Abstract
Mediastinal gray zone lymphoma, B-cell lymphomas with intermediate features between classical Hodgkin lymphoma and primary mediastinal B-cell lymphoma, have not been well described in the literature. We report the clinical characteristics and outcomes of a large retrospective series of 99 cases centrally reviewed by a panel of hematopathologists, with a consensus established for the diagnosis. Cases were defined as classical Hodgkin lymphoma-like morphology (64.6%) with primary mediastinal B-cell lymphoma immunophenotype, primary mediastinal B-cell lymphoma-like morphology (30.3%) with classical Hodgkin lymphoma or composite (5.1%) (synchronous occurrence of classical Hodgkin lymphoma and primary mediastinal B-cell lymphoma). The median age was 32 years (13–83 years); 55% were women. Thirteen of 81 evaluable cases (16%) were Epstein-Barr virus-positive. Twenty-eight percent of patients presented primary refractory disease (progression under first-line treatment or relapse within one year). The 3-year event-free and overall survival rates were 63% and 80%, respectively. Patients treated with a standard regimen (RCHOP/ABVD) had worse event-free survival (P=0.003) and overall survival (P=0.02) than those treated with a dose-intensive chemotherapy (high-dose RCHOP/escalated BEACOPP). Rituximab added to chemotherapy was not associated with better event-free survival (P=0.55) or overall survival (P=0.88). Radiotherapy for patients in complete remission had no impact on event-free survival. In multivariate prognostic analysis, ECOG-PS and anemia were the strongest factors associated with a shorter event-free survival and overall survival, respectively. In conclusion, this report describes the largest series of mediastinal gray zone lymphoma. Our data suggest that a dose-intensive treatment might improve the outcome of this rare and aggressive disease.
Introduction
Mediastinal gray zone lymphoma (MGZL), defined as B-cell lymphoma with intermediate features between primary mediastinal B-cell lymphoma (PMBCL) and classical Hodgkin lymphoma (CHL), is one of the provisional entities in the 2008 World Health Organization (WHO) classification1 based on different retrospective pathological reports from the literature.2–5 Although more recent publications defined a particular methylation profile for this entity,6,7 and a particular immunohistochemistry scoring system to distinguish it from CHL and PMBCL,8 there is a need for clarification of the clinico-pathological diagnostic criteria for MGZL. Previous retrospective studies4,5,9–12 (including from 2 to 112 cases) showed that patients with an MGZL had a poorer outcome than patients with a PMBCL or diffuse large B-cell lymphoma (DLBCL). The poor prognosis was confirmed by the prospective study of the National Cancer Institute that included 24 MGZL cases treated with rituximab and an intensified chemotherapy regimen (namely DA-EPOCH-R: dose adapted etoposide, prednisolone, oncovin, cyclophosphamide, doxorubicin) with a shorter progression-free survival (PFS) and overall survival (OS) as compared with PMBCL.13 The largest previously reported series of GZL between diffuse large B-cell lymphoma (DLBCL) and CHL included 112 patients, 43% with a mediastinal GZL and 57% without mediastinal involvement.11 At a 31-month median follow up, 2-year PFS and OS rates were 40% and 88%, respectively. Furthermore, they observed that rituximab combined with chemotherapy could improve patient prognosis.
To improve the clinico-pathological diagnostic criteria of MGZL from those of PMBCL and CHL, and to better assess treatment results using different therapeutic strategies, we conducted a retrospective analysis with a central pathological review of all the cases by a panel of hematopathologists from the Lymphoma Study Association (LYSA).
Methods
Pathological definition of MGZL and inclusion criteria
We retrospectively identified cases treated in French, Belgian and Portuguese LYSA centers suspected to be MGZL using local pathological records, records from LYSA centers, and from the LYMPHOPATH network, which aims to review all newly diagnosed lymphoma cases in France.14 All the FFPE blocks and immunohistochemistry (IHC) slides (obtained prior to any treatment) were centralized in the LYSA-Pathology (LYSA-P) department located in the Henri Mondor hospital in Paris to perform a central review by a panel of hematopathologists. This central review included 3 different steps. During the first meeting in the LYSA-P, the panel of 12 hematopathologists (ATG, TM, DD, PD, BF, CL, MP, LM, DC, BB, PG, CC) established a consensus for inclusion criteria based on previous pathological descriptions of MGZL from the literature.2–5,9 They identified 4 situations or subgroups, which are presented in the results section, and in Table 1 and Figure 1, to analyze patients’ characteristics and outcomes. As a second step, the panel reviewed all the cases with a multi-head microscope during 3 meetings. When at least 6 experts were in agreement on the diagnosis of MGZL, the case was included. As a third step, all the cases were reviewed a second time by 4 experts (ATG, TM, DD, MP).
Table 1.
Pathological criteria for diagnosis of gray zone lymphoma and subgroups.
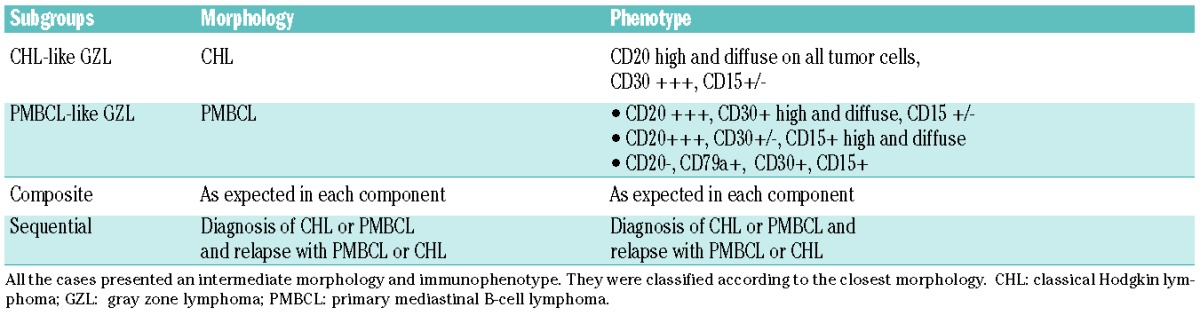
Figure 1.
