Abstract
Background
Discordance in hormone receptors (HR) and human epidermal growth factor receptor 2 (HER2) status between primary tumors and metastatic sites for breast cancer is well established. However, it is uncertain which patient-related factors lead to biopsy when metastases are suspected and whether having a biopsy impacts survival.
Methods
The medical charts of metastatic breast cancer (MBC) patients diagnosed January 2000-August 2014 were retrospectively reviewed. A biopsy was defined as a procedure where tissue was obtained and assessed for both HR and HER2. Both bivariate and multivariate analyses were performed to assess patient characteristics related to biopsy and whether having a biopsy was associated with improved survival.
Results
Of 409 patients suspected of having MBC, 165 (40%) had a biopsy, and 34% of these had discordant HR or HER2 status when compared to the initial diagnosis. In multivariate analysis, having a biopsy was associated with: recurrence in years 2010–2014, disease-free interval of > =3 years, stage 0-IIA at presentation, suspected locoregional recurrence, being HR+/HER2-, or missing HR/HER2 at diagnosis. A similar multivariate analysis revealed that having a biopsy was associated with improved survival (HR = 0.67, p = 0.002). The association of biopsy and improved survival was noted in specific subgroups: patients with missing HR and HER2 data at initial diagnosis (p = 0.001), those without metastases in liver, lung or brain (p = 0.001), and being younger than 70 years old at recurrence (p < 0.001).
Conclusions
Specific clinical factors were associated with biopsy at the time of suspected recurrence. Having a biopsy was associated with reduced mortality.
Keywords: Biopsy, Breast carcinoma, Pathology, Recurrent-metastatic disease, Survival
Background
Breast cancer is the most common cancer among women in the United States, and it is estimated that 246,660 new cancer cases will be diagnosed in 2016 [1]. Despite recent progress in diagnostic and therapeutic approaches of early breast cancer, many patients develop metastases, resulting in about 40,000 deaths annually. Systemic treatment in metastatic breast cancer (MBC), as in early stage breast cancer, is chosen on the basis of estrogen receptor (ER) and progesterone receptor (PR) status and overexpression/amplification of the human epidermal growth factor receptor 2 (HER2). In addition, other factors are routinely used for therapy decisions, such as disease-free interval, site(s) of relapse, and number of metastases [2, 3].
Discordance in hormone receptors and HER2 status between the primary tumor and the metastatic lesion is well documented [4]. A recent review demonstrated a large range of receptor discordance with ER, PR and HER2 discordance of 6–40%, 21–41% and 1–43%, respectively [5]. In addition, a meta-analysis of 48 studies, in which 4200, 2739, and 2987 tumors were evaluated for ER, PR and HER2 discordance, respectively, showed a discordance range of 8–23% [6]. The reasons for discordance may relate to changes in tumor biology between the initial tumor and the metastatic lesion biopsied, heterogeneity within the tumor itself, and/or imperfect accuracy and reproducibility of assays, although the relative contribution of each of these factors to the overall discordance rate is unclear. The unequivocal recommendations for biopsy when metastases are suspected are: solitary metastasis, unusual clinical course, and research [7]. The existent discordance between the primary tumor and recurrent metastasis in ER, PR and HER2 status in portions of tumors has been demonstrated in many studies [5, 8].
The Advanced Breast Cancer (ABC) consensus guidelines recommend biopsying a metastatic lesion, if easily accessible, and confirming the diagnosis, particularly when metastasis is diagnosed for the first time [3]. The National Comprehensive Cancer Network (NCCN) Panel recommended doing a biopsy to confirm recurrence, and repeating ER/PR and HER2 at the metastatic site [2].
Biopsy results may also be related to survival. In a recent Swedish trial, it was concluded that patients with tumor loss of ER or PR during progression have worse survival than patients with retained receptor expression [8]. However, in another retrospective trial, no significant survival difference between patients with discordant versus non-discordant receptors in MBC was seen [9]. Lastly, Curtit et al. reported that patients who had undergone biopsy had survival benefit (median OS 79 months vs 32 months, p = 0.0001) [10].
However, it is still unclear whether patient characteristics are associated with having a biopsy and whether having a biopsy is associated with improved survival. Since prospective trials to address these questions are not likely to be performed, we conducted a large retrospective trial to help answer these questions.
