Abstract
POEMS syndrome is a rare para-neoplastic syndrome secondary to a plasma cell dyscrasia. Effective treatment can control the disease-related symptom complex. We describe the clinical outcome of autologous stem cell transplantation for patients with POEMS syndrome, determining the impact of patient- and disease-specific factors on prognosis. One hundred and twenty-seven patients underwent an autologous stem cell transplantation between 1997–2010 with a median age of 50 years (range 26–69 years). Median time from diagnosis to autologous stem cell transplantation was 7.5 months with 32% of patients receiving an autologous stem cell transplantation more than 12 months from diagnosis. Engraftment was seen in 97% patients and engraftment syndrome was documented in 23% of autologous stem cell transplantation recipients. Hematologic response was characterized as complete response in 48.5%, partial response in 20.8%, less than partial repsonse in 30.7%. With a median follow up of 48 months (95%CI: 38.3, 58.6), 90% of patients are alive and 16.5% of patients have progressed. The 1-year non-relapse mortality was 3.3%. The 3-year probabilities of progression-free survival and overall survival are 84% and 94%, respectively, with 5-year probabilities of progression-free survival and overall survival of 74% and 89%. In a cohort of graft recipients, detailed organ-specific symptom response demonstrated clear symptom benefit after autologous stem cell transplantation especially in relation to neurological symptom control. The data analyzed in this study demonstrate the clinical utility of autologous stem cell transplantation for patients with POEMS syndrome.
Introduction
POEMS syndrome, a rare plasma cell dyscrasia which results in a complex of para-neoplastic clinical manifestations,1,2 is characterized by polyradiculoneuropathy, organomegaly, multiple endocrinopathies, monoclonal protein, and dermopathy. Patients may experience sclerotic bone lesions, extravascular fluid overload (including pleural effusions and ascites), papilledema, thrombocytosis, and pulmonary hypertension. Elevated serum levels of vascular endothelial growth factor (VEGF), which strongly promotes vaso-permeability, are considered to be responsible for the characteristic symptoms of POEMS syndrome3 and can be used for diagnostic purposes. Different angiogenic factors such as basic fibroblast growth factor and hepatocyte growth factor (HGF) were reported to be elevated in patients with POEMS syndrome, suggesting that different angiogenic factors might contribute to the pathogenesis, implying a limitation of clinical effect from direct VEGF blockade alone.4 Interleukin (IL)-12 has been reported to be elevated in the serum of patients with POEMS syndrome, in association with other pro-inflammatory cytokines such as IL-6 and TNFα, which may have an etiological role in the pathogenesis of peripheral nerve demyelination.5
The treatment of POEMS syndrome involves eliminating the plasma cell clone, though the optimal management has yet to be delineated, especially the role of high-dose therapy and the use of novel biological drugs.6 When compared with patients with myeloma (MM) or light chain deposition disease, patients with POEMS syndrome have a superior overall survival (OS). Previously published data from the Mayo Clinic demonstrated a median OS of 13.8 years.1 The therapeutic management has evolved over the years, much in parallel with new drug developments in myeloma. In patients with localized disease, irradiation remains the treatment of choice.2 More recently, steroids and conventional chemotherapy have demonstrated good disease control.7 Anti-VEGF monoclonal antibodies may hold potential efficacy but are associated with significant risks. Immunomodulatory drugs (e.g. thalidomide, lenalidomide and, more recently, pomalidomide) are highly effective in MM, and their use in the POEMS syndrome is evolving, though side effects can be more pronounced, such as neuropathic effects of thalidomide.8–10 Dipenzieri et al. first reported the efficacy of lenalidomide in a patient who did not qualify for high-dose autologous stem cell transplantation (HDM/ASCT).11 More recently, two studies have demonstrated promising results in the phase II trial setting with favorable outcomes.12–14
As with MM, the use of HDT/ASCT in patients with POEMS has yielded encouraging results, though most reported series are small and single center experiences.15–20 We, therefore, sought to characterize the effect of ASCT as therapy for patients with POEMS syndrome, determining the effect on organ-specific dysfunction and delineating key patient- and disease-specific factors predicting for outcome. This multi-center retrospective analysis demonstrates the clinical utility of ASCT when incorporated into the clinical management of this systemic disorder.
Methods
Patients and methods
All patients with POEMS who underwent an ASCT as part of their first-line therapy, reported from all European Group for Blood and Marrow Transplantation (EBMT) centers, were identified from the EBMT database as eligible for this analysis. Patient, disease-, and transplant-related variables were collected according to the data entries in the database based on MED A and MED B forms, including tracking incomplete data entries from participating centers (https://www.ebmt.org/Contents/Data-Management/Registrystructure/MED-ABdatacollectionforms/Pages/MED-AB-data-collection-forms.aspx). Scientific and ethical review of the proposed study was conducted by the Chronic Malignancies Working Party (CMWP) of the EBMT, with final approval of the protocol provided by the Chair of the CMWP, ensuring that all studies were performed in accordance with the principles of the Declaration of Helsinki.
