Abstract
Background
Even though clinical data support a relation between ischemic stroke and venous thromboembolism (VTE), the strength and time dependence of the association remain to be settled at the population level. We therefore aimed to investigate the association between ischemic stroke and VTE in a prospective population‐based cohort.
Methods and Results
Participants (n=30 002) were recruited from 3 surveys of the Tromsø study (conducted in 1994–1995, 2001, and 2007–2008) and followed through 2010. All incident events of ischemic stroke and VTE during follow‐up were recorded. Cox‐regression models with age as time scale and ischemic stroke as a time‐dependent variable were used to calculate hazard ratios (HR) of VTE adjusted for cardiovascular risk factors. During a median follow‐up time of 15.7 years, 1360 participants developed ischemic stroke and 722 had a VTE. The risk of VTE was highest the first month (HR 19.7; 95% CI, 10.1–38.5) and from 1 to 3 months after the stroke (HR 10.6; 95% CI 5.0–22.5), but declined rapidly thereafter. The risk estimates were approximately the same for deep vein thrombosis (HR 19.1; 95% CI, 7.8–38.5), and pulmonary embolism (HR 20.2; 95% CI, 7.4–55.1). Stroke was associated with higher risk for provoked (HR 22.6; 95% CI, 12.5–40.9) than unprovoked VTE (HR 7.4; 95% CI, 2.7–20.1) the first 3 months.
Conclusions
The risk of VTE increased during the first 3 months after an ischemic stroke. The particularly high risk of provoked VTE suggests that additional predisposing factors, such as immobilization, potentiate the VTE risk in patients with ischemic stroke.
Keywords: epidemiology, ischemic stroke, risk factor, venous thromboembolism
Subject Categories: Ischemic Stroke, Epidemiology, Risk Factors, Thrombosis
Introduction
Ischemic stroke is a major challenge to public health and healthcare systems due to frequent hospitalizations, frequent medical complications, disability, dependency, nursing home confinement, and a high mortality rate.1, 2, 3 Even though clinically overt pulmonary embolism (PE) occurs in only 1% of stroke patients during the first 14 days after an acute stroke,4, 5, 6 PE may account for up to 25% to 50% of deaths after acute stroke.6, 7, 8
Venous thromboembolism (VTE), a collective term for deep vein thrombosis (DVT) and PE, is a common disease with serious short‐ and long‐term complications, such as development of the post‐thrombotic syndrome after a DVT, or death due to circulatory collapse secondary to PE.9, 10 VTE is a multifactorial disease, and advancing age and obesity are recognized as shared atherosclerotic risk factors for VTE and ischemic stroke.11 In addition, immobilization, particularly in an in‐hospital setting, is associated with high risk of VTE.12, 13, 14, 15 Therefore, neurological deficits entailing immobilization and other medical complications secondary to acute ischemic stroke may predispose for VTE.2, 16, 17
Several randomized trials including selected patients with acute ischemic stroke have assessed the risk of symptomatic VTE in patients without and with antithrombotic treatment.4, 5 Data from a meta‐analysis displayed that the incidence of asymptomatic and symptomatic DVT was 17% among 1186 patients with stroke, whereas the incidence of symptomatic PE was 1.0% among 10 997 patients who did not receive antithrombotic therapy during follow‐up (controls).5 However, limited data exist regarding the association between ischemic stroke and risk of VTE in the general population. A registry‐based case–control study recruited from the general population in Denmark revealed that patients with a history of stroke had a short‐term 4.4‐fold increased risk of subsequent VTE during the first 3 months after stroke.18 Results from registry‐based studies should, however, be interpreted with caution due to the lack of validation of exposure and outcomes and inability to adjust for obvious confounders such as body mass index (BMI).
The aim of the study was to investigate the overall and time‐dependent risk of VTE by ischemic stroke in a population‐based cohort with validated information on exposure (ischemic stroke), end point (VTE), and potential confounders.