Methods
Study design
After approval of the study protocol by the institutional ethics committee - Helsinki Committee of Rambam Health Care Campus (RHCC) (Certificate no. 0408-13-RMB) - a retrospective analysis was conducted of all medical records of adult patients treated in the Division of Oncology at RHCC in Haifa, Israel, for metastatic breast carcinoma from January 2000 to August 2014. Eligibility for the study included having metastatic/locoregional non-curable disease, previous treatment for early breast cancer with curative intent (including surgery), and an interval of at least 6 months since the end of adjuvant chemotherapy, radiotherapy, and/or surgery (adjuvant hormonal therapy was allowed). Excluded from the study were patients with de novo primary metastatic disease and patients who were not treated with curative intent at first diagnosis (without surgery). Patients were de-identified after data were collected from digital and non-digital records. Data extracted from the medical files included demographics and medical history: age, marital status, number of children, stage (American Joint Committee on Cancer, 7th edition), grade, ER, PR and HER2 status (from initial surgery/biopsy specimen), adjuvant treatment regimen, and type of surgery at initial diagnosis. Data obtained on patients with suspected metastases included the sites of metastasis, if and when a biopsy was done, a pathology report from the biopsy including documentation of metastatic adenocarcinoma, ER, PR and HER2status, and date of death or date of last follow-up. We defined a biopsy as a procedure confirming adenocarcinoma, including reassessment of HR and HER2 status. Patients were defined as not having a biopsy if they did not undergo biopsy, had a biopsy which only histologically confirmed metastases, or did not reassess HR and HER2 status. Positive ER or PR expression was defined as 1% of cells or greater staining for ER or PR. HER2 assessment was scored from 0–3+. In case of overexpression scored at 2+, fluorescent in situ hybridization (FISH) was performed. HER2 was considered positive if overexpression was scored at 3+ in immunohistochemistry, or 2+ in immunohistochemistry, if FISH was positive.
Statistical analysis
Logistic regression was used to calculate the odds ratios (OR) with 95% confidence intervals (95% CI) and p values in bivariate analysis to determine associations between tumor characteristics and likelihood of biopsy. Based on previous theory [11], variables with p < =0.1 were included in subsequent Multivariable Forward Stepwise Logistic Regression analysis. The Hosmer-Lemeshow goodness-of-fit statistic [11] was calculated, and the area under the receiver operating characteristic (ROC) curve was used as a measure of model discrimination. Bivariate Cox regression was then used to calculate hazard ratios (HR) with 95% confidence intervals (CI) and p values for overall survival (OS). Stratified analysis was used to assess confounding. Variables with p < =0.1 were used in multivariate Cox Regression analysis to assess the effect of biopsy on overall survival (OS), as well as the effect of patient and tumor characteristics. Two-tailed p values of 0.05 or less were considered statistically significant. Statistical analyses was performed using SPSS (Statistics Products Solutions Services) 21.0 software for Windows.
Results
Patient characteristics
The medical records of 409 patients diagnosed with MBC between 2000 and 2014 were evaluated. Of these 409 patients, 165 (40%) patients had a biopsy as defined in this study, while 90 (22%) had only histologic confirmation of metastases.
Median follow-up for metastatic disease was 30.2 months (range, 1.1–154.2 months) for all patients and 42.3 months (range, 1.3–155.8 months) for patients who were alive at the time of chart review. Patient characteristics are shown in Table 1. Median age at metastatic diagnosis was 57.4 years (range, 26.6–88.7 years). Of the 409 patients, 42% were initially diagnosed with stage 0-IIA tumors and 57% with stage IIB-III tumors. Also at initial diagnosis, 186 (45%) patients had grade 1–2 tumors, 146 (36%) had grade 3 tumors (19% data missing), 282 (69%) were HR positive and 92 (22%) were HER2 positive.
Table 1.
Patient characteristics, bivariate analysis for biopsy, and overall mortality
| Variable | No. Patients | No Biopsy | Biopsy | All Cause Mortality | |||||
|---|---|---|---|---|---|---|---|---|---|
| No. (%) | No. (%) | P value | OR (95% CI) | % total | P value | HR (95% CI) | |||
| Factors in initial diagnosis | |||||||||
| Year of initial diagnosis | Total | 409 | 244 (60) | 165 (40) | 80.9 | ||||