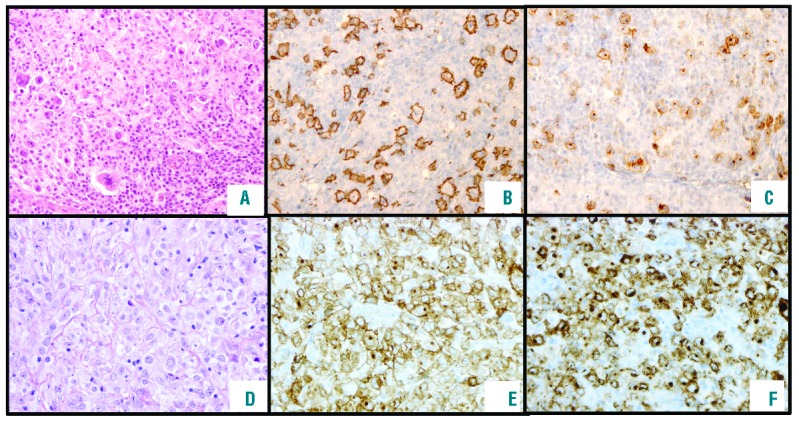
Pathological features of cases included in this series. (A) Classical Hodgkin lymphoma (CHL)-like morphology with tumoral cells resembling Hodgkin lymphoma cells but with less inflammatory background, and a frequent sheet of large cells associated with a phenotype of primary mediastinal B-cell lymphoma (PMBCL) (growth X10). (B) Strong and diffuse CD20 expression in all CHL-like lymphoma cells (growth X10). (C) CD30 expression in lymphoma cells (growth X10). (D) Mediastinal gray zone lymphoma (MGZL) with a primary mediastinal B-cell lymphoma-like morphology (growth X20) with an intermediate morphology with larger and more pleomorphic cells than in typical PMBCL and some cells resemble bi- or multinucleated Reed Sternberg cells (not shown) and sometimes inflammatory background with numerous eosinophils in association with a CHL phenotype. (E and F) High CD30 and CD15 expression in all lymphoma cells (growth X5).
Collection of clinical and outcome data
All cases confirmed by the pathology panel underwent clinical analysis and data were retrospectively collected in the patients’ files and anonymously coded. To limit the analysis to the clinico-pathological entity of MGZL, only cases with mediastinal involvement were included. Sequential cases with CHL at diagnosis and PMBCL at relapse or vice versa were excluded from the clinical and prognostic analysis, as they all presented an event by definition. Cases with a follow up without an event shorter than one year were also excluded (see flow chart in Supplementary Appendix). Treatments were classified as CHL-like chemotherapy and DLBCL-like chemotherapy. Response to treatment was assessed according to the revised and current lymphoma criteria.15,16 EFS was calculated from the date of diagnosis to the date of progression, a change of therapy that was not initially scheduled (radiotherapy, high-dose therapy with autologous stem cell transplantation and other unplanned treatments) or death from any cause. OS was calculated from date of diagnosis to date of death from any cause. Statistical analysis was performed with SAS software. Categorical variables were summarized using frequencies and percentages, and compared using the χ2 test. Univariate prognostic analysis was performed using the log-rank test. Variables with a P<0.05 in univariate analysis were included in a Cox regression model for multivariate prognostic analysis.
The study was conducted with the approval of the LYSA center’s ethics committees and the SUD-EST VI ethics committee (L15-118) according to French law and the Declaration of Helsinki.
Results
Pathological review and case inclusion
Two hundred and four cases were reviewed by the panel of LYSA hematopathologists. Only cases with an intermediate morphology and phenotype between CHL and PMBCL were included. Cases were considered CD20+ if all tumoral cells strongly expressed CD20. Cases of CHL with partial CD20 expression or low expression in tumoral cells were excluded and considered as CD20-positive CHL (Figure 1).
Among the 204 cases, 165 were included in the study as MGZL, and 39 did not fulfill the pathological inclusion criteria for MGZL and were classified as classical PMBCL (12 cases), CHL (20 cases including 15 cases of Nodular Sclerosis Hodgkin Lymphoma BNLI 2), T-cell rich large B-cell lymphoma (3 cases) or nodular lymphocyte predominant Hodgkin lymphoma (4 cases). These MGZL cases were then categorized according to the closest morphological features to well-defined subtypes (PMBCL or CHL): 103 (62%) had CHL-like MGZL, 44 (27%) PMBCL-like MGZL, 6 (4%) a composite (with a morphology of CHL on the one side and of PMBCL on the other side of the same diagnosis biopsy, n=4, or in two different biopsies performed at diagnosis, n=2), and 12 (7%) a sequential form (with a diagnostic biopsy of PMBCL and a relapse biopsy of CHL or vise versa). Among these 165 patients analyzed for pathological features, 23 cases were not further analyzed due to insufficient clinical data at diagnosis, 20 due to the absence of mediastinal involvement at diagnosis and 11 due to a follow-up period shorter than one year without event. Therefore, after exclusion of the 12 sequential patients, a total of 99 MGZL cases were ultimately included in the outcome analysis: 64 CHL-like, 30 PMBCL-like, and 5 composite cases.
Briefly, cases with CHL-like morphology had a morphology closer to CHL than to PMBCL with pleomorphic tumor cells including Reed Sternberg and Hodgkin-like cells in an inflammatory background and fibrotic stroma (Table 1). They had a strong and diffuse CD30 and CD20 expression. They also expressed one or several B-cell transcription factors (OCT2, BOB1, PAX5). Cases with PMBCL-like morphology had morphology closest to PMBCL with an important infiltrate of medium to large tumor cells, sparse fibrosis and more monomorphic background. They were all CD30 positive. CD30 weak cases were all CD15 positive with a B-cell marker. CD20 negative cases had an expression of CD79a or of a B-cell transcription factor with an expression of CD30 or CD15. In all cases, the morphology was always intermediate between CHL and PMBCL (Figure 1): CHL-like with tumoral cells resembling Hodgkin lymphoma cells with less inflammatory background and sheet of large cells or PMBCL-like with larger and more pleomorphic cells than in typical PMBCL, and some cells resemble bi- or multinucleated Reed Sternberg cells (data not shown).
