Abstract
Despite new advances in multiple myeloma treatment and the consequent improvement in overall survival, most patients relapse or become refractory to treatment. This suggests that new molecules and combinations that may further inhibit important survival pathways for these tumor cells are needed. In this context, zalypsis is a novel compound, derived from marine organisms, with a powerful preclinical anti-myeloma effect based on the sensitivity of malignant plasma cells to DNA-damage induction; and it has already been tested in a phase I/II clinical trial in multiple myeloma. We hypothesized that the addition of this compound to the combination of bortezomib plus dexamethasone may improve efficacy with acceptable toxicity. The triple combination demonstrated strong synergy and higher efficacy compared with double combinations; not only in vitro, but also ex vivo and, especially, in in vivo experiments. The triple combination triggers cell death, mainly through a synergistic induction of DNA damage and a decrease in the nuclear localization of nuclear factor kappa B. Our findings support the clinical evaluation of this combination for relapsed and refractory myeloma patients.
Introduction
Multiple myeloma (MM) is the second most common haematological malignancy, with 27,000 new cases recorded in the past year in the USA alone.1 The median survival of these patients has improved from around two to almost four years in the last decade,2 mainly due to improvements in supportive care, the introduction of autologous stem cell transplantation (ASCT), and other therapeutic agents, such as thalidomide, lenalidomide and bortezomib, which have become standards of care in MM. However, once patients become refractory to these agents, their outcome is very poor.2 There is therefore an urgent need to develop novel drugs and new therapeutic strategies that, in monotherapy or, more often, in combination with these standards of care, may overcome resistance and improve their efficacy. In this regard, an arsenal of new compounds with different mechanisms of action have been evaluated in the preclinical setting and in clinical trials.3
Zalypsis is one such molecule. It is a synthetic alkaloid related to substances isolated from marine organisms,4 and has a powerful preclinical anti-myeloma effect based on the sensitivity of malignant plasma cells to DNA-damage induction. Interestingly, this activity is present in cells with a functional, as well as a mutated, p53.5 Moreover, in a recent phase I/II dose-escalation clinical trial carried out in previously refractory MM patients, zalypsis showed a predictable, manageable and reversible toxicity profile with some long-lasting disease stabilization, particularly when dexamethasone was added.6 Some responses (1 PR and 5 MR) were observed with the agent in monotherapy, although when administered at doses above the recommended dose. These preliminary data regarding activity and the improvement observed when adding dexamethasone, prompted us to evaluate the potential synergy of combinations of zalypsis with MM standards of care that could allow an improvement in efficacy while maintaining good tolerability.
In this study, we report the preclinical evaluation of the combination of zalypsis + bortezomib + dexamethasone; in our in vitro, ex vivo and in vivo models it showed remarkable synergy, with strong apoptosis induction through caspase-dependent and caspase-independent mechanisms. We also demonstrate possible mechanisms underlying the synergy, based on a potentiation of the DNA-damaging effect and decreased nuclear factor kappa B (NF-κB) nuclear translocation.
Methods
Reagents and immunochemicals
Zalypsis was provided by PharmaMar SAU (Madrid, Spain), bortezomib was obtained from LC Laboratories (Woburn, MA, USA), and dexamethasone from Sigma-Aldrich (St Louis, MO, USA).
MM cell lines and patient samples
MM.1S human cell line, MM.1S-luc cells with stable expression of luciferase, and the human bone marrow (BM) mesenchymal stromal cell line hMSC-TERT, were used for the in vitro and in vivo experiments. The sources of these cell lines are described in the Online Supplementary Methods. BM samples from patients were obtained following the approval of our Institutional Review Board, and after obtaining informed consent from participating subjects.
Cell viability and mitochondrial membrane potential (ΔΨm) changes
Cell viability and mitochondrial membrane potential (ΔΨm) changes. Viability was evaluated by MTT colorimetric assay by quadruplicates, and ΔΨm by DioC6 (3) staining, as previously described.7
Quantification of in vitro synergism
Synergism was evaluated with Calcusyn software (Biosoft, Ferguson, MO, USA).8
Ex vivo analysis of apoptosis in freshly isolated patient cells
Cytometry analyses of apoptosis in BM tumour plasma cells and lymphocytes were performed as previously described.7
Microenvironment assays
MM.1S cells were incubated for 48 hours with the various drugs in the presence or absence of IL-6 and IGF-1, and co-cultured or not with hMSC-TERT (MM.1S-luc) as described elsewhere.5
Animal models
For the xenograft plasmacytoma model, CB17-SCID mice (The Jackson Laboratory, Bar Harbor, ME) were subcutaneously inoculated into the right flank with 3 × 106 MM.1S cells as previously described.5 When tumors became palpable, mice were randomized to receive different treatments. The control group received the vehicle of zalypsis, which was a dilution containing sucrose, potassium dihydrogen phosphate, phosphoric acid and sterile water for injection. The other groups received: zalypsis (Za), bortezomib (B), dexamethasone (De), and the combinations ZaB, ZaDe, BDe, and ZaBDe. The follow-up of this xenograft model has been described elsewhere.5 The same strain of mice was used to generate the disseminated MM xenograft model by tail vein injection of 3 × 106 MM.1S-luc cells. Tumor development was monitored by non-invasive bioluminescence imaging (BLI) with a Xenogen IVIS 50 system (Caliper Life Sciences, Hopkinton, MA, USA). When tumor burden could be measured by BLI, mice were randomized. Experiments were performed according to institutional guidelines for the use of laboratory animals. All protocols and experiments were approved by the Animal Ethics Committee of the University of Salamanca.