For the analysis of organ-specific disease extent and response to transplant, a POEMS-specific questionnaire-based tool (Online Supplementary Figure S1) was supplied to each study center for completion and for submission for analysis. The tool calculated the extent of key individual organ involvement (neuropathy, organomegaly, volume overload) pre- and post-ASCT at a point where the treating physician considered maximum symptomatic response had been obtained.
Outcome measures
The response criteria of the International Myeloma Working Group21 were modified for use in this study, with investigator-reported response pre- and post-ASCT recorded. Complete response (CR) was defined as: 5% or under neoplastic plasma cells in the bone marrow (BM), and negative immunofixation of the serum and/or urine; partial response (PR) as a 50% or over reduction in paraprotein or 90% or over reduction in urinary light chain excretion compared with the pre-treatment baseline; stable disease (SD: <50% reduction in paraprotein with stable clinical state) and progressive disease (PD: progressive clinical symptoms and/or the re-appearance or progressive rise in the paraprotein level by 25% or more) were considered as treatment failure. Organ-specific symptomatic response (OR) was analyzed according to a treatment-attributable change in the specific score in a time-dependent manner.
Engraftment syndrome (ESy) was defined as previously published.22 The definition was based on the presence of major (a temperature of >38.0°C without an identifiable infectious etiology, rash involving >25% body surface area (BSA), and non-cardiogenic pulmonary edema) and minor [bilirubin ≥2 upper limit of normal (ULN), transaminases ≥2 ULN, weight gain ≥2.5% of baseline, or transient encephalopathy] criteria, with engraftment syndrome being defined as the presence of all 3 major criteria or one major and 2 minor criteria within 96 hours of neutrophil engraftment.
The hematologic response rate at day (d)+100 post ASCT and the maximum response (including time to response) was determined and compared to status at transplant. Relapse was determined as the time point associated with the onset of clinical recurrence or disease progression.
Statistical analysis
Standard descriptive methods were used to report patients’ characteristics, and comparisons in subgroups were performed by the χ2 test or Fisher Exact test (categorical variables) and Mann-Whitney test (continuous variables). OS and progression-free survival (PFS) were computed from transplant respectively to death (OS) or the first event between relapse/progression and death (PFS). Probabilities of OS and PFS were calculated using the Kaplan-Meier estimator, and differences in subgroups were assessed by the log-rank test. Occurrence of relapse/progression and death without this event (non-relapse mortality, NRM) were analyzed in a competing risks framework, applying the proper non-parametric estimator of the cumulative incidence and the Gray test for comparisons. These methods were used also to report the achievement of complete remission (relapse/progression or death were considered competing risks) and of neutrophil engraftment (competing with death). The graphs of time-to-event curves report pointwise 95% confidence intervals (CI) for all probabilities. Organ response (OR) was assessed comparing individual pre- and post-transplant organ scores by the Stuart-Maxwell test to account for dependence between the two measurements observed for each patient. All analyses were performed using the R package v.3.1.0 with the libraries prodlim and cmprsk (for time-to-event end points) and irr (for organ response).
