Introduction
Heart transplantation (HTx) surgically interrupts the parasympathetic vagal neurons and the intrinsic postganglionic sympathetic nerve fibers traveling from the stellate ganglia to the myocardium, causing axonal Wallerian degeneration and thus extrinsic cardiac denervation.1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13 Cardiac denervation causes loss of afferent nerves in the myocardium,which is hypothesized to impair the reflex responses of the sensory endings of the cardiopulmonary region.14 Cardiac denervation occurs in the context of a variable background of nerve density in the donor heart: it has been observed that nerve density differs greatly in all areas of the allograft.15 There was also a significant decrease in total nerve density in cardiac allografts after HTx.15
Early Cardiac Denervation
Cardiac denervation causes the disappearance of neural input to the sinoatrial node and the loss of efferent and afferent nerve signaling into and out of the heart,14, 16, 17, 18 the latter of which makes HTx recipients unable to experience the sensation of angina due to ischemia.19 In addition, cardiac denervation causes the loss of ventricular sensory input, which may play a role in hypotension and bradycardia related to inferior wall infarction.14 It also causes a loss of the presynaptic neuronal uptake‐1 mechanism, thus leading to supersensitivity to the uptake‐1‐dependent catecholamines (CAT), which may have an effect on inotropic responses.20 Furthermore, the exercise capacity in denervated HTx recipients seems to be diminished because denervated hearts must rely on circulating, rather than cardiac, CAT release to adapt to the increased needs of exercise, although this adaptation is insufficient to reach normal heart rate (HR) and contractility.21 Early cardiac denervation in HTx recipients leads to the loss of the normal nocturnal decline in blood pressure.16 It also causes a higher than normal HR at rest, and, in response to exercise, the HR increases more slowly than normal to reach a lower maximal HR, which descends during recovery from exercise at a slower than normal rate. In addition, cardiac denervation causes an abnormal cardiac output (CO) response to exercise.
Therefore, cardiac denervation alters cardiovascular control in HTx recipients.17 It results in lower cardiac index (CI) and heart rate variability (HRV) or heart period variability (HPV), abnormal chronotropic response to exercise, abnormal catecholamine release and hemodynamic responses to exercise and tyramine injection, impaired exercise capacity and physical function, altered diastolic function of the ventricles, presynaptic inotropic supersensitivity and lower inotropic reserve, higher resting HR due to the lack of parasympathetic vagal efferent nerve connections, higher stroke volume (SV), temporary sinus node dysfunction, abnormal cardiopulmonary baroreflexes, depletion of cardiac norepinephrine (NE) within the nerve terminals, higher sensitivity to circulating endogenous CAT due to the lack of presynaptic neuronal uptake capacity which may increase the frequency of arrhythmias, and altered response to adrenergic drugs, which requires adjustments to pharmacotherapy in HTx recipients.1, 2, 3, 4, 5, 6, 8, 9, 11, 19, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39
Cardiac denervation, however, appears not to affect the cardiac production of atrial natriuretic peptide (ANP) and the intrinsic nerve supply in the heart.6, 26 This may be due to cardiac denervation indirectly causing ANP release during exercise by adjusting atrial stretch and increasing the need for elevated CAT and arginine vasopressin (AVP).26 In addition, cardiac denervation seems not to affect the overall density of postsynaptic adrenergic receptors38; however, there appears to be a shift from β1 to β2 receptors.21
Cardiac Reinnervation
Although cardiac denervation occurs immediately after HTx, cardiac reinnervation is a variable phenomenon and is still considered as a controversial issue in the field of cardiac transplantation. Yet, there is ample evidence in literature supporting the idea of cardiac reinnervation, which has been shown to occur in 40% to 70% of recipients late after HTx.40, 41, 42, 43, 44 Sensory reinnervation may occur following HTx as well.45 Sympathetic reinnervation requires the presence of functional nerve terminals occurring outside the heart that are connected to nerve terminals in the transplanted heart.3, 8, 9 Restored presynaptic nerve terminals in HTx recipients were correlated with the reestablishment of interactions between pre‐ and postsynaptic adrenergic components of the synapse.4 Studies using microscopy show sympathetic nerves growing along the coronary blood vessels and the anastomoses between the donor heart and the recipient portions of the myocardium.2 Cardiac reinnervation after HTx reduces the correlation between HR at rest and the predicted intrinsic HR, causes NE release from the cardiac nerve terminals in response to exercise and pharmacological stimuli, allows for the return of neuronal control over HR and ventricular contractility, and improves exercise performance after HTx.2, 22, 23
Because regrowth of nerves takes time, sympathetic reinnervation is observed late following HTx.7 The time between HTx and study participation was longer in reinnervated compared to denervated recipients.21 Sympathetic reinnervation occurs at least 5 to 6 months after HTx, while parasympathetic reinnervation seems to occur more than 1 to 3 years following HTx.1, 22
Time following HTx significantly correlated with the extent of reinnervation.7 Muscle sympathetic nerve activity was higher in long‐term compared with short‐term recipients, showing that cardiac reinnervation occurs later after HTx.46 One study showed that no reinnervation occurred prior to 18 months post‐HTx and that sympathetic reinnervation is progressive and increases with time until 15 years post‐HTx.11 Although some denervated recipients were still observed at 15 years post‐HTx, they were mostly found within the first 18 months post‐HTx.7 Another study showed no evidence of reinnervation for the first year post‐HTx but also showed an increase in reinnervation from the first to third year.3
The heart‐to‐mediastinum ratio (HMR) of 123I‐meta‐iodobenzylguanidine (MIBG) uptake was significantly higher in recipients more than 2 years after HTx when compared with those recipients less than 2 years post‐HTx,47 and no recipient had significant uptake prior to a year post‐HTx.42 Another study showed that none of 19 recipients at 5 months post‐HTx showed MIBG uptake, whereas 11 of 23 recipients at 1 to 2 years post‐HTx showed significant MIBG uptake.42 A third study showed that MIBG uptake was observed at 1 year post‐HTx and continued to increase with time after HTx.47
Of note, ventricular reinnervation was time dependent, whereas sinus node reinnervation was not, and the occurrence of sinus node reinnervation was variable, being detected in some but not all patients.41 Also, some studies focused on the potential functionality of reinnervation rather than the occurrence of reinnervation after transplantation. Although reinnervation reached a higher peak HR and a better HR response at 5 years compared to 1 year post‐HTx, it was functionally insignificant,39 given the observation that the chronotropic response to exercise and the maximal exercise capacity did not improve from 1 to 5 years post‐HTx and remained at subnormal levels.48
Cardiac reinnervation after HTx appears in some but not all HTx recipients and is regionally heterogeneous, for which reason it is sometimes referred to as partial or “patchy” reinnervation.1, 3, 8, 17, 22, 23, 49, 50, 51 Even up to 10 years, cardiac reinnervation was not present in all patients.10 This limited reinnervation may affect the cardiac calcium homeostasis.50 There was also transmural heterogeneity of nerve distribution in allograft hearts.15 The retention of 11C‐meta‐hydroxyephedrine (HED), which is a norepinephrine (NE) analogue, showed heterogeneous “patchy” reinnervation in some HTx recipients, whereas control subjects showed homogeneous retention in the entirety of the left ventricle.7, 8, 9, 33 In addition, MIBG uptake showed only partial sympathetic reinnervation post‐HTx in some but not all recipients, never reaching global cardiac reinnervation.35, 47 Furthermore, heart rate variability (HRV) was noted to be different in transplant recipients from time to time during the day,52 which could be evidence for the heterogeneity of reinnervation.
Sympathetic reinnervation mostly appears in the left ventricular and sinus or sinoatrial node regions of the allograft.12, 22, 23, 24 Left ventricular reinnervation usually occurs over the left atrial connections to the left ventricle, whereas sinus node reinnervation most likely arises over the right atrial connections. Either was also noted to occur without the other.24 Of note, left ventricular reinnervation reached 66% in some individuals.11
Epinephrine (EPI) retention and HED retention, as measures of cardiac reinnervation, were highest in the left anterior descending (LAD) territory, followed by the left circumflex (LCX) territory, and lowest in the right coronary artery (RCA) territory.4, 11, 13, 49 Coronary blood flow during the cold pressor test increased by 46% in the LAD territory but by only 16% in the RCA territory.13 Furthermore, MIBG uptake showed evidence of partial reinnervation in the anterior basal region of the heart, whereas the lower septal, posterolateral, and apical regions of the heart showed no MIBG uptake at all.35, 42 Also, MIBG uptake occurred in the anterolateral and septal regions while being heterogeneous in location and extent among different HTx recipients.41, 47 The anteroseptal left ventricular wall and the LAD territory seem to be the main locations of cardiac reinnervation post‐HTx.4, 9, 21
The retention of HED starts at the base of the anterior wall of the left ventricle and expands to its septal and lateral parts without being detected in the posterior wall.3, 8, 9, 11, 33 Also, after initial cardiac denervation post‐HTx, the basal anterior wall showed the highest reinnervation by HED retention,7 and the distal anterior and basal septal walls showed a less substantial increase in HED retention. The inferior wall, however, remained denervated until the latest time post‐HTx, when only marginal parts of it showed minor reinnervation by HED retention.11 The same study demonstrated that sympathetic reinnervation starts in the basal parts of the heart first and then in the distal parts, whereas the apex shows reinnervation late following HTx. Also, there was a gradient of reinnervation from the base to the apex of the heart in which the anterior and septal walls were reinnervated earlier and the lateral walls were reinnervated later.11 In addition, HED retention demonstrated that ventricular reinnervation occurs prior to sinus node reinnervation.8 No differences in HED retention locations were noted in normal subjects.3, 13
Vagal parasympathetic reinnervation has fewer methods to quantify it,53 appearing most likely in the sinus node area of the allograft.10, 17, 23, 54 However, parasympathetic reinnervation may be more common than sympathetic reinnervation.55 HRV increase, as a sign of parasympathetic reinnervation, starts to appear 3 years post‐HTx.10, 25 Another study showed that significant HRV increase occurred 15 to 37 weeks post‐HTx but was still lower than normal levels.28 In more recent studies, parasympathetic reinnervation appeared to occur at around 2 years post‐HTx,53, 56, 57 given the absence of R‐R interval prolongation following phenylephrine‐induced baroreceptor stimulation at 24 months post‐HTx57 or the significant increase in the high‐frequency (HF) power spectrum within 28 months post‐HTx34 and its association with recovery parameters, which significantly improved within 2 years post‐HTx.53 In other studies, vagal reinnervation appeared to occur as early as 3 to 6 months post‐HTx,28 given the significant increase in HF power spectrum levels that occurred at 6 months post‐HTx.58
In 1 study, measurements at 5 and 8 years did not reflect any evidence of parasympathetic reinnervation,32 and in another study, parasympathetic influence on donor HR did not occur in most recipients up to 8 years post‐HTx.59 Nevertheless, HRV as evidence for parasympathetic reinnervation increased with time after transplantation.25, 28 In response to standing, the presence of rebound bradycardia suggested the presence of vagal reinnervation.27 Parasympathetic reinnervation occurred alongside improvements in HTx recipients postexercise recovery.53
Of note, sympathetic reinnervation may occur without parasympathetic reinnervation, thus causing an “unbalanced” response to stimuli by the cardiac muscle,2 but parasympathetic reinnervation seems to occur only in patients with sympathetic reinnervation.34 It is important to note that parasympathetic reinnervation differs according to the surgical procedure and is more likely to occur in patients who underwent HTx using the bicaval as opposed to the biatrial or standard technique. The bicaval technique interrupts the sympathetic and parasympathetic neuronal connections similarly and will stimulate regeneration of both equally, whereas the biatrial technique interrupts only half of the sympathetic neuronal connections, leaving most of the parasympathetic pathways intact, which may reduce the stimulation to regenerate.40 In general, sympathetic reinnervation of the left ventricle following HTx is needed to improve regulation of blood flow, exercise performance, and ventricular function, but sympathetic reinnervation of the sinus node is needed to restore HR at rest and the chronotropic response to exercise; both processes may not occur simultaneously.41 A pictorial representation of the pattern of reinnervation after cardiac transplantation is illustrated in the Figure.
Figure 1.