Statistical analyses
Analyses were carried out with IBM SPSS Statistics for Windows version 21.0 (IBM Corp., Armonk, NY, USA). Differences in the in vitro experiments, and in tumor volume in the in vivo experiments, were analyzed with the t-student test, defining statistical significance as P<0.05. Differences in survival were analyzed using Kaplan-Meier curves, and the statistical significance was evaluated by the log-rank test, being considered significant when P<0.05.
Comet assay
After each treatment, samples were collected and processed in accordance with the previously described in-house alkaline method.9
Western blot (WB)
WB methods and the preparation of protein lysates from nucleus, cytosol and mitochondria have been described elsewhere.7 The sources of the monoclonal antibodies are described in the Online Supplementary Methods.
Results
Zalypsis synergistically augments the anti-myeloma efficacy of bortezomib plus dexamethasone in in vitro and ex vivo settings
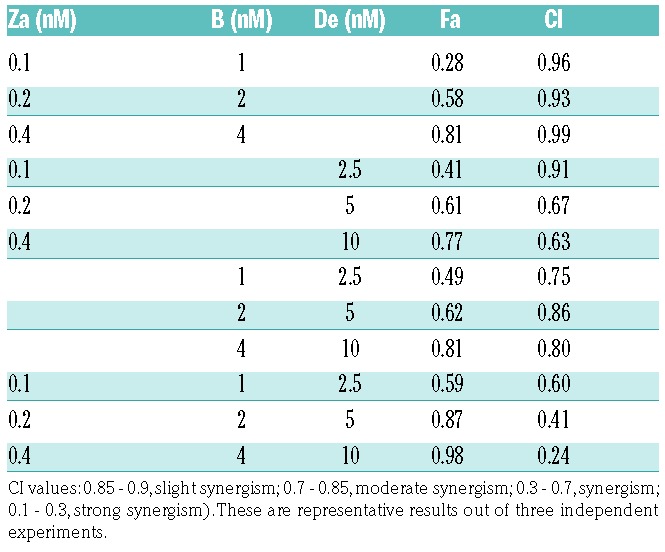
The efficacy of the triple combination, ZaBDe, was compared with the three double combinations (ZaB, ZaDe, BDe) and with the agents in monotherapy in the MM.1S cell line using the MTT assay. The three compounds were combined at suboptimal doses, and increasing combination doses were also tested, maintaining a constant drug ratio. As can be seen in Figure 1A, although all double combinations were more efficient than monotherapies, the triple combination proved notably more powerful than any of the doublets. When synergy was quantified using Calcusyn software, the combination indices (CIs) for the triple combination were in the synergistic range, varying between 0.2 and 0.6 depending on the doses used (Table 1). Interestingly, the synergism in the triple combination was stronger than that resulting from any of the double combinations (Figure 1A and Table 1).
Figure 1.
