Abstract
Background
Exercise is a major nonpharmacological treatment for hypertension, but its underlying mechanisms are still not completely elucidated. Irisin, a polypeptide containing 112 amino acids, which is secreted mainly by skeletal muscle cells during exercise, exerts a protective role in metabolic diseases, such as diabetes mellitus and obesity. Because of the close relationship between irisin and metabolic diseases, we hypothesized that irisin may play a role in the regulation of blood pressure.
Methods and Results
Blood pressures of male Wistar‐Kyoto (WKY) rats and spontaneously hypertensive rats (SHRs) were monitored through the carotid artery. Our study found that acute intravenous injection of irisin reduced blood pressure in SHRs, but not WKY rats. Irisin, by itself, had no direct vasorelaxing effect in phenylephrine‐preconstricted mesenteric arteries from SHRs. However, irisin augmented the acetylcholine‐induced vasorelaxation in mesenteric arteries from SHRs that could be reversed by Nω‐nitro‐l‐arginine‐methyl ester (L‐NAME; 100 μmol/L), indicating a role of nitric oxide (NO) in this action. Indeed, irisin increased NO production and phosphorylation of endothelial nirtic oxide synthase (eNOS) in endothelial cells. 5′‐AMP‐activated protein kinase (AMPK) was involved in the vasorelaxing effect of irisin because compound C (20 μmol/L), an AMPK inhibitor, blocked the irisin‐mediated increase in phosphorylation of eNOS and protein kinase B (Akt) in endothelial cells and vasodilation in mesenteric arteries.
Conclusions
We conclude that acute administration of irisin lowers blood pressure of SHRs by amelioration of endothelial dysfunction of the mesenteric artery through the AMPK‐Akt‐eNOS‐NO signaling pathway.
Keywords: 5‐AMP‐activated protein kinase, hypertension, irisin, nitric oxide, vasorelaxation
Subject Categories: Hypertension, High Blood Pressure
Hypertension is a major public health problem, affecting ≈1 billion people worldwide.1 Exercise, as a nonpharmacological antihypertensive therapy, is able to decrease blood pressure even in subjects with low responsiveness to medical treatment,2 and regular physical exercise is highly recommended by current European and American hypertension guidelines. However, the underlying mechanisms by which exercise decreases blood pressure have not been fully elucidated. Previous studies have provided evidence that endurance aerobic training has an antihypertensive effect, which may be caused by a decrease in the activities of the sympathetic and renin‐angiotensin systems3 and enhancement of baroreceptor sensitivity.4 Additionally, Joham et al have found that aerobic training increases insulin sensitivity.5 Sun et al proposed that moderate levels of exercise enhance vascular endothelial nitric oxide synthase (eNOS) activity resulting in the improvement of endothelium‐dependent vasodilatation.6 Furthermore, a recent study showed that exercise training could even modulate specific miRNAs in the heart, artery, and skeletal muscle to reduce blood pressure.7
The skeletal muscle is the largest endocrine organ that can secrete interleukins, tumor necrosis factor α, leptin, and resistin, and many diseases are closely related to its disorder.8 It has been reported that more than 1000 genes are “activated” by exercise training in human skeletal muscle, all of which may contribute to improvement in health.9, 10 Recently, a newly found exercise‐mediated polypeptide called irisin, the cleavage of extra cellular domain of fibronectin type III domain‐containing 5 protein (FNDC5), has drawn a lot of attention.11 Exercise can upregulate transcription factor PPARγ coactivating factor 1α, which promotes muscle‐derived FNDC5 expression and then releases irisin into the circulation to increase body energy expenditure.12, 13, 14 Both FNDC5 and irisin are decreased in patients with type 2 diabetes mellitus (T2DM), and irisin has been reported to be beneficial in glucose homeostasis, insulin resistance, and related morbidities, including obesity.15 Because of the close relationship between metabolic diseases and hypertension, it is possible that exercise, through the myogenic factor, irisin,16 may lower blood pressure.
Zhang et al and Jiang et al have reported that irisin (0.1–100 μmol/L) caused endothelium‐dependent and ‐independent vasodilation of arteries preconstricted with phenylephrine in mice and rats.17, 18 Zhang et al also reported that bolus injections (2 minutes) of high doses of irisin (0.625–4 μg) decreased the blood pressure of Sprague‐Dawley and spontaneously hypertensive rats (SHRs).17 In humans, the circulating concentration of irisin is 3.6 ng/mL in sedentary individuals and increases to 4.3 ng/mL in individuals undergoing aerobic interval training.11 The circulating concentration of irisin in rats detected by ELISA is around 300 to 600 ng/mL.19, 20, 21 Therefore, in the present study, we studied the effect of low doses of irisin on blood pressure and low concentrations of irisin on arterial relaxation in normotensive Wistar‐Kyoto (WKY) and SHRs.
Mammalian AMP‐activated protein kinase (AMPK) is a serine/threonine protein kinase that has been proposed to function as an intracellular energy sensor and is involved in the regulation of cellular and whole‐body metabolism.22 Nitric oxide (NO) is one of the most important factors for the relaxation of blood vessels and changes in NO bioavailability affect blood flow and arterial blood pressure. In the vasculature, activation of endothelial AMPK has been shown to phosphorylate eNOS1177, stimulating NO release and subsequent vasodilatation of both large conduit and resistance arteries.23 The endothelium‐dependent mesenteric arterial relaxation in mice attributed to high concentrations (0.1–100 μmol/L) of irisin has also been reported to be related to the NO‐cGMP pathway. Therefore, our present study was designed to determine whether the AMPK‐eNOS‐NO pathway is involved in the vasorelaxing effect of irisin in SHRs.
Material and Methods
Blood Pressure Measurement
Male WKY and SHRs (SLRC Laboratory Animals, Shanghai, China), ranging in age from 16 to 18 weeks, were fed a regular and normal sodium (1% NaCl) rat chow. To empty the stomach and prevent food reflux into the respiratory tract under general anesthesia, food, but not water, was withheld 12 hours before the study. Before the performance of the experiments, rats were anesthetized with pentobarbital (50 mg/kg body weight, intraperitoneally), placed on a heated table to maintain rectal temperature between 36° and 37°, and tracheotomized (PE‐240). Catheters (PE‐50) were placed into both external jugular veins, which were used for maintaining anesthesia and irisin injection. Anesthesia was maintained by the infusion of pentobarbital sodium at 0.8 mg/100 g body weight per hour.24 Catheters (PE‐50) were also placed inside the carotid artery for monitoring systemic arterial pressure (Cardiomax II; Columbus Instruments, Columbus, OH). After achieving stable hemodynamic conditions and recording of baseline blood pressures for 5 minutes, rats received an intravenous injection of irisin (0.1, 1, or 10 μg/kg, bolus injection) or heat‐denatured irisin. To determine the role of eNOS on the hypotensive effect of irisin, rats were pretreated with a bolus injection of the eNOS inhibitor, Nω‐nitro‐l‐arginine‐methyl ester (L‐NAME; 30 mg/kg),25 and stable baseline blood pressure and heart rate were recorded for 10 minutes.26 Following the bolus injection of L‐NAME, rats received either the vehicle (1% DMSO in 0.9% NaCl) or an identical series of irisin injections as above; blood pressure and heart rate were recorded for 60 minutes. All studies were approved by the Daping Hospital Animal Care and Use Committee.
Preparation and Study of Small Resistance Arteries
Vascular reactivity was determined as previously described.27 Briefly, the third‐order branches of the mesenteric arteries were dissected and cut in segments of ≈2 mm in length and mounted on 40‐μm stainless‐steel wires in an isometric Mulvany‐Halpern small‐vessel myograph (model 91 M610; J.P. Trading, Aarhus, Denmark). Rings were maintained in physiological saline solution (PSS) at 37°C and continuously bubbled with oxygen (95%) and carbon dioxide (5%; Carbogen). After a 15‐minute equilibration period in oxygenated PSS at 37°C and pH 7.4, arterial segments were stretched to the optimal luminal diameter for active tension development. Then, vessels were rinsed 3 times with fresh PSS and allowed to recover to baseline for 30 minutes. In the first set of experiments, rings were contracted with phenylephrine HCl (PHE; 10 μmol/L) and high‐potassium PSS (125 mmol/L).