| 1980–99 | 134 | 76 (57) | 58 (43) | Ref | 1.00 | 85.1 | Ref. | 1.00 | |
| 2000–09 | 256 | 154 (60) | 102 (40) | 0.512 | 0.87 (0.57–1.33) | 79.7 | 0.000 | 1.54 (1.22–1.94) | |
| 2010–14 | 19 | 14 (74) | 5 (26) | 0.167 | 0.47 (0.16–1.37) | 68.4 | 0.000 | 5.35 (2.95–9.70) | |
| Stage at initial diagnosis | Stage 0-IIA | 172 | 82 (48) | 90 (52) | Ref | 1.00 | 73.8 | Ref. | 1.00 |
| Stage IIB-III | 232 | 161 (69) | 71 (31) | 0.000 | 0.40 (0.27–0.61) | 87.1 | 0.001 | 1.46 (1.17–1.82) | |
| Missing | 5 | 1 (20) | 4 (80) | 40.0 | |||||
| Grade at initial diagnosis | 1.0 | 16 | 5 (31) | 11 (69) | Ref | 1.00 | 62.5 | Ref. | 1.00 |
| 2.0 | 169 | 103 (61) | 66 (39) | 0.028 | 0.29 (0.10–0.88) | 80.5 | 0.167 | 1.57 (0.83–3.00) | |
| 3.0 | 146 | 90 (62) | 56 (38) | 0.026 | 0.28 (0.09–0.86) | 85.6 | 0.009 | 2.37 (1.24–4.52) | |
| Missing | 78 | 46 (59) | 32 (41) | 76.9 | |||||
| HR and HER2 at initial diagnosis | HR+/HER2+ | 48 | 38 (79) | 10 (21) | Ref. | 1.00 | 75.0 | Ref. | 1.00 |
| HR-/HER2- | 49 | 35 (71) | 14 (29) | 0.379 | 1.52 (0.60–3.86) | 87.8 | 0.004 | 1.92 (1.24–3.00) | |
| HR-/HER2+ | 44 | 35 (80) | 9 (20) | 0.964 | 0.98 (0.36–2.69) | 86.4 | 0.032 | 1.65 (1.04–2.61) | |
| HR+/HER2- | 173 | 99 (57) | 74 (43) | 0.007 | 2.84 (1.33–6.07) | 80.3 | 0.090 | 1.38 (0.95–1.99) | |
| Missing HR or HER2 | 95 | 37 (39) | 58 (61) | 0.000 | 5.96 (2.65–13.38) | 78.9 | 0.429 | 1.17 (0.79–1.75) | |
| Factors in metastatic recurrence | |||||||||
| Time to recurrence | <3 years | 145 | 120 (83) | 25 (17) | Ref | 1.00 | 93.1 | Ref | 1.00 |
| > = 3 years | 264 | 124 (47) | 140 (53) | 0.000 | 5.42 (3.31–8.88) | 74.2 | 0.000 | 0.58 (0.47–0.73) | |
| Age at recurrence | <70 years | 348 | 207 (59) | 141 (41) | Ref | 1.00 | 80.7 | Ref | 1.00 |
| > = 70 years | 61 | 37 (60) | 24 (40) | 0.863 | 0.95 (0.55–1.66) | 82.0 | 0.004 | 1.57 (1.16–2.12) | |
| Years of metastatic recurrence | 2000–04 | 116 | 93 (80) | 23 (20) | Ref | 1.00 | 96.6 | Ref. | 1.00 |
| 2005–09 | 165 | 103 (62) | 62 (38) | 0.002 | 2.43 (1.40–4.24) | 90.3 | 0.433 | 1.10 (0.86–1.41) | |
| 2010–14 | 128 | 48 (37) | 80 (63) | 0.000 | 6.74 (3.77–12.04) | 54.7 | 0.596 | 1.09 (0.80–1.48) | |
| No of metastatic sites at recurrence | Single site | 123 | 83 (64) | 40 (36) | Ref | 1.00 | 78.9 | Ref | 1.00 |
| 2–3 sites | 136 | 76 (56) | 60 (44) | 0.056 | 1.64 (0.99–2.72) | 80.1 | 0.011 | 1.43 (1.09–1.89) | |
| >3 sites | 150 | 85 (57) | 65 (43) | 0.068 | 1.59 (0.97–2.61) | 83.3 | 0.001 | 1.57 (1.20–2.06) | |
| Brain metastases | No | 376 | 219 (58) | 157 (42) | Ref | 1.00 | 79.8 | Ref. | 1.00 |
| Yes | 33 | 25 (76) | 8 (24) | 0.054 | 0.45 (0.20–1.02) | 93.9 | 0.000 | 2.60 (1.79–3.79) | |
| Liver metastases | No | 289 | 176 (61) | 113 (39) | Ref | 1.00 | 78.5 | Ref. | 1.00 |
| Yes | 120 | 68 (57) | 52 (43) | 0.427 | 1.19 (0.77–1.83) | 86.7 | 0.007 | 1.38 (1.09–1.74) | |
| Lung metastases | No | 253 | 150 (59) | 103 (41) | Ref | 1.00 | 79.4 | Ref. | 1.00 |
| Yes | 156 | 90 (60) | 62 (40) | 0.846 | 0.96 (0.64–1.44) | 83.3 | 0.015 | 1.32 (1.05–1.64) | |
| HR and HER2 at recurrence | HR+/HER2+ | 26 | 69.2 | Ref | 1.00 | ||||
| HR-/HER2- | 34 | 85.3 | 0.008 | 2.23 (1.24–4.02) | |||||
| HR-/HER2+ | 15 | 53.3 | 0.295 | 1.56 (0.68–3.61) | |||||
| HR+/HER2- | 90 | 60.0 | 0.665 | 1.13 (0.66–1.92) | |||||
| Missing | 244 | 91.0 | 0.004 | 2.01 (1.24–3.26) | |||||
| Site of metastasis at recurrence | No brain, liver or lung | 158 | 89 (56) | 69 (44) | Ref | 1.00 | 75.3 | Ref. | 1.00 |
| Liver or lung | 183 | 113 (62) | 70 (38) | 0.310 | 0.80 (0.52–1.23) | 81.4 | 0.020 | 1.33 (1.05–1.70) | |
| Liver & lung | 35 | 17 (49) | 18 (51) | 0.405 | 1.37 (0.66–2.84) | 91.4 | 0.000 | 2.25 (1.52–3.33) | |
| Brain | 33 | 25 (76) | 8 (24) | 0.043 | 0.41 (0.18–0.97) | 93.9 | 0.000 | 3.22 (2.15–4.81) | |
| LAD at recurrence | No LAD | 239 | 169 (71) | 70 (29) | Ref | 1.00 | 83.7 | Ref. | 1.00 |
| LAD | 170 | 75 (44) | 95 (56) | 0.000 | 3.06 (2.03–4.61) | 77.1 | 0.933 | 0.99 (0.79–1.24) | |
| Biopsy Status | No | 244 | 91.0 | Ref | 1.00 | ||||
| Yes | 165 | 66.1 | 0.000 | 0.64 (0.51–0.81) | |||||
HR hormone receptors, HER2 human epidermal growth factor receptor 2, LAD locally advanced disease, N/A not applicable
All patients underwent surgery with curative intent. More than 75% of the patients received chemotherapy (72% anthracycline-based), either as adjuvant or neoadjuvant treatment. Adjuvant trastuzumab was given to only 8% of patients. Adjuvant hormonal therapy was given to 62% of patients. Biopsy sites were: locoregional recurrence (skin, chest wall, breast or lymph nodes (LN)) 77 (47%), bone 33 (20%), liver 26 (16%), lung 14 (8%), abdomen 5, other sites 10.