Results
Patients’ characteristics and treatments
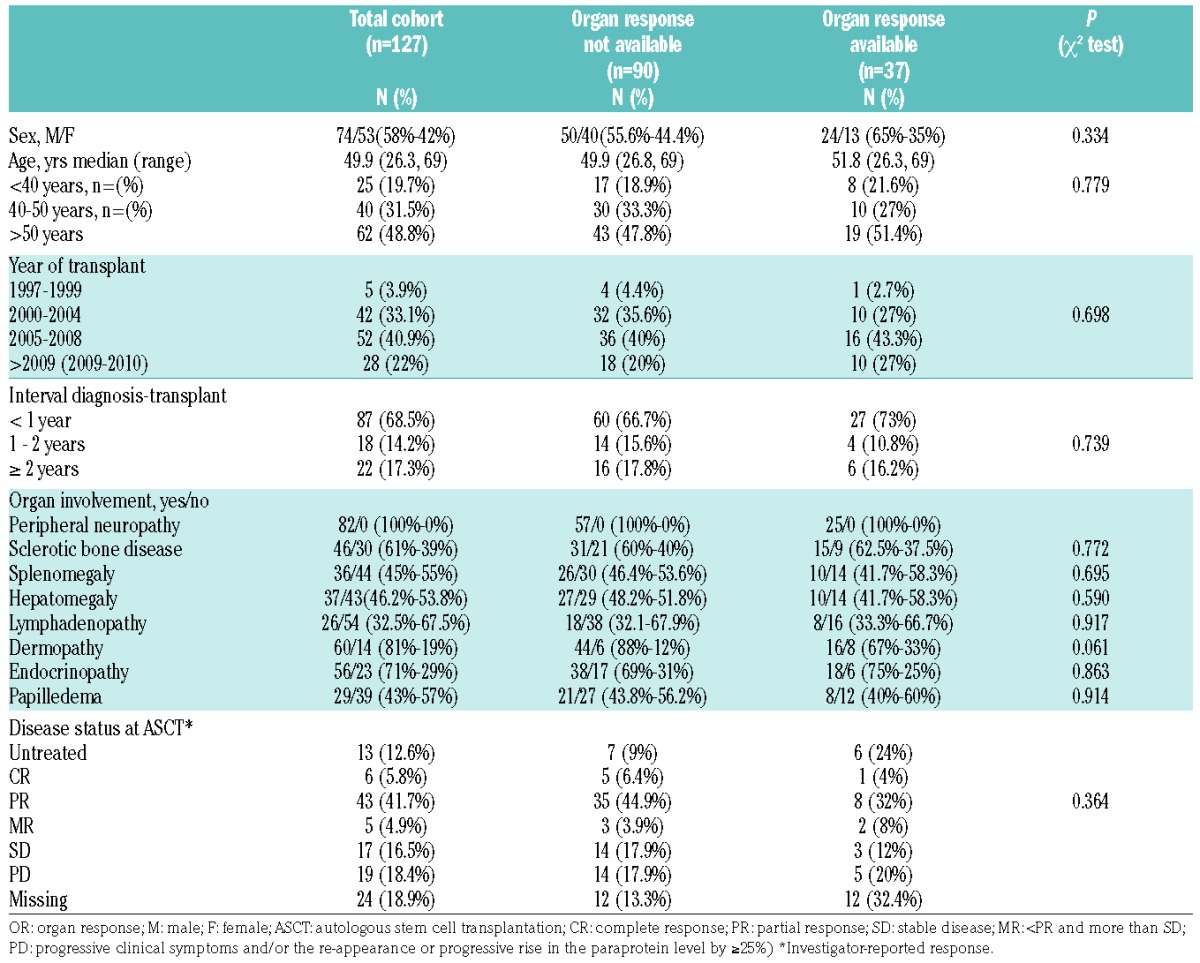
Patients’ characteristics (n=127) are illustrated in Table 1, including presenting features at diagnosis and organ involvement. The median age at transplant was 50 years (range 26–69 years) with a male to female ratio of 1.4:1. 87% of patients underwent an ASCT after initial disease-modifying therapy and the median time from diagnosis to ASCT was 7.5 months (range 0.8–346 months) with most (69%) patients undergoing ASCT within 12 months of diagnosis. The performance score (PS) at transplant was documented in 79% of ASCT recipients, with 40% reporting a good PS, 29% reporting a fair PS and 31% reporting a poor PS. Available data on baseline and pre-ASCT albumin are limited, being available in only 32.2% of patients at diagnosis and 39.4% at ASCT, with a median level of 37 g/L (3–53 g/L) and 36 g/L (2–46 g/L), respectively. The availability of renal function data is similarly limited (36.2% at diagnosis and 42.5% at ASCT). The graft source was peripheral blood stem cells (PBSC) in all reported ASCT with 57% of graft recipients receiving more than 4×106/kg (median CD34+ cell dose re-infused was 4.3×106/kg). One hundred and twenty-three (99.2%) patients were reported as receiving melphalan 200 mg/m2 as conditioning chemotherapy and only one patient (0.8%) received total body irradiation (TBI) as part of the conditioning regimen. The disease status (hematologic response) at transplant was reported as CR/PR 48%, MR/SD 21%, PD 18% and untreated 13%.
Table 1.
Patients’ characteristics (P-value delineates differences between those with and without organ response data).
Transplant results: engraftment and engraftment syndrome
Successful engraftment was documented in 96.8% (n=123) with engraftment failure reported in 3 patients (2 patients with primary graft failure and one with secondary graft failure; one patient with missing data). The median time to neutrophil engraftment was 13 days and the median time to platelet engraftment was 16 days. The estimates of cumulative incidence of neutrophil engraftment are illustrated in Online Supplementary Figure S1A. Engraftment syndrome was reported in 29 patients (23%), manifested by a heterogeneous clinical presentation (Figure 1) and treated with a high dose of corticosteroids. There was no difference in the incidence of engraftment syndrome based on previous treatment, reported in 24% of pre-treated versus 46% untreated patients pre ASCT (P=0.178), nor did we determine any impact on PBSC mobilization regardless of whether this was conducted with or without cyclophosphamide (cyclophosphamide-based mobilization ESy rate of 26.9% compared with non-cyclophosphamide-based mobilization ESy rate of 25.9%; Fisher Exact test P=0.590), though these results must be interpreted with caution due to the limited data availability on the mobilization regimens used. We observed no obvious negative impact on survival in patients who did or did not experience engraftment syndrome: 5-year OS was 90% (95%CI: 79–100) for no ESy compared with 97% (95%CI: 90–100; P=0.7).