To study acetylcholine (Ach)‐induced endothelium‐dependent relaxation, mesenteric arterial segments were rinsed with PSS for 30 minutes and then a cumulative concentration‐response curve to Ach (1 nmol/L to 100 mmol/L) was obtained in PHE‐preconstricted segments preincubated in the absence or presence of irisin (600 ng/mL [48 nmol/L] or 3000 ng/mL [240 nmol/L], 1 hour) and the procedure was repeated with PSS containing sodium nitroprusside (SNP; 1–1000 nmol/L). To study the effect of irisin on vasoconstriction, a cumulative concentration response to PHE (1 nmol/L to 10 μmol/L) was obtained in arterial segments preincubated in the absence or presence of irisin (3000 ng/mL, 1 hour). The possible role of NO in Ach‐mediated vasodilation was investigated in irisin‐treated and ‐untreated arterial segments by preincubation with L‐NAME (100 μmol/L, 30 minutes) before studying concentration response to ACh. In addition, the participation of cyclo‐oxygenase (COX)‐mediated vasorelaxation was investigated in irisin‐treated and ‐untreated segments. Arteries were preincubated with the nonspecific COX inhibitor indomethacin (10 μmol/L) before performing concentration‐response studies to Ach. In addition, the role of endothelium‐derived hyperpolarizing factor (EDHF) in the Ach‐induced relaxation was analyzed. For this purpose, the vasodilator response to Ach in segments precontracted with high K+ solution (60 mmol/L of KCl) was studied.28 In some experiments, the role of AMPK in Ach‐induced relaxation was investigated in irisin‐treated and ‐untreated segments by preincubation with the AMPK inhibitor, compound C (CC; 20 μmol/L)29 for 30 minutes before studying the concentration response to ACh.
Cell Culture and Sample Preparation
Human coronary artery endothelial cells (Pricells, Wuhan, China) were cultured in primary endothelial cell basal medium (Pricells), supplemented with 10% FBS (Gibco, Grand Island, NY), in a humidified incubator at 37°C with 95% air/5% CO2. Before performing experiments, cells were serum starved overnight with reagents at the indicated times and concentrations and then incubated at the indicated times and concentrations. Cells (80% confluence) were flash frozen with liquid nitrogen and homogenized in ice‐cold lysis buffer (5 mL/mg of tissue) containing protease inhibitor cocktail and phosphatase inhibitor cocktail, sonicated, kept on ice for 1 hour, and then centrifuged at 16 000g for 30 minutes. After centrifugation of homogenates, the supernatant was collected and then all samples were stored at −70°C until use.
Western Blot
After boiling the homogenates in sample buffer at 95°C for 5 minutes, 100 μg of protein were separated by SDS‐PAGE (10% polyacrylamide) and then electroblotted onto nitrocellulose membranes (Bio‐Rad Laboratories, Hercules, CA). Blots were blocked overnight with 5% nonfat dry milk in Tris‐PBS with Tween 20 (TBST; 0.05% Tween 20 in 10 mmol/L of phosphate‐buffered [isotonic saline]) at 4°C with constant shaking. Blots were subsequently incubated with antibodies against eNOS (1:800), phosphor (p)‐eNOS (1:800), neural (n)NOS (1:500), p‐nNOS (1:500), AMPK (1:1000), p‐AMPK (1:1000), protein kinase B (Akt; 1:1000), p‐Akt (1:1000), and GAPDH (1:500) overnight in a cold‐room at 4°C. All of the above antibodies were purchased from Cell Signaling Technology (Danvers, MA). Membranes were then further incubated with infrared‐labeled donkey antirabbit IRDye 800 (1:15 000; Li‐Cor Biosciences, Lincoln, NE) at room temperature for 1 hour. Membranes were washed 3 times with TBST. Bound complexes were detected using the Odyssey Infrared Imaging System (Li‐Cor Biosciences). Images were analyzed using the Odyssey Application Software to obtain the integrated intensities.
Evaluation of Intracellular NO Levels With DAF‐2 DA
Human coronary artery endothelial cells were seeded into cell‐culture dishes. After cells achieved 60% confluence, supernatants were removed and then washed 3 times in 1 mL of HEPES buffer (119 mmol/L of NaCl, 20 mmol/L of Na‐HEPES [pH 7.4], 5 mmol/L of NaHCO3, 4.7 mmol/L of KCl, 1.3 mmol/L of CaCl2, 1.2 mmol/L of MgSO4, 1 mmol/L of KH2PO4, 100 μmol/L of l‐arginine, and 5 mmol/L of glucose) at 37°C. Thereafter, cells were incubated with an NO‐sensitive dye, 4,5‐diaminofluorescein diacetate (DAF‐2 DA; 10 μmol/L) for 45 minutes in the dark at 37°C. After loading, cells were rinsed 3 times with HEPES buffer. The concentration of NO in cells was measured using a DAF‐2 DA fluorescence assay. Some assays were performed in the presence of L‐NAME (100 μmol/L)30 throughout the experimental period. Fluorescence was measured with the excitation wavelength set at 495 nm and the emission wavelength at 515 nm, using fluorescence microscopy (Olympus America, Inc., Melville, NY). NO fluorescence was measured every 20 seconds for 10 to 15 minutes in the same area of the endothelial surface. Basal fluorescence intensity was recorded before each experiment.31, 32
NO Assay
Endothelial cells from human coronary artery were grown on 6‐well plates, and experiments were performed 24 hours after cells reached confluence and serum starved for 3 hours, then stimulated with irisin (3000 ng/mL, 10 minutes). Concentrations of NO metabolites nitrite and nitrate in the cell‐culture supernatant were determined using an assay based on the enzymatic conversion of nitrate to nitrite by nitrate reductase, followed by colorimetric detection of nitrite as an azo‐dye product of the Griess reaction (R&D Systems; Minneapolis, MN). All samples were centrifuged to remove particulates at 16 000g for 20 minutes at 4°C.33 One hundred microliters of each supernatant were mixed with 100 μL of the Griess reagent for 10 minutes at 37°C, and absorbance was recorded on a 96‐well plate using Thermo Scientific Varioskan Flash (Thermo LabSystems, Inc., Philadelphia, PA) at 540 nm.34 Total nitrite levels were determined using a standard curve. NO production is expressed as μmol/L.
Additional Materials
PHE, Ach, SNP, L‐NAME and CC, indomethacin, HEPES, and DMSO were obtained from Sigma‐Aldrich (St. Louis, MO). Irisin polypeptide and antibody for irisin were from Phoenix Pharmaceuticals, Inc (Burlingame, CA), and anti‐FNDC5 rabbit polyclonal antibody was from Proteintech (Wuhan, China). Antibodies for total AMPKα1, phosphorylated AMPKα1, total Akt, phosphorylated Akt, total eNOS, phosphorylated eNOS, total nNOS, phosphorylated nNOS, and GAPDH were from Cell Signaling Technology. Infrared‐labeled donkey antirabbit IRDye 800 was from Li‐Cor Biosciences. DAF‐2 DA was from Calbiochem (San Diego, CA). Cell‐culture dishes were from NEST Biotechnology Co. LTD (Rahway, NJ). The Griess reagent system was from R&D Systems.
Statistical Analyses
Data are expressed as mean±SD. For assays involving arterial rings, the number (n) refers to the number of rats, each providing 2 to 3 rings. Relaxation in each arterial segment is expressed as the percentage of the contraction induced by PHE (10 μmol/L). PHE‐induced contraction in each arterial segment is expressed as the percentage of the contraction induced by 60 mmol/L of KCl. Comparison within groups was made by repeated‐measures ANOVA (or paired t test when only 2 groups were compared), and comparison among groups was made by factorial ANOVA with the Holm‐Sidak test (or t test when only 2 groups were compared). A value of P<0.05 was considered significant.
Results
Irisin Lowered Blood Pressure by Improvement of Endothelial Dysfunction in SHRs
Irisin decreased blood pressure in a dose‐dependent (0.1, 1, and 10 μg/kg) manner in SHRs. By contrast, in WKY rats, irisin had no effect on blood pressure (Figure 1A). Zhang et al also did not find an effect of 1 μg (equivalent to 4 μg/kg in a 250‐g rat), but found that 2 μg (equivalent to 8 μg/kg in a 250‐g rat) of irisin slightly decreased blood pressure of normotensive Sprague‐Dawley rats.17 Zhang et al also found that irisin decreased blood pressure in a dose‐dependent fashion in SHRs (2, 4, and 8 μg, equivalent to 8, 16, 32 μg/kg in a 250‐g rat).17 In SHRs, the bolus intravenous injection of irisin (10 μg/kg) started to decrease blood pressure after 5 minutes, reached significance after 10 minutes, with the maximum effect noted after 20 minutes; the vasodepressor effect of irisin was no longer evident at 90 minutes. Heat‐denatured irisin had no effect on blood pressure (Figure 1B). Irisin, also, had no effect on heart rate (Figure 1C).
Figure 1.
