Abstract
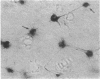
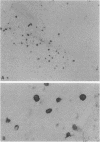
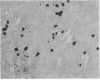
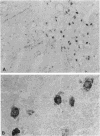
The presence of T lymphocytes bearing the gamma delta T-cell receptor (TCR gamma delta) has been studied immunocyto-chemically in central nervous system (CNS) tissue from 13 patients with multiple sclerosis (MS), 4 with inflammatory non-MS CNS disorders, 6 with other neurological diseases, and 3 with nonneurologic conditions. Twenty-eight of 43 MS lesions contained TCR gamma delta cells and these were most frequently found in chronically demyelinated areas, in contrast to the previously studied CD4+, CD8+ (TCR alpha beta) population, which predominated in more active lesions. Some TCR gamma delta lymphocytes had an unusual morphology with long dendritic processes, which were sometimes interconnected forming a network. Because it is generally believed that TCR gamma delta lymphocytes function in a cytotoxic fashion in association with heat shock proteins (hsp), we examined the colocalization of TCR gamma delta cells with 65- and 70-kDa hsp (hsp65 and hsp70) in MS lesions. Hsp65 was expressed on immature oligodendrocytes at the margins of chronic lesions containing TCR gamma delta lymphocytes. The coexpression of these molecules might imply functional relationships perhaps of significance to the chronicity of the MS disease process and the failure of CNS remyelination.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ang S. L., Seidman J. G., Peterman G. M., Duby A. D., Benjamin D., Lee S. J., Hafler D. A. Functional gamma chain-associated T cell receptors on cerebrospinal fluid-derived natural killer-like T cell clones. J Exp Med. 1987 May 1;165(5):1453–1458. doi: 10.1084/jem.165.5.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balbi B., Moller D. R., Kirby M., Holroyd K. J., Crystal R. G. Increased numbers of T lymphocytes with gamma delta-positive antigen receptors in a subgroup of individuals with pulmonary sarcoidosis. J Clin Invest. 1990 May;85(5):1353–1361. doi: 10.1172/JCI114579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Band H., Hochstenbach F., McLean J., Hata S., Krangel M. S., Brenner M. B. Immunochemical proof that a novel rearranging gene encodes the T cell receptor delta subunit. Science. 1987 Oct 30;238(4827):682–684. doi: 10.1126/science.3672118. [DOI] [PubMed] [Google Scholar]
- Bories J. C., Loiseau P., d'Auriol L., Gontier C., Bensussan A., Degos L., Sigaux F. Regulation of transcription of the human T cell antigen receptor delta chain gene. A T lineage-specific enhancer element is located in the J delta 3-C delta intron. J Exp Med. 1990 Jan 1;171(1):75–83. doi: 10.1084/jem.171.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Born W., Happ M. P., Dallas A., Reardon C., Kubo R., Shinnick T., Brennan P., O'Brien R. Recognition of heat shock proteins and gamma delta cell function. Immunol Today. 1990 Feb;11(2):40–43. doi: 10.1016/0167-5699(90)90015-2. [DOI] [PubMed] [Google Scholar]
- Borst J., van de Griend R. J., van Oostveen J. W., Ang S. L., Melief C. J., Seidman J. G., Bolhuis R. L. A T-cell receptor gamma/CD3 complex found on cloned functional lymphocytes. Nature. 1987 Feb 19;325(6106):683–688. doi: 10.1038/325683a0. [DOI] [PubMed] [Google Scholar]
- Bottino C., Tambussi G., Ferrini S., Ciccone E., Varese P., Mingari M. C., Moretta L., Moretta A. Two subsets of human T lymphocytes expressing gamma/delta antigen receptor are identifiable by monoclonal antibodies directed to two distinct molecular forms of the receptor. J Exp Med. 1988 Aug 1;168(2):491–505. doi: 10.1084/jem.168.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner M. B., McLean J., Scheft H., Riberdy J., Ang S. L., Seidman J. G., Devlin P., Krangel M. S. Two forms of the T-cell receptor gamma protein found on peripheral blood cytotoxic T lymphocytes. Nature. 1987 Feb 19;325(6106):689–694. doi: 10.1038/325689a0. [DOI] [PubMed] [Google Scholar]
- Brenner M. B., McLean J., Scheft H., Warnke R. A., Jones N., Strominger J. L. Characterization and expression of the human alpha beta T cell receptor by using a framework monoclonal antibody. J Immunol. 1987 Mar 1;138(5):1502–1509. [PubMed] [Google Scholar]
