukemia undergoing allogeneic hematopoietic stem cell transplant. Leuk Lymphoma. 2013;54(11):2474–2479. [DOI] [PubMed] [Google Scholar]
- 19.Christopoulos P, Bertz H, Ihorst G, et al. Radiation-free allogeneic conditioning with fludarabine, carmustine, and thiotepa for acute lymphoblastic leukemia and other hematologic malignancies necessitating enhanced central nervous system activity. Biol Blood Marrow Transplant. 2012; 18(9):1430–1437. [DOI] [PubMed] [Google Scholar]
- 20.Holowiecki J, Krawczyk-Kulis M, Giebel S, et al. Status of minimal residual disease after induction predicts outcome in both standard and high-risk Ph-negative adult acute lymphoblastic leukaemia. The Polish Adult Leukemia Group ALL 4-2002 MRD Study. Br J Haematol. 2008;142(2):227–237. [DOI] [PubMed] [Google Scholar]
- 21.Bassan R, Spinelli O, Oldani E, et al. Improved risk classification for risk-specific therapy based on the molecular study of minimal residual disease (MRD) in adult acute lymphoblastic leukemia (ALL). Blood 2009;113(18):4153–4162. [DOI] [PubMed] [Google Scholar]
- 22.Giebel S, Labopin M, Mohty M, et al. The impact of center experience on results of reduced intensity: allogeneic hematopoietic SCT for AML. An analysis from the Acute Leukemia Working Party of the EBMT. Bone Marrow Transplant. 2013;48(2):238–242. [DOI] [PubMed] [Google Scholar]
- 23.Speziali C, Paulson K, Seftel M. Hematopoietic Cell Transplantation for Acute Lymphoblastic Leukemia in Adults. Curr Hematol Malig Rep. 2016;11(3):175–184. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