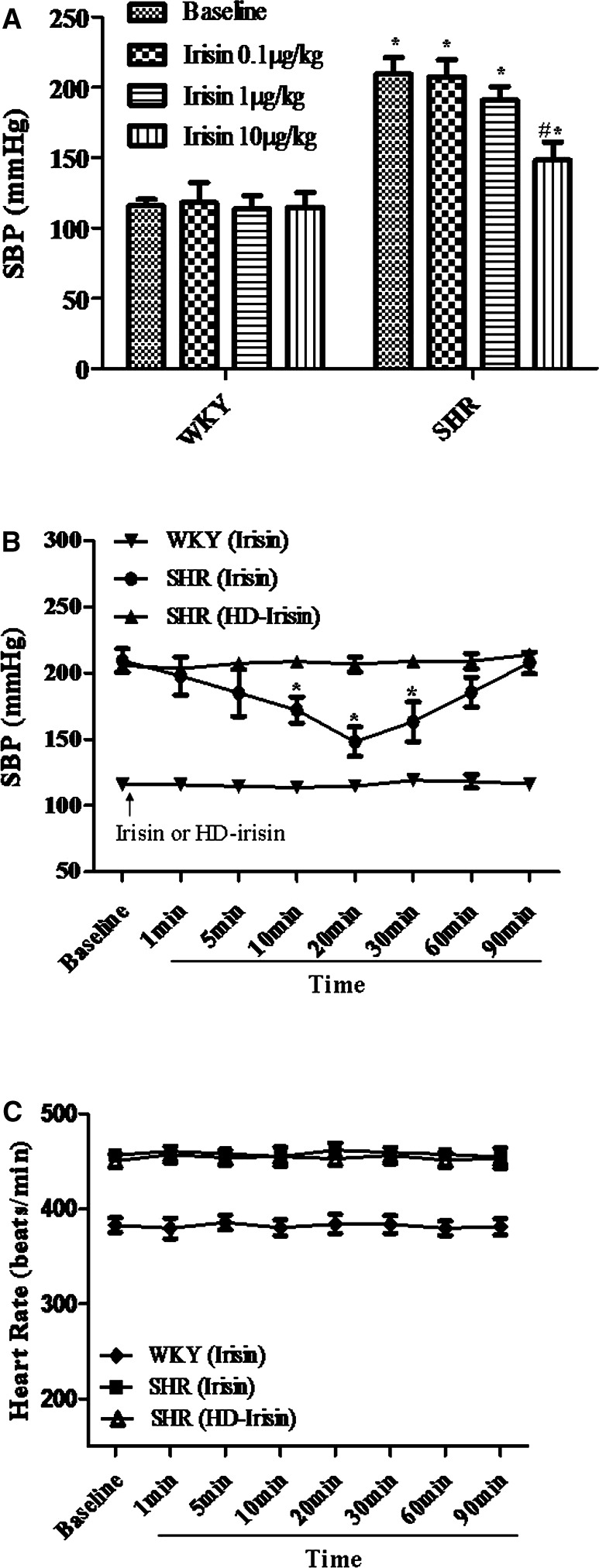
Effect of a bolus intravenous injection of irisin on blood pressure. Irisin decreases systolic blood pressure (SBP) in a concentration‐ (0.1, 1, and 10 μg/kg) (A) and time‐dependent (B) manner in spontaneously hypertensive rats (SHRs), but not Wistar‐Kyoto (WKY) rats. Irisin does not affect the heart rate in either WKY or SHR (C). Irisin (10 μg/kg) and the same dose of heat‐denatured irisin were injected, as a bolus, into the femoral vein. A, *P<0.05 versus WKY; # P<0.05 versus baseline of SHR; n=4/group). B, HD, Heat‐denatured (*P<0.05 vs baseline or other groups at the same time point; n=6/group).
We next determined whether irisin has any vasorelaxant effect in mesenteric arteries. Irisin (3000 ng/mL), by itself, had no direct vasorelaxant effect in mesenteric arteries from SHRs (Figure 2A1) and WKY rats (Figure 2A2), preconstricted with PHE. However, it augmented Ach‐mediated vasorelaxation in mesenteric arteries from SHRs (Figure 2B), but not WKY rats (Figure 2E). We also found that irisin could decrease the vasoconstriction induced by PHE in the mesenteric artery of SHRs (Figure 2D). SNP, an exogenous NO donor, induces endothelium‐independent vasorelaxation.35 We found that there was no additive effect of irisin on SNP‐induced vasorelaxation in mesenteric arteries from both SHRs (Figure 2C) and WKY rats (Figure 2F). Those results indicate that tvascular dysfunction in SHRs can be ameliorated by irisin in an endothelium‐dependent mechanism.
Figure 2.
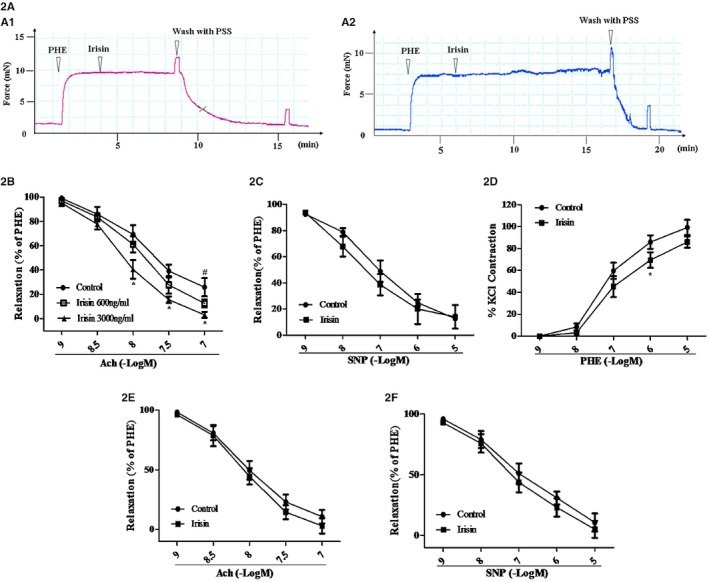
Effect of irisin on mesenteric arterial vasodilation in spontaneously hypertensive rats (SHRs) and Wistar‐Kyoto (WKY) rats. A, Irisin (3000 ng/mL) does not vasodilate the mesenteric artery precontracted with phenylephrine (PHE; 10 μmol/L) of either SHRs (A1) or WKY rats (A2). B and C, Preincubation of mesenteric arteries with irisin (600 or 3000 ng/mL for 1 hour) augments Ach‐mediated (1–100 nmol/L), (B) but not SNP‐mediated (1 nmol/L to 10 μmol/L), (C) vasodilation in PHE‐preconstricted mesenteric arterial segments from the SHR. D, Preincubation of mesenteric arteries with irisin (3000 ng/mL for 1 hour) decreases PHE‐mediated (1 nmol/L to 10 μmol/L) vasoconstriction in SHRs. E and F, Preincubation of mesenteric arteries with irisin (3000 ng/mL for 1 hour) neither augments Ach‐ nor SNP‐mediated vasodilation in mesenteric arterial segments from WKY rats (n=6; *P<0.05 vs control; # P<0.05 vs irisin (600 ng/mL). Ach indicates acetylcholine; PSS, physiological saline solution; SNP, sodium nitroprusside.
Irisin‐Mediated Increase in NO Production Decreased Endothelial Dysfunction in the Mesenteric Artery of the SHR
NO produced in Ach‐induced vasodilation is from endothelial cells, and irisin‐evoked relaxation of mesenteric arteries from mice has been reported to be partially blocked by the NOS inhibitor, L‐NAME.18 Because the irisin sequence is highly conserved among species,36 we determined the effect of irisin on NO production in human coronary endothelial cells. Irisin increased NO production, measured by DAF‐2 DA fluorescence28, 32 staining, in a time‐ and concentration‐dependent (Figure 3A1 and 3A2) manner. L‐NAME (100 μmol/L), an NOS inhibitor, completely abrogated the irisin‐induced increase in NO production (Figure 3B1 and 3B2). To further confirm the results, another method to measure NO metabolites (ie, nitrite and nitrate) was used; consistent with results in Figure 3B, irisin (3000 ng/mL, 10 minutes) increased NO production, whereas pretreatment with L‐NAME (100 μmol/L) abolished the stimulatory effect of irisin on NO production (Figure 3C). In additional studies, human coronary endothelial cells were preincubated with irisin (3000 ng/mL) for 1 hour, washed with HEPES buffer, and then treated with Ach (100 nmol/L). We found that irisin (3000 ng/mL) increased the ability of Ach (100 nmol/L) to increase NO production after 240 seconds of incubation (Figure 3D1 and 3D2).
Figure 3.