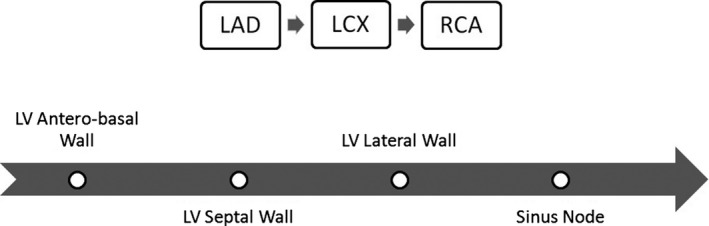
Reinnervation of the heart following cardiac transplantation. Cardiac reinnervation is highest in the left anterior descending (LAD) territory, followed by the left circumflex (LCX) territory, and lowest in the right coronary artery (RCA) territory.4, 11, 13, 49 It starts at the base of the anterior wall of the left ventricle (LV) and expands to the septal and lateral walls.3, 8, 9, 11, 33 The magnitude of reinnervation is highest at the anterobasal wall followed by the septal wall.7, 11 There is a gradient of reinnervation from the base to the apex of the heart.11 Ventricular reinnervation occurs prior to sinus node reinnervation.8
Heart Rate at Rest
HR at rest was higher in HTx and heart‐lung transplant (HLTx) recipients when compared with control subjects.5, 16, 17, 48, 49, 50, 60, 61, 62, 63, 64 This may relate to the lack of sinus node parasympathetic control because of parasympathetic denervation.49 Also, the resting HR was higher in HTx recipients, specifically early following HTx, because of their denervated status because the absence of vagal and β‐adrenergic nervous input allows a faster (intrinsic) HR.65 The absence of vagal reinnervation and the presence of sympathetic reinnervation may explain the association between increased HR at rest and increased leptin levels through cardiac leptin receptors.66 A resting HR ≥90 or 100 beats per minute (bpm) in early recipients at 1 year post‐HTx was found in multivariate analyses to be an independent predictor of mortality following transplantation.67, 68 Recipients with higher baseline HR ≥90 bpm at 1 year post‐HTx had lower survival rates at 3, 5, and 10 years post‐HTx when compared with recipients with HR <90 bpm.68
Although some early (denervated) and late (possibly reinnervated) recipients were found to have a higher HR at rest when compared with controls,21, 23, 27, 29 and others showed a similar resting HR,4, 12 HR measurements were found to be higher in recipients within 3 years post‐HTx when compared with recipients more than 3 years post‐HTx.69 Similarly, our group observed a higher resting HR in early HTx recipients, followed by late HTx recipients, and lowest in control subjects,1 consistent with the evolution of cardiac reinnervation in later years after HTx. HR was also different among late HTx recipients,25 supporting the idea of heterogeneity in cardiac reinnervation after HTx.
In summary, HR at rest seems to be higher early post‐HTx because of the sympathetic and parasympathetic denervation of the transplanted heart, which relates to the surgical removal of the heart from the donor, and this denervation renders the intrinsic HR, which by default is high, in control of resting heart rate. As reinnervation from the recipient occurs over time post‐HTx, the intrinsic HR assumes a lesser contribution to the resting heart rate, reducing the HR to a lower value and leading to a partially normalized resting rate. A summary of the findings in these and other studies70, 71 regarding resting HR early and late after HTx is shown in Table 1.
Table 1.
Heart Rate Measurements Following HTx
| Study Reference | N of Study (N of HTx) | Time Period Post‐HTx (Months) | Variable Hemodynamics/ Intervention | Results Summary |
|---|---|---|---|---|
| Pflugfelder et al,61 1987 | 33 (18) | 11 [1‐25] | HR at rest | Higher in HTx compared to controls |
| HR at peak exercise | Lower in HTx compared to controls | |||
| HR in response to exercise | Slower increase in HTx compared to controls | |||
| HR following exercise | Slower deceleration in HTx compared to controls | |||
| Banner et al,5 1990a | 16 (8) | 20 [4‐30] | HR at rest | Higher in HTx compared to controls |
| HR in response to head‐up tilt | Similar increase in HTx and controls | |||
| Wilson et al,2 1991 | 70 (62) | 3.1±0.4 [2‐5] and 37±3 [12‐102] | HR at rest | Lower in late HTx compared to early HTx |
| HR in response to IV tyramine injection | Lower rise in late HTx compared to controls, no rise in early HTx | |||
| HR in response to IC tyramine injection | No rise in controls and early or late HTx | |||
| HR in response to handgrip | Lower rise in late HTx compared to controls, no rise in early HTx | |||
| Braith et al,16 1992 | 22 (11) | 18±12 [7‐41] | HR at rest | Higher in HTx compared to controls |
| HR in response to exercise | Increase in controls, no rise in HTx | |||
| HR at peak exercise | Lower in HTx compared to controls | |||
| Kaye et al,23 1993 | 40 (15) | 5.5 [2.1‐13.3] and 48.3 [26.7‐96] | HR at rest | Higher in HTx (early and late) compared to controls |
| HR in response to exercise | Attenuated in early HTx, intermediate rise in late HTx, normal rise in controls | |||
| Wilson et al,24 1993 | 57 (50) | 3.0±0.4 [2‐4] and 37±3 [12‐86] | HR in response to IC tyramine injection | Lower rise in late HTx compared to controls, no rise in early HTx |
| Rudas et al,27 1993b | 55 (40) | 2±2 [0.5‐5] and 49±18 [33‐108] | HR at rest | Higher in HTx (early and late) compared to controls |
| HR in response to standing | Slower rise in late HTx compared to controls, no rise in early HTx | |||
| Burke et al,12 1995 | 70 (57) | 3.2 [2‐4] and 37.9 [12‐96] | HR at rest | Similar in HTx (early, late denervated, late reinnervated) and controls |
| HR in response to IC tyramine injection | Similar in HTx (early, late denervated, late reinnervated) and controls | |||
| Doering et al,1 1996 | 49 (33) | 2.28±1.55 and 28.3±13.4 | HR at rest | Highest in early HTx followed by late HTx and lowest in controls |
| HR in response to passive upright tilt | Slower acceleration in early HTx compared to late HTx and controls | |||
| HR at peak exercise | Slower to reach in early HTx compared to late HTx and controls | |||
| HR following exercise | Slower deceleration in HTx (early and late) compared to controls | |||
| Lord et al,71 1996 | 36 (25) | 40 [22‐96] | HR following exercise (at 6 minutes) | Lower recovery in HTx compared to controls |
| Braith et al,60 1998 | 14 (7) | 15±5 | HR at rest | Higher in HTx compared to controls |
| HR in response to exercise | Lower and slower increase in HTx compared to controls | |||
| Doering et al,22 1999 | 49 (33) | 2.28±1.55 and 28.3±13.4 | HR in response to handgrip (at 1 minute) | Lower increase in late HTx compared to controls, no increase in early HTx |
| HR following handgrip (immediate) | Lower deceleration in early HTx compared to controls, intermediate in late HTx | |||
| HR in response to supine‐to‐upright tilt | Similar acceleration in late HTx and controls, diminished acceleration in early HTx | |||
| HR in response to upright‐to‐supine tilt | Lower deceleration in early HTx compared to controls, intermediate in late HTx | |||
| Wilson et al,29 2000 | 61 (41) | 3.8±0.4 [2‐6] and 30±4 [12‐60] | HR at rest | Higher in HTx (early and late) compared to controls |
| HR in response to IC tyramine injection | No increase in early HTx, varying levels for different reinnervation in late HTx | |||
| Piquard et al,63 2000 | 14 (7) | 35.5±8.3 | HR at rest | Higher in HTx compared to controls |
| HR at peak exercise | Lower in HTx compared to controls | |||
| Bengel et al,49 2001 | 48 (27) | 37.2±26.4 [6‐98.4] | HR at rest | Higher in HTx compared to controls |
| Bengel et al,21 2001 | 39 (29) | 38.4±25.2 [6‐98.4] | HR at rest | Higher in HTx (reinnervated and denervated) compared to controls |
| HR at peak exercise | Similar in reinnervated HTx and controls, lower in denervated HTx | |||
| Ferretti et al,62 2002 | 26 (17) | 41±8 | HR at rest | Higher in HTx compared to controls |
| HR at peak exercise | Lower in HTx compared to controls | |||
| Toledo et al,17 2002 | 27 (13) | [0.5‐62.5] | HR at rest | Higher in HTx compared to controls |
| HR in response to change of posture | Fast rise in controls, varying rates for HTx (no or slight rise, slow or fast rise) | |||
| Bengel et al,4 2004 | 12 (12) | 32.4±32.4 [6‐106.8] | HR at rest | Similar in reinnervated and denervated HTx |
| HR in response to exercise | Higher increase in reinnervated compared to denervated HTx | |||
| HR at peak exercise | Higher in reinnervated compared to denervated HTx | |||
| HR at peak exercise during B‐blockade | Higher reduction in reinnervated compared to denervated HTx | |||
| Scott et al,50 2009 | 20 (9) | 96±72 | HR at rest | Higher in HTx compared to controls (DM and RM) |
| HR at peak exercise | Lower in HTx compared to DM controls, similar in HTx and RM controls | |||
| Guimarães et al,64 2010 | 38 (20) | 102±39.6 | HR at rest | Higher in HTx compared to controls |
| HR following 6‐minute walk (immediate) | Higher in HTx compared to controls | |||
| Hayman et al,18 2010 | 18 (9) | 9±2 | HR in response to limb movement | Increase in controls, no rise in HTx |
Time period post‐HTx displayed as mean±standard deviation or standard error [range]. DM indicates donor‐age matched; HLTx, heart/lung transplantation; HR, heart rate; HTx, heart transplantation; IC, intracoronary; IV, intravenous; RM, recipient‐age matched.
HLTx recipients.
HTx or HLTx recipients.
Heart Rate in Response to Physical Stress
Of note, the denervated heart shows a blunted increase in HR at exercise onset, a maximum HR in early recovery after peak exercise, and a plateau in HR after its peak.37 At onset of exercise, HR normally increases via sympathetic input in order to deliver blood to the body at a faster rate and then goes down following exercise in order to return the increased exercise HR to resting values.70 As shown in Table 1, HR increased in response to physical stress at a slower rate or showed a lower increase in HTx recipients when compared with control subjects.60, 61, 63 This could be the result of the higher resting HR in HTx recipients.71 Many studies also showed a lower or no rise in HR in HTx recipients compared to control subjects.16, 18, 60 Other studies showed a lower and a slower increase in HR in response to physical stress in late HTx recipients when compared with controls, whereas early HTx recipients showed either no rise or an attenuated slower rise in HR.2, 23, 27 Our group showed similar findings as well.1, 22 The HR response to postural change should be understood by taking into account the fact that normal subjects respond to postural change by increasing HR at a fast rate through the withdrawal of vagal input because the sympathetic nervous system plays a secondary role in HR control during the first 20 seconds of postural change.17
HR at peak exercise or peak HR during exercise was lower in HTx recipients when compared with control subjects.16, 48, 50, 61, 62, 63 Also, HR reserve at peak exercise was lower in HTx recipients when compared with control subjects.50, 71 However, HR reserve was associated with time after transplantation.50 Similarly, our group found that early HTx recipients reached peak HR at a slower rate during exercise when compared with late HTx recipients and control subjects,1 suggestive of cardiac reinnervation late after HTx. This rate of HR increase during postural change turned from a slower to a faster rate at the 24‐month mark post‐HTx.17 Similarly, denervated HTx recipients had a lower HR at peak exercise when compared with reinnervated HTx recipients and control subjects.3, 4, 21 Similar results were found comparing early HTx recipients to late denervated or reinnervated HTx recipients and control subjects.29 Of note, 96.3% of recipients had an abnormal HR response to exercise at 2 months post‐HTx, and 40.7% of the recipients showed a normalized HR response to exercise at 6 months post‐HTx,37 thus showing that cardiac reinnervation occurs over time, which is evident in the improved exercise capacity and HR response to exercise later post‐HTx.23, 37
In the recovery period after physical stress, early HTx recipients or HTx recipients in general had a lower rate of deceleration compared to late HTx recipients and control subjects,1, 22, 61, 71 which could be due to the absence of vagal reinnervation early after HTx or the delayed humoral CAT release, which makes HR rise even after end of exercise.29 Clearly, HR response to exercise significantly correlated with time after transplantation.17 HR deceleration following physical stress in control subjects and late HTx recipients is probably due to vagal reinnervation, causing baroreceptor activation, for which reason early HTx denervated recipients had a lower rate of deceleration than normal.22, 29 The HR response in late HTx recipients more closely resembles that in control subjects due to sympathetic reinnervation and to some degree the remaining abnormality in the CAT uptake‐1 mechanism.23, 29
Heart Rate in Response to Pharmacotherapy
Tyramine causes degranulation of neuronal vesicles containing NE in the myocardium and coronary vasculature.2 Although some studies found similar HR response to tyramine injection in HTx recipients and control subjects,12 other studies (Table 1) found a lower rise in HR in response to tyramine injection in late HTx recipients compared to controls, with no rise in early HTx recipients.2, 24 Indeed, the HR increase due to tyramine injection was significantly correlated with time after transplantation,24 which is considered as evidence for sinus node reinnervation following HTx.21, 29 Similarly, HTx recipients had different HR responses to tyramine injection based on their varying levels of reinnervation.29 Further studies showed that the levels of HR increase in response to tyramine injection varied among HTx recipients,71 consistent with the heterogeneity in cardiac reinnervation after HTx.