Clinical characteristics associated with biopsy
Clinical characteristics found to be significantly associated with having a biopsy are shown in Table 1. Overall, the percentage of patients who underwent biopsy significantly increased between 2000 and 2014. Factors related to having a biopsy included: not having HR or HER 2 status at initial diagnosis (OR = 5.96, p < 0.0001), disease-free interval longer than > =3 years (OR = 5.42, p < 0.0001), and locally advanced disease (in the breast, LN, skin, or chest wall) (OR = 3.06, p < 0.0001). Advanced stages IIB-III (OR = 0.40, p < 0.0001) and high grade (grade 3 compared to grade 1 (OR = 0.28, p = 0.026)) were associated with fewer biopsies. Of note, patient age at time of suspected metastases was not associated with the biopsy decision.
In multivariate analysis (Table 2), variables that remained significantly associated with biopsy were: year of recurrence (OR = 10.67, p < 0.0001 for 2010–2014), disease-free interval of > =3 years (OR = 3.20, p < 0.001), stage 0-IIA (OR = 1.89, p = 0.012), locally advanced disease (OR = 1.90, p = 0.011), HR+/HER2- at initial diagnosis (OR = 2.83, p = 0.021), and unknown HR or HER2 at initial diagnosis (OR = 6.78, p < 0.001).
Table 2.
Multivariate analysis : Factors predictive of biopsy
| Variable | P value | Adjusted OR (95.0% CI for OR) | |
|---|---|---|---|
| Years of metastatic recurrence | 2000–04 | Ref. | |
| 2005–09 | .000 | 3.79 (1.94–7.40) | |
| 2010–14 | .000 | 10.67 (5.13–22.22) | |
| Time to recurrence(years) | <3 years | Ref. | |
| > = 3 years | .000 | 3.20 (1.78–5.76) | |
| Stage at initial diagnosis | Stage IIB-III | Ref. | |
| Stage 0- IIA | .012 | 1.89 (1.15–3.10) | |
| Site of metastasis at recurrence | No locally advanced disease | Ref. | |
| Locally advanced disease | .011 | 1.90 (1.16–3.10) | |
| HR &HER2 at initial diagnosis | HR+/HER2+ | Ref. | |
| HR-/HER2- | .349 | 1.68 (0.57–4.93) | |
| HR-/HER2+ | .813 | 1.15 (0.35–3.74) | |
| HR+/HER2- | .021 | 2.83 (1.17–6.83) | |
| Missing HR or/and HER2 | .000 | 6.78 (2.49–18.45) |
All other variables were not significant
HR hormone receptors, HER2 human epidermal growth factor receptor 2
Discordance in hormone receptor and HER2 status among primary and metastatic tumors
In 179 patients whose tumor HR status was evaluated both at primary diagnosis and upon recurrence, HR status changed from positive to negative in 27 (15%) patients, and from negative to positive in 14 (8%) patients. HER2 status was evaluated in 114 patients at both diagnosis and recurrence. HER2 changed from positive to negative in 5 (4%) patients, and from negative to positive in 10 (9%) patients. Figure 1 describes changes from initial pathology to biopsy in 107 patients whose HR and HER2 were measured in both biopsies.
Fig. 1.