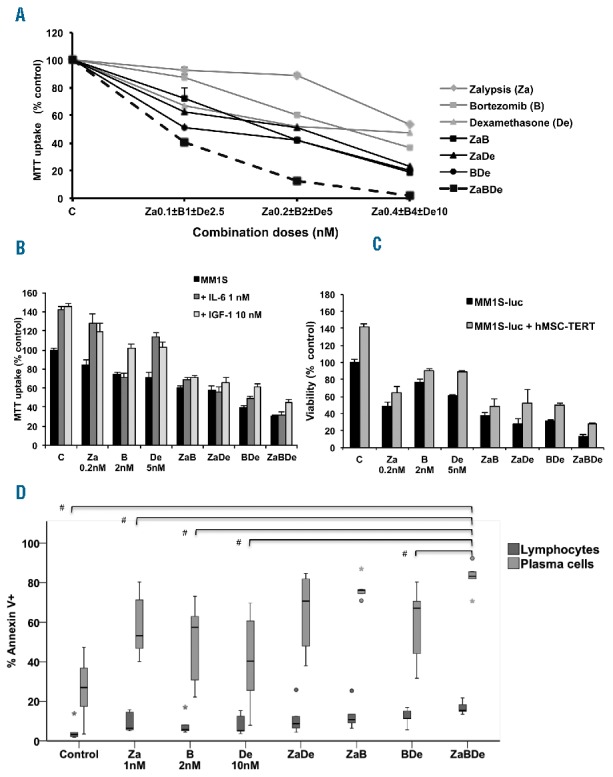
The triple combination ZaBDe is effective in the in vitro and ex vivo settings and overcomes the protective effect of the BM microenvironment. (A) The MM.1S cell line was incubated with increasing doses and combinations of zalypsis, bortezomib and dexamethasone at the indicated doses for 48 hours, keeping a constant ratio. Cell viability was evaluated using the MTT assay. Data presented are means ± SD (n= 3). (B) MM.1S cells were treated with the drugs and doses indicated for 48 hours, in the presence or absence of IL-6 and IGF-1. Data presented are means ± SD (n= 3). (C) MM.1S-luc cells were co-cultured, or not, with the mesenchymal stromal cell line hMSC-TERT, and treated with the drugs and doses indicated. After 48 hours, MM.1S-luc growth was assessed by luciferase bioluminescence signal, which was normalized to growth of MM.1S-luc cells alone and in absence of drug treatment. Data presented are means ± SD (n= 3). (D) BM cells from 5 MM patients were incubated with the indicated doses of the drugs alone and in the different combinations for 48 hours. Apoptosis induction was analyzed by Annexin-V staining by flow cytometry assay, in plasma cells and lymphocytes that were identified based on the surface expression of CD38, CD56 and CD45. Statistically significant differences (#P<0.05) were found between plasma cells treated with ZaBDe and the indicated treatments. Error bars or whiskers indicate the last “non-outlier” value at each side. Outliers are indicated as dots, and extreme outliers as asterisks.
Table 1.
Combination index (CI) and fraction of cells affected by the treatment (Fa) for the different combinations.
In order to evaluate this synergy in a more physiological context, ZaBDe efficacy was assessed in a MM cell line protected by the presence of components of the bone marrow microenvironment. The triple combination maintained its efficacy in the MM.1S cell line in the presence of cytokines IL-6 and IGF-1 (Figure 1B), and also when co-cultured with stromal hMSC-TERT BM cells (Figure 1C). Finally, to evaluate whether this effect could be extended to primary MM cells, the efficacy of the triple combination was tested ex vivo using BM plasma cells from 5 MM patients: three at diagnosis (one with delp53), and two at relapse, including one which also had p53 and had been very heavily pre-treated with six prior lines of therapy and refractory to polychemotherapy, bortezomib, lenalidomide, and allogeneic transplantation. The triple combination ZaBDe was very effective in all patients, even in the two patients with MM plasma cells bearing p53 deletion, further improving the results of the double combinations (Figure 1D) with statistically significant differences as compared with all other treatments except for ZaB and ZaDe. With regard to toxicity, the triple combination was found to minimally affect the viability of normal lymphocytes in the BM from the same MM patients in the ex vivo experimental setting, thereby providing further evidence of the existence of a clear therapeutic window for the ZaBDe combination (Figure 1D).
The ZaBDe triple combination significantly prolongs survival in two murine models of multiple myeloma
The anti-myeloma activity of this combination was next evaluated in vivo in two murine xenograft models of MM: a subcutaneous plasmacytoma model, and a model of disseminated myeloma.
In the first model, CB17-SCID mice bearing human subcutaneous plasmacytomas were randomized to receive vehicle (control group), zalypsis (0.75 mg/kg i.v., weekly, for three total doses), bortezomib (0.1 mg/kg i.p., 5 days per week, indefinitely), dexamethasone (1 mg/kg i.p., 5 days per week, indefinitely), and the respective double and triple combinations (n=10 per group). As observed in Figure 2A, the triple combination induced a clear inhibition of tumor growth and growth control was maintained for more than 75 days (compared with less than 25 days in the most active double combination). Differences in tumor volume of the mice receiving the triple combination compared to the remaining groups were highly statistically significant from day 29 on (P<0.001). These results also translated into improved survival of the mice treated with the triple combination, with a median survival of 102 days (range, 27 to 191 days), compared with 72 days (45 to 105 days) for the double combination ZaDe, 59 days (36 to 109) for BDe, and 58 days for ZaB (41 to 86) (Figure 2B). Regarding survival, statistically significant differences were found between the triple combination and the remaining treatment groups (log-rank test, P<0.05 for all of them). With respect to toxicity, a loss of body weight was observed in all mice receiving combinations that included zalypsis; two of the 10 mice treated with the combination ZaBDe actually died due to toxicity. However, the 8 surviving mice in the ZaBDe group almost completely recovered their initial body weight (Figure 2C).
Figure 2.
