Abstract
Background
The contribution of functionally disturbed coronary autoregulation and structurally impaired microvascular vasodilatory function to reduced coronary flow velocity reserve, reflecting impaired coronary microcirculation in diabetes mellitus (DM), has not been clearly elucidated. The objective of this study was to identify the mechanism of coronary microvascular impairment in DM in relation to duration of disease.
Methods and Results
Coronary flow velocities in the anterior descending coronary artery were assessed by transthoracic echocardiography following angiography revealing normal epicardial coronary arteries in 55 diabetic and 47 nondiabetic patients. Average peak flow velocities, coronary flow velocity reserve, and microvascular resistance in baseline and hyperemic conditions (baseline and hyperemic microvascular resistance, respectively) were assessed. Reduced coronary flow velocity reserve in patients with short duration (<10 years) of DM compared with nondiabetic patients was primarily driven by increased baseline average peak flow velocity (26.50±5.6 versus 22.08±4.31, P=0.008) in the presence of decreased baseline microvascular resistance (3.69±0.86 versus 4.34±0.76, P=0.003). In contrast, decreased coronary flow velocity reserve in patients with long‐standing (≥10 years) DM compared with nondiabetic patients was predominantly driven by reduced hyperemic average peak flow velocity (41.57±10.01 versus 53.47±11.8, P<0.001) due to increased hyperemic microvascular resistance (2.13±0.42 versus 1.69±0.39, P<0.001).
Conclusions
Both altered coronary autoregulation and impaired microvascular vasodilatory function contribute to DM‐related coronary microvascular impairment in a time‐dependent manner. DM‐induced early functional microvascular autoregulatory impairment seems to evolve into structural microvascular impairment in the initially overperfused microvascular territory at the later stage of disease.
Keywords: coronary arterioles, coronary microcirculation, coronary microvascular function, coronary microvascular resistance, diabetes mellitus
Subject Categories: Coronary Circulation, Mechanisms, Pathophysiology
Introduction
Impaired coronary flow velocity reserve (CFVR) suggesting microvascular structural and/or functional impairment has been demonstrated repeatedly in patients with diabetes mellitus (DM) and normal epicardial coronary arteries, using intracoronary Doppler and thermodilution‐derived techniques,1, 2, 3, 4 positron emission tomography,5, 6, 7 echocardiography,8 and chromatographic methods.9 In this patient subset, impaired CFVR, which is mostly attributed to diabetic microangiopathy5, 6, 10 and altered myocardial metabolism,11, 12 was shown to be strongly associated with development of impaired left ventricular (LV) systolic13 and diastolic function14 and adverse long‐term patient outcome including cardiac mortality.15, 16
In the presence of angiographically normal epicardial arteries, diminished CFVR reflecting coronary microvascular impairment in patients with DM can be mainly driven by 2 mechanisms. The first is presumably a result of increased baseline coronary flow in the presence of reduced coronary microvascular resistance at rest (baseline microvascular resistance [BMR]). In this situation, BMR can decrease to compensate for either increased myocardial oxygen requirements due to diabetes‐induced altered myocardial metabolic demand or inappropriately reduced coronary microvascular tonus due to autonomous dysfunction. The second mechanism is the reduction of hyperemic coronary flow due to high microvascular resistance under maximal hyperemia (hyperemic microvascular resistance [HMR]) and may be ascribed to impaired vasodilatory function of the coronary microcirculation due to structural remodeling. Moreover, studies to date reported inconsistent findings in terms of the relative contributions of these 2 mechanisms to impaired CFVR and myocardial flow reserve in diabetic patients with normal epicardial coronary arteries. Although most of the studies performed in patients with DM reported reduced hyperemic flow, representing impaired microvascular vasodilator reserve as the predominant mechanism behind disturbed myocardial or coronary flow reserve,4, 5, 6, 7, 8, 9 only a few studies reported increased baseline flow as the primary contributor.3, 17 None of these studies, however, used baseline and hyperemic coronary flow and microvascular resistance as integrative only, which would have allowed identification of individual contributions of disturbed autoregulatory mechanisms and vasodilatory impairment to impaired CFVR. Furthermore, a possible role has not yet been elucidated for the duration of DM in the evolution of these functional (increased baseline flow, decreased BMR) and structural (decreased hyperemic flow, increased HMR) coronary microvascular abnormalities.
The aim of this study was to elucidate the mechanism underlying impaired CFVR in diabetic patients and to determine the influence of disease duration on the pattern of microvascular involvement in DM.
Methods
The study was performed at the Istanbul Faculty of Medicine Department of Cardiology between March 2014 and July 2015. We prospectively enrolled patients with normal coronary arteries who underwent coronary angiography during evaluation of their chest pain syndrome. Patients with DM (type 2) and controls in same age range with chest pain and normal epicardial arteries on coronary angiogram were included prospectively. Patients with previous myocardial infarction, prior coronary revascularization, or other significant myocardial or valvular heart diseases were excluded. The study was approved by the local ethics committee. All patients gave fully informed written consent. Smoking history, noninvasive blood pressure values, lipid profile, and drug history were recorded in line with current American Diabetes Association guidelines. DM was defined as a fasting glucose level ≥7 mmol/L, hemoglobin A1c ≥6.5%, or a past history of diabetes. Patients who did not meet this definition were defined as nondiabetic. Duration of DM was reported by the patients and reviewed in the patients’ records at our institution's diabetes clinic (Istanbul Faculty of Medicine, Department of Diabetes).
Echocardiographic Protocol
All participants underwent standard 2‐dimensional imaging assessing LV volumes and function, standard and tissue Doppler imaging, and coronary flow evaluation using a digital ultrasound system (Vivid 7; GE Medical Systems Inc).
Assessment of coronary flow velocity pattern
Coronary flow velocity pattern was assessed in the distal left anterior descending coronary artery using transthoracic echocardiography (M.S. and B.U.). Doppler spectral tracings of coronary flow velocity were obtained by positioning a sample volume (1.5 mm wide) on the color signal in the descending anterior coronary artery and trying to align the ultrasound beam as close to parallel to the flow as possible using a modified foreshortened apical 2‐chamber view. After recording of coronary flow, without changing the transducer's position or the Doppler sample volume, a dose of 0.56 mg/kg of dipyridamole (up to 0.84 mg/kg) was infused in 6 minutes under continuous 12‐lead ECG monitoring. Blood pressure and heart rate were recorded every 3 minutes until the end of the test. Coronary flow velocity envelopes taken throughout the infusion period were recorded and analyzed offline (Figure 1A).
Figure 1.