Clinical and biological characteristics
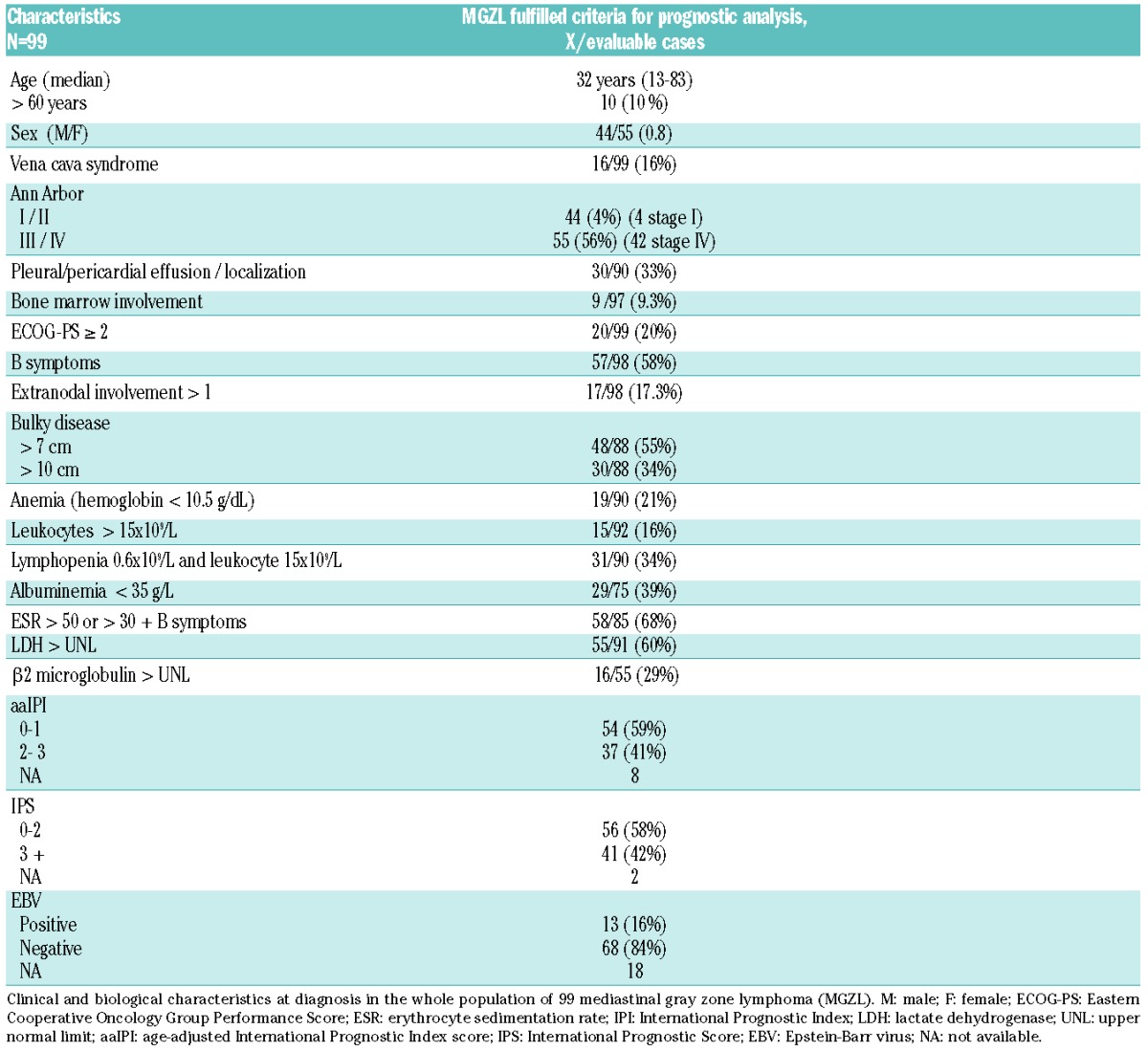
Clinical and biological characteristics are shown in Table 2. Among these 99 cases, 13 of 81 (16%) were associated with Epstein-Barr virus (EBV), as demonstrated by EBER in situ hybridization analysis, with a similar proportion of EBV+ cases among CHL-like and PMBCL-like subtypes. The median age of patients was 32 years (range 13–83 years), 55% were female, and 10 patients (10%) were over 60 years of age at diagnosis. The EBV-related cases were of similar age to the others (median age 29 vs. 32 years for EBV+ vs. EBV− cases, respectively). More than one-third of the patients (34%) had bulky disease with a threshold of 10 cm, and 16 (16%) suffered from superior vena cava syndrome at presentation. Fifty-five patients (55%) had Ann Arbor stage III–IV disease, 40 had stage II, and 4 had stage I. Anemia (<10.5 g/dL) was present in 21% of the patients and lymphopenia (<600/mm3) was present in 34%. The erythrocyte sedimentation rate (ESR) was elevated in 68% and the albumin level was low (<35 g/L) in 39%. The age-adjusted International Prognostic Index score (aaIPI) was low (0 or 1 factor) in 59% of the cases (54 patients) and high (2 or 3 factors) in 37 patients. The Hodgkin Lymphoma International Prognostic Score (HL IPS) score was low (0–2) in 56 patients (58%) and high (>2) in 41 patients.
Table 2.
Clinical and biological characteristics at diagnosis.
Treatments and outcome
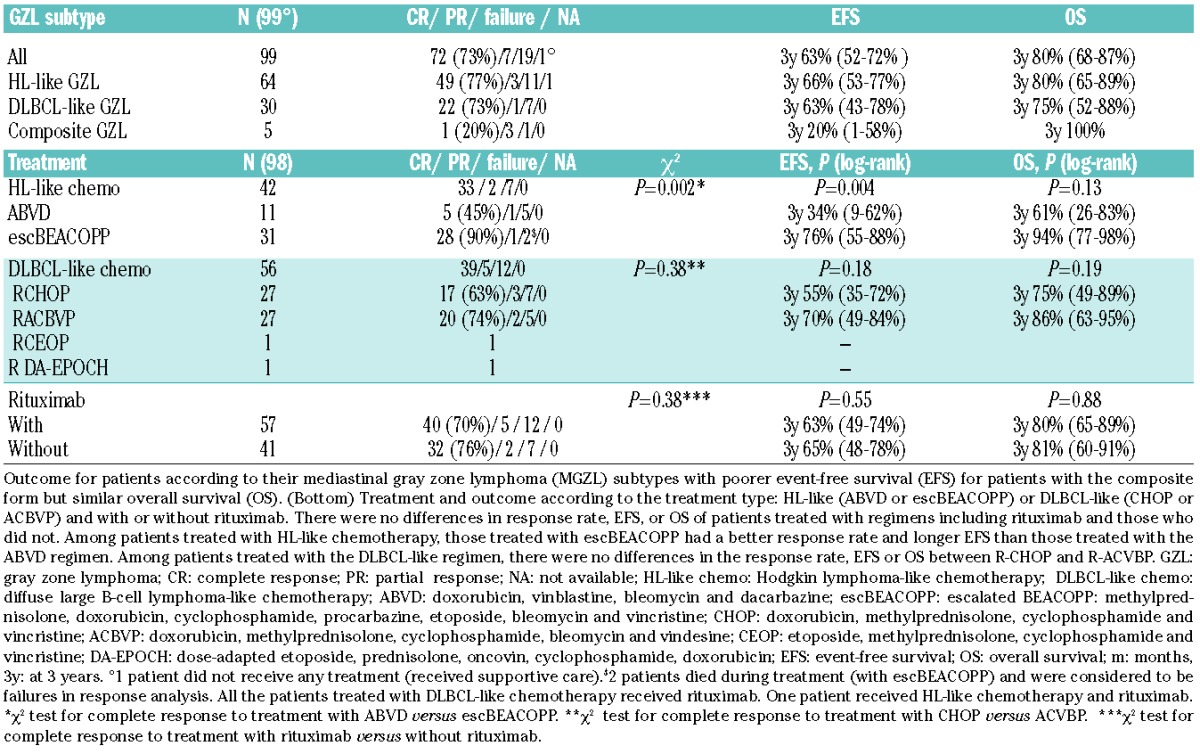
Treatments and outcome are shown in Table 3. Of all patients, 42 cases (43%) were treated with a CHL-like regimen and 56 (57%) with a DLBCL-like regimen. One 78-year old patient did not receive chemotherapy, only supportive care, due to poor performance status and comorbidities. The CHL-like regimen consisted of ABVD (doxorubicin, vinblastine, bleomycin, dacarbazine) or escBEACOPP (escalated BEACOPP: methylprednisolone, doxorubicin, cyclophosphamide, procarbazine, etoposide, bleomycin, vincristine). DLBCL-like treatments consisted of rituximab and chemotherapy, most frequently CHOP (doxorubicin, methylprednisolone, cyclophosphamide and vincristine) or a dose-intensive regimen, namely ACBVP17 (doxorubicin, methylprednisolone, cyclophosphamide, bleomycin, vindesine). Two patients received other DLBCL regimens: one patient DA-EPOCH-R (classified as dose-intensive) and one R-CEOP (doxorubicin replaced by etoposide, classified as non-dose-intensive) due to a contraindication to anthracycline. Among treated patients, the overall response rate was 81% (79 of 98), including 72 complete responses (CR, 73%) and 7 partial responses (PR, 7%). Seventeen patients had stable or progressive disease. Finally, 2 patients died during the treatment period from treatment-related toxicities and were considered as failures in response analysis. There were no differences in the CR rate between regimens including rituximab and those that did not (P=0.38) (Table 3). There were no differences in the CR rate between the R-CHOP and R-ACVBP regimen (P=0.38). Patients treated with ABVD had lower CR rates than those treated with escBEACOPP (45% vs. 90%; P=0.002, χ2 test).
Table 3.
Treatment and outcome according to gray zone subtypes.
Thirty-three patients exhibited disease progression or refractory disease within a median of 6.8 months from diagnosis (range 1.4–37.2 months). Among them, 28 had primary refractory disease; 23 had no CR to initial treatment (6 patients in PR that progressed within a year and 17 stable or progressive diseases). Five additional patients achieved a CR after initial treatment but relapsed within the first year after diagnosis. Among the 7 patients in PR, 6 progressed within a year, and one with a persistent mediastinal mass reached CR with additional radiotherapy (36 Gy) (median FU in CR of 6 years). Finally, 5 patients experienced a relapse more than one year after diagnosis but with a median time of only 14 months between diagnosis and relapse.
After a median follow up of 34 months (0.4–198.5 months), the estimated EFS at three years was 63% (95%CI:52%–72%) and OS 80% (95%CI: 68%–87%) (Figure 2A and B). A total of 20 patients died, 17 of them from a cause related to lymphoma and 2 (10%) from treatment-related toxicities (both received escBEACOPP regimen). Three-year EFS was 34% (95%CI: 9%–62%), 76% (95%CI: 55%–88%), 55% (95%CI: 35%–72%), and 70% (95%CI: 49%–84%) for ABVD, escBEACOPP, R-CHOP and R-ACVBP regimens, respectively. escBEACOPP regimen offered a longer EFS than ABVD regimen (P=0.004). The 3-year OS for patients treated with ABVD, escBEACOPP, R-CHOP or R-ACVBP, was 61% (95%CI: 26%–83%), 94% (95%CI: 77%–98%), 75% (95%CI: 49%–89%), and 86% (95%CI: 63%–95%), respectively, without a significant difference between them. There were no differences in EFS or OS for patients treated with or without rituximab (P=0.55 and 0.88 for EFS and OS, respectively).
Figure 2.
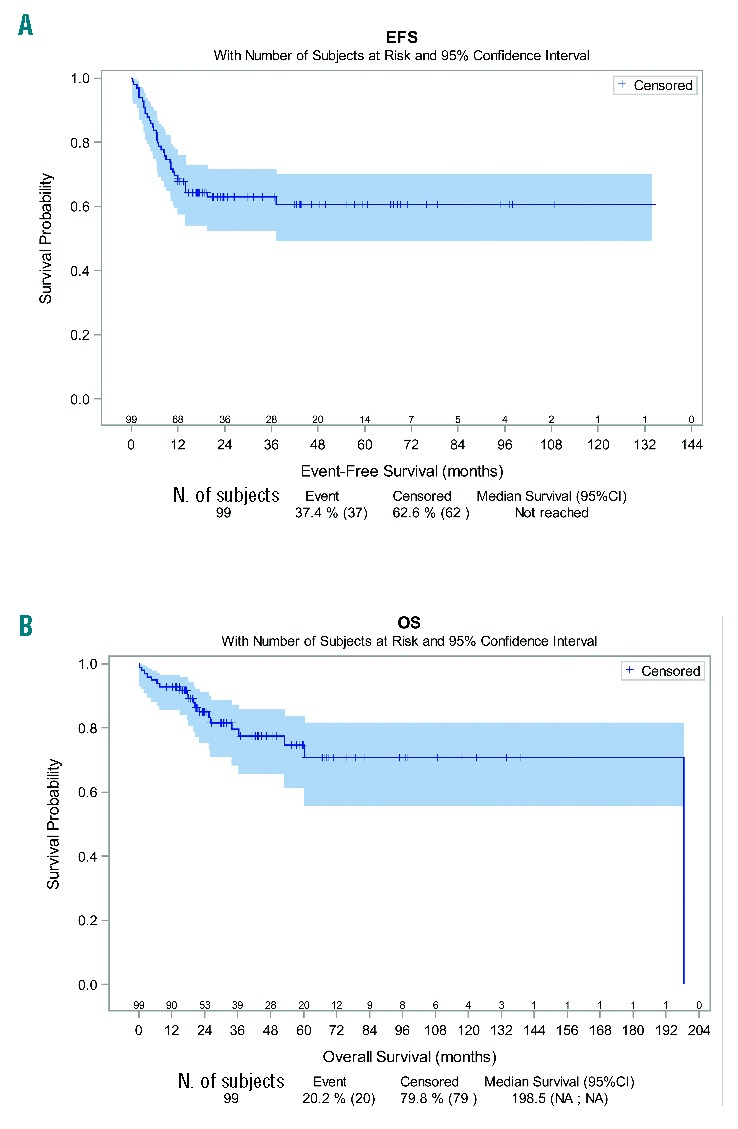
Event-free survival (EFS) and overall survival (OS) in the global population of mediastinal gray zone lymphoma (MGZL). After a median follow up of 34 months (range 0.4–198.5 months) according to reverse Kaplan-Meier method, the estimated EFS at three years was 63% (95%CI: 52%–72%) and OS was 80% (95%CI: 68–87%). NA: not available.