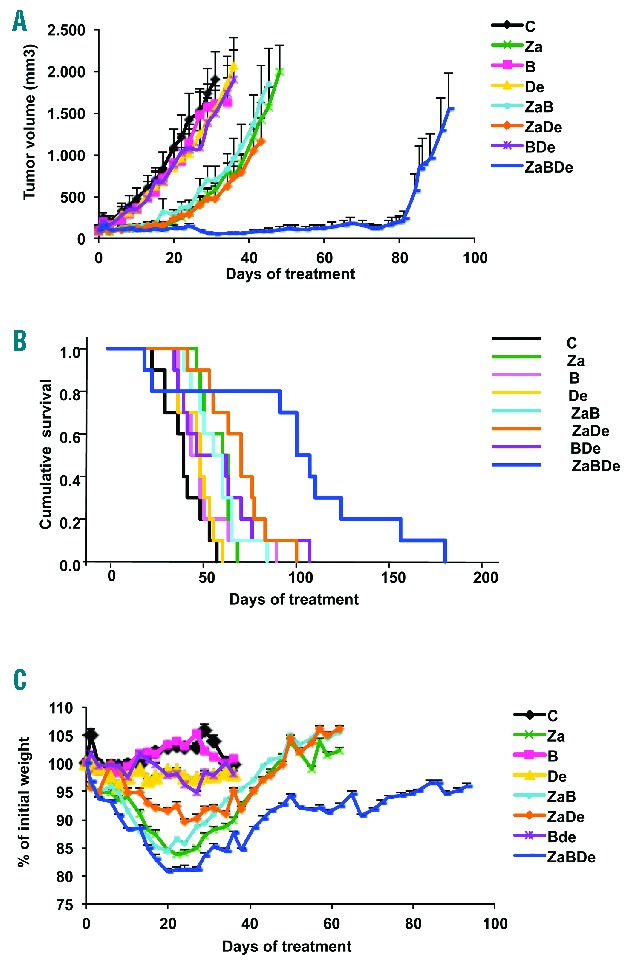
The triple combination ZaBDe has superior anti-MM activity and improves median survival compared with single agents and double combinations in a subcutaneous plasmacytoma model. (A) Tumor volume evolution since the initiation of treatment of CB17-SCID mice bearing human subcutaneous plasmacytomas, randomly assigned to receive vehicle (control group), zalypsis (0.75 mg/kg i.v., weekly, for three doses), bortezomib (0.1 mg/kg i.p., 5 days per week, indefinitely), dexamethasone (1 mg/kg i.p., 5 days per week, indefinitely), and the respective double and triple combinations (n=10 per group). Statistically significant differences (P<0.05) between ZaBDe and all other groups were observed from day 29. (B) Kaplan-Meier curves representing the survival of each treatment group. Statistically significant differences (log-rank test P<0.05) were found between ZaBDe and all other treatments. (C) Percentage of mouse body weight variation during the study in the xenograft plasmacytoma model. Each point represents the mean of all values, and bars stand for standard error.
Based on this initial efficacy at the cost of high toxicity, doses and schemas of treatment were adapted for the disseminated MM model. For this experiment, MM.1S-luc cells were i.v. administered. After 4 weeks, the mice were randomly assigned to the same treatment groups (n=5 per group, except for n=4 for the Za and ZaB groups), with the following modifications: dexamethasone was administered two, rather than five, days per week, and the bortezomib dose was increased from 0.1 to 0.5 mg/kg, five days per week, both indefinitely. In this experiment, the triple combination ZaBDe showed a statistically significant benefit from day 27 on (P<0.01) in terms of delayed tumor dissemination compared with the double combinations ZaDe, BDe and the single agents (Figure 3A–B). The double combination ZaB was also very effective at inhibiting tumor growth; however, the triple combination ZaBDe was even more effective, showing statistically significant differences from day 62 of treatment (P=0.04). Moreover, a significantly better median survival of 99 days (range, 92 to 135 days) for ZaBDe compared with 69 days (range, 65 to 93 days) for ZaB (log-rank test, P=0.007) was observed (Figure 3C). In this model, mice treated with schemes containing zalypsis also showed a reduction of body weight, which was recovered slightly earlier after the administration of the three planned doses of the compound (Figure 3D).
Figure 3.