Figure 1.
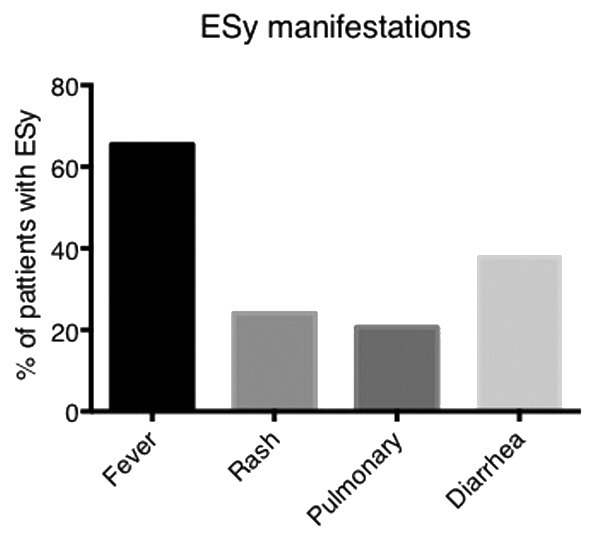
The clinical presentation of engraftment syndrome (ESy) in POEMS syndrome patients undergoing autologous stem cell transplantation (ASCT) (n=29).
Organ-specific responses and durability of response
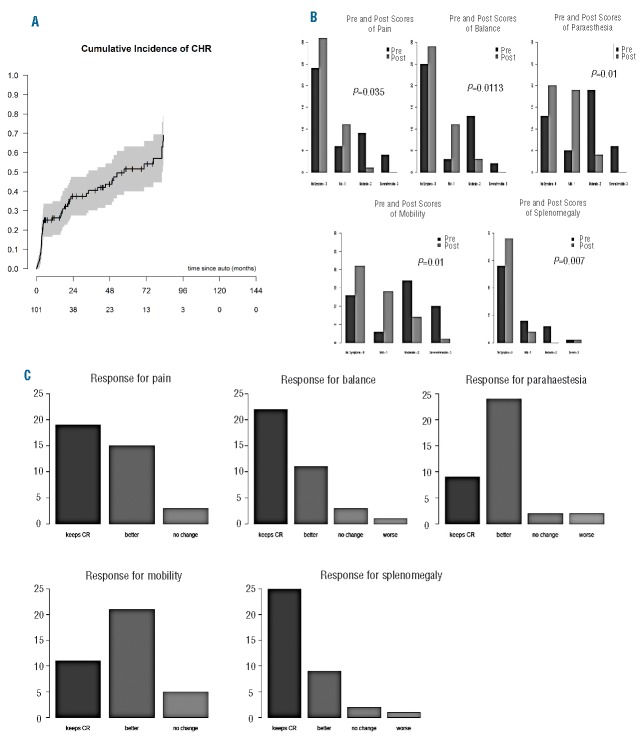
The hematologic response (HR) in evaluable patients (n=101) post ASCT was CRHR in 49 patients (48%) with a median time to CRHR of 4.6 months (Figure 2A), PRHR in 21 patients (21%), less than PRHR in 31 patients (31%). Sixteen patients up-graded their response to CRHR following ASCT, with 9 patients up-grading their response to PRHR following ASCT. Using the clinical scoring tool (Online Supplementary Figure S2), the organ-specific responses pre- and post-ASCT were determined; no difference in PFS was seen between the cohort with and that without OR information (Online Supplementary Figure S2). Organ symptom response was variable across the domains. For example, in the neurological symptom domain, where all patients experienced at least one symptom complex, 7 (16%) patients had resolution of pain, 7 (17%) had resolution of paraesthesia, and 8 (19%) had resolution of mobility limitations with 4 (11%) patients resolving balance issues (Figure 2B). In the cohort with reported organ-specific response (n=37), the majority of patients reported an improvement in their symptoms post ASCT, with patients achieving a symptomatic CR with prior therapy maintaining this in the post-ASCT follow-up period (Figure 2C).
Figure 2.