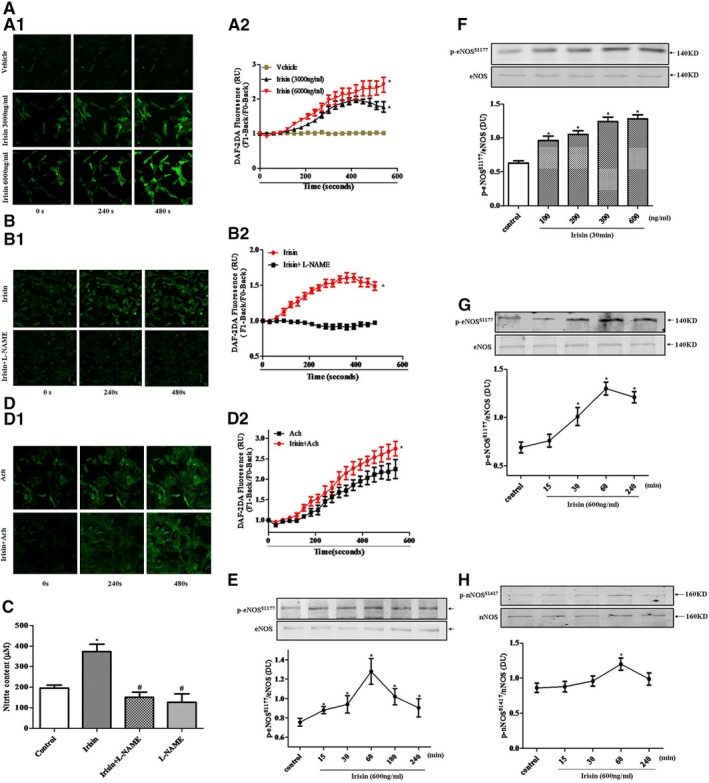
Effect of irisin on NO production and eNOS phosphorylation in mesenteric arteries from spontaneously hypertensive rats (SHRs) and human coronary endothelial cells. A, Effect of irisin on NO production in human coronary endothelial cells. NO production was examined after irisin or vehicle treatment of endothelial cells. Representative experiments are shown at time point 0, 240, and 480 seconds in (A1). The summary of the data and statistical analysis is shown in (A2) (*P<0.05 vs vehicle; n=4). B, Effect of eNOS on irisin‐mediated NO production in human coronary endothelial cells. Human coronary endothelial cells were preincubated with L‐NAME (100 μmol/L, 30 minutes) and then treated with irisin (3000 ng/mL). Representative experiments are shown in (B1). The summary of the data and statistical analysis is shown in (B2) (*P<0.05 vs L‐NAME‐treated group; n=4). C, Effect of irisin on NO production, determined by measurement of nitrite and nitrate contents in the cell‐culture medium of human coronary endothelial cells. Endothelial cells were treated with irisin (3000 ng/mL, 10 minutes) in the presence or absence of an NOS inhibitor (L‐NAME, 100 μmol/L) for 30 minutes. Medium was then collected for NO metabolite nitrite and nitrate detection (*P<0.05 vs control group; # P<0.05 vs irisin; n=4). D, Effect of irisin on Ach‐stimulated NO production in human coronary endothelial cells. Preincubation of irisin (3000 ng/mL) for 1 hour in human coronary endothelial cells increases Ach‐induced (100 nmol/L) production of NO. Representative experiments are shown in (D1). The summary of the data and statistical analysis is shown in (D2) (*P<0.05 vs Ach group; n=4). E and F, Effect of irisin on eNOS phosphorylation in the mesenteric artery from SHRs. Mesenteric arteries were incubated with irisin at the indicated concentrations and periods. Results are expressed as the ratio of phosphorylated eNOSS 177 to total eNOS (*P<0.05 vs control group; n=4). G and H, Effect of irisin on eNOS phosphorylation (G) and nNOS phosphorylation (H) in human coronary endothelial cells. Endothelial cells were incubated with irisin at the indicated concentrations and periods. Results are expressed as the ratio of phosphorylated eNOS and nNOS to total eNOS and nNOS (*P<0.05 vs control group, n=4). Ach indicates acetylcholine; DAF‐2 DA, 4,5‐diaminofluorescein diacetate; DU, density units; eNOS, endothelial nitric oxide synthase; L‐NAME, Nω‐nitro‐L‐arginine methyl ester; nNOS, neural nitric oxide synthase; NO, nitric oxide. RU, relative units.
We, next, evaluated the effect of irisin on eNOS‐ser1177 phosphorylation (p‐eNOS) levels in rat mesenteric arteries from SHRs and human coronary endothelial cells. In the mesenteric arteries, as compared to controls, irisin (600 ng/mL) incubation for 30 minutes significantly stimulated eNOS‐ser1177 phosphorylation26 as early as 15 minutes, peaked at 60 minutes, and then gradually decreased close to the basal level at 240 minutes (Figure 3E). Irisin (30‐minute incubation) also increased eNOS‐ser1177 phosphorylation in a concentration‐dependent manner (Figure 3F). Irisin (600 ng/mL) incubation also stimulated eNOS‐ser1177 phosphorylation in human coronary endothelial cells similar to the rat mesenteric arteries, but the effect occurred later, that is, 30 minutes (Figure 3G). Although eNOS is the isoform of NOS that is mainly expressed in endothelial cells,37, 38 we also assessed the effects of irisin on nNOS phosphorylation in human aortic endothelial cells, and found that, although the expression of nNOS was weaker than eNOS, irisin, at a 600‐ng/mL concentration, also increased nNOS phosphorylation at 60 minutes (Figure 3H).
The effect of irisin on NO production is physiologically significant, because the synergistic vasorelaxant effect of irisin and Ach was blocked by the NOS inhibitor, L‐NAME (100 μmol/L; Figure 4A) but not by inhibitors of COX and EDHF, indomethacin (10 μmol/L), and KCl (60 mmol/L), respectively (Figure 4B and 4C). Moreover, the blood‐pressure–lowering effect of irisin in SHRs was almost completely blocked by pretreatment with L‐NAME (30 mg/kg, bolus injection; Figure 4D).
Figure 4.
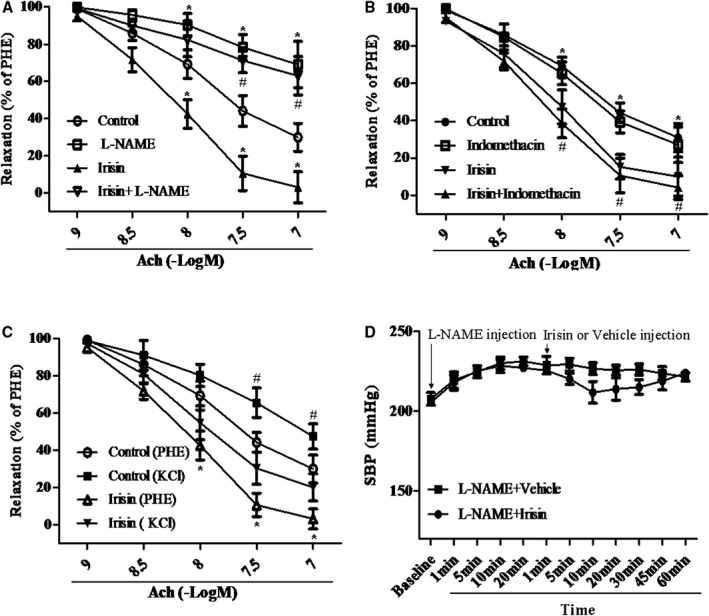
Role of NO, PGI 2, and EDHF on the irisin‐mediated augmentation of Ach‐mediated effect in the mesenteric artery of the spontaneously hypertensive rat (SHR). A and B, Role of NO (A) or PGI 2 (B) in the augmented effect of irisin on Ach‐mediated vasodilation in the mesenteric artery of the SHR. PHE‐precontracted mesenteric artery segments from SHRs were preincubated with irisin (3000 ng/mL, 1 hour) and then incubated with different concentrations of Ach (1–100 nmol/L). The irisin‐mediated augmentation of Ach‐mediated vasodilation was evaluated with or without L‐NAME (100 μmol/L, 30 minutes) (A) or indomethacin (10 μmol/L) (B). (A, n=6; *P<0.05 vs control; # P<0.05 vs irisin; B, *P<0.05 vs irisin; # P<0.05 vs indomethacin; n=6). C, Role of EDHF in the augmented effect of irisin on Ach‐mediated vasodilation in the mesenteric artery of the SHR. Mesenteric artery was precontracted with PHE in the presence or absence of KCl (60 mmol/L) and then treated with Ach with or without preincubation with irisin (3000 ng/mL; 1 hour; # P<0.05 vs irisin [KCl]; *P<0.05 vs control group; n=6). D, Role of eNOS on irisin‐mediated blood pressure lowering effect in the SHR. SHRs were treated with L‐NAME (30 mg/kg, bolus injection) and then irisin (10 μg/kg, bolus injection). The effect of irisin on systolic blood pressure (SBP) was monitored (n=5; P= NS). Ach indicates acetylcholine; EDHF, endothelium‐derived hyperpolarizing factor; eNOS, endothelial nitric oxide synthase; L‐NAME, Nω‐nitro‐L‐arginine methyl ester; NO, nitric oxide; NS, not significant. PHE, phenylephrine.