Event-free survival of the 59 patients treated with dose-intensive regimens (R-ACVBP, n=27 patients, DA-EPOCH-R, n=1 patients, or escBEACOPP, n=31 patients) (60%) was better than the 39 patients treated with a less intensive regimen (R-CHOP, n=27 patients, ABVD, n=11 patients, or R-CEOP, n=1 patient) (40%); estimated EFS at three years was 74% (95%CI: 60%–83%) versus 48% (95%CI: 31%–63%) for patients treated with a dose-intensive versus standard regimen, respectively, (HR=0.38, 95%CI: 0.20–0.75; P=0.003). Patients treated with a dose-intensive regimen also had a longer OS [3-year estimate of 90% (95%CI: 78%–96%) versus 67% (95%CI: 47%–81%) for patients treated with a standard regimen, respectively (HR=0.33, 95%CI: 0.13–0.89; P=0.02)]. As we observed that 9 and 0 patients were older than 60 years of age in standard-dose and intensive regimens, respectively, we restricted our analysis to patients under 60 years of age. The 59 patients treated with a dose-intensive regimen maintained a longer EFS than the 30 treated with a less intensive regimen, with a 3-year EFS estimate of 74% (95%CI: 60%–83%) versus 46% (95%CI: 28%–63%), respectively [(HR=0.37, 95%CI: (0.18–0.74); P=0.003)]. There was a trend for a better OS with a 3-year estimated OS of 90% (95%CI: 78%–96%) versus 72% (95%CI: 49%–86%) for patients treated with a dose-intensive and a standard regimen, respectively (HR=0.39, 95%CI: 0.14–1.10; P=0.06) (Figure 3A and B).
Figure 3.
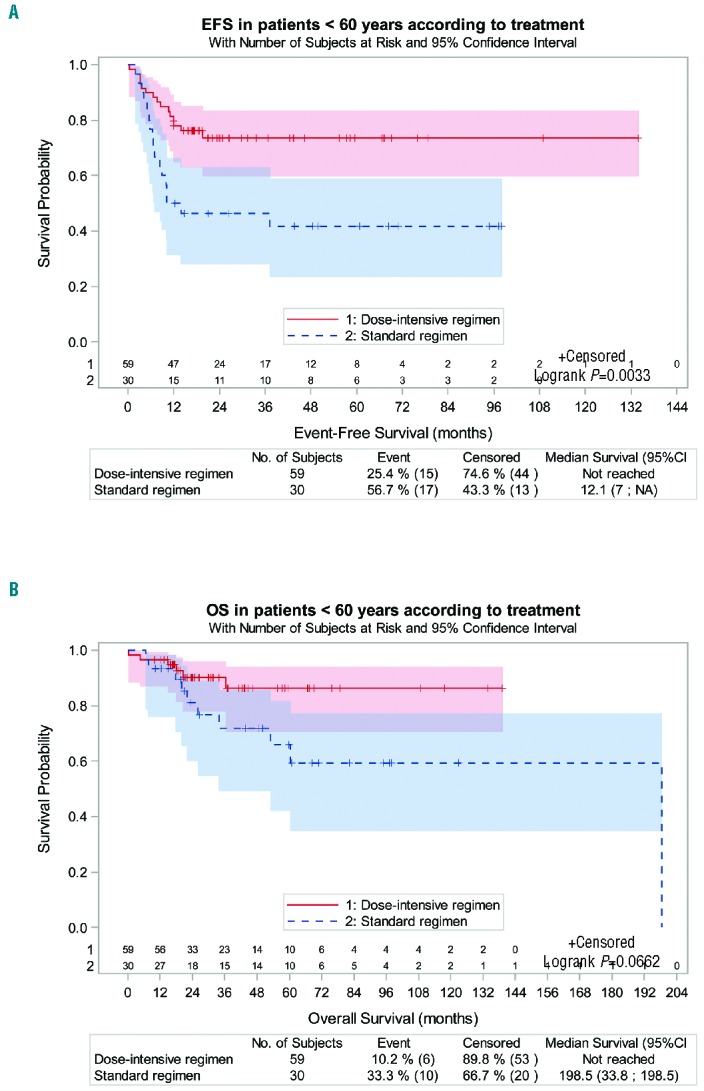
(A and B) Event-free survival (EFS) and overall survival (OS) in the population of patients under 60 years of age according to whether a dose-intensive chemotherapy regimen was administered. To avoid age-related biases, only patients under 60 years of age for whom a dose-intensive regimen could be proposed were included in this analysis. The dose-intensive regimen consisted of escalated (esc)BEACOPP (n=31), R-ACVBP (n=27) or DA-EPOCH-R (n=1). The standard regimen consisted of R-CHOP (n=19), ABVD (n=10) and R-CEOP (n=1). The 3-year estimated EFS was 74% (95%CI: 60%–84%) for patients treated with a dose-intensive regimen versus 46% (95%CI: 28%–63%) for those treated with a standard regimen, with a statistically significant difference [P=0.003, HR=0.37, (95%CI: 018–0.74)]. The 3-year estimated OS was 90% (95%CI: 78%–96%) for patients treated with a dose-intensive regimen, which was higher than for patients treated with a standard regimen [72% (95%CI: 49%–86%)], although the difference was not statistically significant; P=0.06, Hazard Ratio 0.39, (95%CI: 0.14–1.1). ABVD: doxorubicin, vinblastine, bleomycin, dacarbazine; escBEACOPP, escalated BEACOPP: methylprednisolone, doxorubicin, cyclophosphamide, procarbazine, etoposide, bleomycin, vincristine; CHOP: doxorubicin, methylprednisolone, cyclophosphamide, vincristine; ACBVP: doxorubicin, methylprednisolone, cyclophosphamide, bleomycin, vindesine; CEOP: etoposide, methylprednisolone, cyclophosphamide, vincristine; DA-EPOCH: dose-adapted etoposide, prednisolone, oncovin, cyclophosphamide, doxorubicin; R: rituximab; NA: not available.
Seventeen patients of 72 (24%) in CR received the initially planned radiation therapy, with no differences in terms of OS (P=0.38) or EFS (P=0.74) between patients treated without radiation after CR. Only 2 patients were treated with high-dose chemotherapy followed by autologous stem cell transplant (ASCT) as a consolidation therapy after first-line treatment and both are in persistent CR (at 69 and 19 months of follow up, respectively).