Methods
Study Population
The Tromsø Study is a single‐center, prospective, population‐based study, with repeated health surveys of the inhabitants of Tromsø, Norway. Study participants were recruited from the fourth, fifth, and sixth survey of the Tromsø Study, conducted in 1994–1995, 2001, and 2007–2008, respectively. The overall attendance rates were high: 77% in the fourth, 78% in the fifth, and 66% in the sixth survey. In total, 30 586 unique participants aged 25 to 97 years took part in at least 1 of the surveys, and of these, 21 529 subjects participated in 2 or all 3 surveys. Participants who did not consent to medical research (n=225) and participants not officially registered as inhabitants of the municipality of Tromsø at date of study enrollment (n=47) were excluded. Furthermore, participants with a history of VTE (n=78) or ischemic stroke (n=234) were excluded. Consequently, 30 002 participants were included in the study, and followed from the date of enrollment to the end of follow‐up, December 31, 2010 (Figure 1). The regional committee for medical and health research ethics in North Norway approved the study, and all participants gave their informed written consent.
Figure 1.
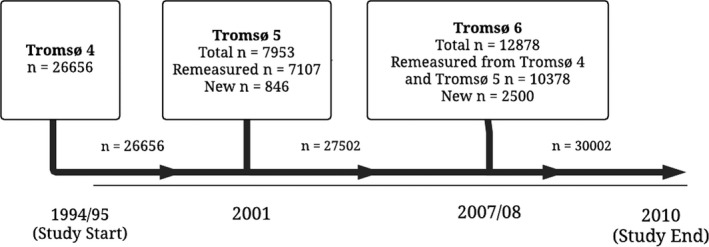
Inclusion of study participants from the fourth (1994–1995), fifth (2001–2002), and sixth (2007–2008) surveys of the Tromsø Study.
Baseline Measurements
Information about the study participants was collected by physical examinations, blood samples, and self‐administered questionnaires at each survey. Systolic and diastolic blood pressures were measured 3 three times with 1‐minute intervals with an automatic device (Dinamap Vital Signs Monitor, 1846; Critikon Inc, Tampa, FL) with participants in a sitting position after 2 minutes of rest, and defined as the mean of the last 2 readings. Nonfasting blood samples were collected from an antecubital vein, serum was prepared by centrifugation after 1‐hour respite at room temperature and analyzed at the Department of Clinical Chemistry, University Hospital of North Norway, Tromsø, Norway. Serum total cholesterol was analyzed by an enzymatic colorimetric method using a commercially available kit (CHOD‐PAP; Boehringer‐Mannheim, Mannheim, Germany). Serum high‐density lipoprotein cholesterol was measured after precipitation of lower‐density lipoproteins with heparin and manganese chloride. Height and weight were measured with participants wearing light clothes and no shoes. BMI was calculated as weight in kilograms divided by the square of height in meters (kg/m2). Obesity (BMI ≥30 kg/m2) was classified according to the World Health Organization definition.19 Hypertension was classified as mean systolic blood pressure ≥140 mm Hg, mean diastolic blood pressure ≥90 mm Hg, or self‐reported use of blood pressure–lowering drugs. Hypercholesterolemia was classified as total serum cholesterol ≥6.5 mmol/L or self‐reported use of lipid‐lowering drugs. Information on family history of myocardial infarction, diabetes mellitus, physical activity, and education level was collected from a self‐administered questionnaire.