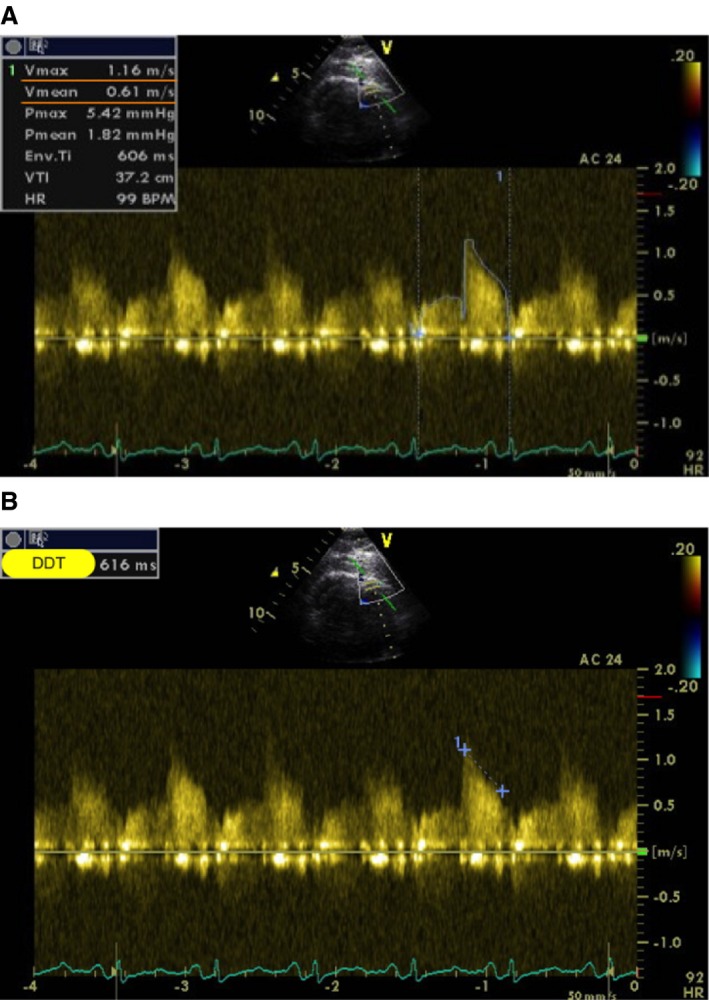
Assessment of average peak coronary velocity and deceleration time of diastolic coronary flow velocity using transthoracic echocardiography. A, Measurement of average peak coronary velocity (Vmean=0.61 m/s). B, Measurement of deceleration time of diastolic coronary flow velocity (DDT 616 ms).
Calculations
Average peak velocities (APVs) and average peak diastolic velocities were measured from spectral Doppler signal recordings obtained by transthoracic echocardiography. CFVR was calculated by dividing transthoracic Doppler‐derived mean hyperemic APV (APVh) by mean baseline APV (APVb):
Because mean pressure in coronary arteries without epicardial stenosis should be equal to the mean aortic pressure, coronary driving pressure can be obtained by measuring mean arterial pressure with the use of a sphygmomanometer. Consequently, microvascular resistance (in mm Hg.s/cm) was calculated from mean blood pressure measured in the arm by sphygmomanometer (mean pressure=[2×diastolic+systolic]/3) divided by APV. Microvascular resistance was calculated in both baseline (BMR) and hyperemic (HMR) states. The arteriolar resistance index (ARI), which represents autoregulatory function, was expressed as BMR minus HMR.18
The difference between BMR and HMR indicates vasodilatory capacity or potential (to dilate under maximal hyperemia) of the arteriolar resistance vessels:
From the diastolic coronary flow velocity spectrum, diastolic deceleration time (in ms) was measured as the time from the peak diastolic velocity to the point at which the extrapolated APV line intersected the baseline from 3 cardiac cycles and then averaged (Figure 1B).
Statistical Analysis
Statistical tests were performed with SPSS version 21.0 software (IBM Corp). Continuous variables were expressed as mean±SD. Group proportions and categorical variables were compared by chi‐square or Fisher exact tests. The Levene test was used to determine whether the standard deviations of the group means were the same. Group means for coronary physiology indices with normal and nonnormal distributions were compared between diabetic and nondiabetic groups with the use of the Student t test and the Mann–Whitney U test for independent groups, respectively. Comparisons of mean values of physiology indices among multiple groups (short and long‐term DM and control groups) were performed with the use of a 1‐way ANOVA test with Bonferroni correction. Group means in diabetic and nondiabetic groups were also adjusted for potential confounders using ANCOVA. In this multivariate adjustment, age, LV mass, presence and absence of hypertension, and angiotensin‐converting enzyme inhibitor and statin usage were included in the model while comparing coronary flow–based parameters and microvascular resistance values between diabetic and nondiabetic groups. Pearson correlation and linear regression analysis were used as appropriate. In the UK Prospective Diabetes Study, the prevalence of microvascular complications in patients with DM was shown to be significantly increased after 10 years.19 Consequently, we empirically chose 10 years as the cutoff for DM duration, and diabetic patients were divided into 2 groups based on this cutoff value (<10 or ≥10 years) with the assumption that diabetic patients with disease duration ≥10 years may have developed microvascular complications significantly more frequently than those with disease duration <10 years. To delineate the independent effect of DM and its duration on microvascular resistance and coronary flow parameters, in addition to statistical adjustment made for controlling potential confounders, analyses were repeated in the presence or absence of hypertension and of LV hypertrophy (LVH). Statistical significance was assigned at P<0.05.
Results
Study Population and Patient Characteristics
We studied 102 consecutive patients (55 with DM and 47 controls). It was not possible to obtain interpretable Doppler envelopes in 10 patients; therefore, 92 patients (50 diabetic) constituted the final study population. There were no significant differences between diabetic and nondiabetic patients in terms of baseline clinical and laboratory characteristics; however, diabetic patients more frequently received angiotensin receptor antagonist and statin therapy compared with controls (Table 1).
Table 1.