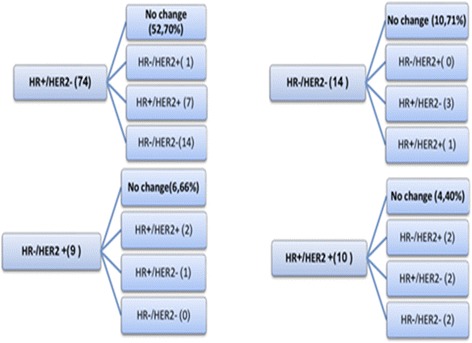
Discordance between initial histology and biopsy (n = 107)
Factors associated with overall mortality
In bivariate analysis, clinical characteristics associated with overall mortality are summarized in Table 1. At diagnosis, advanced stage (IIB, III), higher grade (grade 3), and having a triple negative or HR-/HER2+ tumor was associated with worse survival. In turn, at the time of metastases, age > =70 years, a disease-free interval of <3 years, multiple metastatic sites, brain, liver and lung metastases, or having triple negative disease was associated with worse survival. Of note, having a biopsy upon recurrence was associated with longer survival (HR = 0.64, p < 0.0001).
Multivariate analysis of factors associated with shortened overall survival at initial diagnosis (Table 3) included advanced stage (stage IIB-III) (HR = 1.40, p = 0.004), triple negative phenotype (HR = 2.14, p = 0.001), and HR+/HER2- phenotype (HR = 1.61, p = 0.013). At the time of metastases, age ≥70 years (HR = 1.65, p = 0.002), and brain or liver and lung metastasis (HR = 2.85, HR = 2.46, respectively, p < 0.001) were associated with shortened overall survival. As in bivariate analysis, not having a biopsy was associated with shorter survival as compared to having a biopsy (HR = 0.67, p = 0.002).
Table 3.
Multivariate analysis: Factors predictive of All Cause mortality
| Variable | P value | HR Adjusted(95.0% CI for HR) | |
|---|---|---|---|
| HR & HER2 at initial diagnosis | HR+/HER2+ | Ref. | |
| HR-/HER2- | .002 | 2.14 (1.36–3.39) | |
| HR-/HER2+ | .179 | 1.36 (0.86–2.27) | |
| HR+/HER2- | .013 | 1.61 (1.11–2.29 | |
| HR and/or HER2 missing | .065 | 1.49 (0.98–2.21) | |
| Site of metastasis at recurrence | No brain, liver or lung metastasis | Ref. | |
| Liver & lung involvement | .000 | 2.46 (1.68–3.60) | |
| Brain involvement | .000 | 2.85 (1.88–4.31) | |
| Age at metastatic recurrence (years) | <70 | Ref. | |
| > = 70 | .002 | 1.64 (1.19–2.24) | |
| Stage at initial diagnosis | Stage 0-IIA | Ref. | |
| Stage IIB-III | .004 | 1.40 (1.12–1.76) | |
| Biopsy | No biopsy | Ref. | |
| Biopsy | .002 | 0.67 (0.52–0.86) |
All other variables were not significant
HR hormone receptors, HER2 human epidermal growth factor receptor 2
Association of overall survival and biopsy in selected subgroups
Exploratory analysis of the interaction of biopsy and survival was performed in selected subgroups (Table 4). A significant survival benefit was found in patients with missing data on receptor status (p = 0.001) at the initial diagnosis, no metastasis in liver and lung or brain (p = 0.001), or younger than age 70 (p = 0.0001). There was no significant effect on survival among different tumor phenotypes; however, this might be due to the small number of patients in each group.
Table 4.
Effect of biopsy on survival for selected clinical and pathological variables
| No. Pts. | All Cause Mortality | |||||
|---|---|---|---|---|---|---|
| No. | % total | P value | HR (95%CI) | |||
| TOTAL | 409 | 331 | 80.9 | |||
| Biopsy at recurrence | ||||||
| Biopsy | No Bx | 244 | 222 | 91.0 | 1.00 | |
| Bx | 165 | 109 | 66.1 | 0.000 | 0.64 (0.51–0.81) | |
| HR & HER2at initial diagnosis | ||||||
| HR+/HER2+ | No Bx | 38 | 30 | 78.9 | 1.00 | |
| Bx | 10 | 6 | 60.0 | 0.499 | 0.74 (0.30–1.79) | |
| HR-/HER2- | No Bx | 35 | 33 | 94.3 | 1.00 | |
| Bx | 14 | 10 | 71.4 | 0.236 | 0.65 (0.32–1.33) | |
| HR-/HER2+ | No Bx | 35 | 33 | 94.3 | 1.00 | |
| Bx | 9 | 5 | 55.6 | 0.562 | 0.75 (0.29–1.98) | |
| HR+/HER2- | No Bx | 99 | 90 | 90.9 | 1.00 | |
| Bx | 74 | 49 | 66.2 | 0.097 | 0.74 (0.52–1.06) | |
| HR and/or HER2 missing | No Bx | 37 | 36 | 97.3 | 1.00 | |
| Bx | 58 | 39 | 67.2 | 0.001 | 0.45 (0.28–0.71) | |
| Site of metastasis at recurrence | ||||||
| No liver & lung or brain metastases | No Bx | 202 | 182 | 90.1 | 1.00 | |
| Bx | 139 | 86 | 61.9 | 0.001 | 0.63 (0.49–0.82) | |
| Liver & lung metastases | No Bx | 17 | 15 | 88.2 | 1.00 | |
| Bx | 18 | 17 | 94.4 | 0.539 | 0.80 (0.39–1.63) | |
| Brain metastases | No Bx | 25 | 25 | 100.0 | 1.00 | |
| Bx | 8 | 6 | 75.0 | 0.196 | 0.55 (0.22–1.36) | |
| Age at metastatic recurrence | ||||||
| Age <70 years | No Bx | 207 | 189 | 91.3 | 1.00 | |
| Bx | 141 | 92 | 65.2 | 0.000 | 0.62 (0.48–0.80) | |
| Age >=70 years | No Bx | 37 | 33 | 89.2 | 1.00 | |
| Bx | 24 | 17 | 70.8 | 0.587 | 0.85 (0.47–1.54) | |
| Stage at initial diagnosis | ||||||
| Stage 0-IIA | No Bx | 82 | 71 | 86.6 | 1.00 | |