- Casorati G., De Libero G., Lanzavecchia A., Migone N. Molecular analysis of human gamma/delta+ clones from thymus and peripheral blood. J Exp Med. 1989 Nov 1;170(5):1521–1535. doi: 10.1084/jem.170.5.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccone E., Viale O., Bottino C., Pende D., Migone N., Casorati G., Tambussi G., Moretta A., Moretta L. Antigen recognition by human T cell receptor gamma-positive lymphocytes. Specific lysis of allogeneic cells after activation in mixed lymphocyte culture. J Exp Med. 1988 Apr 1;167(4):1517–1522. doi: 10.1084/jem.167.4.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falini B., Flenghi L., Pileri S., Pelicci P., Fagioli M., Martelli M. F., Moretta L., Ciccone E. Distribution of T cells bearing different forms of the T cell receptor gamma/delta in normal and pathological human tissues. J Immunol. 1989 Oct 15;143(8):2480–2488. [PubMed] [Google Scholar]
- Goodman T., Lefrançois L. Expression of the gamma-delta T-cell receptor on intestinal CD8+ intraepithelial lymphocytes. Nature. 1988 Jun 30;333(6176):855–858. doi: 10.1038/333855a0. [DOI] [PubMed] [Google Scholar]
- Groh V., Porcelli S., Fabbi M., Lanier L. L., Picker L. J., Anderson T., Warnke R. A., Bhan A. K., Strominger J. L., Brenner M. B. Human lymphocytes bearing T cell receptor gamma/delta are phenotypically diverse and evenly distributed throughout the lymphoid system. J Exp Med. 1989 Apr 1;169(4):1277–1294. doi: 10.1084/jem.169.4.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossi C. E., Ciccone E., Migone N., Bottino C., Zarcone D., Mingari M. C., Ferrini S., Tambussi G., Viale O., Casorati G. Human T cells expressing the gamma/delta T-cell receptor (TcR-1): C gamma 1- and C gamma 2-encoded forms of the receptor correlate with distinctive morphology, cytoskeletal organization, and growth characteristics. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1619–1623. doi: 10.1073/pnas.86.5.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas W., Kaufman S., Martinez C. The development and function of gamma delta T cells. Immunol Today. 1990 Oct;11(10):340–343. doi: 10.1016/0167-5699(90)90133-t. [DOI] [PubMed] [Google Scholar]
- Haregewoin A., Soman G., Hom R. C., Finberg R. W. Human gamma delta+ T cells respond to mycobacterial heat-shock protein. Nature. 1989 Jul 27;340(6231):309–312. doi: 10.1038/340309a0. [DOI] [PubMed] [Google Scholar]
- Hofman F. M., Hinton D. R., Johnson K., Merrill J. E. Tumor necrosis factor identified in multiple sclerosis brain. J Exp Med. 1989 Aug 1;170(2):607–612. doi: 10.1084/jem.170.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holoshitz J., Koning F., Coligan J. E., De Bruyn J., Strober S. Isolation of CD4- CD8- mycobacteria-reactive T lymphocyte clones from rheumatoid arthritis synovial fluid. Nature. 1989 May 18;339(6221):226–229. doi: 10.1038/339226a0. [DOI] [PubMed] [Google Scholar]
- Itoyama Y., Sternberger N. H., Kies M. W., Cohen S. R., Richardson E. P., Jr, Webster H. Immunocytochemical method to identify myelin basic protein in oligodendroglia and myelin sheaths of the human nervous system. Ann Neurol. 1980 Feb;7(2):157–166. doi: 10.1002/ana.410070211. [DOI] [PubMed] [Google Scholar]
- Janeway C. A., Jr, Jones B., Hayday A. Specificity and function of T cells bearing gamma delta receptors. Immunol Today. 1988 Mar;9(3):73–76. doi: 10.1016/0167-5699(88)91267-4. [DOI] [PubMed] [Google Scholar]
- Jindal S., Dudani A. K., Singh B., Harley C. B., Gupta R. S. Primary structure of a human mitochondrial protein homologous to the bacterial and plant chaperonins and to the 65-kilodalton mycobacterial antigen. Mol Cell Biol. 1989 May;9(5):2279–2283. doi: 10.1128/mcb.9.5.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi H., Liu C. C., Zheng L. M., Joag S. V., Bayne N. K., Holoshitz J., Young J. D. Expression of perforin and serine esterases by human gamma/delta T cells. J Exp Med. 1991 Feb 1;173(2):499–502. doi: 10.1084/jem.173.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafaille J. J., DeCloux A., Bonneville M., Takagaki Y., Tonegawa S. Junctional sequences of T cell receptor gamma delta genes: implications for gamma delta T cell lineages and for a novel intermediate of V-(D)-J joining. Cell. 1989 Dec 1;59(5):859–870. doi: 10.1016/0092-8674(89)90609-0. [DOI] [PubMed] [Google Scholar]
- Lee S. C., Raine C. S. Multiple sclerosis: oligodendrocytes in active lesions do not express class II major histocompatibility complex molecules. J Neuroimmunol. 1989 Dec;25(2-3):261–266. doi: 10.1016/0165-5728(89)90145-8. [DOI] [PubMed] [Google Scholar]
- McFarlin D. E. The lymphocyte saga continues. Ann Neurol. 1989 May;25(5):503–505. doi: 10.1002/ana.410250514. [DOI] [PubMed] [Google Scholar]