We then analyzed the outcome data according to the different MGZL subtypes. Among the 64 patients with CHL-like MGZL, 34 (53%) were treated with a DLBCL-like regimen (including rituximab in all patients), 29 (47%) with a CHL-like regimen and one patient received palliative care. Their 3-year estimated EFS was 66% (95%CI: 53%–77%). The majority of patients with PMBCL-like MGZL (23 of 30, 77%) received a DLBCL-like regimen and 7 received a CHL-like regimen. Their 3-year estimated EFS was comparable to that of CHL-like GZL: 63% (95%CI: 43%–78%). The 5 patients with a composite MGZL treated with RCHOP (n=3), RACVBP (n=1) and ABVD (n=1) had a shorter EFS compared to the 94 patients with the other MGZL subtypes [3-year EFS of 20% (95%CI: 1–58%) vs. 65% (95%CI: 54%–74%)] but had a similar OS (80%, 75% and 100% for CHL, PMBCL-like and composite MGZL, respectively), although the low number of patients with a composite MGZL limits evaluation.
Outcome after progression and salvage options
The estimated median OS post relapse was 30 months (95%CI: 15–189), with 17 deaths among 33 progressive and relapsed patients. Twenty-nine patients received a chemotherapy-based salvage regimen (19 associated with rituximab) and 4 received palliative care. Fifteen patients responded to first-line salvage, 2 had a stable disease and 12 progressed. Eighteen patients received high-dose therapy after salvage (first or second line of salvage): 16 followed by ASCT and 2 followed by allotransplant. Among them, 11 (69%, 9 ASCT and 2 allotransplant) achieved a CR with a follow up of 30 months after relapse. Among the 11 patients who received salvage chemotherapy without transplantation, 3 (20%) were in CR after 2, 30 and 60 months of follow up, respectively. Finally, among the 33 relapsed/refractory patients, 11 patients had a prolonged second CR longer than one year after salvage therapy.
Prognostic factors
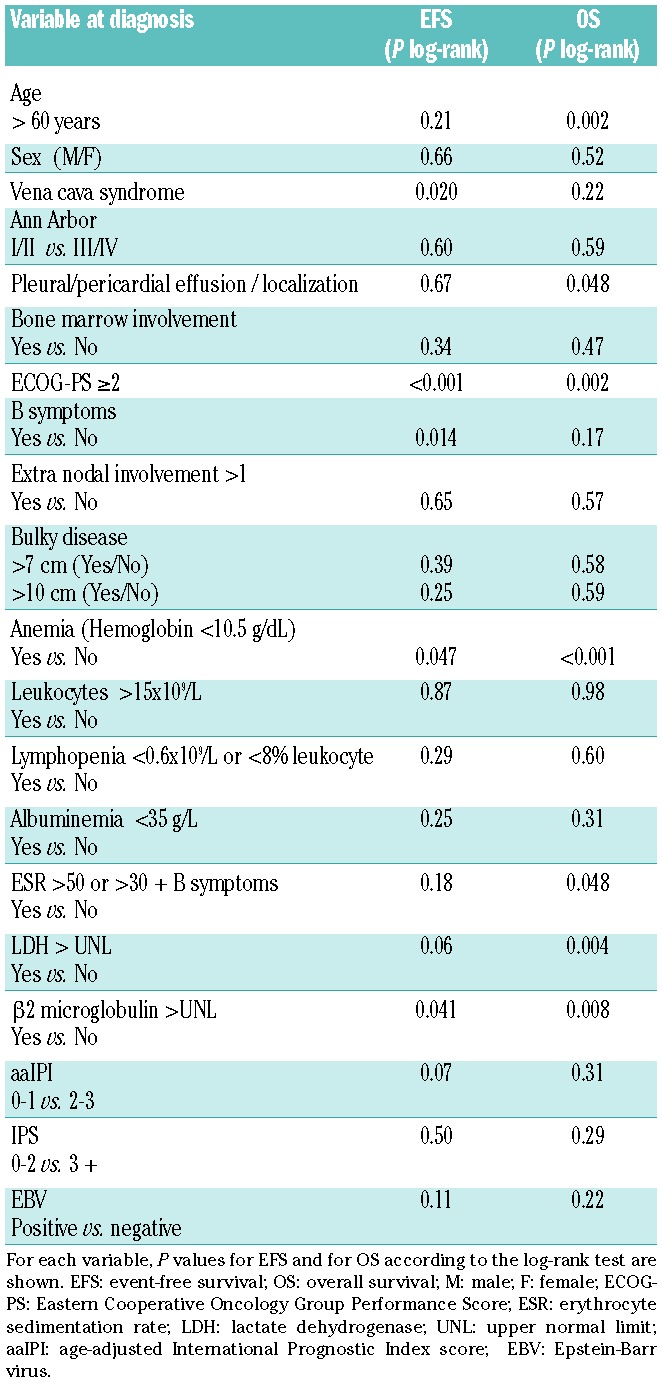
In the univariate prognostic analysis, a vena cava syndrome, an Eastern Cooperative Oncology Group Performance Score (ECOG-PS) over 1, anemia (<10.5 g/dL), presence of B symptoms, and high β2 microglobulin level at diagnosis were all associated with a shorter EFS. Age over 60 years, presence of pleural or pericardial effusion, ECOG-PS over 1, anemia, high lactate dehydrogenase (LDH) level, high β2 microglobulin level and ESR (>50 or >30 with B symptoms) were associated with a shorter OS (Table 4). In a multivariate analysis (n=88 patients with available data), including the relevant prognostic factors for EFS and OS in univariate analysis (vena cava syndrome, ECOG-PS, anemia, B symptoms for EFS and age, ECOG-PS, anemia and LDH for OS) and the treatment strategy (dose-intensive regimen or standard regimen), ECOG-PS was the only factor significantly associated with shorter EFS [P=0.04, HR 2.4, CI (1.1–5.7)]. A trend for an association with EFS was observed for B symptoms (HR=2.1, 95%CI: 0.9–4.9; P=0.07) and dose-intensive treatment (HR=0.5, 95%CI: 0.2–1.3; P=0.1). Anemia was the only factor associated with OS [P=0.02, HR=4.4, 95%CI: (1.2–16)].
Table 4.
Prognostic factors for event-free survival (EFS) and overall survival (OS) in univariate analysis.