| Bx | 90 | 56 | 62.2 | 0.025 | 0.67 (0.47–0.95) | |
| Stage IIB-III | No Bx | 161 | 151 | 93.8 | 1.00 | |
| Bx | 71 | 51 | 71.8 | 0.034 | 0.71 (0.51–0.97) | |
HR hormone receptors, HER2 human epidermal growth factor receptor 2, Bx biopsy
Discussion
The primary purposes in doing a biopsy when MBC is suspected are to confirm the diagnosis of metastasis and to reassess HR and HER2 status, two key factors that influence subsequent treatment. In a prospective trial that included 64 women with a history of prior breast cancer and suspected new metastases, a non-breast cancer diagnosis was found in 14, and 10% had benign findings [12]. Of the 205 patients prospectively included in the BRITS trial, 9% did not have recurrent disease on biopsy [13]. In our study, 33% of patients had a biopsy only for histological confirmation of MBC. We also found that, in recent years, physicians ordered significantly more biopsies (OR = 10.67, p < 0.0001 for 2010–2014), perhaps due to the fact that recent studies have emphasized that receptor status can change [10, 14].
The main purpose of a biopsy is to reassess the HR and HER2 status of the metastatic disease. In our study, a biopsy that included confirmation of HR and HER2 status was associated with a significant survival benefit (p = 0.001). In a study by Amir et al. [14], there were discordant findings between findings at initial diagnosis and findings after metastasis in 37.6% of patients, resulting in a treatment change in 14% of patients. However, no change in OS was noted between concordant and discordant groups in that study [14]. Different results were seen in a study in China, which showed worse OS for patients who had receptors discordance in the biopsy [15]. However, in a review of 13 recent major studies regarding biopsy in MBC, the impact of this procedure on survival was not clear [16].
Improved survival for patients whose metastases retained ER or PR expression as compared to those with loss of ER or PR expression was shown by Karlsson et al. in a cohort of 177 patients with MBC [8], and in a pooled analysis of two prospective studies (BRITS and DESTINY studies) that included 289 MBC patients, where a gain of HR or HER2 expression led to most changes in treatment approaches [17]. We demonstrated that a biopsy that included confirmation of HR and HER2 was associated with lower mortality. However, the major benefit was seen mainly in patients who had missing data on HR or HER2 status at initial diagnosis (p = 0.001).
We offer some explanations for the association of undergoing biopsy and improved survival: biopsy may show discordance with initial findings that may be associated with improved treatment selection, in turn, resulting in a higher response rate, longer progression free interval, and possibly improved survival. This explanation is supported by recent data showing that molecular subtyping and gene modules of post-relapse biopsy are associated with survival [18]. In our study, 8% of patients who were initially HR- became HR+, suggesting that endocrine therapy should be considered. At the same time, 15% of patients who were HR+ at diagnosis became HR- at biopsy and would likely not have benefitted from endocrine therapy; for these patients, chemotherapy would be a better choice. In 9% of patients, HER2- changed to HER2+, a change that can modify the treatment plan to include anti-HER2 directed therapy; anti-HER2 directed therapies significantly improved survival for patients with HER2+ MBC [19, 20]. Of note, a small group, 4% of patients, changed from HER2+ to HER2- and most likely would not have benefitted from anti-HER2 therapy. Another possible explanation for what we observed in our sample is that physicians choose to biopsy patients whose clinical scenarios are associated with improved survival, such as stage at initial presentation and longer disease-free intervals (Table 2). To test this scenario, a prospective randomized control trial would be needed.
Although no difference was found in biopsy percentages by age groups, patients <70 years who had a biopsy were found to have improved survival in subgroup analysis (p < 0.0001). This finding might be partially due to competing causes of mortality in older patients and the fact that older patients are more likely to have HR+ tumors and HR+ metastases [21]. In a large study with a long follow-up period (28 years), 30% of metastatic breast cancer patients >70 years died from non-breast cancer causes [22].