Assessment of Ischemic Stroke
Ischemic stroke was defined according to the World Health Organization definition when computed tomography or magnetic resonance imaging scans or autopsy had ruled out brain hemorrhage.20 An independent end‐point committee performed validation of hospitalized and out‐of‐hospital events of ischemic stroke based on data from hospital and out‐of‐hospital journals, autopsy records, and death certificates. The Norwegian national 11‐digit identification number allowed linkage to national and local diagnosis registries. Cases of possible incident ischemic stroke were identified by linkage to the hospital discharge diagnosis registry at the University Hospital of North Norway with a broad search for the International Classification of Diseases (ICD), 9th Revision codes 430 to 438 in the period 1994 to 1998, and thereafter for the ICD, 10th Revision codes I60 to I69. Manual and/or electronic text searches were performed in paper versions (used until 2001) and digital versions of hospital records for notes on ischemic stroke in all participants with 1 or more of these diagnoses for case validation.
Assessment of VTE
All incident VTE events during follow‐up were identified by searching the hospital discharge diagnosis registry, the autopsy registry, and the radiology procedure registry at the University Hospital of North Norway as previously described.21 The University Hospital of North Norway is the only hospital in the region, and all diagnostic radiology and hospital care is provided exclusively by this hospital. The medical record for each potential case of VTE was reviewed by trained personnel, and a VTE event was considered verified and recorded when presence of clinical signs and symptoms of DVT or pulmonary embolism were combined with objective confirmation tests (by compression ultrasonography, venography, spiral computed tomography, perfusion–ventilation scan, pulmonary angiography, autopsy), and resulted in a VTE diagnosis that required treatment, as previously described in detail.21 VTE cases from the autopsy registry were recorded when the death certificate indicated VTE as cause of death or a significant condition associated with death. The VTE events were classified as provoked and unprovoked, depending on the presence of provoking factors at the time of diagnosis. Provoking factors were recent surgery or trauma within the previous 8 weeks, acute medical conditions (acute myocardial infarction, ischemic stroke, or major infectious disease), active cancer, immobilization (bed rest >3 days, wheelchair use, or long‐distance travel exceeding 4 hours within the last 14 days prior to the event) or any other factors described by a physician in the medical record (eg, intravascular catheter).
Statistical Analysis
Participants who developed ischemic stroke during the study period contributed with nonexposed person‐time from the inclusion date to the date of a diagnosis of ischemic stroke, and then with exposed person‐time from the date of ischemic stroke onwards. For each participant, nonexposed and exposed person‐years were counted from the date of enrollment to the date of an incident diagnosis of VTE, the date the participant died or moved from Tromsø, or until the end of the study period, December 31, 2010, whichever came first. Participants who died or moved from the municipality during follow‐up were censored at the date of death or migration.
Statistical analyses were performed using STATA version 14.0 (Stata Corporation, College Station, TX). Crude incidence rates (IR) of VTE were calculated and expressed as number of events per 1000 person‐years at risk. Cox proportional hazards regression models were used to calculate hazard ratios (HR) with 95% CI of VTE, DVT, and PE after ischemic stroke. Age was used as time scale in the Cox model, with the age of the participants at study enrollment defined as entry time, and the age at the VTE event or censoring event (ie, death, migration, or the date of study end) defined as exit time. Ischemic stroke was included as a time‐dependent covariate in the Cox model. Therefore, participants who developed ischemic stroke during follow‐up contributed with person‐years in both the unexposed and exposed group (ie, unexposed person‐years from baseline inclusion to stroke and exposed person‐years from stroke to end of follow‐up). In those who participated in several surveys, information on potential confounders was updated at each survey. HRs for VTE were estimated with 3 different models. The first model was adjusted for age (as time scale) and sex, while the second model was additionally adjusted for BMI. Model 3 was adjusted for age (as time scale), sex, BMI, diabetes mellitus, smoking, systolic blood pressure, high‐density lipoprotein cholesterol, physical activity, and education. The proportional hazard assumption was tested using Schoenfeld residuals and found not violated. Statistical interactions between ischemic stroke and sex were tested by including cross‐product terms in the proportional hazards models, and no interactions were found. Finally, 1‐Kaplan–Meier curves were estimated to visualize the cumulative incidence of VTE over time in subjects without and with incident ischemic stroke.