Heart Rate Variability and R‐R Intervals
HRV is defined as the beat‐to‐beat variation in HR and is used as a means to noninvasively measure the autonomic nervous system influence on the heart.10, 32 The absence of HRV in response to the deep breathing test reflects the absence of parasympathetic reinnervation for several years post‐HTx.22 HRV or HPV at rest or during physical exercise was found to be lower in HTx recipients compared to control subjects.17, 22, 23, 28
In early HTx recipients, HRV is lower than normal levels, probably because of the denervation of the transplanted heart, which leaves the heart under hormonal and internal control loops only.17 However, HRV is shown to increase with time after transplantation,25, 28 consistent with cardiac reinnervation later following HTx. This increase in HRV started to appear after 3 years post‐HTx.10, 25 Another study showed that a HRV increase in recipients experiencing atrial reinnervation occurred 15 to 37 weeks post‐HTx and was a sign of parasympathetic reinnervation, given that it was still subnormal to controls.28 In addition, this HRV increase seemed to differ among HTx recipients,25 which confirms the heterogeneity of cardiac reinnervation after HTx. These findings about HRV could be explained either by the concept of “patchy” reinnervation or by the inability of regenerating nerves to reestablish the connections between the cardiac afferent and efferent fibers that are responsible for the reflexive responses of HRV in normal subjects.23
Furthermore, R‐R interval variance was similar between early and late HTx recipients or HTx recipients and control subjects.41, 46 However, R‐R intervals in the supine position were lower in reinnervated HTx recipients compared to control subjects who had similar R‐R intervals as denervated HTx recipients,41 consistent with cardiac reinnervation. Of note, however, all HTx recipients showed low values of R‐R intervals and R‐R‐interval variability at rest,40 which could be interpreted as incomplete reinnervation. This is also supported by the observation that the R‐R interval decreased significantly from initial values at 1 to 48 months post‐HTx to follow‐up values at 119 to 172 months post HTx.72
Low‐Frequency Power Spectrum
The low‐frequency (LF) 0.1‐Hz peak power spectrum represents the carotid baroreflex modulation of R‐R intervals, reflecting sympathetic reinnervation.17, 73 The LF component of HRV was directly associated with sympathetic reinnervation in the sinus node post‐HTx.74 Sympathetic activity, as a measure of LF power spectrum, increased in HTx recipients but remained lower than normal levels.32 Of note, the LF spectrum obtained for denervated ventricles showed a narrow distribution compared to a wider range of values for reinnervated ventricles.8 The LF function of HRV was lower in HTx recipients than in control subjects,17, 34, 41, 75 as illustrated in Table 2. Comparing reinnervated or late HTx recipients to denervated or early HTx recipients, LF power or normalized LF power was higher in the reinnervated and late HTx recipients.8, 25, 41, 46 Additionally, the LF spectrum was similar between late HTx recipients and control subjects,46 supporting the hypothesis of cardiac reinnervation late after HTx. All recipients more than 3 years post‐HTx showed an increased LF range.25 This is further supported by evidence showing an increasing correlation between LF power spectrum density of ventricular and sinus node reinnervation and time after transplantation.8, 17, 34, 40, 41, 75
Table 2.
Heart Rate Variability Following HTx
| Study Reference | N of Study (N of HTx) | Time Period Post‐HTx (Months) | Variable Hemodynamics/ Intervention | Results Summary |
|---|---|---|---|---|
| Smith et al,28 1990 | 34 (18) | [0.23‐0.92] and [3.45‐8.51] | HPV at rest | Lower in HTx (early and late) compared to controls |
| Kaye et al,23 1993 | 40 (15) | 5.5 [2.1‐13.3] and 48.3 [26.7‐96] | HRV at rest | Lower in HTx compared to controls |
| Bernardi et al,75 1995 | 42 (26) | 21±4 [2‐63] | LF function without atropine; with neck suction | Lower in HTx compared to controls |
| LF function without atropine; without neck suction | Lower in HTx compared to controls | |||
| LF power in SBP | Similar in HTx and controls | |||
| LF power in DBP | Similar in HTx and controls | |||
| HF function without atropine; with neck suction | Lower in HTx compared to controls | |||
| HF function without atropine; without neck suction | Lower in HTx compared to controls | |||
| HF power in SBP | Similar in HTx and controls | |||
| HF power in DBP | Higher in HTx compared to controls | |||
| Halpert et al,25 1996 | 37 (37) | [1‐36] and [36‐122] | LF power during daily activity | Higher in late HTx compared to early HTx |
| HF power during daily activity | Higher in late HTx compared to early HTx | |||
| Uberfuhr et al,34 1997 | 31 (13) | 14±5 and 42±8 | LF RR‐intervals | Lower in HTx compared to controls |
| HF RR‐intervals | Lower in HTx compared to controls | |||
| Doering et al,22 1999 | 49 (33) | 2.28±1.55 and 28.3±13.4 | HRV in response to deep breathing | Lower in HTx (early and late) compared to controls |
| Uberfuhr et al,8 2000 | 38 (38) | 55.2±46.8 [2‐163] | LF power spectrum | Higher in reinnervated HTx compared to denervated HTx |
| van De Borne et al,46 2001 | 21 (14) | 5±2 [1‐14] and 138±8 [103‐163] | RR variance | Similar in early and late HTx |
| LF variability | Higher in late HTx compared to early HTx | |||
| Normalized LF variability | Higher in late HTx compared to early HTx | |||
| Normalized LF variability | Similar in late HTx and controls | |||
| Toledo et al,17 2002 | 27 (13) | [0.5‐62.5] | HRV at rest | Lower in HTx compared to controls |
| LF power spectrum | Lower in HTx compared to controls | |||
| LF fluctuations | Lower in HTx compared to controls | |||
| HF power spectrum | Lower in HTx compared to controls | |||
| Lovric et al,41 2004 | 12 | 51.0±22.4 [12‐97] | RR interval in supine position | Lower in reinnervated HTx compared to controls |
| RR interval in supine position | Similar in denervated HTx and controls | |||
| RR interval in upright position | Similar in HTx (denervated and reinnervated) and controls | |||
| LF power in supine position | Lower in HTx (denervated and reinnervated) compared to controls | |||
| LF power in upright position | Lower in HTx (denervated and reinnervated) compared to controls | |||
| Normalized LF power in upright position | Higher in reinnervated HTx compared to denervated HTx | |||
| Normalized LF power in upright position | Lower in HTx (denervated and reinnervated) compared to controls |
Time period post‐HTx displayed as mean±standard deviation or standard error [range]. DBP indicates diastolic blood pressure; HF, high frequency; HPV, heart period variability; HRV, heart rate variability; HTx, heart transplantation; LF, low frequency; SBP, systolic blood pressure.
Detecting that HTx recipients had lower LF power within the first 3 years after HTx further supports the idea of sympathetic reinnervation late after HTx,69 especially after noting that LF power during daily activity significantly increased starting at 4 years post‐HTx and onward.10, 34 Moreover, evidence of the LF power spectrum differed between HTx recipients late following HTx,25 supporting heterogeneous reinnervation. In addition, it has also been observed that some recipients early after HTx could have signs of sympathetic reinnervation, shown by the clear 0.1‐Hz peak of HRV.40 This is consistent with heterogeneous reinnervation because late HTx recipients do not show evidence of a clear LF peak of HRV.40 It is also interesting to note that the LF component of R‐R intervals may differ between bicavally and biatrially transplanted recipients.40
High‐Frequency Power Spectrum
The high‐frequency (HF) 0.2‐Hz peak power spectrum is vagal in origin, reflecting parasympathetic reinnervation.17, 25, 73 The HF power spectrum was significantly associated with HR recovery and the delay of peak HR, which were recovery parameters improving within 2 years post‐HTx and reflecting parasympathetic reinnervation.53 Many studies showed a lower HF function in HTx recipients compared to control subjects, as shown in Table 2.17, 34, 75 In control subjects a high degree of coherence was observed between R‐R interval and neck suction pressure at 0.2 Hz, although HTx recipients lacked such coherence, which translates into lack of vagal reinnervation.75 Also, all recipients more than 3 years post‐HTx did not have a higher HF range, showing no evidence of parasympathetic reinnervation.25
Measurements at 5 and 8 years post‐HTx showed no evidence of reinnervation as well,32 although other measurements showed evidence of parasympathetic reinnervation as early as 6 months28, 58 or 2 years34, 53, 56 post‐HTx. It also seems that HF power spectrum increases with time after HTx,34 but other studies showed no correlation with time after HTx.10, 17 Nevertheless, more HTx recipients showed a response to the 0.2‐Hz HF neck suction later after HTx, indicative of more parasympathetic reinnervation later post‐HTx.40 Of note, not all sympathetically reinnervated HTx recipients showed parasympathetic reinnervation evident by the 0.2‐Hz frequency.73 This might be due to differences in the transplantation technique, whether bicaval or biatrial.40 Table 2 shows a summary of the analysis of studies about HRV early and late after HTx.
Systolic Blood Pressure
Table 3 shows a summary of the findings by studies about systolic blood pressure (SBP) at rest early and late post‐HTx. Resting SBP was found to be similar between HTx or HLTx recipients and controls5, 16, 61 for up to 5 years post‐HTx.48 Also, early and late (or denervated and possibly reinnervated) HTx recipients had similar resting SBP compared to control subjects.21, 24, 29 However, late HTx recipients were found to have higher SBP at rest compared to controls.64 Peak SBP and peak increment in SBP with exercise were found to be lower in recipients for up to 5 years post‐HTx compared to controls.48
Table 3.
Pressure Measurements Following HTx
| Study Reference | N of Study (N of HTx) | Time Period Post‐HTx (Months) | Variable Hemodynamics/ Intervention | Results Summary |
|---|---|---|---|---|
| Pflugfelder et al,61 1987 | 33 (18) | 11 [1‐25] | SBP at rest | Similar in HTx and controls |
| DBP at rest | Similar in HTx and controls | |||
| SBP at peak exercise | Lower in HTx compared to controls | |||
| DBP at peak exercise | Lower in HTx compared to controls | |||
| Banner et al,5 1990a | 16 (8) | 20 [4‐30] | SBP at rest | Similar in HTx and controls |
| DBP at rest | Higher in HTx compared to controls | |||
| DBP in response to head‐up tilt | Increase in controls, no significant rise in HTx | |||
| Wilson et al,2 1991 | 70 (62) | 3.1±0.4 [2‐5] and 37±3 [12‐102] | SABP in response to IV tyramine injection | Similar rise in HTx (early and late) and controls |
| DABP in response to IV tyramine injection | Similar rise in HTx (early and late) and controls | |||
| Arterial BP in response to IC tyramine injection | No rise in controls and early or late HTx | |||
| SBP in response to handgrip | Similar rise in HTx (early and late) and controls | |||
| DBP in response to handgrip | Similar rise in HTx (early and late) and controls | |||
| Aortic BP in response to handgrip | Similar rise in HTx (early and late) and controls | |||
| Schwaiger et al,33 1991 | 16 (11) | 4.4±2.3 and 42±22 | BP at rest | Higher in HTx (early and late) compared to controls |
| BP in response to HED injection | No significant rise in controls and early or late HTx | |||
| Braith et al,16 1992 | 22 (11) | 18±12 [7‐41] | SBP at rest | Similar in HTx and controls |
| SBP at peak exercise | Lower in HTx compared to controls | |||
| DBP at rest | Higher in HTx compared to controls | |||
| DBP at peak exercise | Higher in HTx compared to controls | |||
| Kaye et al,23 1993 | 40 (15) | 5.5 [2.1‐13.3] and 48.3 [26.7‐96] | MAP at rest | Higher in HTx (early and late) compared to controls |
| Wilson et al,24 1993 | 57 (50) | 3.0±0.4 [2‐4] and 37±3 [12‐86] | SBP at rest | Similar in HTx (early and late) and controls |
| DBP at rest | Higher DBP in early HTx compared to controls | |||
| Aortic BP in response to IC tyramine injection | No significant change in controls and early or late HTx | |||
| Rudas et al,27 1993b | 55 (40) | 2±2 [0.5‐5] and 49±18 [33‐108] | BP at rest | Higher in HTx (early and late) compared to controls |
| SBP in response to head‐up tilt | No change in controls and early or late HTx | |||
| DBP in response to head‐up tilt | Similar increase in HTx (early and late) and controls | |||
| SBP drop following standing | Similar in HTx (early and late) and controls | |||
| SBP overshoot following standing | Lower in late HTx compared to controls | |||
| Burke et al,12 1995 | 70 (57) | 3.2 [2‐4] and 37.9 [12‐96] | LVSP at rest | Similar in HTx (early, late denervated, late reinnervated) and controls |
| LVEDP at rest | Similar in HTx (early, late denervated, late reinnervated) and controls | |||
| LVSP in response to IC tyramine injection | Higher increase in late reinnervated compared to late denervated and controls | |||
| LVEDP in response to IC tyramine injection | Similar change in HTx (early, late denervated, late reinnervated) and controls | |||
| MAP at rest | Similar in HTx (early, late denervated, late reinnervated) and controls | |||
| MAP in response to tyramine injection | No change in controls and early or late denervated or reinnervated HTx | |||
| Wilson et al,29 2000 | 61 (41) | 3.8±0.4 [2‐6] and 30±4 [12‐60] | DBP at rest | Higher in HTx (denervated and reinnervated) compared to controls |
| DBP in response to exercise | Similar decrease in HTx (denervated and reinnervated) and controls | |||
| SBP at rest | Similar in HTx (denervated and reinnervated) and controls | |||
| SBP in response to exercise | Higher increase in reinnervated HTx compared to denervated HTx | |||
| Piquard et al,63 2000 | 14 (7) | 35.5±8.3 | BP at rest | Higher in HTx compared to controls |
| BP at peak exercise | Similar in HTx and controls | |||
| Bengel et al,21 2001 | 39 (29) | 38.4±25.2 [6‐98.4] | DBP at rest | Higher in HTx (denervated and reinnervated) compared to controls |
| SBP at rest | Similar in HTx (denervated and reinnervated) and controls | |||
| SBP in response to exercise | Higher in reinnervated HTx compared to denervated HTx | |||
| DBP in response to exercise | Similar in HTx (denervated and reinnervated) and controls | |||
| Scott et al,50 2009 | 20 (9) | 96±72 | ESP at rest | Similar in HTx and controls (DM and RM) |
| Guimarães et al,64 2010 | 38 (20) | 102±39.6 | SBP at rest | Higher in HTx compared to controls |
| SBP following 6‐minute walk (immediate) | Similar in HTx and controls | |||
| DBP at rest | Higher in HTx compared to controls | |||
| DBP following 6‐minute walk (immediate) | Similar in HTx and controls |
Time period post‐HTx displayed as mean±standard deviation or standard error [range]. BP indicates blood pressure; DABP, diastolic aortic blood pressure; DBP, diastolic blood pressure; DM, donor‐age matched; ESP, end‐systolic pressure; HED, 11C‐meta‐hydroxyephedrine; HTx, heart transplantation; IC, intracoronary; IV, intravenous; LVEDP, left ventricular end diastolic pressure; LVSP, left ventricular systolic pressure; MAP, mean arterial pressure; RM, recipient‐age matched; SABP, systolic aortic blood pressure; SBP, systolic blood pressure.