Our study has several limitations. First, it is retrospective and we assume but cannot be certain that experienced clinicians chose to biopsy patients with suspected metastases with a more favorable prognosis. Second, we do not have data on how treatment decisions were affected in patients with discordant biopsy findings. Third, our study was not sufficiently powered to check discordance of HR and HER2 for different metastatic sites. In one study that examined discordance in 233 distant breast cancer metastases at different sites (76 skin, 63 liver, 43 lung, 44 brain, 7 gastro-intestinal), receptor conversion seemed to occur mostly in liver and brain metastases; however, this was significant only for PR conversion [23].
Conclusions
Our data confirm the high discordance rate between findings at initial breast cancer diagnosis and findings at metastases. Our findings suggest that patients most likely to benefit from biopsy are those without liver, lung or brain metastases, are younger than 70 years of age, and with missing receptors status at initial diagnosis. Recent American Society of Clinical Oncology (ASCO) guidelines [24] suggest that, in patients with accessible metastases, biopsy for confirmation of metastases and retesting of estrogen receptor, progesterone receptor, and HER2 status should be offered. However, evidence is lacking to determine whether modifying anticancer therapy on the basis of a change in receptor status influences clinical outcomes [24]. The ASCO panel consensus was to preferentially use the ER, PR, and HER2 status of the metastasis to direct therapy, if supported by the clinical scenario and the patient’s goals. In addition, tissues from metastases may be used to assess molecular differences not limited to receptor level but also to investigate DNA, RNA, protein and functional pathway levels and to look for actionable mutations that might allow for novel new therapies [5, 25]. Our findings support current recommendations and show that, in selected patients, a biopsy at metastatic recurrence that included HR and HER2 status was associated with significantly reduced mortality.
Acknowledgements
The authors thank Mrs. Myrna Perlmutter for her help in the preparation of this paper. Funding for this service was provided by the Division of Oncology, Rambam Health Care Campus, Haifa, Israel.
Funding
The project was funded by the Division of Oncology, Rambam Health Care Campus, Haifa, Israel.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Authors’ contributions
SS wrote the manuscript, collected and analyzed the data; TM did the statistical analysis; GF treated the patient and helped to draft the manuscript; KD treated the patient and helped to draft the manuscript; NS did the data collection and analysis; HM helped to draft the manuscript; GBS was the study initiator, helped in the data analysis, and drafted the manuscript. All authors have read and approved the manuscript as submitted.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Page 4 – This study was approved by the ethics committee of Rambam Health Care Campus, Haifa, Israel (Certificate no. 0408-13-RMB); Due to the retrospective nature of this study, consent for participation was not requested.
Abbreviations
- CI
Confidence intervals
- ER
Estrogen receptor
- FISH
Fluorescent in situ hybridization
- HER2
Human epidermal growth factor receptor 2
- HR
Hazard ratio
- HR
Hormone receptor
- MBC
Metastatic breast cancer
- OR
Odds ratio
- OS
Overall survival
- PR
Progesterone receptor
- RHCC
Rambam Health Care Campus
Contributor Information
Shlomit Strulov Shachar, Email: S_shachar@rambam.health.gov.il.
Tanya Mashiach, Email: t_mashiach@rambam.health.gov.il.
Georgeta Fried, Email: g_fried@rambam.health.gov.il.
Karen Drumea, Email: k_drumea@rambam.health.gov.il.
Noa Shafran, Email: noa@shafran.co.il.
Hyman B. Muss, Email: muss@med.unc.edu
Gil Bar-Sela, Phone: 972-4-7776409, Email: g_barsela@rambam.health.gov.il.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.NCCN (1AD) NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Breast Cancer. Version 12015 1–187. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf.