Irisin Phosphorylates eNOS Through Upregulation of AMPK and Akt Phosphorylation in Human Coronary Endothelial Cells
To elucidate the mechanisms underlying the increase in eNOS phosphorylation in response to irisin, AMPK and Akt, the upstream transducers of eNOS phosphorylation, were evaluated. As shown in Figure 5A through 5C, irisin increased AMPK (Thr172) and Akt (Ser 473) phosphorylation in a concentration‐ and time‐dependent manner, but had no effect on total AMPK and Akt. An additional study showed that in the presence of CC (20 μmol/L), an AMPK inhibitor, the irisin‐mediated increase in phosphorylations of Akt and eNOS were blocked (Figure 6A1 through 6A4). Moreover, pretreatment with CC partially blocked the synergistic vasorelaxant effect of irisin and Ach (Figure 6B).
Figure 5.
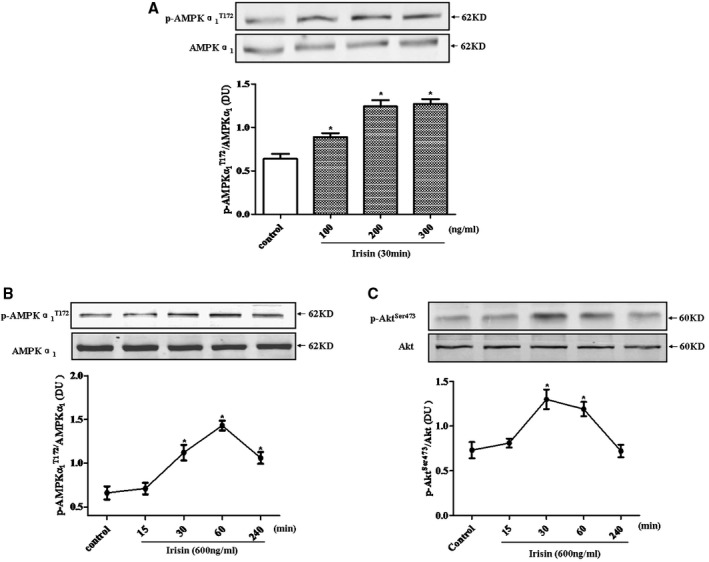
Effect of irisin on AMPK and Akt phosphorylation in human coronary endothelial cells. Endothelial cells were incubated with irisin at the indicated concentrations and times. Phospho‐AMPKα1 (A and B) and phospho‐Akt (C) results were normalized by total AMPKα1 and Akt, respectively (*P<0.05 vs control group; n=4). Akt indicates protein kinase B; AMPK, AMP‐activated protein kinase; DU, density units; p, phospho.
Figure 6.
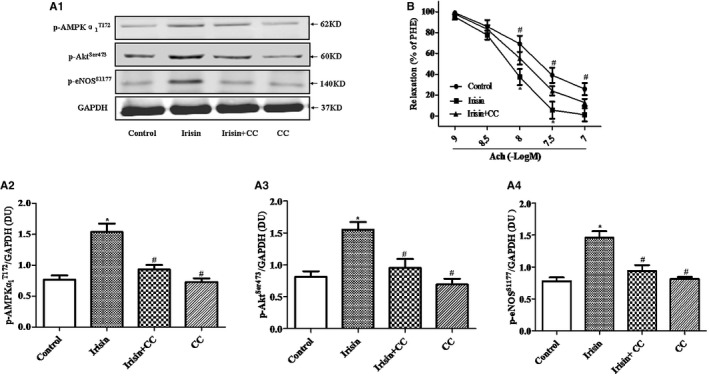
Role of AMPK on irisin‐mediated stimulation of Akt and eNOS phosphorylation in human coronary endothelial cells and vasorelaxation in mesenteric artery from the spontaneously hypertensive rat (SHR). A, Endothelial cells were treated with irisin (600 ng/mL, 1 hour) in the presence or absence of an AMPK inhibitor (compound C [CC], 20 μmol/L) for 30 minutes. Representative immunoblots of phosphorylated AMPK, Akt, and eNOS are shown in (A1). The summary of the quantified blots and statistical analyses are shown in (A2 through A4). Phospho‐AMPKα1, ‐Akt, and ‐eNOS results were then normalized to GAPDH (*P<0.05 vs control group; # P<0.05 vs irisin; n=4). B, Role of AMPK on the irisin‐mediated augmentation of Ach‐induced vasodilation in mesenteric artery from the SHR. Mesenteric artery was preincubated with irisin (3000 ng/mL, 1 hour) and then treated with Ach in the presence or absence of compound C (CC, 20 μmol/L, 30 minutes) (*P<0.05 vs irisin+ CC; # P<0.05 vs irisin; n=6). Ach indicates acetylcholine; Akt, protein kinase B; AMPK, AMP‐activated protein kinase; DU, density units; eNOS, endothelial nitric oxide synthase; p, phospho; PHE, phenylephrine.
Discussion
Exercise training lowers blood pressure and is a recommended nonpharmacological therapy for hypertension, but the mechanisms involved remain elusive. Studies have shown that exercise training attenuates aortic remodeling and improves endothelial function caused by skeletal muscle‐cell–derived factors.3, 39 Since its discovery, irisin has gained great interest as an agent to combat obesity, T2DM, and other metabolic diseases.40, 41, 42, 43 Irisin has been reported to promote human umbilical vein endothelial cell (HUVEC) proliferation and angiogenesis through the extracellular signal‐related kinase signaling pathway and partially suppress high‐glucose–induced apoptosis.44, 45 Circulating irisin levels are positively associated with endothelium‐dependent vasodilation in patients with newly diagnosed T2DM without clinical angiopathy.46 Because metabolic diseases and endothelial dysfunction are associated with hypertension, we studied the effect of irisin in the regulation of blood pressure. We found that bolus intravenous administration of irisin decreases blood pressure, but had no direct vasorelaxing effect. Instead, as shown in Figure 7, irisin ameliorates the endothelial dysfunction of the mesenteric artery of SHRs, by increasing in NO production and activating the AMPK‐Akt‐eNOS pathway.
Figure 7.
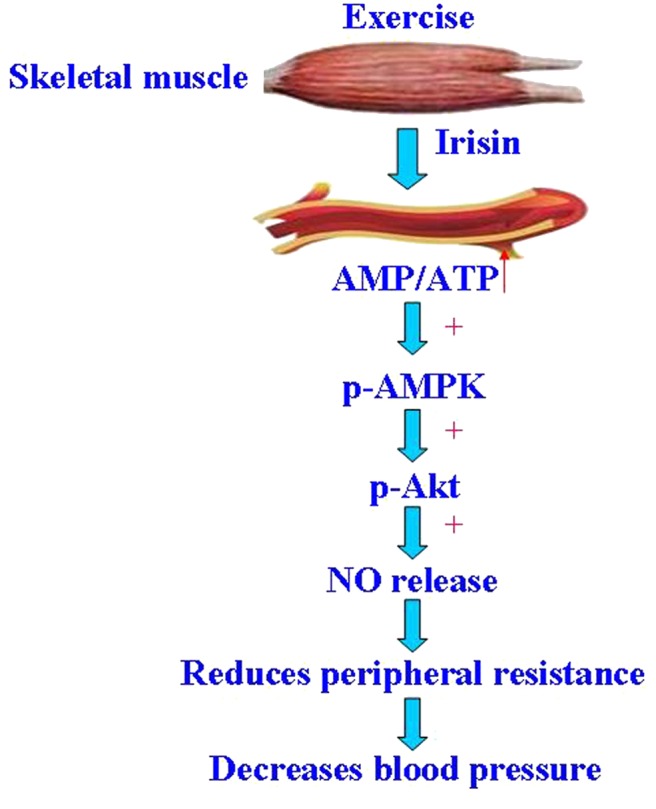
Schematic illustration of the proposed signaling mechanism involved in irisin‐mediated vasorelaxation and lowering blood pressure in the spontaneously hypertensive rat (SHR). During exercise, the skeletal muscles release irisin into the circulation, which acts on arterial endothelial cells to increase AMP/ATP levels and NO release by activation of AMPK and Akt. The upregulation of NO ameliorates endothelial dysfunction and, ultimately, decreases blood pressure. Akt indicates protein kinase B; AMPK, AMP‐activated protein kinase; NO, nitric oxide; p, phospho.