Baseline Demographic and Clinical Characteristics and Laboratory Findings of the Study Groups
| Diabetic Patients | Nondiabetic Patients (n=42) | P Values | ||||
|---|---|---|---|---|---|---|
| All DM patients (n=50) | DM <10 Years (n=28) | DM ≥10 Years (n=22) | All DM vs Nondiabetic | DM <10 vs ≥10 Years | ||
| Demographics, history, findings | ||||||
| Sex, male | 60% | 60.7% | 59% | 63.8% | 0.668 | 0.98 |
| Age, y | 55.4±8.3 | 56.37±7.13 | 59.04±9.3 | 55.0±8.3 | 0.268 | 0.25 |
| Body mass index | 30.9±4.4 | 30.69±4.66 | 31.19±4.36 | 29.6±4.8 | 0.163 | 0.62 |
| Cigarettes | 22% | 21.4% | 22.7% | 25% | 0.852 | 0.92 |
| Hypertension | 68% | 67.8% | 68.6% | 62% | 0.834 | 0.73 |
| Systolic blood pressure | 125.8±18.4 | 124.4±19.1 | 126.3±17.9 | 126.7±10.2 | 0.798 | 0.49 |
| Diastolic blood pressure | 72.3±8.5 | 72.8±7.5 | 74.4±8.1 | 74.7±10.2 | 0.231 | 0.67 |
| Heart rate | 73.2±8.4 | 74.4±8.2 | 73.5±7.9 | 73.1±7.5 | 0.973 | 0.87 |
| DM duration, y | 8.9±6.7 | — | — | |||
| Laboratory | ||||||
| Total cholesterol | 194.4±42.2 | 194.25±41.62 | 194.62±43.98 | 200.5±30.2 | 0.481 | 0.97 |
| LDL‐C | 124.1±33.9 | 122.14±35.41 | 127.16±32.22 | 126.4±25.8 | 0.725 | 0.58 |
| HDL‐C | 45.8±11.7 | 46.00±12.30 | 45.62±11.00 | 46.5±13.3 | 0.798 | 0.90 |
| Creatinine | 0.92±0.45 | 0.83±0.16 | 1.03±0.67 | 0.84±0.16 | 0.325 | 0.16 |
| HbA1c | 7.7±1.7 | 7.64±1.60 | 7.68±1.67 | 5.2±0.3 | <0.001 | 0.91 |
| Treatment | ||||||
| Insulin | 46% | 44.4% | 48.4% | — | — | 0.84 |
| Insulin plus OAD | 98% | 97% | 100% | — | — | 0.98 |
| Calcium channel blocker | 25.4% | 25.7% | 24.9% | 22.9% | 0.437 | 0.54 |
| Beta blocker | 25.4% | 24.6% | 25.6% | 22.9% | 0.437 | 0.86 |
| ACEI/ARB | 76.4% | 78.5% | 74.3% | 52.8% | 0.010 | 0.72 |
| Statin | 52.5% | 51.5% | 53.5% | 22.4% | 0.008 | 0.74 |
ACEI indicates angiotensin‐converting enzyme inhibitors; ARB, angiotensin receptor blockers; DM, diabetes mellitus; HbA1c, hemoglobin A1c; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; OAD, oral antidiabetic medication.
In standard echocardiographic evaluation, there were no significant differences between diabetic patients and controls with respect to LV volume indexes, ejection fraction, and LV mass index; however, diastolic indexes tended to be worse in those with DM (Table 2).
Table 2.
Standard Echocardiographic Findings
| Diabetic (n=50) | Nondiabetic (n=42) | P Value | |
|---|---|---|---|
| Septal wall thickness | 1.16±0.13 | 1.12±0.13 | 0.387 |
| Posterior wall thickness, cm | 1.08±0.90 | 1.04±0.07 | 0.528 |
| LV end‐diastolic diameter, cm | 4.70±0.43 | 4.48±0.43 | 0.080 |
| LV end‐systolic diameter, cm | 2.84±0.28 | 2.75±0.36 | 0.190 |
| LV end‐diastolic volume index | 52.54±12.7 | 54.24±10.48 | 0. 245 |
| LV end‐systolic volume index | 16.07±5.52 | 17.17±5.83 | 0.359 |
| LV ejection fraction, % | 70.76±6.50 | 73.15±5.70 | 0.212 |
| LV mass index | 98.81±18.51 | 98.08±18.45 | 0.852 |
| Mitral Ee′ | 10.92±2.70 | 9.90±2.64 | 0.085 |
| Mitral E/A | 0.86±0.23 | 1.061±0.28 | 0.001 |
LV indicates left ventricular.
Impact of DM on Coronary Microvascular Functional and Structural Integrity
Patients with DM, compared with nondiabetic patients, had significantly lower CFVR (1.80±0.34 versus 2.49±0.42, P<0.001), lower BMR (3.77±0.83 versus 4.32±0.72 mm Hg/cm−1 per s−1, P=0.002), higher HMR (2.02±0.51 versus 1.68±0.39 mm Hg.s/cm, P=0.002), lower ARI, and steeper deceleration of diastolic coronary flow. In addition, compared with nondiabetic patients, APVb was significantly higher and APVh was significantly lower in diabetic patients (Table 3).
Table 3.
Effect of DM on Coronary Microcirculation
| DM (n=50) | No DM (n=42) | Mean Difference | 95% CI | P Value | |
|---|---|---|---|---|---|
| CFVR | 1.80±0.34 | 2.49±0.42 | −0.687 | −0.85 to −0.52 | <0.001 |
| APVbas | 24 (15–38) | 22 (16–40) | 0.012 | ||
| APVhyp | 45.54±12.10 | 53.65±12.64 | −8.11 | −13.52 to 2.71 | 0.004 |
| BMR | 3.77±0.83 | 4.32±0.72 | 0.26 | 0.05–0.46 | 0.002 |
| HMR | 2.02±0.51 | 1.69±0.39 | 0.332 | 0.12–0.53 | 0.002 |
| ARI | 1.74±0.55 | 2.65±0.53 | −0.906 | −1.14 to −0.66 | <0.001 |
| DDTbas | 979 (400/2506) | 1018 (809–1498) | 0.016 | ||
| DDThyp | 709.68±174.12 | 807.77±131.53 | −98.09 | 167.38–28.80 | 0.006 |
Due to nonnormal distribution, median values and minimum and maximum values were provided for APVbas and DDTbas. APVbas indicates basal average peak velocity; APVhyp, hyperemic average peak velocity; ARI, arteriole resistance index; BMR, baseline microvascular resistance; CFVR, coronary flow velocity reserve; DDTbas, baseline diastolic deceleration time; DDThyp, hyperemic diastolic deceleration time; DM, diabetes mellitus; HMR, hyperemic microvascular resistance.