HLTx recipients.
HTx or HLTx recipients.
SBP at peak exercise was lower in HTx recipients compared to control subjects.16, 61 SBP increase during exercise was higher in reinnervated compared to denervated HTx recipients.21, 29 SBP increased more during exercise in controls and late reinnervated HTx recipients compared to early and late denervated HTx recipients.29 In response to the handgrip test, there was a similar increase in SBP in early and late HTx recipients compared to controls.2 Following standing, there was a similar drop in SBP between early and late HTx recipients and control subjects.27 Therefore, SBP increase during exercise seems to be a sign of normalizing reinnervated myocardium late post‐HTx.
Diastolic Blood Pressure
Table 3 shows a summary of the findings by studies about diastolic blood pressure (DBP) at rest early and late post‐HTx. In 1 study, resting DBP was similar between HTx recipients and controls.61 Other studies, however, showed a higher resting DBP in HTx recipients, HLTx recipients, or early recipients compared to controls.5, 16, 24, 64 It was also found to be higher in denervated and reinnervated recipients compared to controls.21, 29
At peak exercise, DBP was lower in HTx recipients compared to controls.61 On the same note, DBP in response to head‐up tilt increased in controls but not in HLTx recipients.5 However, another study showed DBP to be higher in HTx recipients compared to controls at peak exercise.16 In response to exercise, DBP decrease was similar between denervated and reinnervated recipients and controls29 or decreased to a similar reading between both groups.21 Finally, there was similar rise in DBP in response to the handgrip test in early and late HTx recipients and control subjects.2
Other Pressure Measurements
Blood pressure (BP) at rest was higher in HTx recipients or early and late HTx recipients compared to controls.27, 33, 63 This observation may be related to the elevated levels of CAT or the use of cyclosporine immunosuppression post HTx.49 At peak exercise there was a similar BP between HTx recipients and controls.63 During exercise, however, BP increased to a greater extent in controls compared to HTx recipients.61 Another study, however, showed a similar BP increase in HTx recipients and controls, suggesting that adrenomedullin (ADM) might participate in BP regulation in HTx recipients during exercise.63 Aortic BP was also similar between early and late HTx recipients and controls in response to the handgrip test.2
One study showed that resting mean arterial pressure (MAP) was higher in early and late HTx recipients compared to control subjects.23 Another study, however, found similar MAP between controls and early, late denervated, or late reinnervated HTx recipients.12 One study noted differences in baseline MAP between controls and HTx recipients.18 And another study showed that arterial pressure increased significantly in the first month post‐HTx and increased more in late HTx.28
In summary, denervation post‐HTx seems to cause a loss in the daily nocturnal decline in BP, which in turn causes an overall higher BP at rest throughout a 24‐hour period. As reinnervation occurs, some return of the nocturnal decline in BP may occur, thus reducing the overall blood pressure.
Catecholamine Levels: Norepinephrine
Table 4 shows a summary of the findings of many studies regarding norepinephrine (NE) levels early and late post‐HTx. The presence of NE within the cardiac nerve terminals implies reinnervation, given that the nuclei of the cardiac sympathetic nerves are located outside the transplanted heart2 and that the left ventricular NE stores are reduced early after HTx but seem to increase gradually with time post‐HTx.24, 29 This is shown by the observation that coronary blood flow increase in response to sympathetic stimulation correlated with the regional NE content in the myocardial sympathetic nerve terminal.13 Also, the increase in arterial NE in HTx recipients is a sign of increased sympathetic activity.1 Resting plasma NE levels (venous, arterial, aortic, or coronary sinus) were similar between HTx or HLTx recipients, early and late recipients, or denervated and reinnervated recipients and control subjects.2, 5, 16, 21, 23, 24, 29, 60, 64. Interestingly though, it was speculated that the similarity between NE levels at rest in late HTx recipients and controls could be the result of the reinnervation process, which renders a higher NE release at rest unnecessary.64 Our group, however, found higher plasma NE levels at rest in early and late HTx recipients compared to controls.1 Higher plasma CAT levels at rest were noted in HTx recipients by another study as well; however, plasma CAT levels at rest were independent of time post‐HTx.62
Table 4.
Catecholamine Levels Following HTx
| Study Reference | N of Study (N of HTx) | Time Period Post‐HTx (Months) | Variable Hemodynamics/ Intervention | Results Summary |
|---|---|---|---|---|
| Banner et al,5 1990a | 16 (8) | 20 [4‐30] | Plasma NE at rest | Similar in HTx and controls |
| Plasma NE in response to head‐up tilt | Higher increase in HTx compared to controls | |||
| Plasma EPI at rest | Similar in HTx and controls | |||
| Plasma EPI in response to head‐up tilt | Similar increase in HTx and controls | |||
| Wilson et al,2 1991 | 70 (62) | 3.1±0.4 [2‐5] and 37±3 [12‐102] | Coronary sinus NE at rest | Similar in HTx (early and late) and controls |
| Aorta NE at rest | Similar in HTx (early and late) and controls | |||
| Cardiac NE release/uptake at rest | Similar in HTx (early and late) and controls | |||
| Cardiac NE release/uptake in response to IV tyramine injection | Lower in late HTx compared to controls, no release in early HTx | |||
| Cardiac NE release/uptake in response to handgrip | Higher release in late compared to early HTx | |||
| Braith et al,16 1992 | 22 (11) | 18±12 [7‐41] | Plasma NE at rest | Similar in HTx and controls |
| Plasma NE in response to exercise | Higher increase in HTx compared to controls | |||
| Plasma NE at peak exercise | Higher in HTx compared to controls | |||
| Kaye et al,23 1993 | 40 (15) | 5.5 [2.1‐13.3] and 48.3 [26.7‐96] | Plasma arterial NE at rest | Similar in HTx (early and late) and controls |
| Total NE spillover rate to plasma at rest | Similar in HTx (early and late) and controls | |||
| NE clearance rate from plasma at rest | Similar in HTx (early and late) and controls | |||
| Cardiac NE spillover rate at rest | Similar in late HTx and controls, lower in early HTx | |||
| Plasma arterial NE in response to exercise | Trend toward higher values in HTx compared to controls | |||
| Total NE spillover rate to plasma in response to exercise | Similar in HTx (early and late) and controls | |||
| Cardiac NE spillover rate in response to exercise | Similar in late HTx and controls, lower in early HTx | |||
| Plasma EPI at rest | Lower in HTx (early and late) compared to controls | |||
| EPI release rate to plasma at rest | Lower in HTx (early and late) compared to controls | |||
| EPI clearance rate from plasma at rest | Similar in HTx (early and late) and controls | |||
| Plasma arterial EPI in response to exercise | Trend toward lower values in HTx compared to controls | |||
| EPI secretion rate in response to exercise | Similar in HTx (early and late) and controls | |||
| Wilson et al,24 1993 | 57 (50) | 3.0±0.4 [2‐4] and 37±3 [12‐86] | Plasma NE at rest | Similar in HTx (early and late) and controls |
| Left ventricular NE release in response to IC tyramine injection | Lower in late HTx compared to controls, no release in early HTx | |||
| Doering et al,1 1996 | 49 (33) | 2.28±1.55 and 28.3±13.4 | Plasma NE at rest | Higher in HTx (early and late) compared to controls |
| Plasma NE in response to upright tilting | Higher in late HTx compared to early HTx and controls | |||
| Plasma NE in response to supine‐to‐upright tilt | Higher increase in late HTx compared to early HTx and controls | |||
| Plasma EPI in response to upright tilting | Similar response in HTx (early and late) and controls | |||
| Braith et al,60 1998 | 14 (7) | 15±5 | Plasma NE at rest | Similar in HTx and controls |
| Plasma NE in response to exercise | Similar increase and rate of increase in HTx and controls | |||
| Doering et al,22 1999 | 49 (33) | 2.28±1.55 and 28.3±13.4 | Plasma NE immediately before upright‐to‐supine tilt | Higher in late HTx compared to early HTx and controls |
| Wilson et al,29 2000 | 61 (41) | 3.8±0.4 [2‐6] and 30±4 [12‐60] | Plasma venous NE at rest | Similar in HTx (early and late) and controls |
| Plasma venous NE at peak exercise | Similar in HTx (early and late) and controls | |||
| Plasma EPI at rest | Lower in HTx (early and late) compared to controls | |||
| Plasma EPI at peak exercise | Lower increase in late reinnervated HTx compared to controls | |||
| Bengel et al,21 2001 | 39 (29) | 38.4±25.2 [6‐98.4] | Plasma NE at rest | Similar in reinnervated and denervated HTx |
| Plasma NE following peak exercise | Similar in reinnervated and denervated HTx | |||
| Plasma EPI at rest | Similar in reinnervated and denervated HTx | |||
| Plasma EPI following peak exercise | Similar in reinnervated and denervated HTx | |||
| Odaka et al,9 2001 | 17 (17) | [2‐163.2] | Cardiac NE release in response to IV tyramine injection | Higher in reinnervated compared to denervated HTx |
| Ferretti et al,62 2002 | 26 (17) | 41±8 | Plasma CAT at rest | Higher in HTx compared to controls |
| Plasma CAT at peak exercise | Higher in HTx compared to controls | |||
| Bengel et al,4 2004 | 12 (12) | 32.4±32.4 [6‐106.8] | Global EPI retention at rest | Higher in reinnervated compared to denervated HTx |
| Maximal EPI retention at rest | Higher in reinnervated compared to denervated HTx | |||
| Guimarães et al,64 2010 | 38 (20) | 102±39.6 | Plasma NE at rest | Similar in HTx and controls |
| Plasma NE immediately following 6‐minute walk | Higher in HTx compared to controls |
Time period post‐HTx displayed as mean±standard deviation or standard error [range]. CAT indicates catecholamine; EPI, epinephrine; HLTx, heart‐lung transplantation; HTx, heart transplantation; IC, intracoronary; IV, intravenous; NE, norepinephrine.
HLTx recipients.