Response to autologous stem cell transplantation (ASCT) in patients with POEMS syndrome. (A) Cumulative incidence of complete hematologic response (CHR) post ASCT. (B) Individual symptomatic domain score modification pre- and post-ASCT, where the Y-axis represents the absolute number of patients (n). Comparative analysis was performed using the Stuart-Maxwell test to determine statistically significant differences on comparative groups of outcomes. (C) Domain-specific response grading post-ASCT, where the Y-axis represents the absolute number of patients (n). CR: complete response; “Keeps CR”: no symptoms pre- and remain asymptomatic post ASCT; “No change”: mild, moderate and severe before ASCT remain in a similar category post ASCT.
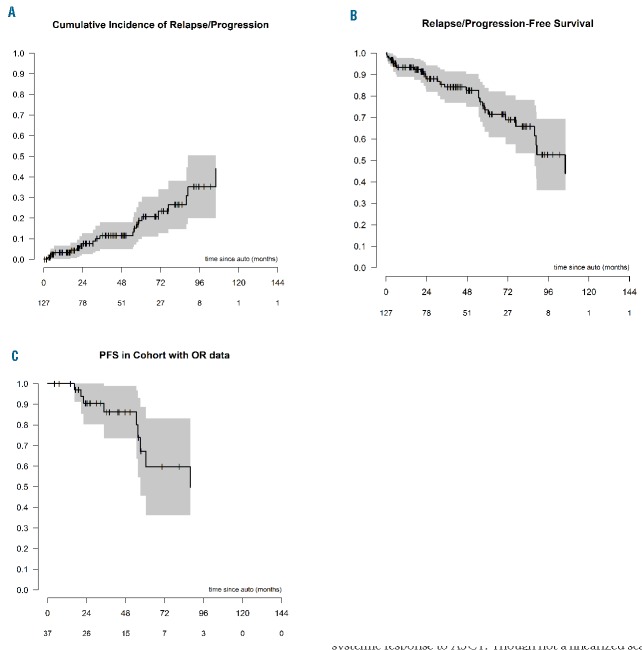
At last follow up, 21 (17%) patients demonstrated progressive disease. The 3-year relapse rate (RR) for the cohort was 12% (95%CI: 5, 18%) (Figure 3A). Median PFS for the cohort was 106 months [95% CI: 87.8, not reached (NR)] with a 5-year PFS of 74% (95%CI: 63.2, 83.7%) (Figure 3B). Median PFS for patients in whom OR data were available was 88 months (95%CI: 51, NR) with a 5-year PFS of 67% (95%CI: 45.7, 88.7) (Figure 3C). In a univariate analysis, the only factor to demonstrate significance in relation to PFS was PS: patients with a good score (0–1: median PFS NR, 95%CI: 92, NR) compared to those with fair (2: median 77 months 95%CI: 14, NR) and poor (≥3: median 89 months 95%CI: 57, NR) scores (log-rank P=0.032) (Online Supplementary Figure S3). No impact on PFS was noted either from the time to transplant (<1 year, 1–2 years or >2 years), use of pre-transplant therapy, or the status at transplant (CR vs. <CR).
Figure 3.
(A) Cumulative rate of disease progression and (B) progression-free survival (PFS) of patients with POEMS syndrome undergoing an autologous stem cell transplantation (ASCT). (C) PFS in sub-group assessed for organ response. Shaded area represents 95% confidence intervals.
Survival
A total of 114 (90%) patients are alive at last follow up with a median follow up of 48 months (95%CI: 38.3, 58.6). Thirteen patients have died, of whom 4 (31%) resulted from disease progression, 3 (23%) died from infection, with 6 patients dying from other causes. There were 3 reported incidences of secondary primary malignancy (2.9%; data missing in 9 patients): one patient was diagnosed with myelodysplastic syndrome (MDS), one patient with myelodysplasia, and one patient with colorectal carcinoma. NRM rates at d+30 and d+100 were 1.6% (95%CI: 0, 3.8) and 2.4% (95%CI: 0, 5.0). The 1- and 5-year NRM were 3.3% (95%CI: 0.1, 6.4) and 7.7% (95%CI: 1.9, 13.6), respectively (Figure 4). The 5-year OS for the cohort was 88.6% (95%CI: 81.5, 95.8%) (Figure 5). No impact on OS was seen either on the time to transplant (<1 year, 1–2 years or >2 years), use of pre-transplant therapy, or status at transplant (CR vs. <CR).
Figure 4.