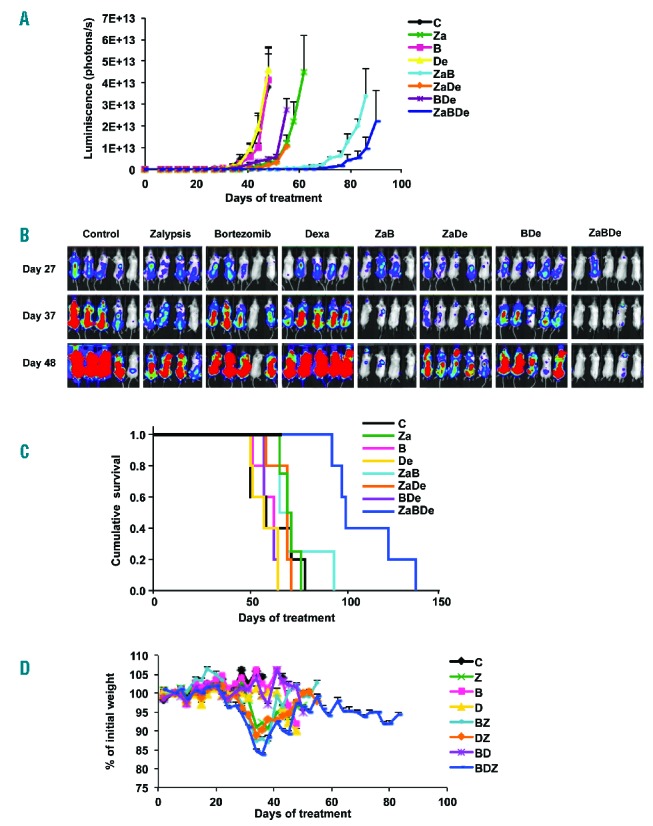
The triple combination ZaBDe has superior anti-MM activity and improves median survival compared with single agents and double combinations in a model of disseminated multiple myeloma. (A) Tumor burden evolution since the initiation of treatment of mice bearing the MM.1S-luc xenograft model. Mice were randomly assigned to receive vehicle (control group), zalypsis (0.75 mg/kg i.v., weekly for three doses), bortezomib (0.5 mg/kg i.p., 5 days per week, indefinitely), dexamethasone (1 mg/kg i.p., 2 days per week, indefinitely) and the respective double and triple combinations (n=5 per group, except n=4 in Za and ZaB groups). Statistically significant differences (P<0.05) were observed from day 27 comparing ZaBDe with single agents (Za, B, De) and double combinations (ZaDe, BDe); and between ZaBDe and ZaB from day 62. (B) Images representing the bioluminescence of each mouse by treatment group at days 27, 37 and 48 after initiation of treatment. (C) Statistically significant differences (log-rank test P<0.05) were found between ZaBDe and all other treatments. (D) Percentage of mouse body weight variation during the study in the disseminated MM model. Each point represents the mean of all values, and bars stand for standard error.
The triple combination triggers DNA damage and decreases NF-κB translocation into the nucleus
We had previously shown that zalypsis, at least partially, acts via the induction of DNA damage, using p53-dependent and p53-independent mechanisms.5 In this study, ZaBDe increased DNA fragmentation, as suggested by the comet assay (Figure 4A). Triple combination as well as dexamethasone in monotherapy and all the doubles, induced H2AX phosphorylation, a marker of DNA double-strand breaks (DSBs) (Fig. 4B). Treatment of MM.1S with the triple combination also induced an early increase in p53 (Figure 4B and Online Supplementary Figure S1A), an indicator of DNA damage response. This effect was more evident than that induced by zalypsis and bortezomib on their own (Figure 4B), and similar to that induced by the combination ZaB (Figure 4B). Some p53 targets, such as p16 and p21, were also induced after only 12 h of treatment (Online Supplementary Figure S1A), more than in any of the double combinations (Figure 4B). No major changes were observed in the p53 target PUMA, or in levels of the p53 inhibitor MDM2 (Figure 4B and Online Supplementary Figure S1A). Despite the increasing levels of the cell cycle regulators, p16 and p21, the cell cycle profile did not show any significant modifications (Figure 4C and Online Supplementary Figure S1B).
Figure 4.
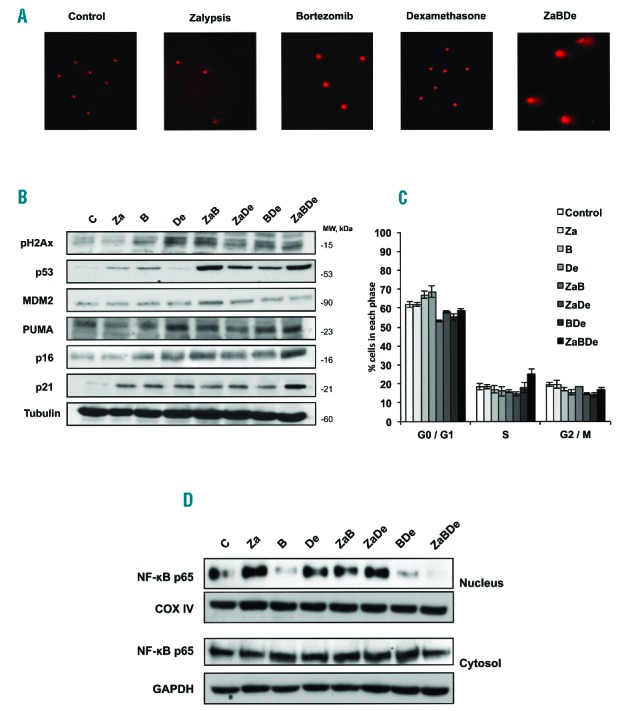
ZaBDe triggers DNA damage and prevents NF-κB translocation into the nucleus. (A) Comet assay of MM.1S cells after treatment with the vehicle (control), zalypsis (0.2 nM), bortezomib (2 nM), dexamethasone (5 nM) and the triple combination at the same doses. The image is representative one from at least 20 optic fields evaluated for each condition. (B) Western Blot of different proteins implicated in the DNA damage response after treatment of MM.1S cells with vehicle (control), zalypsis (0.2 nM), bortezomib (2 nM), dexamethasone (5 nM) and the double and triple combinations at the same doses. (C) Cell cycle distribution examined by flow cytometry after propidium iodide staining of MM.1S cells incubated with the same agents and combinations at equal doses as above. Data represent means ± SD of three independent experiments. (D) Western blot of NF-κB distribution in nuclear and cytosolic fractions of MM.1S cells treated with the drugs in monotherapy and in the double and triple combinations. All these experiments were performed after 48 hours of incubation.