In humans, the circulating concentration irisin detected by mass spectrometry is 3.6 ng/mL in sedentary individuals and increases to 4.3 ng/mL in individuals undergoing aerobic interval training.11 The circulating concentration of irisin in the rat detected by ELISA is around 300 to 600 ng/mL.19, 20, 21 Therefore, we chose 600 ng/mL to study the effect of irisin on the function of rat mesenteric artery.
It is well known that Ach induces vasorelaxation through endothelium‐derived relaxing factors that include NO, prostacyclin (prostaglandin I2; PGI2), and EDHF.47 Therefore, the NO inhibitor, L‐NAME, and COX inhibitor, indomethacin, were used to determine whether or not the increase in ACh‐induced vasodilation induced by irisin is attributed to NO or prostaglandins. The vasorelaxation induced by EDHF is endothelium‐dependent opening of K+ channels that leads to hyperpolarization of vascular smooth muscle cells.48 In order to determine whether or not the increase in ACh‐induced vasodilation induced by irisin is attributed to an increase in EDHF activity, mesenteric resistance arteries were precontracted with a high K+ solution (60 mmol/L).28 Our results suggest that the vasodilatory synergism of irisin and Ach can be blocked by L‐NAME, indicating the involvement of NO. After preincubation with indomethacin or 60 mmol/L of K+ solution, ACh‐induced relaxation was decreased to a similar extent in both experimental conditions, indicating that the synergistic vasorelaxation effect of irisin with Ach in SHR is independent of EDHF and COX pathways. Although irisin has been reported to dilate rat mesenteric arteries through ATP‐sensitive potassium channels, this effect was noted at micromolar concentrations of irisin,17 much higher than the nanomolar concentrations of circulating irisin, used in the current report.44, 45, 46 The ability of higher concentrations of irisin to relax mouse mesenteric arteries has also been reported to be independent of PGI2.18 In additional studies, we found that irisin concentration‐ and time‐dependently enhanced the phosphorylation of eNOS from endothelial cells and mesenteric arteries, an effect that was blocked by L‐NAME. Moreover, pretreatment with L‐NAME to block NOS almost completely prevented the blood‐pressure–lowering effect of irisin in SHRs. Endothelial cells express eNOS to a greater extent than other NOS isoforms, including nNOS.37, 49 Although we also found that irisin could stimulate the phosphorylation of nNOS, its effect was weaker than eNOS. Thus, all pieces of evidence show a role of NO, presumably generated mainly by eNOS, in the irisin‐mediated amelioration of endothelial dysfunction and high blood pressure.
At present, various methods have been proposed to detect NO production inside or outside living organisms. Fluorescent dyes, like DAF‐2DA, are the direct way to quantify NO production and are still widely used,50, 51 although doubts about its specificity have recently been raised.51, 52 Those studies provide evidence that DAF‐2DA dyes react not only with NO, but also with peroxidase enzyme and hydrogen peroxide; both are secreted in the case of elicitation of suspension cells, with a fluorescence increase mimicking NO release from cells. Besides, NO has an extremely short half‐life53 therefore, it is difficult to detect NO production in so short a time. Because of the above‐mentioned limitation, scientists realize that measurement of NO metabolites (ie, nitrite and nitrate) might be an alternative method, determined by the Griess reaction method,54 chemiluminescence,55 or high‐performance liquid chromatography (HPLC).56 Among these methods, HPLC, with the advantages of high sensitivity, was widely applied recently. Because of the lack of HPLC equipment, we used DAF‐2 DA fluorescent probes and the Griess reaction method to determine NO production instead in the present study. We found that irisin increased NO production, whereas pretreatment with L‐NAME abolished the stimulatory effect of irisin on NO production, which is consisted with other reports; for example, Han et al found that irisin could stimulate NO production in HUVECs.54
AMPK has been characterized as an energy sensor (sensitive to the AMP/ATP ratio) in the regulation of glucose uptake and fatty acid oxidation in the whole body.22, 57 AMPK is involved in endothelial cell homeostasis.58, 59 The principal AMPK catalytic subunit isoform contributing to AMPK activity in endothelial cells is the α1 isoform.60 Previous studies have shown that AMPK induces phosphorylation of eNOS at serine‐1177 and activates NO generation in endothelial cells.61, 62 A recent study also found that high concentrations of AMPK agonist also dilate resistance arteries through activation of SERCA and BKCa channels in smooth muscle.63 Irisin promotes the synthesis of uncoupling protein 1 (UCP1) in brown fat cells,12 and UCP1 causes bioenergetically uncoupled energy dissipation (heat production, thermogenesis).64
Exercise activates AMPK in skeletal muscle and endothelial cells.65, 66 In our present study, we found that exogenous irisin dose‐ and time‐dependently enhances the phosphorylation of AMPK. Inhibition of AMPK prevents the irisin‐mediated phosphorylation of eNOS and Akt. We also found that inhibition of AMPK partially blocks the irisin‐mediated increase in Ach‐induced relaxation of mesenteric artery. Therefore, AMPK/Akt/eNOS/NO is involved in the vasodilatory effect of irisin.
In conclusion, we found that low doses or physiological concentrations of irisin did not lower blood pressure or dilate the mesenteric artery of WKY rats. By contrast, irisin decreased blood pressure of SHRs in a concentration‐dependent manner. Physiological concentrations of irisin (48 and 240 nmol/L) did not dilate the mesenteric artery of SHRs precontracted by PHE. However, the same concentration of irisin ameliorated the impaired‐endothelial relaxation response to Ach in the mesenteric artery of the SHR. The vasodilatory effect of irisin was caused by the stimulation of arterial endothelial cells to increase AMP/ATP levels and NO release by activation of AMPK and Akt. Upregulation of NO production improved the endothelial dysfunction in the SHR and ultimately decreased blood pressure, which may be helpful to normalize the high blood pressure of hypertensive patients.
Sources of Funding
These studies were supported, in part, by grants from the National Natural Science Foundation of China (31430043, 31130029, National International Technology Special Grant (2014DFA31070), and National Basic Research Program of China (2013CB531104).
Disclosures
None.
(J Am Heart Assoc. 2016;5: e003433 doi: 10.1161/JAHA.116.003433)
Contributor Information
Lin Zhou, Email: zhoulin@mail.tmmu.com.cn.
Chunyu Zeng, Email: chunyuzeng01@163.com.