- Mehra V., Sweetser D., Young R. A. Efficient mapping of protein antigenic determinants. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7013–7017. doi: 10.1073/pnas.83.18.7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modlin R. L., Pirmez C., Hofman F. M., Torigian V., Uyemura K., Rea T. H., Bloom B. R., Brenner M. B. Lymphocytes bearing antigen-specific gamma delta T-cell receptors accumulate in human infectious disease lesions. Nature. 1989 Jun 15;339(6225):544–548. doi: 10.1038/339544a0. [DOI] [PubMed] [Google Scholar]
- Nakata M., Smyth M. J., Norihisa Y., Kawasaki A., Shinkai Y., Okumura K., Yagita H. Constitutive expression of pore-forming protein in peripheral blood gamma/delta T cells: implication for their cytotoxic role in vivo. J Exp Med. 1990 Dec 1;172(6):1877–1880. doi: 10.1084/jem.172.6.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien R. L., Happ M. P., Dallas A., Palmer E., Kubo R., Born W. K. Stimulation of a major subset of lymphocytes expressing T cell receptor gamma delta by an antigen derived from Mycobacterium tuberculosis. Cell. 1989 May 19;57(4):667–674. doi: 10.1016/0092-8674(89)90135-9. [DOI] [PubMed] [Google Scholar]
- Oksenberg J. R., Stuart S., Begovich A. B., Bell R. B., Erlich H. A., Steinman L., Bernard C. C. Limited heterogeneity of rearranged T-cell receptor V alpha transcripts in brains of multiple sclerosis patients. Nature. 1990 May 24;345(6273):344–346. doi: 10.1038/345344a0. [DOI] [PubMed] [Google Scholar]
- Parker C. M., Groh V., Band H., Porcelli S. A., Morita C., Fabbi M., Glass D., Strominger J. L., Brenner M. B. Evidence for extrathymic changes in the T cell receptor gamma/delta repertoire. J Exp Med. 1990 May 1;171(5):1597–1612. doi: 10.1084/jem.171.5.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel V. L., Evans D. A., Kaufman D. R. Reasoning strategies and the use of biomedical knowledge by medical students. Med Educ. 1990 Mar;24(2):129–136. doi: 10.1111/j.1365-2923.1990.tb02511.x. [DOI] [PubMed] [Google Scholar]
- Poser C. M., Paty D. W., Scheinberg L., McDonald W. I., Davis F. A., Ebers G. C., Johnson K. P., Sibley W. A., Silberberg D. H., Tourtellotte W. W. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983 Mar;13(3):227–231. doi: 10.1002/ana.410130302. [DOI] [PubMed] [Google Scholar]
- Prineas J. W., Kwon E. E., Goldenberg P. Z., Ilyas A. A., Quarles R. H., Benjamins J. A., Sprinkle T. J. Multiple sclerosis. Oligodendrocyte proliferation and differentiation in fresh lesions. Lab Invest. 1989 Nov;61(5):489–503. [PubMed] [Google Scholar]
- Raine C. S., Scheinberg L. C. On the immunopathology of plaque development and repair in multiple sclerosis. J Neuroimmunol. 1988 Dec;20(2-3):189–201. doi: 10.1016/0165-5728(88)90160-9. [DOI] [PubMed] [Google Scholar]
- Raine C. S., Scheinberg L., Waltz J. M. Multiple sclerosis. Oligodendrocyte survival and proliferation in an active established lesion. Lab Invest. 1981 Dec;45(6):534–546. [PubMed] [Google Scholar]
- Rajasekar R., Sim G. K., Augustin A. Self heat shock and gamma delta T-cell reactivity. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1767–1771. doi: 10.1073/pnas.87.5.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins D. S., Shirazi Y., Drysdale B. E., Lieberman A., Shin H. S., Shin M. L. Production of cytotoxic factor for oligodendrocytes by stimulated astrocytes. J Immunol. 1987 Oct 15;139(8):2593–2597. [PubMed] [Google Scholar]
- Selmaj K. W., Raine C. S. Tumor necrosis factor mediates myelin and oligodendrocyte damage in vitro. Ann Neurol. 1988 Apr;23(4):339–346. doi: 10.1002/ana.410230405. [DOI] [PubMed] [Google Scholar]
- Selmaj K., Raine C. S., Cannella B., Brosnan C. F. Identification of lymphotoxin and tumor necrosis factor in multiple sclerosis lesions. J Clin Invest. 1991 Mar;87(3):949–954. doi: 10.1172/JCI115102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinnick T. M., Vodkin M. H., Williams J. C. The Mycobacterium tuberculosis 65-kilodalton antigen is a heat shock protein which corresponds to common antigen and to the Escherichia coli GroEL protein. Infect Immun. 1988 Feb;56(2):446–451. doi: 10.1128/iai.56.2.446-451.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y. J., Tian W. T., Snider R. M., Rittershaus C., Rogers P., LaManna L., Ip S. H. Signal transduction of gamma/delta T cell antigen receptor with a novel mitogenic anti-delta antibody. J Immunol. 1988 Sep 1;141(5):1476–1479. [PubMed] [Google Scholar]
- Wucherpfennig K. W., Ota K., Endo N., Seidman J. G., Rosenzweig A., Weiner H. L., Hafler D. A. Shared human T cell receptor V beta usage to immunodominant regions of myelin basic protein. Science. 1990 May 25;248(4958):1016–1019. doi: 10.1126/science.1693015. [DOI] [PubMed] [Google Scholar]