In addition to p53, NF-κB is known to be activated as part of the DNA damage response to orchestrate a cell survival pathway.10 In our studies, according to this hypothesis, dexamethasone and, in particular, zalypsis increased the nuclear translocation of NF-κB, indicating a potential mechanism of resistance to these agents, while bortezomib induced a decrease in this translocation. Though attenuated, this resistance mechanism was still present in both doublets containing bortezomib (ZaB and BDe). The triple combination, however, was able to completely abrogate this nuclear translocation and subsequent activation of NF-κB, which could constitute another explanation for the synergy (Figure 4D).
The triple combination induces apoptosis through caspase-dependent and caspase-independent mechanisms
As a final event leading to cell death, ZaBDe induced apoptosis, as demonstrated by an increase in annexin V-positive cells by flow cytometry (Figure 5A), and the cleavage of caspases-8, -9, -3 and PARP (Figure 5B) as compared with the double combinations. These changes were also detected at early time points in the triple combination (Online Supplementary Figure S2A and S2B), and indicate the involvement of the extrinsic and intrinsic pathways of apoptosis. However, pre-treatment with the pan-caspase inhibitor Z-VAD-FMK only partially rescued cells from apoptosis, indicating the involvement of other caspase-independent mechanisms (Figure 5C).
Figure 5.
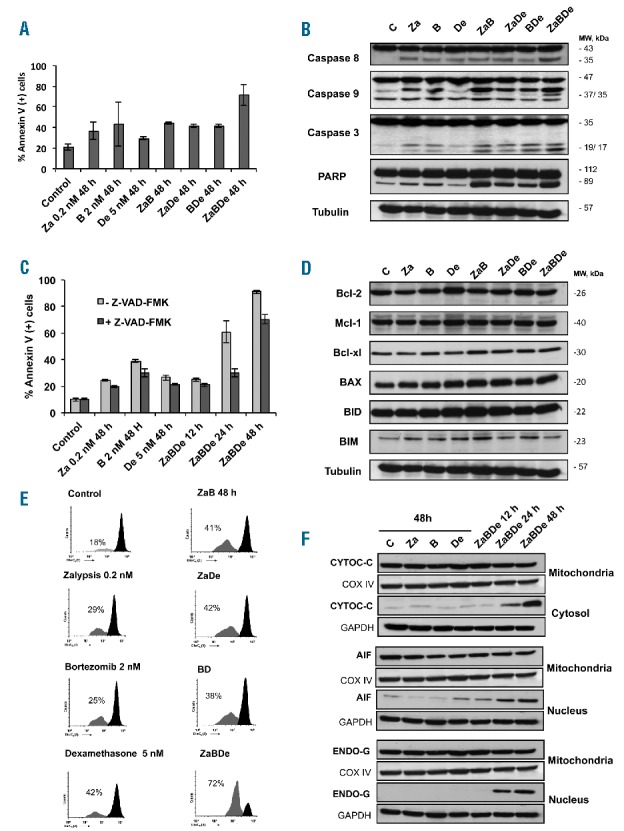
The triple combination ZaBDe induces apoptosis through caspase dependent and independent mechanisms. (A) Apoptosis induction, as evaluated by Annexin V+ staining, of MM.1S cells treated with the vehicle (control), zalypsis (0.2 nM), bortezomib (2 nM), dexamethasone (5 nM) and the double and triple combinations. Data represent means ± SD of three independent experiments. (B) Western blot showing cleavage of caspases-8, -9, -3 and PARP in MM.1S cells after incubation with the indicated treatments at the same doses as before. (C) Cells were pre-incubated, or not, with Z-VAD-FMK for 24 hours (50μM administered every 24 hours) and subsequently treated with the agents as indicated. Apoptosis was evaluated by Annexin-V staining by flow cytometry. Data are presented as mean ± SD (D) Western blot evaluation of Bcl-2 family members after treatment of MM.1S cells with the drugs. (E) Representative examples of mitochondrial membrane potential (ΔΨm) changes evaluated by DioC6 (3) staining after incubation with the drugs alone and in double and triple combinations in the same conditions as above. (F) Western blot showing subcellular localization of several mitochondrial proteins implicated in cell death after treatment of MM.1S cells with the agents as indicated in the figure. All these experiments were performed after 48 hours of incubation unless otherwise specified.