After multivariate adjustment made for potential confounders (age, LV mass, presence or absence of hypertension, angiotensin‐converting enzyme inhibitor and statin usage), compared with nondiabetic controls, diabetic patients had significantly lower CFVR (1.86 versus 2.46, adjusted P=0.001) (Figure 2A), which was mainly driven by significantly reduced APVh in diabetic compared with nondiabetic patients (45.44 versus 54.51, adjusted P=0.006) (Figure 2B). In accordance with these findings, HMR was significantly higher (1.98 versus 1.70 mm Hg.s/cm, adjusted P=0.019) and ARI was significantly lower (1.82 versus 2.51, adjusted P=0.0001) in diabetic patients compared with nondiabetic patients after multivariable adjustment (Figure 2A); however, APVb value did not differ between diabetic and nondiabetic patients (24.61 versus 22.61, adjusted P=0.11) (Figure 2B). Accordingly, values for BMR (3.98 versus 4.27 mm Hg.s/cm, adjusted P=0.16) and baseline diastolic deceleration time (1004 versus 1061, adjusted P=0.363) also did not differ between diabetic and nondiabetic patients.
Figure 2.
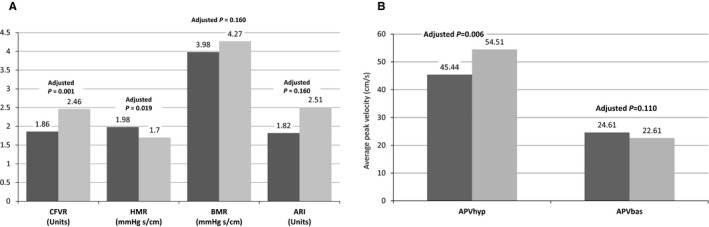
Indices of coronary microcirculation in diabetic (dark gray) and nondiabetic (light gray) patients. Comparison made after multivariate adjustment. A, Parameters used for assessing functional status of coronary microcirculation. B, Hyperemic and baseline coronary average peak velocities. APVbas indicates baseline average peak velocity; APVhyp, hyperemic average peak velocity; ARI, arteriolar resistance index; BMR, baseline microvascular resistance; CFVR, coronary flow velocity reserve; HMR, hyperemic microvascular resistance.
Four groups were constituted based on the presence or absence of DM and hypertension. In the absence of accompanying hypertension, compared with non‐DM patients (n=26), patients with DM (n=16) had significantly lower CFVR (1.93±0.48 versus 2.49±0.34, P<0.001) that was primarily driven by decreased APVh (41.75±14.49 versus 56.58±11.22, P=0.001) due to increased HMR (2.20±0.69 versus 1.57±0.38 mm Hg.s/cm, P<0.001) (Figure 3A). No differences were observed in the values for APVb (22.88±4.11 versus 21.50±5.39, P=0.353) and BMR (4.07±0.79 versus 4.34±1.06 mm Hg.s/cm, P=0.35) between the diabetic and nondiabetic patient groups in the absence of hypertension. Among the 4 groups, the mean value of CFVR was lowest (1.77±0.33) in the group composed of patients who had both DM and hypertension (Figure 3B).
Figure 3.
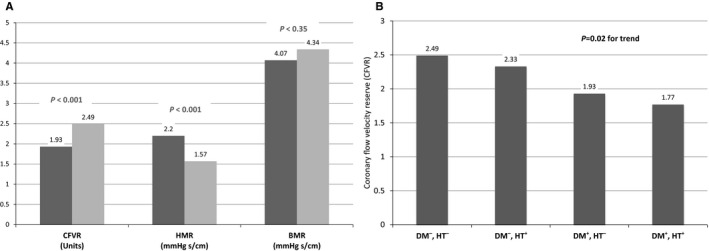
A, Comparison of coronary flow velocity reserve (CFVR) with hyperemic microvascular resistance (HMR) and baseline microvascular resistance (BMR) in nonhypertensive in diabetic (dark gray) and nondiabetic (light gray) patients. B, CFVR trend among 4 groups based on the presence and absence of diabetes mellitus (DM) and hypertension (HT).
Influence of DM Duration on Coronary Flow and Microvascular Perfusion Parameters
In this prespecified analysis, duration of DM was found to be inversely correlated with APVh (r=−0.371, P=0.008) (Figure 4A), CFVR (r=−0.378, P=0.007), HMR (r=0.292, P=0.04), and ARI (r=−0.298, P=0.034); however, there was no correlation between DM duration and APVb (r=0.03) (Figure 4B).
Figure 4.
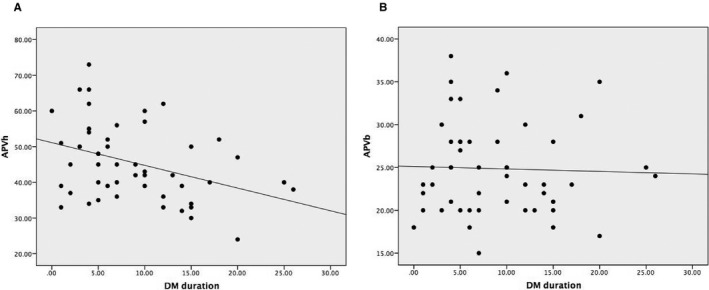
Relationship between duration of diabetes mellitus (DM) and coronary flow parameters. A, DM duration and hyperemic average peak velocity (APVh). B, DM duration and baseline average peak velocity (APVb).
Diabetic patients were then divided into 2 groups based on the duration of the disease (≥10 versus <10 years; duration of DM ≥10 years indicated long‐standing DM). In total, 22 patients were classified as having long‐standing DM, and 28 had short duration of disease (<10 years).
Short‐term DM (<10 years) versus nondiabetic patients
CFVR was significantly lower in patients with history of DM <10 years compared with nondiabetic patients. This difference was observed to be primarily driven by increased APVb (P=0.014) due to significantly decreased BMR in short‐term diabetic patients compared with nondiabetic patients (3.69±0.86 versus 4.34±0.76 mm Hg.s/cm, P=0.005) (Table 4 and Figure 5). APVh, HMR, and diastolic deceleration time were not different between short‐term DM and nondiabetic patients (Table 4 and Figure 5).
Table 4.
Microvascular Perfusion Parameters in DM Patients Based on the Duration of Disease
| DM <10 Years (n=28) | DM ≥10 Years (n=22) | Nondiabetic (n=42) | P Values for Differences | |||
|---|---|---|---|---|---|---|
| DM <10 vs ≥10 Years | DM <10 Years vs Nondiabetic | DM ≥10 Years vs Nondiabetic | ||||
| APVbas | 26.50±5.6 | 24.00±5.23 | 22.08±4.31 | 0.32 | 0.014 | 0.49 |
| APVhyp | 48.00±12.8 | 41.57±10.01 | 53.47±11.8 | 0.041 | 0.052 | <0.001 |
| CFVR | 1.89±0.37 | 1.71±0.25 | 2.43±0.42 | 0.045 | <0.001 | <0.001 |
| HMR | 1.89±0.53 | 2.13±0.42 | 1.69±0.39 | 0.16 | 0.223 | 0.002 |
| BMR | 3.69±0.86 | 3.98±0.69 | 4.34±0.76 | 0.59 | 0.005 | 0.056 |
| ARI | 1.80±0.63 | 1.67±0.44 | 2.65±0.53 | 0.707 | <0.001 | <0.001 |
| DDTbas | 1037.64±354.27 | 924.86±172.02 | 1086.80±177.27 | 0.834 | 0.476 | 0.003 |
APVbas indicates basal average peak velocity; APVhyp, hyperemic average peak velocity; ARI, arteriole resistance index; BMR, baseline microvascular resistance; CFVR, coronary flow velocity reserve; DDTbas, baseline diastolic deceleration time; DM, diabetes mellitus; HMR, hyperemic microvascular resistance.