Results
During a median follow‐up of 15.7 years, 1360 (4.5%) subjects developed ischemic stroke and 722 (2.4%) subjects developed VTE. Baseline characteristics of the study participants are shown in Table 1. The mean age and BMI, as well as the proportions of men and subjects with hypertension and hypercholesterolemia were higher in stroke patients than in those without stroke (Table 1).
Table 1.
Baseline Characteristics of Participants Without and With Ischemic Stroke (n=30 002)
| No Ischemic Stroke (n=28 642) | Ischemic Stroke (n=1360) | |
|---|---|---|
| Age, y | 46±14 | 63±13 |
| Sex (male) | 47.1 (13 497) | 54.0 (734) |
| BMI, kg/m2 | 25.3±3.9 | 26.6±4.1 |
| Total cholesterol, mmol/L | 5.94±1.29 | 6.74±1.30 |
| HDL cholesterol, mmol/L | 1.49±0.41 | 1.47±0.42 |
| Triglycerides, mmol/L | 1.53±1.04 | 1.84±1.13 |
| Systolic blood pressure, mm Hg | 133±20 | 153±25 |
| Diastolic blood pressure, mm Hg | 77±12 | 87±14 |
| Hypertensiona | 32.7 (9380) | 72.6 (988) |
| Hypercholesterolemiab | 31.8 (9110) | 55.2 (752) |
| Smokingc | 35.7 (10 247) | 34.0 (463) |
| Physical activityd | 32.9 (9411) | 19.5 (265) |
| Educatione | 28.3 (8116) | 14.6 (198) |
| Self‐reported diabetes mellitus | 1.6 (468) | 6.0 (82) |
The Tromsø Study 1994–2010. Values are % (n) or mean±SD. BMI indicates body mass index; HDL, high‐density lipoprotein.
Mean systolic/diastolic blood pressure ≥140/≥90 mm Hg, use of antihypertensives, or self‐reported hypertension.
Total cholesterol ≥6.5 mmol/L, use of lipid‐lowering drugs, or self‐reported hypercholesterolemia.
Self‐reported daily smoking, yes/no.
≥1 hours of moderate or hard physical activity per week, yes/no.
>10 years of education.
Characteristics of the VTE events without and with ischemic stroke with regard to anatomical localization and predisposing factors are shown in Table 2. In total, 57 of the 722 VTEs occurred in patients with an ischemic stroke. VTE patients with an ischemic stroke had a higher proportion of provoked events compared to those without stroke. Moreover, the proportion of patients that had been immobilized before the VTE event was substantially higher in those with stroke (51% versus 15%).
Table 2.
Characteristics of VTE Events (n=722)
| No Ischemic Stroke (n=665) % (n) | Ischemic Stroke (n=57) % (n) | |
|---|---|---|
| Clinical characteristics | ||
| Deep vein thrombosis | 58.0 (386) | 50.9 (29) |
| Pulmonary embolism | 42.0 (279) | 49.1 (28) |
| Provoked | 50.0 (332) | 63.2 (36) |
| Unprovoked | 50.0 (333) | 36.8 (21) |
| Clinical risk factors | ||
| Estrogena, b | 5.7 (38) | 5.2 (3) |
| Pregnancy/puerperiuma | 0.9 (6) | — |
| Heredityc | 3.6 (24) | — |
| Provoking factors | ||
| Surgery | 15.9 (106) | 7.0 (4) |
| Trauma | 7.8 (52) | 8.8 (5) |
| Cancer | 24.5 (163) | 17.5 (10) |
| Immobilityd | 16.1 (107) | 43.9 (25) |
| Othere | 5.3 (35) | 1.8 (1) |
The Tromsø Study 1994–2010. DVT indicates deep vein thrombosis; PE, pulmonary embolism; VTE, venous thromboembolism.
Only women included in the analysis.
Current or previous use of hormone replacement therapy or oral contraceptives.
Venous thromboembolism in a first‐degree relative before 60 years of age.