Discussion
We report the largest series of MGZL with a central pathological review in the LYSA-P center performed by a panel of hematopathologists. Based on the literature data and according to the advice of different experts in hematopathology, a consensus on pathological criteria was established for the inclusion of cases. This series, like others,2,3,6 includes a few cases that present both CHL and PMBCL features on the same biopsy (composite lymphoma) or in 2 biopsies at different times (sequential lymphoma). These cases should be considered as the extremities of the spectrum, as described in the WHO classification and in other GZL series.1–3,6,18 Indeed, 2 sequential cases were previously reported to have identical lymphoma clones in both biopsies,3 and both components of composite lymphoma had the same methylation profile as other MGZL in another report.6 Clinico-pathological characteristics of the 99 patients showed that patients were young, as previously reported,2 but with a small female predominance, in contrast to other reports.2,11,13 This difference might be related to the limited number of patients in each study. The majority of the cases had CHL-like morphology. Only 6 cases were composite with 2 distinct patterns (CHL and PMBCL in the same biopsy or 2 distinct biopsies at diagnosis). Except for these cases, morphology was always intermediate between CHL and PMBCL and the immune phenotype was inversely correlated with the closest morphology (CHL-like morphology with a PMBCL-like phenotype and vice versa). Thirteen cases were EBV positive. Among them, 4 had a morphology closer to PMBCL than CHL and the diagnosis of CHL was clearly ruled out. Nine had a morphology closer to CHL than PMBCL. They were not considered as CHL because they had conserved B-cell program markers on each tumor cell (a strong CD20 expression on all tumor cells and expression of B-cell transcription factors). Nevertheless, the diagnosis of these EBV-associated CHL-like cases remain challenging. Half of the patients in the CHL-like subgroup were treated with a CHL-like regimen and half with a DLBCL-like regimen. The majority of the patients in the PMBCL-like subgroup received a DLBCL-like treatment. The variability in treatment administered within each subgroup demonstrates inter-clinician variability and the poor reproducibility of diagnosis over the past 15 years.
The overall response rate was 80% in our report, including 73% CR, as compared to 71% ORR and 59% CR in the study by Evens et al.11 The 2-year estimated EFS in the present report is slightly higher than in Evens et al. (63% vs. 40%), but the 2-year OS was similar in both reports (85% vs. 88%). The difference might be related to the first-line strategy with more dose-intensive regimen administered in our series. Most of the cases of progression observed here (85% of the relapses) occurred within the first year after diagnosis. These data suggest that MGZL is an aggressive disease that requires an efficient first-line therapeutic strategy.
Rituximab did not appear to provide any added benefit either in the whole population or in each different subgroup. This lack of benefit might be related to the utilization of a dose-intensive regimen or to the lack of statistical power with a relatively low number of patients included in this retrospective cohort. These data differ from those previously reported11 and may require further investigation. Radiotherapy for patients in CR did not seem to add any benefit in terms of EFS or OS; nevertheless, one patient in PR converted to CR thanks to mediastinal radiation. Regarding the different chemotherapy regimens, ABVD seems to be the less efficient regimen, with a significantly lower CR rate and EFS when compared with escBEACOPP (CR rate 45% vs. 90%; EFS 4% vs. 76% at 3 years for the ABVD and escBEACOPP regimen, respectively), and a strong trend toward shorter OS (61% vs. 94% at 3 years for ABVD and escBEACOPP, respectively). According to Evens et al., patients treated with ABVD also showed an inferior CR rate and PFS, with 23% PFS at two years,11 and a prolonged PFS for the DA-EPOCH-R regimen (68% at 2 years). The difference in EFS for the whole population between our report and the study by Evens et al. might be related to the different treatment strategies, with 51% and 30% of the patients treated with ABVD and R-CHOP, respectively, in their analysis compared to 11% and 28%, respectively, in our report where the other patients (60%) were treated with more intensive regimens. With the dose-intensive DA-EPOCH-R regimen, Wilson et al.13 reported a 5-year EFS and OS of 62% and 74%, respectively, similar to our results (61% EFS and 75% OS at 5 years). The comparison of dose-intensive versus standard regimens presented in our series is in line with the Wilson et al. report and suggests that MGZL patients might benefit from a dose-intensive chemotherapy that allowed longer EFS and OS (P=0.003 and 0.02, respectively), even in the young and fit population (P=0.003 and 0.06, respectively). We report 2 treatment-related deaths (9.5%) with the escBEACOPP regimen. Finally, after salvage, when achievable, ASCT seemed to be of benefit to the responsive patients.
Taken together, these data suggested that MGZL is an aggressive disease with a high rate of primary refractory patients. Therefore, according to our data, and considering the possible biases related to the retrospective aspect of the study, a dose-intense regimen should be proposed whenever possible for younger patients and whenever classical regimens such as ABVD or R-CHOP appear inadequate. However, the best intensive regimen needs to be defined. New regimens including targeted therapies should be developed for unfit and older patients for whom intensive treatment is inadequate. Indeed, for example, checkpoint inhibitors (as in CHL), or XPO1 inhibitors (as in PMBCL) should be evaluated in MGZL. All these cases also expressed CD30 that could be targeted with Brentuximab vedotin.
In the univariate prognostic analysis including clinico-biological markers, the presence of vena cava syndrome, pleural or pericardial effusion, anemia, B symptoms, ECOG-PS over 1, high β2 microglobulin level and ESR were associated with poor EFS and/or OS. The significance of vena cava syndrome and pleural or pericardial effusion is a specific characteristic of PMBCL19 that might explain why these factors were not relevant in the report by Evens et al. that included less than 50% of patients with a mediastinal presentation. The presence of an altered ECOG-PS was the most important prognostic factor for EFS in our series, as in Evens et al.11 In contrast to other reports,11,13 disease stage or lymphopenia were not associated with outcome, but anemia was the only factor independently associated with poor OS. There was no significant difference in outcome between the 3 different MGZL subgroups.
The most important weakness of our study is inherent to its retrospective nature and the possible lack of uniformity of the patients’ clinical evaluation, treatment and follow up. However, we present here the largest series of MGZL with a central pathological review performed by an expert panel.
In conclusion, according to the consensus on the criteria established by the literature data and confirmed by our panel of hematopathologists, a large series of MGZL, intermediate between PMBCL and CHL, could be identified. The rate of primary refractory disease and poor results of the standard chemotherapy regimen suggest that an alternative dose-intensive treatment may be required for these patients. The pathological diagnosis remains challenging and requires a large immunohistochemical panel (especially B-cell transcription factors) and expert review. In our series, rituximab did not seem to improve patients’ outcome. A meta-analysis including all cases reported in the literature could be helpful to determine whether rituximab should be systematically combined with CT for these patients. There is a need to design a prospective trial with inclusion criteria based on pathological review in order to define the best type of treatment for these patients. Biological analyses are warranted to improve the accuracy of MGZL diagnosis and find new biological pathways for therapeutic objectives.
Supplementary Material
Acknowledgments
LYSA and the LYSA Pathology group: Véronique Jalloux, Nadine Vaihen, Stéphanie Cox, Aurélie Gaultier, and Anne-Sophie Veillard.