Figure 5.
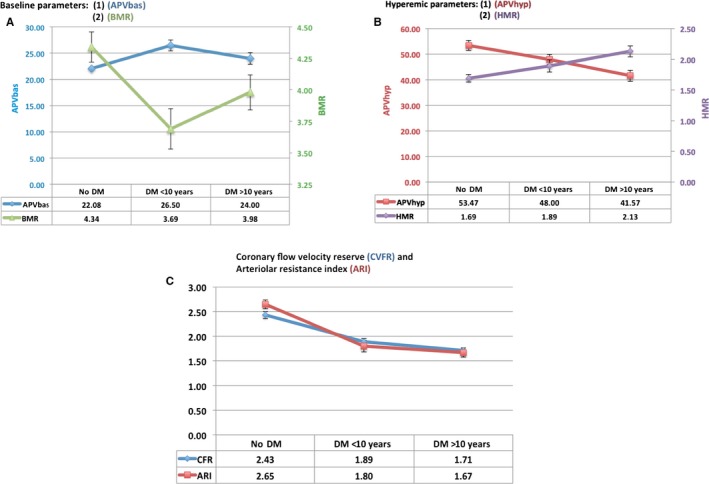
Average baseline and hyperemic coronary flow and microvascular resistance parameters with standard error of the mean in patients with short‐ and long‐duration diabetes mellitus and in nondiabetic controls. A, Baseline coronary flow velocity and microvascular resistance values. B, Hyperemic coronary flow velocity and microvascular resistance values. C, Comparison of the coronary flow velocity reserve and arteriolar resistance index values in nondiabetic and diabetic patients stratified according to duration of disease (≥10 versus <10 years). APVbas indicates baseline average peak velocity; APVhyp, hyperemic average peak velocity; BMR, baseline microvascular resistance; DM, diabetes mellitus; HMR, hyperemic microvascular resistance.
Long‐standing DM (≥10 years) versus nondiabetic patients
In contrast, there were no significant differences between patients with long‐standing DM and nondiabetic patients with respect to APVb and BMR; however, compared with nondiabetic patients, HMR was found to be significantly elevated in those with long‐standing DM (2.13±0.42 versus 1.69±0.39, P=0.002). Significantly decreased APVh (41.57±10.01 versus 53.47±11.8, P<0.001) was consistently observed in the long‐standing DM group compared with the nondiabetic group. Diastolic deceleration time was also significantly faster in those with long‐term DM than in nondiabetic patients (Table 4 and Figure 5).
Short‐duration versus long‐standing DM
Demographic and laboratory findings and treatment used did not differ between patients with long‐standing DM and those with short duration of disease (Table 1). Patients with history of DM <10 years had significantly higher CFVR (1.89±0.37 versus 1.71±0.25, P=0.045) than patients with long‐standing DM. This difference seemed to be driven by significantly decreased APVh in the long‐term DM group (Table 4 and Figure 5). Although it did not reach statistical significance (P=0.16), HMR was also higher in long‐standing DM patients than in those with short duration of disease. In contrast, there were no differences between these 2 groups with respect to APVb, BMR, and ARI (Table 4 and Figure 5).
In observing nondiabetic controls and long‐standing DM patients, a progressive increase in HMR was noted over time, along with a corresponding progressive decrease in APVh (Figure 5B).
Effect of DM duration on microvascular parameters in the absence of LVH
In addition to statistical adjustment controlling for the presence and absence of LVH, coronary flow, and microvascular resistance, comparisons performed between diabetic patients (n=37) and control participants (n=17) were repeated selectively in the absence of LVH. In this analysis, again, CFVR was found to be significantly lower in diabetic patients than in nondiabetic controls (1.87±0.36 versus 2.43±0.30, P<0.001).
In the absence of LVH, compared with nondiabetic controls, significantly increased APVb (25.69±4.88 versus 21.57±3.57, P=0.02) due to decreased BMR (3.72±0.56 versus 4.21±0.58 mm Hg.s/cm, P=0.04) was observed in patients with short‐duration DM (n=17). Nevertheless, there were no differences in APVh (49.69±11.47 versus 52.50±11.85, P=0.49) and HMR (1.93±0.57 versus 1.69±0.42 mm Hg.s/cm, P=0.22) between patients with short duration of DM and nondiabetic controls.
In contrast, APVh (37.12±5.13 versus 52.50±11.85, P=0.002), due to increased HMR (2.21±0.37 versus 1.69±0.42 mm Hg.s/cm, P=0.009), was significantly lower in patients with long‐standing DM (n=20) compared with nondiabetic controls in the absence of LVH. APVb, however, did not differ between long‐standing DM patients and nondiabetic controls (21.00±2.13 versus 21.57±3.57, P=0.65).
Discussion
The findings of this study revealed that reduced CFVR in DM, implying coronary microvascular impairment, was predominantly driven by the combination of decreased hyperemic coronary flow velocity and increased HMR. In addition, the underlying mechanism of the impairment in coronary microvascular function in DM was observed to change in a time‐dependent manner. In patients with short duration of DM (<10 years), impaired CFVR was primarily driven by increased baseline coronary flow velocity accompanied by decreased BMR, suggesting DM‐induced autoregulatory dysfunction and/or increased metabolic needs. Alternatively, significantly reduced hyperemic coronary flow velocity accompanied by increased HMR, implying disturbed microvascular vasodilatory function, seemed to be the prevailing mechanism behind impaired CFVR in patients with long‐standing DM (≥10 years). Importantly, these findings were shown to be independent of the presence or absence of hypertension or LVH. Progressively impaired arteriolar dilatory reserve, as measured by the ARI, resulting from either decreased BMR or increased HMR seemed to be the predominant mechanism underlying coronary microvascular abnormalities in DM. Confirmation of the result of this hypothesis‐generating study will require a longitudinal trial including a larger number of patients.