In addition, cardiac NE release/uptake ratio at rest was similar between early and late HTx recipients and controls2; however, the cardiac NE spillover rate at rest was similar between late HTx recipients and controls but not early HTx recipients, who showed a lower rate.23 On the other hand, NE levels entering and exiting the myocardium were similar between early and late HTx recipients and controls.2 Of note, the total NE spillover rate to plasma and NE clearance rate from plasma at rest were both found to be similar in early and late HTx recipients and controls despite the observation that the spillover NE rate showed a logarithmic progression toward control values with time post‐HTx.23 Use of MIBG to identify and localize cardiac reinnervation post‐HTx in imaging studies revealed that there was a net transmyocardial NE release in all 10 HTx recipients with MIBG uptake, whereas the 6 recipients with no MIBG uptake experienced no net transmyocardial NE release.42
Plasma NE or CAT levels at peak exercise were higher in HTx recipients compared to controls.16, 62 In response to exercise, there was a higher increase in plasma NE levels and a trend toward higher plasma arterial NE levels in HTx recipients compared to controls.16, 23 Other studies showed a similar increase in plasma NE levels in response to exercise and at peak exercise in control subjects and HTx recipients, early and late recipients, or denervated and reinnervated recipients.21, 29, 60 On the other hand, plasma NE levels immediately after a 6‐minute walk were higher in HTx recipients than in control subjects.64 In support, CAT concentration increase with relative exercise power levels was higher in HTx recipients compared to controls.62 The increase of cardiac NE spillover during exercise was described to be a sign of reinnervation.23
Plasma NE levels in response to head‐up tilt increased more in HLTx recipients compared to controls.5 Our group showed that plasma NE levels in response to upright tilting and supine‐to‐upright tilting were higher in late HTx recipients compared to early recipients or controls.1 We also showed that immediately before upright‐to‐supine tilting, plasma NE levels were higher in late HTx recipients compared to their counterparts.22 Of note, there was a higher cardiac NE release in response to the handgrip test in late compared to early HTx recipients.2
In response to intravenous (IV) tyramine injection, cardiac NE release was higher in reinnervated compared to denervated HTx recipients.9 Nevertheless, cardiac NE release/uptake ratio was lower in late HTx recipients compared to controls, whereas early HTx recipients experienced no cardiac NE release.2 Also in response to intracoronary (IC) tyramine injection, the left ventricular NE release was lower in late HTx recipients compared to controls with no release in early recipients.24 It is noteworthy that the likelihood and magnitude of tyramine‐induced cardiac NE release increased with time after transplantation, reaching a maximum value after 4 years post‐HTx.2 Cardiac reinnervation21 and HED retention9 were associated with increased tyramine‐induced NE spillover. Furthermore, there was a strong linear relationship between plasma NE release and time post‐HTx during the first 5 months and then until 2 to 3 years following HTx.1 Significant left ventricular NE release in response to tyramine injection was noted in 9 out of 13 late HTx recipients, indicating cardiac reinnervation in left ventricular regions.12
Epinephrine
Table 4 shows a summary of the effects of HTx on epinephrine (EPI) levels. Of note, the inotropic response to EPI was attenuated in early compared to late HTx recipients, thus providing evidence for the time‐dependent manner of cardiac reinnervation.20 It was observed that the mRNA levels of phenylethanolamine N‐methyltransferase (PNMT), which is the enzyme synthesizing EPI, were higher in the first 3 years post‐HTx, which reflects “autonomous sympathicotrophy” and started to decrease with longer times post‐HTx, which is a result of sympathetic reinnervation because of the lesser need for high CAT levels.69 This PNMT expression was localized to the right ventricle. In general, HTx recipients seem to have a greater sympathetic activity than normal subjects, probably because of the supersensitivity of the presynaptic origin in response to EPI infusion.22, 23 This supersensitivity to circulating CAT can be the result of either the upregulation of postsynaptic β‐adrenergic receptors or the more likely reason of denervation‐associated loss of presynaptic neuronal uptake.36
Resting EPI plasma levels were found to be similar between HLTx recipients and controls.5 Other studies found early and late HTx recipients to have lower plasma EPI levels at rest compared to control subjects.23, 29 Resting plasma EPI levels were shown to be similar between reinnervated and denervated recipients,21 even though global and maximal EPI retentions at rest were higher in reinnervated compared to denervated recipients while significantly correlating with HR increase, peak HR, and peak rate‐pressure product during exercise.4 Although resting EPI release rate to plasma was lower in early and late HTx recipients compared to controls, resting EPI clearance rate from plasma was found to be similar between those groups.23
In response to exercise, EPI secretion rate was similar between early and late recipients and control subjects even though there was a trend toward lower plasma arterial EPI levels in HTx recipients compared to controls.23 At peak exercise, there was a lower plasma EPI increase in late reinnervated HTx recipients compared to controls.29 However, plasma EPI levels following peak exercise were similar between reinnervated and denervated HTx recipients.21 Of note, EPI levels in response to head‐up tilting increased similarly between HLTx recipients and control subjects.5 Our group found similar results with HTx recipients as well.1 This increase in EPI levels in response to the head‐up tilt could contribute to the HR response mechanism in transplant recipients because of the higher potency of EPI compared to NE.5
In summary, denervation of the transplanted heart causes lowered cardiac CAT stores, which in turn lowers the cardiac CAT release in response to stimuli. Because reinnervation occurs late post‐HTx, the cardiac CAT stores may increase in the nerve terminal, which in turn increases the cardiac CAT release, making the cardiac muscle depend more on its own CAT stores (and neural input) and less on the circulating plasma CAT levels in response to stimuli.
Imaging Techniques: 11C‐meta‐Hydroxyephedrine (HED)
Some scintigraphic imaging techniques use uptake or retention of HED to study the extent of cardiac innervation because it is a CAT analogue that is taken up and stored in the presynaptic sympathetic nerve terminals.21 Retention of HED was observed in 10 (59%) of 17 HTx recipients.9 A study showed no evidence of HED uptake early following HTx until up to a year; however, a steady increase in uptake was noticeable late post‐HTx until up to 5 years.3 Although not all recipients showed cardiac reinnervation post‐HTx, HED retention increased over the following 3 years.11 Maximal HED retention, or left ventricular HED retention, significantly correlated with time after HTx,3, 7, 21, 49 given that maximal HED retention correlated significantly with reinnervation post‐HTx.11 This uptake or retention was not homogeneous as in control subjects or early HTx recipients, who showed reduced homogeneous retention.3, 33 Early HTx recipients showed only 28% of HED retention compared to control subjects, whereas late HTx recipients showed higher retention, especially in the basal segment and anteroseptal left ventricular wall.33 It is interesting to note that maximal HED retention was higher in HTx recipients with idiopathic cardiomyopathy and younger recipients, as well as in cases of shorter cross‐clamp time and fewer episodes of transplant rejection.7
123I‐meta‐Iodobenzylguanidine (MIBG)
Other scintigraphic imaging techniques use uptake of MIBG to study the extent of cardiac innervation. HTx recipients with higher MIBG heart/mediastinum quotient (HMQ) had a higher relative density of myocardial nerve fibers by use of the PGP 9.5 neuronal marker.44 Significant uptake of MIBG was found in 6 (50%) of 12 recipients,41 11 (48%) of 23 recipients,42 18 (40%) of 45 recipients,43 and 70% of recipients,44 showing a degree of reinnervation post‐HTx. The HMR of MIBG uptake intensity, or MIBG uptake in general, correlated with time post‐HTx.41, 43, 47 The uptake of MIBG was observed following the first year post‐HTx and started to increase with time after transplantation.47 The 12‐month post‐HTx MIBG HMR levels were higher than the 2‐month levels of the same HTx recipients.43 In addition, those recipients with significant MIBG uptake had been transplanted for a longer time and had improved exercise tolerance, longer duration of exercise, and higher maximum VO2 compared to recipients with lower MIBG uptake.44
Exercise Tolerance
Table 5 shows the exercise performance variables used to analyze reinnervation post‐HTx. It is noteworthy that there was no difference in the exercise capacity between patients transplanted using the biatrial versus the bicaval technique, given that biatrially transplanted recipients were studied later than bicavally transplanted recipients.70 Exercise performance, in general, was associated with a reduced peak HR in denervated HTx recipients.21 Also, exercise training and moderate physical activity following transplantation could improve the state of cardiac reinnervation,50, 76 as shown by improving HRV.77
Table 5.
Exercise Performance Variables Following HTx
| Study Reference | N of Study (N of HTx) | Time Period Post‐HTx in Months | Variable Hemodynamics/ Intervention | Results Summary |
|---|---|---|---|---|
| Braith et al,16 1992 | 22 (11) | 18±12 [7‐41] | Peak power output | Lower in HTx compared to controls |
| Kaye et al,23 1993 | 40 (15) | 5.5 [2.1‐13.3] and 48.3 [26.7‐96] | Maximum exercise capacity | Lower in HTx (early and late) compared to controls |
| Lord et al,71 1996 | 36 (25) | 40 [22‐96] | Exercise duration | Lower in HTx compared to controls |
| Workload | Lower in HTx compared to controls | |||
| Braith et al,60 1998 | 14 (7) | 15±5 | Peak power output | Lower in HTx compared to controls |
| Uberfuhr et al,3 2000 | 47 (47) | 43.2±40.8 [2‐163.2] | Workload | Higher in reinnervated compared to denervated HTx |
| Anaerobic threshold | Higher in reinnervated compared to denervated HTx | |||
| Wilson et al,29 2000 | 61 (41) | 3.8±0.4 [2‐6] and 30±4 [12‐60] | Exercise duration | Lower in HTx (early and late) compared to controls |
| Anaerobic threshold | Lower in HTx (early and late) compared to controls | |||
| Piquard et al,63 2000 | 14 (7) | 35.5±8.3 | Maximum tolerated power | Lower in HTx compared to controls |
| Bengel et al,21 2001 | 39 (29) | 38.4±25.2 [6‐98.4] | Exercise duration | Lower in HTx compared to controls; Higher in reinnervated compared to denervated HTx |
| Ferretti et al,62 2002 | 26 (17) | 41±8 | Maximum aerobic power | Lower in HTx compared to controls |
| Peak power | Lower in HTx compared to controls | |||
| Exercise duration at exhaustion | Lower in HTx compared to controls | |||
| Bengel et al,4 2004 | 12 (12) | 32.4±32.4 [6‐106.8] | Exercise duration | Higher in reinnervated compared to denervated HTx |
| Workload | Similar in denervated and reinnervated HTx | |||
| Exercise duration due to β‐blockade | Higher reduction in reinnervated compared to denervated HTx |
Time period post‐HTx displayed as mean±standard deviation or standard error [range]. HTx indicates heart transplantation.
It seems that peak power output remains lower in HTx recipients compared to controls.16, 60, 62 Exercise duration was found to be lower in early and late HTx recipients compared to controls for up to 5 years post‐HTx,21, 29, 48, 62, 71 whereas it seems to be higher in reinnervated compared to denervated recipients with higher reduction in reinnervated recipients in response to β‐blockade.4, 21 This exercise duration was obviously found to improve at 1 year post‐HTx compared to pre‐HTx but still remained lower than that of controls.78 Maximum exercise capacity was also lower in early and late HTx recipients compared to controls.23 However, the exercise capacity appeared to be higher 6 months compared to 2 months post‐HTx.37
Workload was lower in HTx recipients compared to controls,71 higher in reinnervated recipients, and lowest in denervated recipients.3, 21 Recipients showed a steady increase in maximum workload for up to 60 months but then went back to first‐year measures in the fifth year post‐HTx.3 In addition, sympathetic reinnervation of the sinus node was associated with an increased total workload in HTx recipients.71 Although maximal aerobic power, maximal tolerated power, and anaerobic threshold were lower in HTx recipients compared to controls,29, 62, 63 anaerobic threshold was higher in reinnervated compared to denervated recipients.3 Recipients also showed a steady increase in anaerobic threshold for up to 60 months but then went back to first‐year measures in the fifth year post‐HTx.3
Oxygen Consumption
Table 6 shows the oxygen consumption variables used to analyze reinnervation post‐HTx. Peak systemic oxygen consumption was lower in HTx recipients compared to controls at rest and during exercise.16, 60 Maximum oxygen consumption (VO2) was higher in reinnervated compared to denervated recipients during exercise,3 but it was still lower in HTx recipients compared to controls even though it showed a steady increase up to 5 years post‐HTx before leveling off at first‐year measures.29 Although this peak oxygen consumption still remained lower in HTx recipients than in controls, it was found to improve at 1 year post‐HTx compared to pre‐HTx values,78 given that any improvement at 6 months post‐HTx did not improve any further for up to 9 years posttransplantation.79 In addition, oxygen consumption or uptake was similar between early and late HTx recipients and controls at rest or lower in HTx recipients during exercise.29, 63 Also during exercise, peak VO2 was lower in HTx recipients for up to 5 years compared to control subjects.48 This maximal VO2 showed a weak but significant relationship with peak HR at 1 year post‐HTx, given that maximal oxygen uptake improved in HTx recipients for 2 years posttransplantation and then seemed not to change significantly for up to 5 years post‐HTx.39
Table 6.