The role of Bcl-2 family members was also studied due to their involvement in the regulation of the intrinsic apoptotic pathway. In this regard, an early increase in the BH3 only protein, BIM, (which was reversed thereafter), and a slight increase in Bax were observed after treatment with the triple combination (Online Supplementary Figure S2C). In this case, however, no differences were observed when compared with the double combinations (Figure 5D). BIM and BAX are both pro-apoptotic proteins closely associated with the permeabilization of the outer mitochondrial membrane. Probably partially due to the activity of these proteins, a loss of mitochondrial membrane potential (ΔΨm) was noted, as measured by flow cytometry after DioC6(3) staining; again, this was clearly superior to that induced by the agents in monotherapy and in double combinations (Figure 5E and Online Supplementary Figure S2D). Consistent with the permeabilization of the outer mitochondrial membrane, increased cytosol levels of cytochrome C were observed after treatment with ZaBDe (Figure 5F). Moreover, some other proteins involved in the caspase-independent apoptotic pathway, such as AIF (apoptosis-inducing factor) and Endo-G (endonuclease G),11 were also found to accumulate in the nucleus, where they execute their proapoptotic functions (Figure 5F).
Discussion
The incurable nature of MM and the dismal outcome of patients who become refractory to the current standards of care make it necessary to search for novel compounds and combinations that, by targeting multiple pathways simultaneously, are able to overcome the acquired resistance. In this work, we have evaluated the combination of bortezomib and dexamethasone with zalypsis, a novel DNA-damaging agent derived from marine organisms, for which an anti-MM effect has been demonstrated in preclinical settings5 and in an early-phase clinical trial.6
The ZaBDe combination was effective in different preclinical models and patient samples; the most significant results being the differences in terms of tumor growth control and survival in the two xenograft models evaluated. This synergistic effect suggests that even with low doses of the agents in the triple combination, we can obtain significant clinical benefit. Regarding toxicity, our ex vivo studies in patient samples have shown that the triple combination induces plasma cell apoptosis whilst preserving lymphocytes, thereby ensuring treatment tolerability. This is important since one of the main toxicities with zalypsis in monotherapy is hematological; our data suggest that this toxicity would be lower with the combination as we can reduce the doses of the drugs achieving higher efficacy ex vivo. In fact, in the previously published study of zalypsis in monotherapy,5 the agent was quite toxic to normal lymphocytes in BM from patients ex vivo. In the present work, due to the lower doses of zalypsis used, the toxicity to lymphocytes was much lower. Regarding the in vivo data, we observed some toxicity in the initial experiment based on the subcutaneous plasmacytoma model. In fact, two toxic early deaths were observed with the triple combination in the plasmacytoma model, which prompted the adaptation of doses in the disseminated MM model, in which efficacy was maintained and tolerability slightly improved with an earlier recovery of the body weight loss.
With respect to the mechanism of action, we have shown that the triple combination displayed a synergic effect that relies on the convergence of several mechanisms; including the enhancement of DNA damage and the blockade of NF-κB translocation into the nucleus. Regarding the first point, DSBs are the most severe forms of DNA damage, and they affect genomic stability. The tumor suppressor p53 plays an important role in the DNA-damage response (DDR), either by inducing cell cycle arrest, in an attempt to repair DNA damage, or by inducing the apoptosis of severely damaged cells.12 From our ex vivo studies, and building on previous results with zalypsis in monotherapy,5 we found that the triple combination is also effective in plasma cells with p53 deletion, a signature of adverse outcome in MM patients.13,14 The DNA damage induced by ZaBDe seems to be severe, since a preferential apoptotic effect, rather than cell cycle arrest, was detected. Moreover, the impairment of some mechanisms of DDR described for bortezomib15 could also play an important role in this synergy. The apoptotic induction after DNA damage can be counteracted by the activation of NF-κB,16 a heterodimer composed of p50 and p65 subunits, which is the main pro-survival transcription factor in the DDR.10 Our results suggest that the nuclear translocation of NF-κB induced by zalypsis and dexamethasone could be a potential mechanism of resistance to these compounds. The addition of the proteasome inhibitor bortezomib partially reverted this mechanism in double combinations and, most interestingly, was completely overcome in the triple combination. In fact, we consider that this effect of bortezomib could be important in explaining the synergy observed in the triple combination.
The mitochondrial pathway seems to play an important role in the apoptotic mechanism of ZaBDe activity, with the concurrent activation of caspase-dependent and caspase-independent routes. On one hand, cytochrome C, a major caspase activator, seems to be liberated into the cytosol where it ultimately exerts its activity through caspase mechanisms. On the other hand, two proteins implicated in caspase-independent apoptosis, AIF and endo-G, are translocated from the mitochondria into the nucleus. The latter two proteins have been linked to the induction of DNA fragmentation, nuclear condensation and cell death,17 even in the presence of caspase inhibitors.18
In conclusion, we have demonstrated the synergy of the ZaBDe triple combination in several preclinical models of MM. The anti-myeloma activity of this combination partly relies on the induction of DNA damage and on the decrease of NF-κB translocation into the nucleus, resulting in both caspase-dependent and caspase-independent apoptosis. These results indicate the value of the clinical development of the combination ZaBDe for the treatment of relapsed and refractory MM patients.