Our findings suggest that there may be a bimodal pattern in involvement of coronary microcirculation in diabetic patients. In patients with short‐term DM, the combination of increased baseline coronary flow velocity and decreased BMR despite normal perfusion pressure distinguishes DM‐induced impaired autoregulation and/or adaptive response to altered myocardial energy metabolism in the diabetic heart as the initial functional insult in diabetic microcirculation. In the relatively early phase of the disease, DM‐induced defective coronary autoregulation20 may fail to adapt distal vascular tone to regulate coronary flow, and baseline coronary flow may have increased disproportionately.11, 17, 21 In addition, in the diabetic heart, altered myocardial energy metabolism due to increased baseline oxygen requirement20, 21, 22 can also be physiologically coupled with increased coronary flow at rest. Consequently, in the first decade, DM‐induced impaired adaptive autoregulatory response of coronary resistance vessels20 may cause persistently increased myocardial blood flow despite adequately perfused myocardium and may expose coronary microcirculation to a constant high‐flow situation.
Decrease in CFVR due to decreased BMR and increased coronary flow velocity at rest has also been shown in different settings. First, impaired CFVR after angioplasty and stenting was demonstrated to be related to increased baseline coronary flow velocity and decreased BMR.23, 24, 25 Second, a recently published study elegantly demonstrated that in patients undergoing percutaneous intervention, primary determinants of impaired CFVR measured in reference vessels without flow‐limiting stenosis were increased baseline flow in combination with decreased BMR, implying preexisting disturbance of coronary autoregulation in adequately perfused myocardium.26
In the long term, it seems that DM leads to a significant increase in HMR in combination with reduced hyperemic coronary flow velocity. These findings suggest that disturbed vasodilatory function of coronary microcirculation may be the prevailing mechanism behind impaired microcirculation as those with the disease age. Significantly faster deceleration of the diastolic coronary flow velocity (diastolic deceleration time), which reflects an impeding effect of increased microvascular resistance on diastolic coronary flow due to structural microvascular damage,27 was observed consistently only in patients with long‐standing DM compared with nondiabetic patients. Consequently, initial functional impairment in coronary autoregulatory function detected in patients with short duration of disease seemed to evolve into structural impairment of the coronary microcirculation in the long term, as shown by increased HMR and decreased hyperemic coronary flow velocity.
From a pathophysiological point of view, on the temporal scale, initial functional impairment of the autoregulatory mechanism leading to an inappropriately increased baseline coronary and microvascular flow may induce structural microvascular remodeling or rarefaction in the related microvascular territory exposed by continuous insult of increased blood flow. This structurally remodeled microcirculation may subsequently pave the way for an increase in HMR and consequent decrease in hyperemic flow in the long term. In line with this evolution, duration of DM was found to be inversely correlated with hyperemic, but not baseline, coronary flow velocity. Similarly, capillary rarefaction in kidney,28, 29 which is the hallmark of diabetic nephropathy, is known to be linked with glomerular hyperfiltration or hyperperfusion that occurs in early DM.30, 31 These pathophysiological processes may also share common pathways in diabetic heart and kidney.
From a mechanistic point of view, our findings indicating that increased baseline coronary flow in early DM would be thought, contradictorily, to be associated with decreased risk of coronary atherosclerosis in diabetic patients via increasing baseline shear stress. It is well known, however, that diabetic patients have a high and accelerated atherosclerosis risk beginning from the early stages of the disease. In general, in the coronary tree, distal narrower epicardial arteries are relatively spared from atherosclerotic processes mainly because of high shear stress in these segments. Nevertheless, a pattern of diabetic atherosclerosis is characterized by widespread involvement of the coronary arteries without sparing distal segments. This involvement pattern suggests that shear stress–related vascular‐protection mechanisms may not be working properly or may be defective in DM. Reports indicating impaired flow‐mediated dilatation in diabetic patients also support this thought.32, 33, 34 To this end, the protective effect of shear stress on the vessel wall may well be stimulated predominantly by peak shear stress episodes induced by peak hyperemic flow velocities rather than by baseline shear stress. Accordingly, physical activity covering only ≈2% of daily routine is known to be enough to generate substantial functional and structural vascular improvements. Consequently, in diabetic patients, increased baseline shear stress at early stages induced by increased baseline flow velocity may not protect patients from developing diffuse epicardial atherosclerosis at later stages. In addition, progressive decrease in vasodilatory reserve, which can potentially lead to a reduction in difference between hyperemic and baseline peak shear stress, may also contribute to acceleration of macrovascular complications. In diabetic patients, rigorous physical activity—even more intense than for nondiabetic patients—can be a pivotal measure to prevent DM‐specific micro‐ and macrovascular complications.
Without being stratified by duration of disease, our findings indicating impaired CFVR based predominantly on the disturbed vasodilatory reserve in diabetic patients were consistent with most previous studies.4, 5, 6, 7, 9 Furthermore, this finding remained consistent not only after statistical adjustment to control potential confounders but also in comparisons made between purely diabetic patients without hypertension and/or LVH and matched nondiabetic controls. In the literature, only 2 studies reported a substantial contribution of increased baseline coronary flow velocity to impaired CFVR in DM.3, 17 Moreover, most studies did not control or exclude the possible substantial contribution of concurrently presented hypertension and LVH to coronary blood flow.3, 17 Only in 1 study, which included diabetic but not hypertensive patients, decreased hyperemic flow was shown to be the prevailing mechanism behind reduced CFVR in DM.5 In the current study, in addition to statistical adjustment, analyses comparing flow and resistance data between diabetic and nondiabetic groups were also performed in the absence of concurrent hypertension and LVH. In both comparisons, presence of DM was consistently associated with decreased hyperemic coronary flow velocity and increased HMR, implying disturbed vasodilatory reserve as the prevailing mechanism behind microvascular dysfunction.
Limitations
Some limitations of this study deserve mention. First, the population of this study can be thought to be relatively limited; however, the new noninvasive method that we used in assessing coronary microvascular resistance enabled us to scrutinize coronary flow velocity patterns in combination with microvascular resistance values in a reasonable number of patients who would be quite difficult to reach using invasive methods.
Second, in the present study, no intracoronary pressure measurements were performed, and coronary microvascular resistance was calculated using noninvasive parameters, which are quite comparable to invasive indices in assessing microvascular integrity.35 Nevertheless, we included only patients with angiographically documented normal left anterior descending artery, in whom mean systemic blood pressure measured by sphygmomanometer is expected to be almost equal to the mean intracoronary pressure. In addition, echocardiographic coronary flow analyses were performed on the day following coronary angiography demonstrating normal conduit artery.