Bed rest >3 days, journeys of >4 hours by car, boat, train, or air within the last 14 days, or other types of immobilization.
Other provoking factor described by a physician in the medical record (eg, intravascular catheter).
IR and HR for VTE among participants without and with incident ischemic stroke during follow‐up are shown in Table 3. In participants without stroke, 665 VTE‐events were identified during 361 634 person‐years of follow‐up, corresponding to IR of 1.8 per 1000 person‐years. In subjects with incident ischemic stroke, there were 57 VTEs identified during 6482 person‐years of follow‐up equivalent to IR of 10.3 per 1000 person‐years. Ischemic stroke was associated with a 3‐times (HR 3.2; 95% CI 2.4–4.4) higher risk of VTE compared to those without ischemic stroke. The IR of VTE was highest during the first month after an ischemic stroke (IR 82.1 per 1000 person‐years) with a 20‐fold higher risk (HR 19.7; 95% CI, 10.1–38.5) compared to those without ischemic stroke. In the period from 1 to 3 months after the stroke, the risk of VTE was 11‐fold increased in stroke patients (HR 10.6; 95% CI 5.0–22.5). The risk declined rapidly thereafter, and was in the period more than 3 months only 1.5 times increased (HR 1.5; 95% CI, 1.1–2.2). Separate analyses of DVT and PE showed that the risk of both outcomes was highest during the first 3 months after the incident ischemic stroke (Table 3). The multivariable HRs were 19.1 (95% CI, 7.8–46.9) and 10.3 (95% CI, 3.8–28.0) for DVT and 20.2 (95% CI, 7.4–55.1) and 11.0 (95% CI, 3.5–35.5) for PE during the first month and in the period 1 to 3 months after stroke, respectively. The risk estimates for both DVT (HR 1.3; 95% CI, 0.8–2.3) and PE (HR 1.8; 95% CI, 1.0–3.0) were no longer significant after the first 3 months.
Table 3.
Incidence Rates and Hazard Ratios for VTE, DVT, and PE According to Ischemic Stroke Exposure
| Person‐Years | VTE Events | Crude IR (95% CI)a | Model 1b HR (95% CI) | Model 2c HR (95% CI) | Model 3d HR (95% CI) | |
|---|---|---|---|---|---|---|
| Total VTE | ||||||
| No stroke | 361 634 | 665 | 1.8 (1.7–2.0) | Reference | Reference | Reference |
| <1 month | 122 | 10 | 82.1 (44.2–152.5) | 16.4 (8.7–30.8) | 15.8 (8.4–29.8) | 19.7 (10.1–38.5) |
| 1 to 3 months | 172 | 8 | 46.5 (23.2–92.9) | 9.5 (4.7–19.2) | 9.2 (4.5–18.5) | 10.6 (5.0–22.5) |
| >3 months | 5193 | 39 | 7.5 (5.5–10.3) | 1.5 (1.1–2.1) | 1.4 (1.0–2.0) | 1.5 (1.1–2.2) |
| DVT | ||||||
| No stroke | 361 634 | 386 | 1.1 (1.0–1.2) | Reference | Reference | Reference |
| <1 month | 122 | 6 | 49.2 (22.1–109.6) | 17.7 (7.8–39.9) | 17.4 (7.7–39.2) | 19.1 (7.8–46.9) |
| 1 to 3 months | 172 | 4 | 23.2 (8.7–61.9) | 8.7 (3.2–23.4) | 8.5 (3.1–22.9) | 10.3 (3.8–28.0) |
| >3 months | 5193 | 19 | 3.7 (2.3–5.7) | 1.3 (0.8–2.1) | 1.2 (0.8–2.0) | 1.3 (0.8–2.3) |
| PE | ||||||
| No stroke | 361 634 | 279 | 0.8 (0.7–0.9) | Reference | Reference | Reference |
| <1 month | 122 | 4 | 32.8 (12.3–87.5) | 14.8 (5.5–40.0) | 14.0 (5.2–37.0) | 20.2 (7.4–55.1) |
| 1 to 3 months | 172 | 4 | 23.2 (8.7–61.9) | 10.4 (3.9–28.3) | 10.0 (3.7–27.1) | 11.2 (3.5–35.5) |
| >3 months | 5193 | 20 | 3.9 (2.5–6.0) | 1.7 (1.1–2.7) | 1.6 (1.0–2.5) | 1.8 (1.0–3.0) |
The Tromsø Study 1994–2010. DVT indicates deep vein thrombosis; HR, hazard ratio; IR, incidence rates; PE, pulmonary embolism; VTE, venous thromboembolism.