Supplementary Material
Acknowledgments
The authors would like to thank to Isabel Isidro, Teresa Prieto and Almudena Martín (Cancer Research Center and Universitary Hospital of Salamanca) for their help and excellent technical work.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/102/1/168
Funding
This work was in part funded by the Spanish ISCIII-FIS (PI 15/0067 and PI15/02156) and FEDER, the Spanish RTICC (RD12/0036/0058), “Asociación Española Contra el Cancer” (AECC, GCB120981SAN), the regional Council from “Castilla y León” (GRS 1175/A/15 and FIC335U14) and a research grant from Pharmamar SAU. MMS were also supported by the Network of Centers for Regenerative Medicine and Cellular Therapy from Castilla y León, Spain. A-A López-Iglesias was supported by a grant from the Spanish Society of Hematology and Hemotherapy.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA. Cancer J Clin. 2015;65(1): 5–29. [DOI] [PubMed] [Google Scholar]
- 2.Kumar SK, Rajkumar SV, Dispenzieri A, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111(5):2516–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ocio EM, Richardson PG, Rajkumar SV, et al. New drugs and novel mechanisms of action in multiple myeloma in 2013: a report from the International Myeloma Working Group (IMWG). Leukemia. 2014;28(3): 525–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petek B, Jones R. PM00104 (Zalypsis®): A Marine Derived Alkylating Agent. Molecules. 2014;19(8):12328–12335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ocio EM, Maiso P, Chen X, et al. Zalypsis: a novel marine-derived compound with potent antimyeloma activity that reveals high sensitivity of malignant plasma cells to DNA double-strand breaks. Blood. 2009; 113(16):3781–3791. [DOI] [PubMed] [Google Scholar]
- 6.Ocio EM, Oriol A, Bladé J, et al. Phase I/II study of weekly PM00104 (Zalypsis®) in patients with relapsed/refractory multiple myeloma. Br J Haematol. 2016;172(4): 625–628. [DOI] [PubMed] [Google Scholar]
- 7.Maiso P, Carvajal-Vergara X, Ocio EM, et al. The histone deacetylase inhibitor LBH589 is a potent antimyeloma agent that overcomes drug resistance. Cancer Res. 2006;66(11): 5781–5789. [DOI] [PubMed] [Google Scholar]
- 8.Chou TC. Drug combination studies and their synergy quantification using the choutalalay method. Cancer Res. 2010; 70(2): 440–446. [DOI] [PubMed] [Google Scholar]
- 9.Olive PL, Banáth JP. The comet assay: a method to measure DNA damage in individual cells. Nat Protoc. 2006;1(1):23–29. [DOI] [PubMed] [Google Scholar]
- 10.Poltz R, Naumann M. Dynamics of p53 and NF-kB regulation in response to DNA damage and identification of target proteins suitable for therapeutic intervention. BMC Syst Biol. 2012;6:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kroemer G, Martin SJ. Caspase-independent cell death. Nat Med. 2005; 11(7):725–730. [DOI] [PubMed] [Google Scholar]
- 12.Cann KL, Hicks GG. Regulation of the cellular DNA double-strand break response. Biochem. Cell Biol. 2007;85(6):663–674. [DOI] [PubMed] [Google Scholar]
- 13.Fonseca R, Blood E, Rue M, et al. Clinical and biologic implications of recurrent genomic aberrations in myeloma. Blood. 2003;101(11):4569–4575. [DOI] [PubMed] [Google Scholar]
- 14.Drach J, Ackermann J, Fritz E, et al. Presence of a p53 gene deletion in patients with multiple myeloma predicts for short survival after conventional-dose chemotherapy. Blood. 1998;92(3):802–809. [PubMed] [Google Scholar]
- 15.Neri P, Ren L, Gratton K, et al. Bortezomib-induced “BRCAness” sensitizes multiple myeloma cells to PARP inhibitors. Blood. 2011;118(24):6368–6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacobs MD, Harrison SC. Structure of an IB/NF-kB Complex. Cell. 1998;95(6): 749–758. [DOI] [PubMed] [Google Scholar]
- 17.Yu S-W, Wang H, Poitras MF, et al. Mediation of poly(ADP-ribose) polymerase-1-dependent cell death by apoptosis-inducing factor. Science. 2002;297(5579):259–263. [DOI] [PubMed] [Google Scholar]
- 18.Li LY, Luo X, Wang X. Endonuclease G is an apoptotic DNase when released from mitochondria. Nature. 2001;412(6842):95–99. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