We thank the lymphopath consortium for sending their samples and for their participation in the consensus meeting: Prof. P. Gaulard and C. Copie-Bergman (Hôpital Henri Mondor, Créteil, France); Prof. V. Costes Martineau and V Slazweski (CHU de Montpellier, France); Dr. J Brière and V Meignan (CHU Saint Louis, Paris, France); Dr. I. Soubeyran (Institut Bergonié, Bordeaux, France); Dr. C Chassagne-Clement (Centre Léon Bérard, Lyon, France); Prof. L. Xerri (Institut Paoli Calmettes, Marseille, France); Dr. A Moreau and Dr. C Bossard (CHU de Nantes, France); Prof. M.C. Coppin and Dr. Bouchindhonne (CHU de Lille, France); Dr. J.M. Picquenot (Centre Henri Becquerel, Rouen, France); Dr. F Charlotte (CHU la Pitié Salpétrière, Paris, France); Dr. M Patey (CHU Reims); Dr. T Petrella and Dr. P Tas (CHU Dijon, France).
We thank the LYSA center for collecting clinical data: Dr. Anne Bannos (Bayonne), Dr. Julien Lazarovici (IGR), Dr. Jérome Cornillon (Saint Etienne), Prof. Olivier Casasnovas (Dijon), Prof. Guillaume Cartron (Montpellier), Dr. Rémy Gressin (Grenoble), Dr. Alexandre Morel (Necker), Dr. Sylvain Carras (Grenoble), Dr. Nicolas Daguindau (Annecy), Dr. Reman (Caen), Dr. Bruno Anglaret (Valence), Dr. Morgane Cheminant and Dr. Richard Delarue (CHU Necker, Paris), Prof. Roch Houot and Dr. Tony Marchand (Rennes), Prof. Steven LeGouill (Nantes), Prof. Arnaud Jaccard (Limoges), Dr. Luc Fornecker (Strasbourg), Dr. Sophie Cereja (CH Sud Francilien), Dr. Jérôme Cornillon (CH Saint Etienne), Dr Richard Lemal and Prof. Olivier Tournillac (Clermont-Ferrand).
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/102/1/150
References
- 1.Swerdlow SH, Campo E, Harris NL, et al. WHO classification of tumors of haematopoietic and lymphoid tissues. 4th ed. Lyon: International Agency for Research on Cancer, 2008. [Google Scholar]
- 2.Traverse-Glehen A, Pittaluga S, Gaulard P, et al. Mediastinal Gray Zone Lymphoma: The Missing Link Between Classic Hodgkin’s Lymphoma and Mediastinal Large B-Cell Lymphoma. Am J Surg Pathol. 2005;29(11):1411–1421. [DOI] [PubMed] [Google Scholar]
- 3.Quintanilla-Martinez L, Fend F. Mediastinal gray zone lymphoma. Haematologica. 2011;96(4):496–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Minami J, Dobashi N, Asai O, et al. Two cases of mediastinal gray zone lymphoma. J Clin Exp Hematop. 2010;50(2):143–149. [DOI] [PubMed] [Google Scholar]
- 5.Gualco G, Natkunam Y, Bacchi CE. The spectrum of B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma and classical Hodgkin lymphoma: a description of 10 cases. Mod Pathol. 2012;25(5):661–674. [DOI] [PubMed] [Google Scholar]
- 6.Eberle FC, Rodriguez-Canales J, Wei L, et al. Methylation profiling of mediastinal gray zone lymphoma reveals a distinctive signature with elements shared by classical Hodgkin’s lymphoma and primary mediastinal large B-cell lymphoma. Haematologica. 2011;96(4):558–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eberle FC, Salaverria I, Steidl C, et al. Gray zone lymphoma: chromosomal aberrations with immunophenotypic and clinical correlations. Mod Pathol. 2011;24(12):1586–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Malley DP, Fedoriw Y, Weiss LM. Distinguishing Classical Hodgkin Lymphoma, Gray Zone Lymphoma, and Large B-cell Lymphoma: A Proposed Scoring System. Appl Immunohistochem Mol Morphol. 2016;24(8):535–540. [DOI] [PubMed] [Google Scholar]
- 9.Dunleavy K, Grant C, Eberle F, Pittaluga S, Jaffe E, Wilson W. Gray zone lymphoma: better treated like hodgkin lymphoma or mediastinal large B-cell lymphoma? Curr Hematol Malig Rep. 2012;7(3):241–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunleavy K, Wilson WH. Primary mediastinal B-cell lymphoma and mediastinal gray zone lymphoma: do they require a unique therapeutic approach? Blood. 2015; 125(1):33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evens AM, Kanakry JA, Sehn LH, et al. Gray zone lymphoma with features intermediate between classical hodgkin lymphoma and diffuse large B-cell lymphoma: Characteristics, outcomes, and prognostication among a large multicenter cohort. Am J Hematol. 2015;90(9):778–783 [DOI] [PubMed] [Google Scholar]
- 12.Garcia JF, Mollejo M, Fraga M, et al. Large B-cell lymphoma with Hodgkin’s features. Histopathology. 2005;47(1):101–110. [DOI] [PubMed] [Google Scholar]
- 13.Wilson WH, Pittaluga S, Nicolae A, et al. A prospective study of mediastinal gray-zone lymphoma. Blood. 2014;124(10):1563–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laurent C, Delas A, Gaulard P, et al. Breast implant-associated anaplastic large cell lymphoma: two distinct clinicopathological variants with different outcomes. Ann Oncol. 2016;27(2):306–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheson B, Pfistner B, Juweid M, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;10;25(5): 579–586. [DOI] [PubMed] [Google Scholar]
- 16.Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for Initial Evaluation, Staging, and Response Assessment of Hodgkin and Non-Hodgkin Lymphoma: The Lugano Classification. J Clin Oncol. 2014;32(27):3059–3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Recher C, Coiffier B, Haioun C, et al. Intensified chemotherapy with ACVBP plus rituximab versus standard CHOP plus rituximab for the treatment of diffuse large B-cell lymphoma (LNH03-2B): an open-label randomised phase 3 trial. Lancet. 2011;26;378(9806):1858–1867. [DOI] [PubMed] [Google Scholar]
- 18.Quintanilla-Martinez L, de Jong D, de Mascarel A, et al. Gray zones around diffuse large B cell lymphoma. Conclusions based on the workshop of the XIV meeting of the European Association for Hematopathology and the Society of Hematopathology in Bordeaux, France. J Hematopathol. 2009;2(4):211–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aoki T, Izutsu K, Suzuki R, et al. Prognostic significance of pleural or pericardial effusion and the implication of optimal treatment in primary mediastinal large B-cell lymphoma: A multicenter retrospective study in Japan. Haematologica. 2014; 99(12):1817–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