Third, measurement of dipyridamole‐derived CFVR does not provide satisfactory or sensitive information about overall human coronary microvascular function. Assessment of myocardial flow reserve using positron emission tomography, which allows overall assessment of the coronary microcirculation, would have provided more conclusive results; however, its relatively limited availability, ionizing radiation exposure, and cost might limit its widespread use for this purpose.
Finally, compared with invasive measurement of coronary flow velocity by Doppler wire, noninvasive imaging of coronary blood flow envelopes by transthoracic Doppler echocardiography is less operator dependent and is a reliable technique with a high success rate but is mostly limited to interrogation of left anterior descending artery flow. Consequently, our results were derived only from interrogation of microvascular territory supplied by the left anterior descending artery.
Future Directions
Diffuse subclinical (occult) atherosclerosis is associated with decreased coronary hyperemic flow,36 as we observed in our diabetic population with normal epicardial arteries. Consequently, microvascular changes—initially functional and subsequently structural—may be a precursor of overt coronary macrovascular pathologies that may develop at later stages of DM. In patients with DM, therapeutic targeting of functional coronary microvascular dysfunction at early stages may prevent both structural microvascular impairment and potentially interlinked macrovascular complications at later stages. In diabetic patients with angiograms showing normal epicardial coronary arteries, assessment of microvascular status using noninvasive methods can easily identify the presence and extent of microvascular dysfunction and may help identify high‐risk diabetic patients in timely manner.
Conclusions
As underlying mechanisms, either altered or disturbed coronary autoregulation and impaired microvascular vasodilatory function contribute to DM‐related coronary microvascular impairment in a time‐dependent manner. DM‐induced early functional microvascular autoregulatory impairment resulting in increased coronary flow at rest seems to evolve into structural microvascular impairment in the initially overperfused microvascular territory that is presented with reduced hyperemic flow and increased microvascular resistance (disturbed vasodilatory function) at later stages of the disease.
Sources of Funding
This study was partly supported by Turkish Academy of Sciences (Dr Sezer).
Disclosures
None.
(J Am Heart Assoc. 2016;5:e003995 doi: 10.1161/JAHA.116.003995)
References
- 1. Melikian N, Kearney MT, Thomas MR, De Bruyne B, Shah AM, MacCarthy PA. A simple thermodilution technique to assess coronary endothelium dependent microvascular function in humans: validation and comparison with coronary flow reserve. Eur Heart J. 2007;28:2188–2194. [DOI] [PubMed] [Google Scholar]
- 2. Nitenberg A, Valensi P, Sachs R, Dalim M, Aptecar E, Attali JR. Impairment of coronary vascular reserve and ACh‐induced coronary vasodilation in diabetic patients with angiographically normal coronary arteries and normal ventricular systolic function. Diabetes. 1993;32:1017–1023. [DOI] [PubMed] [Google Scholar]
- 3. Picchi A, Limbruno U, Focardi M, Cortese B, Micheli A, Boschi L, Severi S, De Caterina R. Increased basal coronary blood flow as a cause of reduced coronary flow reserve in diabetic patients. Am J Physiol Heart Circ Physiol. 2011;301:H2279–H2284. [DOI] [PubMed] [Google Scholar]
- 4. Nahser PJ Jr, Brown RE, Oskarsson H, Winnifold MD, Rossen JD. Maximal coronary flow reserve and metabolic coronary vasodilation in patients with diabetes mellitus. Circulation. 1995;91:635–640. [DOI] [PubMed] [Google Scholar]
- 5. Yokoyama I, Momomura S, Ohtake T, Yonekura K, Nishikawa J, Sasaki Y, Omata M. Reduced myocardial flow reserve in non‐insulin dependent diabetes mellitus. J Am Coll Cardiol. 1997;30:1472–1477. [DOI] [PubMed] [Google Scholar]
- 6. Yokoyama I, Yonekura K, Ohtake T, Yang W, Shin WS, Yamada N, Ohtomo K, Nagai R. Coronary microangiopathy in type 2 diabetic patients: relation to glycemic control, sex, and microvascular angina rather than to coronary artery disease. J Nucl Med. 2000;41:978–985. [PubMed] [Google Scholar]
- 7. Pitkanen OP, Nuutila P, Raitakari OT, Ronnemaa T, Koskinen PJ, Iida H, Lehtimaki TJ, Laine HK, Takala T, Viikari JS, Knuuti J. Coronary flow reserve is reduced in young men with IDDM. Diabetes. 1998;47:248–254. [DOI] [PubMed] [Google Scholar]
- 8. Atar A, Altuner TK, Bozbas H, Korkmaz ME. Coronary flow reserve in patients with diabetes mellitus and pre‐diabetes. Echocardiography. 2012;29:634–640. [DOI] [PubMed] [Google Scholar]
- 9. Strauer BE, Motz W, Vogt M, Schwarzkopff B. Evidence for reduced coronary flow reserve in patients with insulin‐dependent diabetes. A possible cause for diabetic heart disease in man. Exp Clin Endocrinol Diabetes. 1997;105:15–20. [DOI] [PubMed] [Google Scholar]
- 10. Tasca C, Stefaneanu L, Vasilescu C. The myocardial microangiopathy in human and experimental diabetes mellitus: a microscopic, ultra‐structural, morphometric and computer‐assisted symbolic‐logic analysis. Endocrinologie. 1986;24:59–69. [PubMed] [Google Scholar]
- 11. Stanley WC, Lopaschuk GD, McCormack JG. Regulation of energy substrate metabolism in the diabetic heart. Cardiovasc Res. 1997;34:25–33. [DOI] [PubMed] [Google Scholar]
- 12. Shivu GN, Phan TT, Abozguia K, Ahmed I, Wagenmakers A, Henning A, Narendran P, Stevens M, Frenneaux M. Relationship between coronary microvascular dysfunction and cardiac energetics impairment in type 1 diabetes mellitus. Circulation. 2010;121:1209–1215. [DOI] [PubMed] [Google Scholar]
- 13. Yonaha O, Matsubara T, Naruse K, Ishii H, Murohara T, Nakamura J, Amano T, Hotta N. Effects of reduced coronary flow reserve on left ventricular function in type 2 diabetes. Diabetes Res Clin Pract. 2008;82:98–103. [DOI] [PubMed] [Google Scholar]