- 3.Cardoso F, Costa A, Norton L, Senkus E, Aapro M, André F, et al. ESO-ESMO 2nd international consensus guidelines for advanced breast cancer (ABC2) Ann Oncol. 2014;25:1871–87. doi: 10.1093/annonc/mdu385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brennan MJ, Donegan WL, Appleby DE. The variability of estrogen receptors in metastatic breast cancer. Am J Surg. 1979;137:260–2. doi: 10.1016/0002-9610(79)90159-4. [DOI] [PubMed] [Google Scholar]
- 5.Criscitiello C, André F, Thompson AM, De Laurentiis M, Esposito A, Gelao L, et al. Biopsy confirmation of metastatic sites in breast cancer patients: clinical impact and future perspectives. Breast Cancer Res. 2014;16:205. doi: 10.1186/bcr3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aurilio G, Disalvatore D, Pruneri G, Bagnardi V, Viale G, Curigliano G, et al. A meta-analysis of oestrogen receptor, progesterone receptor and human epidermal growth factor receptor 2 discordance between primary breast cancer and metastases. Eur J Cancer. 2014;50:277–89. doi: 10.1016/j.ejca.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Pusztai L, Viale G, Kelly CM, Hudis CA. Estrogen and HER-2 receptor discordance between primary breast cancer and metastasis. Oncologist. 2010;15:1164–8. doi: 10.1634/theoncologist.2010-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karlsson E, Appelgren J, Solterbeck A, Bergenheim M, Alvariza V, Bergh J. Breast cancer during follow-up and progression – a population based cohort on new cancers and changed biology. Eur J Cancer. 2014;50:2916–24. doi: 10.1016/j.ejca.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 9.Karagöz Özen DS, Ozturk MA, Aydin Ö, Aydin Ö, Turna ZH, Ilvan S, Özgüroglu M. Receptor expression discrepancy between primary and metastatic breast cancer lesions. Oncol Res Treat. 2014;37:622–6. doi: 10.1159/000368312. [DOI] [PubMed] [Google Scholar]
- 10.Curtit E, Nerich V, Mansi L, Chaigneau L, Cals L, Villanueva C, et al. Discordances in estrogen receptor status, progesterone receptor status, and HER2 status between primary breast cancer and metastasis. Oncologist. 2013;18:667–74. doi: 10.1634/theoncologist.2012-0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katz MH. Multivariable analysis: a practical guide for clinicians. 2 2007. [Google Scholar]
- 12.Qu Q, Zong Y, Fei XC, Chen XS, Xu C, Lou GY, et al. The importance of biopsy in clinically diagnosed metastatic lesions in patients with breast cancer. World J Surg Oncol. 2014;12:93. doi: 10.1186/1477-7819-12-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thompson AM, Jordan LB, Quinlan P, Anderson E, Skene A, Dewar JA, Breast Recurrence in Tissues Study Group et al. Prospective comparison of switches in biomarker status between primary and recurrent breast cancer: the Breast Recurrence In Tissues Study (BRITS) Breast Cancer Res. 2010;12:R92. doi: 10.1186/bcr2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amir E, Miller N, Geddie W, Freedman O, Kassam F, Simmons C, et al. Prospective study evaluating the impact of tissue confirmation of metastatic disease in patients with breast cancer. J Clin Oncol. 2012;30:587–92. doi: 10.1200/JCO.2010.33.5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang YF, Liao YY, Yang M, Peng NF, Xie SR, Xie YF. Discordances in ER, PR and HER2 receptors between primary and recurrent/metastatic lesions and their impact on survival in breast cancer patients. Med Oncol. 2014;31:214. doi: 10.1007/s12032-014-0214-2. [DOI] [PubMed] [Google Scholar]
- 16.Vignot S, Besse B, André F, Spano JP, Soria JC. Discrepancies between primary tumor and metastasis: a literature review on clinically established biomarkers. Crit Rev Oncol Hematol. 2012;84:301–13. doi: 10.1016/j.critrevonc.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 17.Amir E, Clemons M, Purdie CA, Miller N, Quinlan P, Geddie W, et al. Tissue confirmation of disease recurrence in breast cancer patients: pooled analysis of multi-centre, multi-disciplinary prospective studies. Cancer Treat Rev. 2012;38:708–14. doi: 10.1016/j.ctrv.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 18.Tobin NP, Harrell JC, Lovrot J, Egyhazi Brage S, Frostvik Stolt M, Carlsson L, TEX Trialists Group et al. Molecular subtype and tumor characteristics of breast cancer metastases as assessed by gene expression significantly influence patient post-relapse survival. Ann Oncol. 2015;26:81–8. doi: 10.1093/annonc/mdu498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Swain SM, Baselga J, Kim S-B, Ro J, Semiglazov V, Campone M, CLEOPATRA Study Group et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372:724–34. doi: 10.1056/NEJMoa1413513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–92. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 21.Diab SG, Elledge RM, Clark GM. Tumor characteristics and clinical outcome of elderly women with breast cancer. J Natl Cancer Inst. 2000;92:550–6. doi: 10.1093/jnci/92.7.550. [DOI] [PubMed] [Google Scholar]
- 22.Schairer C, Mink PJ, Carroll L, Devesa SS. Probabilities of death from breast cancer and other causes among female breast cancer patients. J Natl Cancer Inst. 2004;96:1311–21. doi: 10.1093/jnci/djh253. [DOI] [PubMed] [Google Scholar]
- 23.Hoefnagel LD, van de Vijver MJ, van Slooten HJ, Wesseling P, Wesseling J, Westenend PJ, et al. Receptor conversion in distant breast cancer metastases. Breast Cancer Res. 2010;12:R75. doi: 10.1186/bcr2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Poznak C, Somerfield MR, Bast RC, Cristofanilli M, Goetz MP, Gonzalez-Angulo AM, et al. Use of biomarkers to guide decisions on systemic therapy for women with metastatic breast cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2015;33:2695–704. doi: 10.1200/JCO.2015.61.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toss A, Cristofanilli M. Molecular characterization and targeted therapeutic approaches in breast cancer. Breast Cancer Res. 2015;17:60. doi: 10.1186/s13058-015-0560-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.