Per 1000 persons‐years.
Model 1: Age as timescale, adjusted for sex.
Model 2: Model 1+body mass index.
Model 3: Model 2+systolic blood pressure, diabetes mellitus, high‐density lipoprotein cholesterol, smoking, physical activity, and education level.
The cumulative incidences of VTE in subjects without and with ischemic stroke are shown in Figure 2. There was a notable increase in the cumulative incidence of VTE during the initial 3 months following an incident stroke as displayed by the substantially steeper slope in the incidence curve for subjects with ischemic stroke compared to those without ischemic stroke. The cumulative incidence of VTE was 15% during the first 3 months in subjects with ischemic stroke, compared with 0.2% in the general population during the same time period. The incidence curves for VTE remained essentially parallel in the period more than 6 months after the incident ischemic stroke event (Figure 2).
Figure 2.

Overall cumulative incidence of venous thromboembolism (VTE) in subjects with and without ischemic stroke. The Tromsø Study 1994–2010.
In analyses stratified for the presence of provoking factors, ischemic stroke displayed a higher risk for provoked VTE (HR 22.6; 95% CI, 12.5–40.9) than for unprovoked VTE (HR 7.4; 95% CI, 2.7–20.1) during the first 3 months (Table 4). In subgroup analyses, ischemic stroke was associated with a 20‐fold (HR 19.7; 95% CI, 9.1–42.7) higher risk of provoked DVT and a 29‐fold (HR 29.0; 95% CI, 11.5–73.6) higher risk of provoked PE compared with subjects without ischemic stroke (Table 4). The risk estimates for provoked VTE (HR 1.9; 95% CI, 1.1–3.0), and provoked PE (HR 2.8; 95% CI, 1.3–5.7) remained significantly increased more than 3 months after ischemic stroke, whereas the risk estimate for provoked DVT was no longer statistically significant (HR 1.4; 95% CI, 0.7–2.8).
Table 4.
Incidence Rates and Hazard Ratios for VTE, DVT, and PE According to Ischemic Stroke Exposure by the Presence of Predisposing Factors
| Person‐Years | VTE Events | Crude IR (95% CI)a | Model 1b HR (95% CI) | Model 2c HR (95% CI) | Model 3d HR (95% CI) | |
|---|---|---|---|---|---|---|
| Provoked VTE | ||||||
| No stroke | 361 634 | 332 | 0.9 (0.8–1.0) | Reference | Reference | Reference |
| <3 months | 294 | 14 | 47.6 (28.2–80.4) | 19.3 (11.2–33.2) | 18.8 (10.9–32.4) | 22.6 (12.5–40.9) |
| >3 months | 5193 | 22 | 4.2 (2.8–6.4) | 1.1 (1.1–2.6) | 1.5 (1.0–2.4) | 1.9 (1.1–3.0) |
| Unprovoked VTE | ||||||
| No stroke | 361 634 | 333 | 0.9 (0.8–1.0) | Reference | Reference | Reference |
| <3 months | 294 | 4 | 13.6 (5.1–36.2) | 5.5 (2.0–14.8) | 5.3 (1.9–14.2) | 7.4 (2.7–20.1) |
| >3 months | 5193 | 17 | 3.3 (2.0–5.3) | 1.3 (0.8–2.2) | 1.3 (0.8–2.1) | 1.4 (0.8–2.5) |
| Provoked DVT | ||||||
| No stroke | 361 634 | 216 | 0.6 (0.5–0.7) | Reference | Reference | Reference |