Oxygen Uptake or Consumption Following HTx
| Study Reference | N of Study (N of HTx) | Time Period Post‐HTx (Months) | Variable Hemodynamics/ Intervention | Results Summary |
|---|---|---|---|---|
| Braith et al,16 1992 | 22 (11) | 18±12 [7‐41] | Peak systemic oxygen consumption during exercise | Lower in HTx compared to controls |
| Burke et al,12 1995 | 70 (57) | 3.2 [2‐4] and 37.9 [12‐96] | Transcardiac arteriovenous oxygen difference at rest | Similar in HTx (early and late) and controls |
| Braith et al,60 1998 | 14 (7) | 15±5 | Peak systemic oxygen consumption at rest | Lower in HTx compared to controls |
| Peak oxygen uptake during graded exercise | Lower in HTx compared to controls | |||
| Uberfuhr et al,3 2000 | 47 (47) | 43.2±40.8 [2‐163.2] | Maximum oxygen consumption during exercise | Higher in reinnervated HTx compared to denervated HTx |
| Wilson et al,29 2000 | 61 (41) | 3.8±0.4 [2‐6] and 30±4 [12‐60] | Oxygen consumption at rest | Similar in HTx (early and late) and controls |
| Maximum oxygen consumption during exercise | Lower in HTx compared to controls | |||
| Piquard et al,63 2000 | 14 (7) | 35.5±8.3 | Oxygen uptake at rest | Similar in HTx and controls |
| Oxygen uptake at peak exercise | Lower in HTx compared to controls | |||
| Scott et al,50 2009 | 20 (9) | 96±72 | Oxygen consumption at rest | Lower in HTx compared to controls (DM and RM) |
| Guimarães et al,64 2010 | 38 (20) | 102±39.6 | Oxygen consumption following 6‐minute walk (immediate) | Similar in HTx and controls |
Time period post‐HTx displayed as mean±standard deviation or standard error [range]. DM indicates donor‐age matched; HTx, heart transplantation; RM, recipient‐age matched.
Hemodynamic and Echocardiographic Variables
Resting CI did not differ between controls and HTx recipients but was higher in controls compared to HTx recipients at peak exercise.50, 61 Resting CO was found to be similar between early and late HTx recipients and controls.1, 18 It was also noted that resting CO of HTx recipients, although within the normal range, was less than that of normal subjects, whereas exercise CO was significantly higher in controls.60 Another study showed that HLTx recipients had a greater decrease in CO in response to the head‐up tilt compared to controls.5 In response to passive limb movement, HTx recipients showed a significant increase in CO but that was attenuated compared to controls.18 The attenuation of CO in HTx recipients may be related to the inability to relatively increase HR as necessary combined with the subnormal increase in SV, resulting in chronotropic and inotropic incompetence in HTx recipients, which leads to a reduced exercise capacity.39 The increase in CO during exercise, however, seemed to depend on increased SV and HR in controls but only on increased SV in HTx recipients.18 CO seems to increase in early exercise by increasing preload, and in later exercise by the chronotropic and inotropic effects of circulating CAT,34 taking into account the observation that HTx recipients had impaired preload and vascular reserves during exercise.50 The rise in ventricular filling pressure greater than normal showed that HTx recipients seem to rely on Frank‐Starling mechanisms to increase their CO23 rather than circulating NE.60 Studies also related the CO observations in HTx recipients to a higher resting HR and lower peak SV.10
Resting SV was found to be either higher in controls than early followed by late HTx recipients or higher in controls compared to HTx recipients in general,1, 5, 49, 60, 61 although 1 study showed resting SV to be similar in both groups.18 The lower SV values at rest in HTx recipients were attributed to the increased heart rate and decreased diastolic period.60 In response to exercise, SV dropped in normal subjects but seemed to not change in early and late HTx recipients.1 Others showed that SV in exercise significantly increased more rapidly and to a greater extent compared to resting SV in HTx recipients compared to controls.60, 61 This could be because resting SV is reduced in HTx recipients, so a greater relative increase is permitted during exercise.60 In response to the head‐up tilt, HLTx recipients experienced a greater decrease in SV compared to controls by means of reducing venous return and ventricular preload, which results in an increased HR and contractility with vasoconstriction due to the cardiopulmonary receptors and baroreceptors.5 In addition, both groups experienced a similar increase in SV in response to limb movement.18 SV increase during upright exercise in HTx recipients is due to an increase in either plasma CAT concentration or CAT sensitivity.60
Interestingly, resting left ventricular ejection fraction (LVEF) did not differ between reinnervated and denervated recipients or HTx recipients and controls.4, 49, 50, 61 Global and regional EF at rest did not also differ among controls and denervated and reinnervated recipients.49 During exercise, LVEF was higher and reached peak values in reinnervated compared to denervated recipients4; however, it did not differ in general between controls and HTx recipients even at peak exercise.50, 61 Global and regional EF during peak exercise were lower in denervated recipients compared to controls or reinnervated recipients.49 LVEF recovery after exercise did not differ between reinnervated and denervated recipients.4 However, in response to β‐blockade there was a higher increase in LVEF in reinnervated compared to denervated HTx recipients.4 In a univariate analysis the increase in EF correlated with time after transplantation, HR increase, overall exercise time, and maximal workload, and HED retention significantly correlated with the change in global EF in response to stress.21
The left ventricular end‐diastolic volume index (LVEDVI) at rest was lower in HTx recipients compared to controls.49, 61 From rest to exercise, LVEDVI increased in HTx recipients but did not change in normal subjects.61 The left ventricular end‐systolic volume index (LVESVI) at rest was either similar to or slightly lower than that in HTx recipients compared to controls.49, 61 From rest to exercise, the LVESVI decreased in controls but not in HTx recipients.61 An additional study found similar LVEDVI, LVESVI, and SV index between donor age‐matched and recipient age‐matched HTx recipients.50
Additional Variables
Resting systemic vascular resistance (SVR) was found to be higher in late recipients compared to early recipients, followed by controls,1 and higher in reinnervated recipients compared to normal subjects.49 SVR index, however, was found to be similar between HTx recipients and controls.5 In response to the head‐up tilt, SVR increased in HTx recipients more than in controls5 and was also higher in HTx recipients at peak exercise.50 Additional values include work metabolic index, which was similar between denervated and reinnervated recipients and comparable to controls.49 The same study found that stroke work index at rest was higher in normal subjects compared to HTx recipients. Finally, 11C‐acetate clearance constant did not differ between denervated and reinnervated recipients and was comparable to that in controls.49
Rate‐pressure product (RPP) at rest did not differ between reinnervated and denervated recipients4 but was higher in them compared to controls.21 RPP during exercise was higher in reinnervated recipients and controls compared to denervated recipients21; however, another study found exercising RPP to be lower in recipients compared to controls for up to 5 years post‐HTx.48 In support, a study found that RPP at peak exercise was 31% higher in controls compared to HTx recipients.16 Peak RPP in exercise was found to be higher in reinnervated compared to denervated recipients.4 RPP recovery after exercise did not differ between reinnervated and denervated recipients, but peak RPP following β‐blockade decreased more in reinnervated compared to denervated recipients.4
Resting peak filling rate did not differ between reinnervated and denervated or early and late recipients.4, 47 Peak filling rate increase during exercise was higher in reinnervated compared to denervated or late compared to early HTx recipients.4, 47 However, peak filling rate recovery after exercise did not differ between denervated and reinnervated recipients.4 Resting peak emptying rate was lower in recipients 2 to 12 years post‐HTx compared to recipients 0.5 to 2 years post‐HTx, although the later transplant group had higher peak emptying rate during exercise.47
Blood and plasma volumes were greater in HTx recipients compared to controls normalized for body weight, and that volume increase was not associated with any difference in plasma sodium concentration or osmolality.60 During passive limb movement, the femoral blood volume entering the leg increased but was attenuated in HTx recipients compared to controls, given that peripheral vascular function seemed to be similar between the 2 groups.18 Blood volume could be higher in HTx recipients as a means to maintain CO and BP in a compensatory way, evading cardiac denervation.60 Coronary blood flow at rest was higher in HTx recipients compared to normal subjects.13 After tyramine injection, it was found to be lower in late reinnervated compared to early denervated recipients and was lower in controls compared to both groups.12 In response to the cold pressor test, the magnitude of coronary blood flow was higher in the LAD territory compared to the RCA and circumflex artery territories.13 Resting peripheral blood flow did not differ between HTx recipients and controls in the passive and the control legs.18 In addition, resting brachial artery blood flow did not differ between controls and HTx recipients.18
For parasympathetic reinnervation, a shorter intraoperative cardiopulmonary bypass time was associated with higher HF power spectrum values 6 months post‐HTx, which in turn was associated with recovery of tachycardia.58 For cardiac reinnervation in general, younger recipients were more likely to be reinnervated compared to older recipients.3, 7 Also, younger donor age correlated with cardiac reinnervation post‐HTx, given that donors of reinnervated recipients were younger than those of denervated recipients.7, 21, 55 This can possibly be due to the lower availability of target‐derived neurotrophic factors.7 Reinnervated HTx recipients were on average younger than nonreinnervated recipients by 5.5 years.3 In addition, shorter aortic cross‐clamp time correlated with more cardiac reinnervation post‐HTx, which could be due to the morphological alterations in scar tissue as a result of surgery and its impact on the regrowth of sympathetic fibers.7 The same study also showed a lower degree of reinnervation with a higher frequency of cardiac rejection episodes, which could be related to the lower availability and synthesis of neurotropins. It is interesting to note that recipients with prior ischemic as opposed to dilated cardiomyopathy showed better cardiac reinnervation post‐HTx42 and had more nerve sprouting as well.15
Peptides and Hormones
Baseline atrial natriuretic peptide (ANP) concentration was found to be higher in HLTx recipients compared to controls, and it decreased similarly in both groups in response to exercise, which may be modulated by physiological factors rather than the central nervous system.5 Another study, however, showed an increase in ANP concentration in response to exercise, and the increase was higher in HTx recipients.16 In healthy humans, ANP increases during exercise because of an increase in atrial stretch due to increased venous return.26 Some related the stimulation in the natriuretic system following HTx to the volume or pressure overload causing diastolic cardiac and vascular dysfunction.26 Brain natriuretic peptide (BNP) release was increased in exercise in HTx recipients compared to controls, probably because of the increased atrial stretch, whereas healthy humans show no release of BNP in exercise.26 In addition, adrenomedullin (ADM) as a natriuretic peptide was shown to increase following HTx and might be involved in the BP response to exercise in HTx recipients.63 It was also correlated with a higher left ventricular mass index following transplantation.
Resting plasma renin activity (PRA) was similar in both HLTx recipients and control groups; however, in response to the head‐up tilt, it increased in controls but not in HLTx recipients, which could be related to cyclosporine treatment.5 On the other hand, another study found that baseline PRA was higher in HTx recipients compared to controls, and in response to exercise, PRA increased similarly in both groups.16 Arginine vasopressin (AVP) baseline concentration was similar between HTx recipients and controls.5, 16 In response to the head‐up tilt, AVP concentration did not significantly increase in either group, but in response to exercise, AVP levels increased more in HTx recipients than in controls.5, 16 Plasma aldosterone levels and, likewise, plasma angiotensin II levels at rest were similar in both groups at rest and increased similarly in response to exercise.16 Finally, cardiac release of dopamine at rest was observed to be significantly reduced in early post‐HTx and seemed to be higher in late HTx while being still lower in normal subjects.23 In general, plasma levels of neuroendocrine hormones at rest and during exercise did not correlate with time after HTx, given that HTx recipients showed an exaggerated endocrine response during exercise compared to controls.16
Immunohistochemistry
The cardiac allografts had relatively fewer immunostained nerves compared to age‐matched recipient hearts with intact innervation6; however, the magnitude of nerve sprouting increased with time after HTx and varied among patients,66 although most occurred near blood vessels.15 Also, the donor aorta and pulmonary and coronary arteries showed a general loss of nerves in the adventitia and around the vasa vasorum. In addition, neuropeptide tyrosine and tyrosine hydroxylase were localized in innervated myocardium, endocardium, and the small branches of the coronary artery; however, recipient hearts showed rare presence of both.6 Tyrosine hydroxylase stained heterogeneously in only a few HTx recipients in the endocardium, myocardium, and around the blood vessels,15 providing evidence for sympathetic reinnervation in transplanted hearts. Somatostatin and vasoactive intestinal peptide were localized in innervated atrial myocardium, endocardium, and around the intramyocardial arteries but were rarely detected in transplanted hearts.6 Moreover, calcitonin‐gene‐related peptide, substance P, and neurokinin were localized in the neural cell bodies in intrinsic ganglia and nerve trunks, whereas transplanted hearts showed rare localization.6 Furthermore, the growth‐associated protein‐43 (GAP‐43) stained positive in the endocardium, myocardium, and mostly around the blood vessels within 6 months post‐HTx.15
Summary and Conclusion
Cardiac denervation occurs immediately following heart transplantation (HTx) because of surgical interruption of the sympathetic and parasympathetic nerve fibers, which results in cardiovascular control alterations. Reinnervation occurs months to years following HTx, is partial and regionally heterogeneous, and does not appear in all recipients. Sympathetic reinnervation can occur as early as 5 to 6 months but is more likely to occur after 18 months post‐HTx. It usually appears first in the left ventricle, followed by the sinus node region, highest in the left anterior descending followed by the left circumflex, and lowest in the right coronary artery territories, while also being concentrated in the anterobasal expanding to the septal and lateral regions of the myocardium. Parasympathetic reinnervation can occur as early as 3 to 6 months post‐TTx but mostly occurs at around 2 years post‐HTx. It is usually concentrated around the sinus node area. Sympathetic reinnervation can occur without parasympathetic reinnervation, but the latter seems to appear only in sympathetically reinnervated recipients.