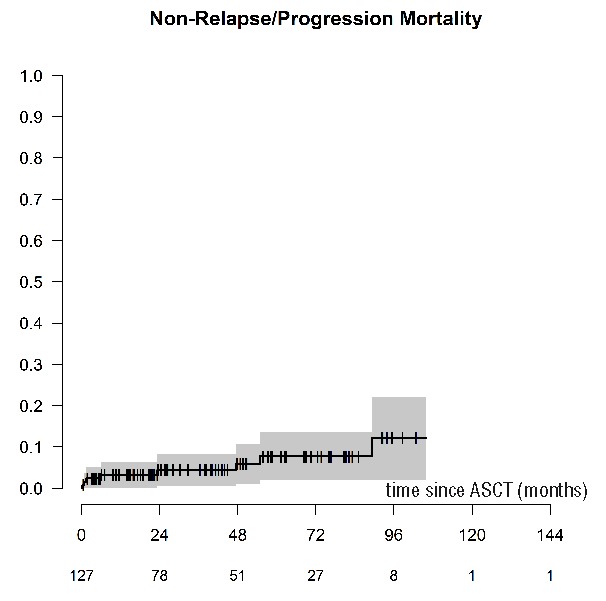
Cumulative non-relapse mortality of patients with POEMS syndrome undergoing an autologous stem cell transplantation (ASCT).
Figure 5.
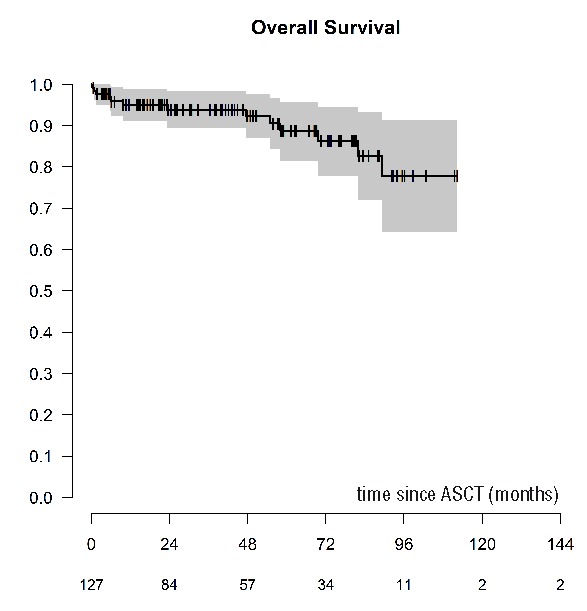
Overall survival of patients with POEMS syndrome undergoing an autologous stem cell transplantation (ASCT).
Discussion
Autologous stem cell transplantation has become standard first-line therapy in patients with plasma cell dyscrasia, and has been reported to be a suitable treatment modality in POEMS syndrome.17,20,23 Although a recently reported non-ASCT treatment modality has shown good disease control, the long-term outcome from ASCT in POEMS syndrome has been shown to be extremely good.7 D’Souza et al. reported the results from long-term follow up, categorized according to responses seen in hematologic, radiological, biochemical (VEGF) and clinical parameters.16 Given that the clinical phenotype relates to organ-specific symptomatology, a clinical correlation with the hematologic response post ASCT is warranted. The results presented in this study demonstrate that ASCT in POEMS syndrome not only results in excellent hematologic control, but also excellent organ-specific disease response.
This present study defines several key clinical issues. Firstly, data regarding POEMS syndrome patients undergoing ASCT are limited, though many case reports provide information on individual responses. In this, the largest multi-center cohort reported so far, we demonstrate that ASCT has a clear impact both in terms of PFS and OS, with good cohort follow up and completion of data. D’Souza et al.16 report a single center experience with excellent follow up, clearly showing the impact of ASCT, with results similar to those presented here. The median overall survival of our cohort has not been reached, which, given the follow-up period, is indicative of the durability of responses in this rare syndrome.
Secondly, delivery of a therapy which can have such a positive impact must not carry with it an unacceptable risk. Our data demonstrate procedural safety with a 1-year NRM of 3.3%, which is somewhat surprising given the extent to which this therapy can compromise patients’ physiological status. In particular, the pro-inflammatory Esy (manifested by fever, weight gain, pulmonary infiltrates, rashes and diarrhea) was reported in 23% of patients, compared to the previously reported 37%.16 It has been suggested that incidence of Esy can be reduced if patients have peripheral blood stem cell mobilization incorporating cyclophosphamide, perhaps relating to the immune-modulatory capacity of high-dose cyclophosphamide. The incidence of ESy in ASCT performed for myeloma patients has seen an increase in association with the introduction of novel agents.24 In the current era of biological disease-modifying agents and pre-transplant treatment strategies, this remains an issue worthy of exploration.