References
- 1. Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–223. [DOI] [PubMed] [Google Scholar]
- 2. Dimeo F, Pagonas N, Seibert F, Arndt R, Zidek W, Westhoff TH. Aerobic exercise reduces blood pressure in resistant hypertension. Hypertension. 2012;60:653–658. [DOI] [PubMed] [Google Scholar]
- 3. Gu Q, Wang B, Zhang XF, Ma YP, Liu JD, Wang XZ. Contribution of renin‐angiotensin system to exercise‐induced attenuation of aortic remodeling and improvement of endothelial function in spontaneously hypertensive rats. Cardiovasc Pathol. 2014;23:298–305. [DOI] [PubMed] [Google Scholar]
- 4. Matsukawa K, Ishii K, Kadowaki A, Liang N, Ishida T. Differential effect of central command on aortic and carotid sinus baroreceptor‐heart rate reflexes at the onset of spontaneous, fictive motor activity. Am J Physiol Heart Circ Physiol. 2012;303:H464–H474. [DOI] [PubMed] [Google Scholar]
- 5. Joham AE, Teede HJ, Hutchison SK, Stepto NK, Harrison CL, Strauss BJ, Paul E, Watt MJ. Pigment epithelium‐derived factor, insulin sensitivity, and adiposity in polycystic ovary syndrome: impact of exercise training. Obesity (Silver Spring). 2012;20:2390–2396. [DOI] [PubMed] [Google Scholar]
- 6. Sun MW, Zhong MF, Gu J, Qian FL, Gu JZ, Chen H. Effects of different levels of exercise volume on endothelium‐dependent vasodilation: roles of nitric oxide synthase and heme oxygenase. Hypertens Res. 2008;31:805–816. [DOI] [PubMed] [Google Scholar]
- 7. Neves VJ, Fernandes T, Roque FR, Soci UP, Melo SF, de Oliveira EM. Exercise training in hypertension: role of microRNAs. World J Cardiol. 2014;6:713–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Iizuka K, Machida T, Hirafuji M. Skeletal muscle is an endocrine organ. J Pharmacol Sci. 2014;125:125–131. [DOI] [PubMed] [Google Scholar]
- 9. Pan X, Zhang Y, Tao S. Effects of Tai Chi exercise on blood pressure and plasma levels of nitric oxide, carbon monoxide and hydrogen sulfide in real‐world patients with essential hypertension. Clin Exp Hypertens. 2015;37:8–14. [DOI] [PubMed] [Google Scholar]
- 10. Venkatesh B, Lee AP, Swann JB, Ohta Y, Flajnik MF, Kasahara M, Boehm T, Warren WC. Venkatesh et al. reply. Nature. 2014;511:E9–E10. [DOI] [PubMed] [Google Scholar]
- 11. Jedrychowski MP, Wrann CD, Paulo JA, Gerber KK, Szpyt J, Robinson MM, Nair KS, Gygi SP, Spiegelman BM. Detection and quantitation of circulating human irisin by tandem mass spectrometry. Cell Metab. 2015;22:734–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Bostrom EA, Choi JH, Long JZ, Kajimura S, Zingaretti MC, Vind BF, Tu H, Cinti S, Hojlund K, Gygi SP, Spiegelman BM. A PGC1‐alpha‐dependent myokine that drives brown‐fat‐like development of white fat and thermogenesis. Nature. 2012;481:463–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Villarroya F. Irisin, turning up the heat. Cell Metab. 2012;15:277–278. [DOI] [PubMed] [Google Scholar]
- 14. Kelly DP. Medicine. Irisin, light my fire. Science. 2012;336:42–43. [DOI] [PubMed] [Google Scholar]
- 15. Sanchis‐Gomar F, Lippi G, Mayero S, Perez‐Quilis C, Garcia‐Gimenez JL. Irisin: a new potential hormonal target for the treatment of obesity and type 2 diabetes. J Diabetes. 2012;4:196. [DOI] [PubMed] [Google Scholar]
- 16. Huh JY, Siopi A, Mougios V, Park KH, Mantzoros CS. Irisin in response to exercise in humans with and without metabolic syndrome. J Clin Endocrinol Metab. 2015;100:E453–E457. [DOI] [PubMed] [Google Scholar]
- 17. Zhang W, Chang L, Zhang C, Zhang R, Li Z, Chai B, Li J, Chen E, Mulholland M. Central and peripheral irisin differentially regulate blood pressure. Cardiovasc Drugs Ther. 2015;29:121–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jiang M, Wan F, Wang F, Wu Q. Irisin relaxes mouse mesenteric arteries through endothelium‐dependent and endothelium‐independent mechanisms. Biochem Biophys Res Commun. 2015;468:832–836. [DOI] [PubMed] [Google Scholar]
- 19. Samy DM, Ismail CA, Nassra RA. Circulating irisin concentrations in rat models of thyroid dysfunction—effect of exercise. Metabolism. 2015;64:804–813. [DOI] [PubMed] [Google Scholar]
- 20. Czarkowska‐Paczek B, Zendzian‐Piotrowska M, Gala K, Sobol M, Paczek L. One session of exercise or endurance training does not influence serum levels of irisin in rats. J Physiol Pharmacol. 2014;65:449–454. [PubMed] [Google Scholar]
- 21. Seo DY, Kwak HB, Lee SR, Cho YS, Song IS, Kim N, Bang HS, Rhee BD, Ko KS, Park BJ, Han J. Effects of aged garlic extract and endurance exercise on skeletal muscle FNDC‐5 and circulating irisin in high‐fat‐diet rat models. Nutr Res Pract. 2014;8:177–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Carling D, Viollet B. Beyond energy homeostasis: the expanding role of AMP‐activated protein kinase in regulating metabolism. Cell Metab. 2015;21:799–804. [DOI] [PubMed] [Google Scholar]
- 23. Zheng Q, Yuan Y, Yi W, Lau WB, Wang Y, Wang X, Sun Y, Lopez BL, Christopher TA, Peterson JM, Wong GW, Yu S, Yi D, Ma XL. C1q/TNF‐related proteins, a family of novel adipokines, induce vascular relaxation through the adiponectin receptor‐1/AMPK/eNOS/nitric oxide signaling pathway. Arterioscler Thromb Vasc Biol. 2011;31:2616–2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen Y, Asico LD, Zheng S, Villar VA, He D, Zhou L, Zeng C, Jose PA. Gastrin and D1 dopamine receptor interact to induce natriuresis and diuresis. Hypertension. 2013;62:927–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lopez RM, Perez T, Castillo C, Castillo MC, Castillo EF. Acute intravenous injection and short‐term oral administration of N(G) ‐nitro‐L‐arginine methyl ester to the rat provoke increased pressor responses to agonists and hypertension, but not inhibition of acetylcholine‐induced hypotensive responses. Fundam Clin Pharmacol. 2011;25:333–342. [DOI] [PubMed] [Google Scholar]
- 26. Fink J, Fan NY, Rosenfeld L, Stier CT Jr. Contribution of endothelin to the acute pressor response of L‐NAME in stroke‐prone spontaneously hypertensive rats. J Cardiovasc Pharmacol. 1998;31:618–622. [DOI] [PubMed] [Google Scholar]
- 27. Fu J, Han Y, Wang H, Wang Z, Liu Y, Chen X, Cai Y, Guan W, Yang D, Asico LD, Zhou L, Jose PA, Zeng C. Impaired dopamine D1 receptor‐mediated vasorelaxation of mesenteric arteries in obese Zucker rats. Cardiovasc Diabetol. 2014;13:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xavier FE, Blanco‐Rivero J, Sastre E, Caracuel L, Callejo M, Balfagon G. Tranilast increases vasodilator response to acetylcholine in rat mesenteric resistance arteries through increased EDHF participation. PLoS One. 2014;9:e100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pyla R, Osman I, Pichavaram P, Hansen P, Segar L. Metformin exaggerates phenylephrine‐induced AMPK phosphorylation independent of CAMKKbeta and attenuates contractile response in endothelium‐denuded rat aorta. Biochem Pharmacol. 2014;92:266–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kim JA, Formoso G, Li Y, Potenza MA, Marasciulo FL, Montagnani M, Quon MJ. Epigallocatechin gallate, a green tea polyphenol, mediates NO‐dependent vasodilation using signaling pathways in vascular endothelium requiring reactive oxygen species and Fyn. J Biol Chem. 2007;282:13736–13745. [DOI] [PubMed] [Google Scholar]
- 31. Yuen CY, Wong WT, Tian XY, Wong SL, Lau CW, Yu J, Tomlinson B, Yao X, Huang Y. Telmisartan inhibits vasoconstriction via PPARgamma‐dependent expression and activation of endothelial nitric oxide synthase. Cardiovasc Res. 2011;90:122–129. [DOI] [PubMed] [Google Scholar]
- 32. Wang Y, Dong J, Liu P, Lau CW, Gao Z, Zhou D, Tang J, Ng CF, Huang Y. Ginsenoside Rb3 attenuates oxidative stress and preserves endothelial function in renal arteries from hypertensive rats. Br J Pharmacol. 2014;171:3171–3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Babbitt DM, Kim JS, Forrester SJ, Brown MD, Park JY. Effect of interleukin‐10 and laminar shear stress on endothelial nitric oxide synthase and nitric oxide in African American human umbilical vein endothelial cells. Ethn Dis. 2015;25:413–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hess DT, Matsumoto A, Kim SO, Marshall HE, Stamler JS. Protein S‐nitrosylation: purview and parameters. Nat Rev Mol Cell Biol. 2005;6:150–166. [DOI] [PubMed] [Google Scholar]