Cardiac denervation after HTx is important clinically because it explains why recipients are unable to experience angina due to ischemia post‐HTx but may have bradycardia and hypotension relating to inferior wall infarction post‐HTx. Cardiac denervation sheds light into why exercise capacity is diminished early post‐HTx, relating to the slower increase in heart rate during exercise because the heart relies on noncardiac circulating catecholamines. Cardiac denervation also explains the loss of the nocturnal decline in blood pressure, and the higher resting heart rate early post‐HTx. Because cardiac denervation depletes the catecholamine stores in the cardiac muscle, HTx recipients may experience more arrhythmias because of dependence on circulating catecholamines. This can also cause an abnormal response to adrenergic agonist or antagonist medications.
Cardiac reinnervation post‐HTx is important clinically because the extent and distribution of reinnervation are usually limited. Incomplete limited cardiac reinnervation causes an abnormally high heart rate at rest early post‐HTx, which has been associated with a lower survival at 3, 5, and 10 years post‐HTx. Finally, sympathetic reinnervation may occur without parasympathetic reinnervation, and it is important to realize that this unbalanced reinnervation affects the heart rate and its response to stimuli.
Methods to assess cardiac reinnervation after HTx include hemodynamic variables, exercise tolerance, oxygen consumption, electrophysiologic parameters, biochemical, peptide, and hormonal levels, scintigraphic and echocardiographic imaging, pathology, and immunohistochemistry. More specifically, methods such as MIBG uptake, HED retention, HRV increase, HR reserve, LF and HF power spectrum, tyramine‐induced HR increase and cardiac NE release, left ventricular NE stores, spillover NE rate, and plasma NE release correlate with time after transplantation, extent of reinnervation, and the magnitude of nerve sprouting. Moderate exercise training may improve the reinnervation process. Future studies are needed to examine whether additional variables, interventions, or lifestyle changes may promote reinnervation following cardiac transplantation.
Disclosures
None.
J Am Heart Assoc. 2016;5:e004070 doi: 10.1161/JAHA.116.004070.
References
- 1. Doering LV, Dracup K, Moser DK, Czer LS, Peter CT. Hemodynamic adaptation to orthostatic stress after orthotopic heart transplantation. Heart Lung. 1996;25:339–351. [DOI] [PubMed] [Google Scholar]
- 2. Wilson RF, Christensen BV, Olivari MT, Simon A, White CW, Laxson DD. Evidence for structural sympathetic reinnervation after orthotopic cardiac transplantation in humans. Circulation. 1991;83:1210–1220. [DOI] [PubMed] [Google Scholar]
- 3. Uberfuhr P, Ziegler S, Schwaiblmair M, Reichart B, Schwaiger M. Incomplete sympathic reinnervation of the orthotopically transplanted human heart: observation up to 13 years after heart transplantation. Eur J Cardiothorac Surg. 2000;17:161–168. [DOI] [PubMed] [Google Scholar]
- 4. Bengel FM, Ueberfuhr P, Karja J, Schreiber K, Nekolla SG, Reichart B, Schwaiger M. Sympathetic reinnervation, exercise performance and effects of beta‐adrenergic blockade in cardiac transplant recipients. Eur Heart J. 2004;25:1726–1733. [DOI] [PubMed] [Google Scholar]
- 5. Banner NR, Williams TD, Patel N, Chalmers J, Lightman SL, Yacoub MH. Altered cardiovascular and neurohumoral responses to head‐up tilt after heart‐lung transplantation. Circulation. 1990;82:863–871. [DOI] [PubMed] [Google Scholar]
- 6. Wharton J, Polak JM, Gordon L, Banner NR, Springall DR, Rose M, Khagani A, Wallwork J, Yacoub MH. Immunohistochemical demonstration of human cardiac innervation before and after transplantation. Circ Res. 1990;66:900–912. [DOI] [PubMed] [Google Scholar]
- 7. Bengel FM, Ueberfuhr P, Hesse T, Schiepel N, Ziegler SI, Scholz S, Nekolla SG, Reichart B, Schwaiger M. Clinical determinants of ventricular sympathetic reinnervation after orthotopic heart transplantation. Circulation. 2002;106:831–835. [DOI] [PubMed] [Google Scholar]
- 8. Uberfuhr P, Frey AW, Ziegler S, Reichart B, Schwaiger M. Sympathetic reinnervation of sinus node and left ventricle after heart transplantation in humans: regional differences assessed by heart rate variability and positron emission tomography. J Heart Lung Transplant. 2000;19:317–323. [DOI] [PubMed] [Google Scholar]
- 9. Odaka K, von Scheidt W, Ziegler SI, Ueberfuhr P, Nekolla SG, Reichart B, Bengel FM, Schwaiger M. Reappearance of cardiac presynaptic sympathetic nerve terminals in the transplanted heart: correlation between PET using (11)C‐hydroxyephedrine and invasively measured norepinephrine release. J Nucl Med. 2001;42:1011–1016. [PubMed] [Google Scholar]
- 10. Beckers F, Ramaekers D, Speijer G, Ector H, Vanhaecke J, Verheyden B, Van Cleemput J, Droogne W, Van de Werf F, Aubert AE. Different evolutions in heart rate variability after heart transplantation: 10‐year follow‐up. Transplantation. 2004;78:1523–1531. [DOI] [PubMed] [Google Scholar]
- 11. Bengel FM, Ueberfuhr P, Ziegler SI, Nekolla S, Reichart B, Schwaiger M. Serial assessment of sympathetic reinnervation after orthotopic heart transplantation. A longitudinal study using PET and C‐11 hydroxyephedrine. Circulation. 1999;99:1866–1871. [DOI] [PubMed] [Google Scholar]
- 12. Burke MN, McGinn AL, Homans DC, Christensen BV, Kubo SH, Wilson RF. Evidence for functional sympathetic reinnervation of left ventricle and coronary arteries after orthotopic cardiac transplantation in humans. Circulation. 1995;91:72–78. [DOI] [PubMed] [Google Scholar]
- 13. Di Carli MF, Tobes MC, Mangner T, Levine AB, Muzik O, Chakroborty P, Levine TB. Effects of cardiac sympathetic innervation on coronary blood flow. N Engl J Med. 1997;336:1208–1215. [DOI] [PubMed] [Google Scholar]
- 14. Mohanty PK, Thames MD, Arrowood JA, Sowers JR, McNamara C, Szentpetery S. Impairment of cardiopulmonary baroreflex after cardiac transplantation in humans. Circulation. 1987;75:914–921. [DOI] [PubMed] [Google Scholar]
- 15. Kim DT, Luthringer DJ, Lai AC, Suh G, Czer L, Chen LS, Chen PS, Fishbein MC. Sympathetic nerve sprouting after orthotopic heart transplantation. J Heart Lung Transplant. 2004;23:1349–1358. [DOI] [PubMed] [Google Scholar]
- 16. Braith RW, Wood CE, Limacher MC, Pollock ML, Lowenthal DT, Phillips MI, Staples ED. Abnormal neuroendocrine responses during exercise in heart transplant recipients. Circulation. 1992;86:1453–1463. [DOI] [PubMed] [Google Scholar]
- 17. Toledo E, Pinhas I, Aravot D, Almog Y, Akselrod S. Functional restitution of cardiac control in heart transplant patients. Am J Physiol Regul Integr Comp Physiol. 2002;282:R900–R908. [DOI] [PubMed] [Google Scholar]
- 18. Hayman MA, Nativi JN, Stehlik J, McDaniel J, Fjeldstad AS, Ives SJ, Walter Wray D, Bader F, Gilbert EM, Richardson RS. Understanding exercise‐induced hyperemia: central and peripheral hemodynamic responses to passive limb movement in heart transplant recipients. Am J Physiol Heart Circ Physiol. 2010;299:H1653–H1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hunt SA. Current status of cardiac transplantation. JAMA. 1998;280:1692–1698. [DOI] [PubMed] [Google Scholar]
- 20. Koglin J, Gross T, Uberfuhr P, von Scheidt W. Time‐dependent decrease of presynaptic inotropic supersensitivity: physiological evidence of sympathetic reinnervation after heart transplantation. J Heart Lung Transplant. 1997;16:621–628. [PubMed] [Google Scholar]
- 21. Bengel FM, Ueberfuhr P, Schiepel N, Nekolla SG, Reichart B, Schwaiger M. Effect of sympathetic reinnervation on cardiac performance after heart transplantation. N Engl J Med. 2001;345:731–738. [DOI] [PubMed] [Google Scholar]
- 22. Doering LV, Dracup K, Moser DK, Czer LS, Peter CT. Evidence of time‐dependent autonomic reinnervation after heart transplantation. Nurs Res. 1999;48:308–316. [DOI] [PubMed] [Google Scholar]
- 23. Kaye DM, Esler M, Kingwell B, McPherson G, Esmore D, Jennings G. Functional and neurochemical evidence for partial cardiac sympathetic reinnervation after cardiac transplantation in humans. Circulation. 1993;88:1110–1118. [DOI] [PubMed] [Google Scholar]
- 24. Wilson RF, Laxson DD, Christensen BV, McGinn AL, Kubo SH. Regional differences in sympathetic reinnervation after human orthotopic cardiac transplantation. Circulation. 1993;88:165–171. [DOI] [PubMed] [Google Scholar]
- 25. Halpert I, Goldberg AD, Levine AB, Levine TB, Kornberg R, Kelly C, Lesch M. Reinnervation of the transplanted human heart as evidenced from heart rate variability studies. Am J Cardiol. 1996;77:180–183. [DOI] [PubMed] [Google Scholar]
- 26. Geny B, Richard R, Mettauer B, Lonsdorfer J, Piquard F. Cardiac natriuretic peptides during exercise and training after heart transplantation. Cardiovasc Res. 2001;51:521–528. [DOI] [PubMed] [Google Scholar]
- 27. Rudas L, Pflugfelder PW, Kostuk WJ. Immediate cardiovascular responses to orthostasis in the early and late months after cardiac transplantation. Int J Cardiol. 1993;38:141–150. [DOI] [PubMed] [Google Scholar]
- 28. Smith ML, Ellenbogen KA, Eckberg DL, Sheehan HM, Thames MD. Subnormal parasympathetic activity after cardiac transplantation. Am J Cardiol. 1990;66:1243–1246. [DOI] [PubMed] [Google Scholar]
- 29. Wilson RF, Johnson TH, Haidet GC, Kubo SH, Mianuelli M. Sympathetic reinnervation of the sinus node and exercise hemodynamics after cardiac transplantation. Circulation. 2000;101:2727–2733. [DOI] [PubMed] [Google Scholar]
- 30. Deng MC. Cardiac transplantation. Heart. 2002;87:177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Geny B, Richard R, Zoll J, Charloux A, Piquard F. Last Word on Point:Counterpoint: cardiac denervation does/does not play a major role in exercise limitation after heart transplantation. J Appl Physiol. 2008;104:568. [DOI] [PubMed] [Google Scholar]
- 32. Keeley EC, Toth ZK, Goldberg AD. Long‐term assessment of heart rate variability in cardiac transplant recipients. J Heart Lung Transplant. 2000;19:310–312. [DOI] [PubMed] [Google Scholar]
- 33. Schwaiger M, Hutchins GD, Kalff V, Rosenspire K, Haka MS, Mallette S, Deeb GM, Abrams GD, Wieland D. Evidence for regional catecholamine uptake and storage sites in the transplanted human heart by positron emission tomography. J Clin Invest. 1991;87:1681–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Uberfuhr P, Frey AW, Fuchs A, Paniara C, Roskamm H, Schwaiger M, Reichart B. Signs of vagal reinnervation 4 years after heart transplantation in spectra of heart rate variability. Eur J Cardiothorac Surg. 1997;12:907–912. [DOI] [PubMed] [Google Scholar]
- 35. Guertner C, Krause BJ, Klepzig H Jr, Herrmann G, Lelbach S, Vockert EK, Hartmann A, Maul FD, Kranert TW, Mutschler E, Hubner K, Hoer G. Sympathetic re‐innervation after heart transplantation: dual‐isotope neurotransmitter scintigraphy, norepinephrine content and histological examination. Eur J Nucl Med. 1995;22:443–452. [DOI] [PubMed] [Google Scholar]
- 36. von Scheidt W, Bohm M, Schneider B, Reichart B, Erdmann E, Autenrieth G. Isolated presynaptic inotropic beta‐adrenergic supersensitivity of the transplanted denervated human heart in vivo. Circulation. 1992;85:1056–1063. [DOI] [PubMed] [Google Scholar]