Thirdly, given the systemic manifestations of POEMS syndrome, there is a need to encompass the systemic nature of the disorder when defining effective therapies rather than reliance on laboratory parameters of response alone. Until now, reported data factoring objective organ response have been particularly limited and no uniform scoring system or patient-reported outcome (PRO) tool has been applied. In this study cohort, we used an objective organ-specific symptom PRO to gain insight into the systemic response to ASCT. Though not a linearized scale, this PRO tool has been able to demonstrate the impact of ASCT on the propagation of systemic symptoms with complete resolution in specific symptoms in 10%–20% of patients but with a general improvement in symptomatic scoring being reported in the majority of patients. What is interesting to note is the relative lack of a connection between objective symptomatic response and laboratory-based response. As previously reported, this observation continues to be valid even in this larger series of patients. It is important to note that patients who do not achieve or who fail to remain in CR, are those patients most at risk of systematic relapse disease.25 It would be very useful to further define PRO tools in this setting, especially in relation to pre-ASCT therapy.
It is important to review the data presented in this manuscript in the light of the limitations of the study. Firstly, the hematologic response post ASCT (Figure 2A) seems to indicate a continuing CR evolution up to 24–72 months post ASCT. There is no recorded post ASCT therapy within the data fields, so further consideration of this phenomenon is warranted. A delayed response has been observed in other monoclonal gammopathies, e.g. in AL amyloidosis27 and Waldenstrom macroglobulinemia.28 Alternatively, from a statistical perspective, the estimated CR rate and its increase in time should be interpreted with caution given the enlarging confidence intervals with each advancing time frame. The observed phenomenon in this study may well reflect both these explanations as a cumulative effect.
Secondly, due to the retrospective nature of the work, as well as the contribution from many international centers, there was no routine monitoring of VEGF serum level or any consistent reporting of newer imaging techniques (e.g. PET/CT scanning). Furthermore, in this transplant registry database, full recording of comorbidities is limited and therefore we were not able to include this in the analysis. The organ-specific response data, whilst very informative on a clinical basis, does represent a sub-group analysis, which is a limitation of the retrospective nature of the study. None the less, the comprehensive analysis of the largest ASCT cohort in POEMS patients does provide key clinical conclusions to aid the physician.
We show the long-term outcomes of patients with POEMS syndrome treated with ASCT, with a sub-group analysis showing the impact of the treatment on the systemic manifestations of this syndrome. Patients can progress/relapse after transplantation, and therefore should be followed periodically. Given the paucity of post-ASCT events, performing univariate and multivariate analysis to establish independent factors of predicting OS remains the main limitation. The analysis to identify risk factors for progression post transplant demonstrated that the PS at time of transplant is the most significant. The use of appropriate pre-transplant therapy to control disease-related symptoms, and thus PS, at the time of transplant warrants further consideration. Though this study is retrospective, given the relative rarity of this syndrome, the data presented are key to the clinician’s decision-making process.
Supplementary Material
Acknowledgments
The authors wish to thank all transplant center leads and data managers for helping with the conduct of this study. The authors would also like to thank the constituent members of the EBMT Chronic Malignancy Working Party for their support throughout the study.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/102/1/1601
References
- 1.Dispenzieri A. POEMS syndrome: update on diagnosis, risk-stratification, and management. Am J Hematol. 2012;87(8):804–814. [DOI] [PubMed] [Google Scholar]
- 2.Dispenzieri A. POEMS syndrome: 2014 update on diagnosis, risk-stratification, and management. Am J Hematol. 2014; 89(2):214–223. [DOI] [PubMed] [Google Scholar]
- 3.Imai N, Kubota M, Saitou M, Yagi N, Serizawa M, Kobari M. Increase of serum vascular endothelial growth factors in wet beriberi: two case reports. Intern Medi. 2012;51(8):929–932. [DOI] [PubMed] [Google Scholar]
- 4.Yamada Y, Sawai S, Misawa S, et al. Multiple angiogenetic factors are upregulated in POEMS syndrome. Anna Hematol. 2013;92(2):245–248. [DOI] [PubMed] [Google Scholar]
- 5.Kanai K, Sawai S, Sogawa K, et al. Markedly upregulated serum interleukin-12 as a novel biomarker in POEMS syndrome. Neurology. 2012;79(6):575–582. [DOI] [PubMed] [Google Scholar]