- 35. Iwatani Y, Kosugi K, Isobe‐Oku S, Atagi S, Kitamura Y, Kawasaki H. Endothelium removal augments endothelium‐independent vasodilatation in rat mesenteric vascular bed. Br J Pharmacol. 2008;154:32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li X, Fang W, Hu Y, Wang Y, Li J. Characterization of fibronectin type III domain‐containing protein 5 (FNDC5) gene in chickens: cloning, tissue expression, and regulation of its expression in the muscle by fasting and cold exposure. Gene. 2015;570:221–229. [DOI] [PubMed] [Google Scholar]
- 37. Bachetti T, Comini L, Curello S, Bastianon D, Palmieri M, Bresciani G, Callea F, Ferrari R. Co‐expression and modulation of neuronal and endothelial nitric oxide synthase in human endothelial cells. J Mol Cell Cardiol. 2004;37:939–945. [DOI] [PubMed] [Google Scholar]
- 38. Baskova IP, Alekseeva A, Kostiuk SV, Neverova ME, Smirnova TD, Veiko NN. Use of the most recent reagent (CUFL) for stimulation of NO synthesis by the medicinal leech salivary cell secretion in the cultures of human endothelium cells (HUVEC) and in rat cardiomiocytes. Biomed Khim. 2012;58:65–76. [DOI] [PubMed] [Google Scholar]
- 39. Gu Q, Wang B, Zhang XF, Ma YP, Liu JD, Wang XZ. Chronic aerobic exercise training attenuates aortic stiffening and endothelial dysfunction through preserving aortic mitochondrial function in aged rats. Exp Gerontol. 2014;56:37–44. [DOI] [PubMed] [Google Scholar]
- 40. Zhu D, Wang H, Zhang J, Zhang X, Xin C, Zhang F, Lee Y, Zhang L, Lian K, Yan W, Ma X, Liu Y, Tao L. Irisin improves endothelial function in type 2 diabetes through reducing oxidative/nitrative stresses. J Mol Cell Cardiol. 2015;87:138–147. [DOI] [PubMed] [Google Scholar]
- 41. Chen JQ, Huang YY, Gusdon AM, Qu S. Irisin: a new molecular marker and target in metabolic disorder. Lipids Health Dis. 2015;14:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Liu TY, Shi CX, Gao R, Sun HJ, Xiong XQ, Ding L, Chen Q, Li YH, Wang JJ, Kang YM, Zhu GQ. Irisin inhibits hepatic gluconeogenesis and increases glycogen synthesis via the PI3K/Akt pathway in type 2 diabetic mice and hepatocytes. Clin Sci (Lond). 2015;129:839–850. [DOI] [PubMed] [Google Scholar]
- 43. Wu J, Spiegelman BM. Irisin ERKS the fat. Diabetes. 2014;63:381–383. [DOI] [PubMed] [Google Scholar]
- 44. Song H, Wu F, Zhang Y, Wang F, Jiang M, Wang Z, Zhang M, Li S, Yang L, Wang XL, Cui T, Tang D. Irisin promotes human umbilical vein endothelial cell proliferation through the ERK signaling pathway and partly suppresses high glucose‐induced apoptosis. PLoS One. 2014;9:e110273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wu F, Song H, Zhang Y, Mu Q, Jiang M, Wang F, Zhang W, Li L, Li H, Wang Y, Zhang M, Li S, Yang L, Meng Y, Tang D. Irisin induces angiogenesis in human umbilical vein endothelial cells in vitro and in zebrafish embryos in vivo via activation of the ERK signaling pathway. PLoS One. 2015;10:e0134662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Xiang L, Xiang G, Yue L, Zhang J, Zhao L. Circulating irisin levels are positively associated with endothelium‐dependent vasodilation in newly diagnosed type 2 diabetic patients without clinical angiopathy. Atherosclerosis. 2014;235:328–333. [DOI] [PubMed] [Google Scholar]
- 47. Kang KT. Endothelium‐derived relaxing factors of small resistance arteries in hypertension. Toxicol Res. 2014;30:141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Triggle CR, Samuel SM, Ravishankar S, Marei I, Arunachalam G, Ding H. The endothelium: influencing vascular smooth muscle in many ways. Can J Physiol Pharmacol. 2012;90:713–738. [DOI] [PubMed] [Google Scholar]
- 49. Karimi Galougahi K, Liu CC, Garcia A, Gentile C, Fry NA, Hamilton EJ, Hawkins CL, Figtree GA. β3 Adrenergic stimulation restores nitric oxide/redox balance and enhances endothelial function in hyperglycemia. J Am Heart Assoc. 2016;5:e002824 doi: 10.1161/JAHA.115.002824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Villalba N, Sonkusare SK, Longden TA, Tran TL, Sackheim AM, Nelson MT, Wellman GC, Freeman K. Traumatic brain injury disrupts cerebrovascular tone through endothelial inducible nitric oxide synthase expression and nitric oxide gain of function. J Am Heart Assoc. 2014;3:e001474 doi: 10.1161/JAHA.114.001474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cheang WS, Tian XY, Wong WT, Lau CW, Lee SS, Chen ZY, Yao X, Wang N, Huang Y. Metformin protects endothelial function in diet‐induced obese mice by inhibition of endoplasmic reticulum stress through 5′ adenosine monophosphate‐activated protein kinase‐peroxisome proliferator‐activated receptor delta pathway. Arterioscler Thromb Vasc Biol. 2014;34:830–836. [DOI] [PubMed] [Google Scholar]
- 52. Ruemer S, Krischke M, Fekete A, Lesch M, Mueller MJ, Kaiser WM. Methods to detect nitric oxide in plants: are DAFs really measuring NO? Methods Mol Biol. 2016;1424:57–68. [DOI] [PubMed] [Google Scholar]
- 53. Kelm M, Schrader J. Control of coronary vascular tone by nitric oxide. Circ Res. 1990;66:1561–1575. [DOI] [PubMed] [Google Scholar]
- 54. Han F, Zhang S, Hou N, Wang D, Sun X. Irisin improves endothelial function in obese mice through the AMPK‐eNOS pathway. Am J Physiol Heart Circ Physiol. 2015;309:H1501–H1508. [DOI] [PubMed] [Google Scholar]
- 55. MacArthur PH, Shiva S, Gladwin MT. Measurement of circulating nitrite and S‐nitrosothiols by reductive chemiluminescence. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;851:93–105. [DOI] [PubMed] [Google Scholar]
- 56. Wu A, Duan T, Tang D, Xu Y, Feng L, Zheng Z, Zhu J, Wang R, Zhu Q. Determination of nitric oxide‐derived nitrite and nitrate in biological samples by HPLC coupled to nitrite oxidation. Chromatographia. 2013;76:1649–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Qi D, Young LH. AMPK: energy sensor and survival mechanism in the ischemic heart. Trends Endocrinol Metab. 2015;26:422–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Fisslthaler B, Fleming I. Activation and signaling by the AMP‐activated protein kinase in endothelial cells. Circ Res. 2009;105:114–127. [DOI] [PubMed] [Google Scholar]
- 59. Dagher Z, Ruderman N, Tornheim K, Ido Y. The effect of AMP‐activated protein kinase and its activator AICAR on the metabolism of human umbilical vein endothelial cells. Biochem Biophys Res Commun. 1999;265:112–115. [DOI] [PubMed] [Google Scholar]
- 60. Zou MH, Hou XY, Shi CM, Nagata D, Walsh K, Cohen RA. Modulation by peroxynitrite of Akt‐ and AMP‐activated kinase‐dependent Ser1179 phosphorylation of endothelial nitric oxide synthase. J Biol Chem. 2002;277:32552–32557. [DOI] [PubMed] [Google Scholar]
- 61. Ikeda Y, Aihara K, Yoshida S, Iwase T, Tajima S, Izawa‐Ishizawa Y, Kihira Y, Ishizawa K, Tomita S, Tsuchiya K, Sata M, Akaike M, Kato S, Matsumoto T, Tamaki T. Heparin cofactor II, a serine protease inhibitor, promotes angiogenesis via activation of the AMP‐activated protein kinase‐endothelial nitric‐oxide synthase signaling pathway. J Biol Chem. 2012;287:34256–34263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Morrow VA, Foufelle F, Connell JM, Petrie JR, Gould GW, Salt IP. Direct activation of AMP‐activated protein kinase stimulates nitric‐oxide synthesis in human aortic endothelial cells. J Biol Chem. 2003;278:31629–31639. [DOI] [PubMed] [Google Scholar]
- 63. Schneider H, Schubert KM, Blodow S, Kreutz CP, Erdogmus S, Wiedenmann M, Qiu J, Fey T, Ruth P, Lubomirov LT, Pfitzer G, Mederos YSM, Hardie DG, Gudermann T, Pohl U. AMPK dilates resistance arteries via activation of SERCA and BKCa channels in smooth muscle. Hypertension. 2015;66:108–116. [DOI] [PubMed] [Google Scholar]
- 64. Shabalina IG, Kalinovich AV, Cannon B, Nedergaard J. Metabolically inert perfluorinated fatty acids directly activate uncoupling protein 1 in brown‐fat mitochondria. Arch Toxicol. 2016;90:1117–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lee‐Young RS, Ayala JE, Hunley CF, James FD, Bracy DP, Kang L, Wasserman DH. Endothelial nitric oxide synthase is central to skeletal muscle metabolic regulation and enzymatic signaling during exercise in vivo. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1399–R1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Huh JY, Mougios V, Kabasakalis A, Fatouros I, Siopi A, Douroudos II, Filippaios A, Panagiotou G, Park KH, Mantzoros CS. Exercise‐induced irisin secretion is independent of age or fitness level and increased irisin may directly modulate muscle metabolism through AMPK activation. J Clin Endocrinol Metab. 2014;99:E2154–E2161. [DOI] [PubMed] [Google Scholar]