- 37. Buendia Fuentes F, Martinez‐Dolz L, Almenar Bonet L, Sanchez‐Lazaro I, Navarro Manchon J, Sanchez‐Gomez JM, Raso Raso R, Aguero‐Ramon‐Llin J, Sancho‐Tello de Carranza MJ, Salvador Sanz A. Normalization of the heart rate response to exercise 6 months after cardiac transplantation. Transplant Proc. 2010;42:3186–3188. [DOI] [PubMed] [Google Scholar]
- 38. Gilbert EM, Eiswirth CC, Mealey PC, Larrabee P, Herrick CM, Bristow MR. Beta‐adrenergic supersensitivity of the transplanted human heart is presynaptic in origin. Circulation. 1989;79:344–349. [DOI] [PubMed] [Google Scholar]
- 39. Carter R, Al‐Rawas OA, Stevenson A, McDonagh T, Stevenson RD. Exercise responses following heart transplantation: 5 year follow‐up. Scott Med J. 2006;51:6–14. [DOI] [PubMed] [Google Scholar]
- 40. Bernardi L, Valenti C, Wdowczyck‐Szulc J, Frey AW, Rinaldi M, Spadacini G, Passino C, Martinelli L, Vigano M, Finardi G. Influence of type of surgery on the occurrence of parasympathetic reinnervation after cardiac transplantation. Circulation. 1998;97:1368–1374. [DOI] [PubMed] [Google Scholar]
- 41. Lovric SS, Avbelj V, Trobec R, Zorman D, Rakovec P, Hojker S, Gersak B, Milcinski M. Sympathetic reinnervation after heart transplantation, assessed by iodine‐123 metaiodobenzylguanidine imaging and heart rate variability. Eur J Cardiothorac Surg. 2004;26:736–741. [DOI] [PubMed] [Google Scholar]
- 42. De Marco T, Dae M, Yuen‐Green MS, Kumar S, Sudhir K, Keith F, Amidon TM, Rifkin C, Klinski C, Lau D, Botvinick EH, Chatterjee K. Iodine‐123 metaiodobenzylguanidine scintigraphic assessment of the transplanted human heart: evidence for late reinnervation. J Am Coll Cardiol. 1995;25:927–931. [DOI] [PubMed] [Google Scholar]
- 43. Buendia‐Fuentes F, Almenar L, Ruiz C, Vercher JL, Sanchez‐Lazaro I, Martinez‐Dolz L, Navarro J, Bello P, Salvador A. Sympathetic reinnervation 1 year after heart transplantation, assessed using iodine‐123 metaiodobenzylguanidine imaging. Transplant Proc. 2011;43:2247–2248. [DOI] [PubMed] [Google Scholar]
- 44. Parry DS, Foulsham L, Jenkins G, Wharton J, Marron K, Banner N, Yacoub M. Incidence and functional significance of sympathetic reinnervation after cardiac transplantation. Transplant Proc. 1997;29:569–570. [DOI] [PubMed] [Google Scholar]
- 45. Stark RP, McGinn AL, Wilson RF. Chest pain in cardiac‐transplant recipients. Evidence of sensory reinnervation after cardiac transplantation. N Engl J Med. 1991;324:1791–1794. [DOI] [PubMed] [Google Scholar]
- 46. van De Borne P, Neubauer J, Rahnama M, Jansens JL, Montano N, Porta A, Somers VK, Degaute JP. Differential characteristics of neural circulatory control: early versus late after cardiac transplantation. Circulation. 2001;104:1809–1813. [DOI] [PubMed] [Google Scholar]
- 47. Estorch M, Camprecios M, Flotats A, Mari C, Berna L, Catafau AM, Ballester M, Narula J, Carrio I. Sympathetic reinnervation of cardiac allografts evaluated by 123I‐MIBG imaging. J Nucl Med. 1999;40:911–916. [PubMed] [Google Scholar]
- 48. Givertz MM, Hartley LH, Colucci WS. Long‐term sequential changes in exercise capacity and chronotropic responsiveness after cardiac transplantation. Circulation. 1997;96:232–237. [DOI] [PubMed] [Google Scholar]
- 49. Bengel FM, Ueberfuhr P, Schiepel N, Nekolla SG, Reichart B, Schwaiger M. Myocardial efficiency and sympathetic reinnervation after orthotopic heart transplantation: a noninvasive study with positron emission tomography. Circulation. 2001;103:1881–1886. [DOI] [PubMed] [Google Scholar]
- 50. Scott JM, Esch BT, Haykowsky MJ, Warburton DE, Toma M, Jelani A, Taylor D, Paterson I, Poppe D, Liang Y, Thompson R. Cardiovascular responses to incremental and sustained submaximal exercise in heart transplant recipients. Am J Physiol Heart Circ Physiol. 2009;296:H350–H358. [DOI] [PubMed] [Google Scholar]
- 51. Esch BT, Scott JM, Warburton DE, Thompson R, Taylor D, Cheng Baron J, Paterson I, Haykowsky MJ. Left ventricular torsion and untwisting during exercise in heart transplant recipients. J Physiol. 2009;587:2375–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lord SW, Senior RR, Das M, Whittam AM, Murray A, McComb JM. Low‐frequency heart rate variability: reproducibility in cardiac transplant recipients and normal subjects. Clin Sci (Lond). 2001;100:43–46. [PubMed] [Google Scholar]
- 53. Imamura T, Kinugawa K, Okada I, Kato N, Fujino T, Inaba T, Maki H, Hatano M, Kinoshita O, Nawata K, Kyo S, Ono M. Parasympathetic reinnervation accompanied by improved post‐exercise heart rate recovery and quality of life in heart transplant recipients. Int Heart J. 2015;56:180–185. [DOI] [PubMed] [Google Scholar]
- 54. Fitzpatrick AP, Banner N, Cheng A, Yacoub M, Sutton R. Vasovagal reactions may occur after orthotopic heart transplantation. J Am Coll Cardiol. 1993;21:1132–1137. [DOI] [PubMed] [Google Scholar]
- 55. Tio RA, Reyners AK, van Veldhuisen DJ, van den Berg MP, Brouwer RM, Haaksma J, Smit AJ, Crijns HJ. Evidence for differential sympathetic and parasympathetic reinnervation after heart transplantation in humans. J Auton Nerv Syst. 1997;67:176–183. [DOI] [PubMed] [Google Scholar]
- 56. Ramaekers D, Ector H, Vanhaecke J, van Cleemput J, van de Werf F. Heart rate variability after cardiac transplantation in humans. Pacing Clin Electrophysiol. 1996;19:2112–2119. [DOI] [PubMed] [Google Scholar]
- 57. Raczak G, La Rovere MT, Mortara A, Assandri J, Prpa A, Pinna GD, Maestri R, D'Armini AM, Vigano M, Cobelli F. Arterial baroreflex modulation of heart rate in patients early after heart transplantation: lack of parasympathetic reinnervation. J Heart Lung Transplant. 1999;18:399–406. [DOI] [PubMed] [Google Scholar]
- 58. Imamura T, Kinugawa K, Fujino T, Inaba T, Maki H, Hatano M, Kinoshita O, Nawata K, Kyo S, Ono M. Recipients with shorter cardiopulmonary bypass time achieve improvement of parasympathetic reinnervation within 6 months after heart transplantation. Int Heart J. 2014;55:440–444. [DOI] [PubMed] [Google Scholar]
- 59. Arrowood JA, Minisi AJ, Goudreau E, Davis AB, King AL. Absence of parasympathetic control of heart rate after human orthotopic cardiac transplantation. Circulation. 1997;96:3492–3498. [DOI] [PubMed] [Google Scholar]
- 60. Braith RW, Plunkett MB, Mills RM Jr. Cardiac output responses during exercise in volume‐expanded heart transplant recipients. Am J Cardiol. 1998;81:1152–1156. [DOI] [PubMed] [Google Scholar]
- 61. Pflugfelder PW, Purves PD, McKenzie FN, Kostuk WJ. Cardiac dynamics during supine exercise in cyclosporine‐treated orthotopic heart transplant recipients: assessment by radionuclide angiography. J Am Coll Cardiol. 1987;10:336–341. [DOI] [PubMed] [Google Scholar]
- 62. Ferretti G, Marconi C, Achilli G, Caspani E, Fiocchi R, Mamprin F, Gamba A, Ferrazzi P, Cerretelli P. The heart rate response to exercise and circulating catecholamines in heart transplant recipients. Pflugers Arch. 2002;443:370–376. [DOI] [PubMed] [Google Scholar]
- 63. Piquard F, Charloux A, Mettauer B, Epailly E, Lonsdorfer E, Popescu S, Lonsdorfer J, Geny B. Exercise‐induced increase in circulating adrenomedullin is related to mean blood pressure in heart transplant recipients. J Clin Endocrinol Metab. 2000;85:2828–2831. [DOI] [PubMed] [Google Scholar]
- 64. Guimarães GV, D'Avila V, Bocchi EA, Carvalho VO. Norepinephrine remains increased in the six‐minute walking test after heart transplantation. Clinics (Sao Paulo). 2010;65:587–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Stinson EB, Griepp RB, Schroeder JS, Dong E Jr, Shumway NE. Hemodynamic observations one and two years after cardiac transplantation in man. Circulation. 1972;45:1183–1194. [DOI] [PubMed] [Google Scholar]
- 66. Winnicki M, Phillips BG, Accurso V, van De Borne P, Shamsuzzaman A, Patil K, Narkiewicz K, Somers VK. Independent association between plasma leptin levels and heart rate in heart transplant recipients. Circulation. 2001;104:384–386. [DOI] [PubMed] [Google Scholar]
- 67. Melero‐Ferrer JL, Sanchez‐Lazaro IJ, Almenar‐Bonet L, Martinez‐Dolz L, Buendia‐Fuentes F, Portoles‐Sanz M, Rivera‐Otero M, Salvador‐Sanz A. Impact of basal heart rate on long‐term prognosis of heart transplant patients. Transplant Int. 2013;26:502–507. [DOI] [PubMed] [Google Scholar]
- 68. Castel MA, Roig E, Rios J, Tomas C, Mirabet S, Cardona M, Brossa V, Lopez L, Vargas L, Sionis A, Vallejos I, Perez‐Villa F. Long‐term prognostic value of elevated heart rate one year after heart transplantation. Int J Cardiol. 2013;168:2003–2007. [DOI] [PubMed] [Google Scholar]
- 69. Goncalvesova E, Micutkova L, Mravec B, Ksinantova L, Krizanova O, Fabian J, Kvetnansky R. Changes in gene expression of phenylethanolamine N‐methyltransferase in the transplanted human heart. Ann N Y Acad Sci. 2004;1018:430–436. [DOI] [PubMed] [Google Scholar]
- 70. Czer LS, Cohen MH, Gallagher SP, Czer LA, Soukiasian HJ, Rafiei M, Pixton JR, Awad M, Trento A. Exercise performance comparison of bicaval and biatrial orthotopic heart transplant recipients. Transplant Proc. 2011;43:3857–3862. [DOI] [PubMed] [Google Scholar]
- 71. Lord SW, Brady S, Holt ND, Mitchell L, Dark JH, McComb JM. Exercise response after cardiac transplantation: correlation with sympathetic reinnervation. Heart. 1996;75:40–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Cornelissen VA, Vanhaecke J, Aubert AE, Fagard RH. Heart rate variability after heart transplantation: a 10‐year longitudinal follow‐up study. J Cardiol. 2012;59:220–224. [DOI] [PubMed] [Google Scholar]
- 73. Uberfuhr P, Frey AW, Reichart B. Vagal reinnervation in the long term after orthotopic heart transplantation. J Heart Lung Transplant. 2000;19:946–950. [DOI] [PubMed] [Google Scholar]
- 74. Lord SW, Clayton RH, Mitchell L, Dark JH, Murray A, McComb JM. Sympathetic reinnervation and heart rate variability after cardiac transplantation. Heart. 1997;77:532–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Bernardi L, Bianchini B, Spadacini G, Leuzzi S, Valle F, Marchesi E, Passino C, Calciati A, Vigano M, Rinaldi M, Martinelli L, Finardi G, Sleight P. Demonstrable cardiac reinnervation after human heart transplantation by carotid baroreflex modulation of RR interval. Circulation. 1995;92:2895–2903. [DOI] [PubMed] [Google Scholar]
- 76. Bernardi L, Radaelli A, Passino C, Falcone C, Auguadro C, Martinelli L, Rinaldi M, Vigano M, Finardi G. Effects of physical training on cardiovascular control after heart transplantation. Int J Cardiol. 2007;118:356–362. [DOI] [PubMed] [Google Scholar]
- 77. Lai FC, Chang WL, Jeng C. The relationship between physical activity and heart rate variability in orthotopic heart transplant recipients. J Clin Nurs. 2012;21:3235–3243. [DOI] [PubMed] [Google Scholar]
- 78. Leung TC, Ballman KV, Allison TG, Wagner JA, Olson LJ, Frantz RP, Edwards BS, Dearani JA, Daly RC, McGregor CG, Rodeheffer RJ. Clinical predictors of exercise capacity 1 year after cardiac transplantation. J Heart Lung Transplant. 2003;22:16–27. [DOI] [PubMed] [Google Scholar]
- 79. Osada N, Chaitman BR, Donohue TJ, Wolford TL, Stelken AM, Miller LW. Long‐term cardiopulmonary exercise performance after heart transplantation. Am J Cardiol. 1997;79:451–456. [DOI] [PubMed] [Google Scholar]