- 6.Dispenzieri A. How I treat POEMS syndrome. Blood. 2012;119(24):5650–5658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li J, Zhang W, Jiao L, et al. Combination of melphalan and dexamethasone for patients with newly diagnosed POEMS syndrome. Blood. 2011;117(24):6445–6449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sinisalo M, Hietaharju A, Sauranen J, Wirta O. Thalidomide in POEMS syndrome: case report. Am J Hematol. 2004;76(1):66–68. [DOI] [PubMed] [Google Scholar]
- 9.Kim SY, Lee SA, Ryoo HM, Lee KH, Hyun MS, Bae SH. Thalidomide for POEMS syndrome. Ann Hematol. 2006;85(8):545–546. [DOI] [PubMed] [Google Scholar]
- 10.Inoue D, Kato A, Tabata S, et al. Successful treatment of POEMS syndrome complicated by severe congestive heart failure with thalidomide. Intern Med. 2010;49(5):461–466. [DOI] [PubMed] [Google Scholar]
- 11.Dispenzieri A, Klein CJ, Mauermann ML. Lenalidomide therapy in a patient with POEMS syndrome. Blood. 2007; 110(3):1075–1076. [DOI] [PubMed] [Google Scholar]
- 12.Jaccard A, Abraham J, Recher C, et al. Lenalidomide therapy in nine patients with poems syndrome. Blood. 2009; 114(22):3872.19717645 [Google Scholar]
- 13.Tomas JF, Giraldo P, Lecumberri R, Nistal S. POEMS syndrome with severe neurological damage clinically recovered with lenalidomide. Haematologica. 2012;97(2):320–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Royer B, Merlusca L, Abraham J, et al. Efficacy of lenalidomide in POEMS syndrome: a retrospective study of 20 patients. Am J Hematol. 2013;88(3):207–212. [DOI] [PubMed] [Google Scholar]
- 15.Chen F, Zhang K, Tan X, et al. Autologous peripheral blood stem cell transplantation in a patient with POEMS syndrome. Ann Hematol. 2008;87(3):247–248. [DOI] [PubMed] [Google Scholar]
- 16.D’Souza A, Lacy M, Gertz M, et al. Long-term outcomes after autologous stem cell transplantation for patients with POEMS syndrome (osteosclerotic myeloma): a single-center experience. Blood. 2012; 120(1):56–62. [DOI] [PubMed] [Google Scholar]
- 17.Diaz-Betancur J, Cuervo-Sierra J, Guzman-Zapata A, Mondragon-Arismendi MC, Cuellar-Ambrosi F. Autologous peripheral blood stem cell transplantation in a patient with POEMS syndrome. Hematol Oncol Stem Cell Ther. 2009;2(1):305–307. [DOI] [PubMed] [Google Scholar]
- 18.Dispenzieri A. Long-Term Outcomes After Autologous Stem Cell Transplantation in Patients With POEMS Syndrome. Clin Adv Hematol Oncol. 2012;10(11):744–746. [PubMed] [Google Scholar]
- 19.Imai N, Kitamura E, Tachibana T, et al. Efficacy of autologous peripheral blood stem cell transplantation in POEMS syndrome with polyneuropathy. Intern Med. 2007;46(3):135–138. [DOI] [PubMed] [Google Scholar]
- 20.Kuwabara S, Misawa S, Kanai K, et al. Autologous peripheral blood stem cell transplantation for POEMS syndrome. Neurology. 2006;66(1):105–107. [DOI] [PubMed] [Google Scholar]
- 21.Durie BG, Harousseau JL, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20(9):1467–1473. [DOI] [PubMed] [Google Scholar]
- 22.Dispenzieri A, Lacy MQ, Hayman SR, et al. Peripheral blood stem cell transplant for POEMS syndrome is associated with high rates of engraftment syndrome. Eur J Haematol. 2008;80(5):397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jaccard A, Royer B, Bordessoule D, Brouet JC, Fermand JP. High-dose therapy and autologous blood stem cell transplantation in POEMS syndrome. Blood. 2002; 99(8):3057–3059. [DOI] [PubMed] [Google Scholar]
- 24.Cornell RF, Drobyski W, Zhong X, et al. Risk factors and clinical features of engraftment syndrome after autologous hematopoietic cell transplantation (AHCT). Biol Blood Marrow Transplant. 2013; 1:S130. [Google Scholar]
- 25.Vannata B, Laurenti L, Chiusolo P, et al. Efficacy of lenalidomide plus dexamethasone for POEMS syndrome relapsed after autologous peripheral stem-cell transplantation. Am J Hematol. 2012;87(6):641–642. [DOI] [PubMed] [Google Scholar]
- 26.Imai N, Taguchi J, Yagi N, Konishi T, Serizawa M, Kobari M. Relapse of polyneuropathy, organomegaly, endocrinopathy, M-protein, and skin changes (POEMS) syndrome without increased level of vascular endothelial growth factor following successful autologous peripheral blood stem cell transplantation. Neuromuscul Disord. 2009;19(5):363–365. [DOI] [PubMed] [Google Scholar]
- 27.Vesole DH, Perez WS, Akasheh M, et al. High-dose therapy and autologous hematopoietic stem cell transplantation for patients with primary systemic amyloidosis: a Center for International Blood and Marrow Transplant Research Study. Mayo Clin Proc. 2006;81(7):880–888. [DOI] [PubMed] [Google Scholar]
- 28.Kyriakou C, Canals C, Sibon D, et al. High-dose therapy and autologous stem-cell transplantation in Waldenstrom macroglobulinemia: the Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. J Clin Oncol. 2010;28(13):2227–2232. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.