- 14. Kawata T, Daimon M, Miyazaki S, Ichikawa R, Maruyama M, Chiang S, Ito C, Sato F, Watada H, Daida H. Coronary microvascular function is independently associated with left ventricular filling pressure in patients with type 2 diabetes mellitus. Cardiovasc Diabetol. 2015;14:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Murthy VL, Naya M, Foster CR, Gaber M, Hainer J, Klein J, Dorbola S, Blankstein S, Di Carli MF. Association between coronary vascular dysfunction and cardiac mortality in patients with and without diabetes mellitus. Circulation. 2012;126:1858–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cortigiani L, Rigo F, Gheraradi S, Galderisi M, Bovenzi F, Sicari R. Prognostic meaning of coronary microvascular disease in type 2 diabetes mellitus: a transthoracic Doppler echocardiographic study. J Am Soc Echocardiogr. 2014;27:742–748. [DOI] [PubMed] [Google Scholar]
- 17. Meyer C, Schwaiger M. Myocardial blood flow and glucose metabolism in diabetes mellitus. Am J Cardiol. 1997;80:94A–101A. [DOI] [PubMed] [Google Scholar]
- 18. Chamuleau SA, Siebes M, Meuwissen M, Koch KT, Spaan JA, Piek JJ. Association between coronary lesion severity and distal microvascular resistance in patients with coronary artery disease. Am J Physiol Heart Circ Physiol. 2003;285:H2194–H2200. [DOI] [PubMed] [Google Scholar]
- 19. Fowler MJ. Microvascular and macrovascular complications of diabetes. Clin Diabetes. 2008;26:77–82. [Google Scholar]
- 20. Tune JD, Yeh C, Setty S, Zong P, Downey HF. Coronary blood flow control is impaired at rest and during exercise in conscious diabetic dogs. Basic Res Cardiol. 2002;97:248–257. [DOI] [PubMed] [Google Scholar]
- 21. Diamant M, Lamb HJ, Groeneveld Y, Endert EL, Smit JW, Bax JJ, Romijn JA, de Roos A, Radder JK. Diastolic dysfunction is associated with altered myocardial metabolism in asymptomatic normotensive patients with well‐controlled type 2 diabetes mellitus. J Am Coll Cardiol. 2003;42:328–335. [DOI] [PubMed] [Google Scholar]
- 22. Taegtmeyer H, McNulty P, Young ME. Adaptation and maladaptation of the heart in diabetes: part I: general concepts. Circulation. 2002;105:1727–1733. [DOI] [PubMed] [Google Scholar]
- 23. van Liebergen RA, Piek JJ, Koch KT, de Winter RJ, Lie KI. Immediate and long‐term effect of balloon angioplasty or stent implantation on the absolute and relative coronary blood flow velocity reserve. Circulation. 1998;98:2133–2140. [DOI] [PubMed] [Google Scholar]
- 24. Nanto S, Kodama K, Hori M, Mishima M, Hirayama A, Inoue M, Kamada T. Temporal increase in resting coronary blood flow causes an impairment of coronary flow reserve after coronary angioplasty. Am Heart J. 1992;123:28–36. [DOI] [PubMed] [Google Scholar]
- 25. Kern MJ, Deligonul U, Vandormael M, Labovitz A, Gudipati CV, Gabliani G, Bodet J, Shah Y, Kennedy HL. Impaired coronary vasodilator reserve in the immediate postcoronary angioplasty period: analysis of coronary artery flow velocity indexes and regional cardiac venous efflux. J Am Coll Cardiol. 1989;13:860–872. [DOI] [PubMed] [Google Scholar]
- 26. Van de Hoef TP, Bax M, Damman P, Delewi R, Hassel MECJ, Piek MA, Chamuleau SAJ, Voskuil M, van Eck‐Smit BL, Verberne HJ, Henriques JP, Koch KT, de Winter RJ, Tijssen JG, Piek JJ, Meuwissen M. Impaired coronary autoregulation is associated with long‐term fatal events in patients with stable coronary artery disease. Circ Cardiovasc Interv. 2013;6:329–335. [DOI] [PubMed] [Google Scholar]
- 27. Claessen B, Bax M, Delewi R, Meuvissen M, Henriques J, Piek JJ. The Doppler flow wire in acute myocardial infarction. Heart. 2010;96:631–635. [DOI] [PubMed] [Google Scholar]
- 28. Rattigan S, Richards SM, Keske MA. Microvascular contributions to insulin resistance. Diabetes. 2013;62:343–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mayer G. Capillary rarefaction, hypoxia, VEGF and angiogenesis in chronic renal disease. Nephrol Dial Transplant. 2011;26:1132–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Christensen JA, Biwer E, Bohle A. Hyperperfusion injury of the human kidney in different glomerular diseases. Am J Nephrol. 1988;8:179–186. [DOI] [PubMed] [Google Scholar]
- 31. Nangaku M. Chronic hypoxia and tubulointerstitial injury: a final common pathway to end‐stage renal failure. J Am Soc Nephrol. 2006;17:17–25. [DOI] [PubMed] [Google Scholar]
- 32. Lochart CJ, Agnew CE, McCann A, Hamilton PK, Quinn CE, McCall DO, Plumb RD, McClenaghan VC, McGivern RC, Harbinson MT, McVeigh GE. Impaired flow‐mediated dilatation response in uncomplicated Type 1 diabetes mellitus: influence of shear stress and microvascular reactivity. Clin Sci. 2011;121:129–139. [DOI] [PubMed] [Google Scholar]
- 33. Ito H, Nakashima M, Meguro K, Furuwaka H, Yamashita H, Takaki A, Yukowa C, Omoto T, Shinozaki M, Nishio S, Abe M, Antoku S, Mifune M, Togane M. Flow mediated dilatation is reduced with the progressive stages of glomerular filtration rate and albuminuria in Type 2 diabetic patients without coronary heart disease. J Diabetes Res. 2015;728127:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Henry RM, Ferreira I, Kostense PJ, Dekker JM, Nijpels G, Heine RJ, Kamp O, Bouter LM, Stehouwer CD. Type 2 diabetes is associated with impaired endothelium‐dependent, flow‐mediated dilation, but impaired glucose metabolism is not; The Hoorn Study. Atherosclerosis. 2004;174:49–56. [DOI] [PubMed] [Google Scholar]
- 35. Okcular I, Sezer M, Aslanger E, Cimen A, Umman B, Nisanci Y, Umman S. The accuracy of deceleration time of diastolic coronary flow measured by transthoracic echocardiography in predicting long‐term left ventricular infarct size and function after reperfused myocardial infarction. Eur J Echocardiogr. 2010;11:823–828. [DOI] [PubMed] [Google Scholar]
- 36. Gould KL, Kirkeeide RL, Buchi M. Coronary flow reserve as a physiologic measure of stenosis severity. J Am Coll Cardiol. 1990;15:459–474. [DOI] [PubMed] [Google Scholar]