| <3 months | 294 | 8 | 27.2 (13.6–54.4) | 17.5 (8.5–35.8) | 17.2 (8.4–35.2) | 19.7 (9.1–42.7) |
| >3 months | 5193 | 12 | 2.3 (1.3–4.1) | 1.4 (0.8–2.6) | 1.3 (0.7–2.4) | 1.4 (0.7–2.8) |
| Unprovoked DVT | ||||||
| No stroke | 361 634 | 170 | 0.5 (0.4–0.5) | Reference | Reference | Reference |
| <3 months | 294 | 2 | 6.8 (1.7–27.2) | 5.9 (1.4–23.8) | 5.8 (1.4–23.4) | 7.2 (1.8–29.6) |
| >3 months | 5193 | 7 | 1.3 (0.6–2.8) | 1.2 (0.5–2.5) | 1.1 (0.5–2.5) | 1.3 (0.6–3.0) |
| Provoked PE | ||||||
| No stroke | 361 634 | 116 | 0.3 (0.3–0.4) | Reference | Reference | Reference |
| <3 months | 294 | 6 | 20.4 (9.2–45.4) | 22.4 (9.7–51.8) | 21.8 (9.4–50.1) | 29.0 (11.5–73.6) |
| >3 months | 5193 | 10 | 1.9 (1.0–3.6) | 2.0 (1.0–3.9) | 1.9 (1.0–3.7) | 2.8 (1.3–5.7) |
| Unprovoked PE | ||||||
| No stroke | 361 634 | 163 | 0.5 (0.4–0.5) | Reference | Reference | Reference |
| <3 months | 294 | 2 | 6.8 (1.7–27.2) | 5.2 (1.3–21.0) | 4.8 (1.2–19.7) | 7.7 (1.9–31.5) |
| >3 months | 5193 | 10 | 1.9 (1.0–3.6) | 1.4 (0.7–2.8) | 1.3 (0.7–2.6) | 1.6 (0.7–3.4) |
The Tromsø Study 1994–2010. DVT indicates deep vein thrombosis; HR, hazard ratio; IR, incidence rate; PE, pulmonary embolism; VTE, venous thromboembolism.
Per 1000 persons‐years.
Model 1: Age as timescale, adjusted for sex.
Model 2: Model 1+body mass index.
Model 3: Model 2+systolic blood pressure, diabetes mellitus, high‐density lipoprotein cholesterol, smoking, physical activity, and education level.
Discussion
In our prospective cohort study, subjects who developed ischemic stroke had an increased risk of VTE, compared to those without ischemic stroke in the general population. The incidence rate and relative risk were especially high during the first 3 months after ischemic stroke and declined rapidly thereafter. Analyses stratified on predisposing factors of VTE displayed higher risk of provoked than unprovoked events, although the confidence intervals for the point estimates overlapped. As stroke was associated with a transient increased risk of both unprovoked and provoked VTE, our findings suggest that mechanisms or conditions related to the ischemic stroke itself contribute substantially to the association between ischemic stroke and VTE.
Comprehensive data from clinical trials of stroke patients have consistently shown that stroke patients are at high risk of VTE, and DVT in particular.5, 6 However, only 1 previous large registry‐based case–control study conducted in Denmark has investigated the risk of VTE in stroke patients compared to the general population.18 Stroke patients had an overall 1.3‐fold increased risk of VTE, but the risk was particularly high during the first 3 months after stroke with a 4.4‐fold increased VTE risk compared to those without stroke. Accordingly, we found that patients with ischemic stroke had an overall 3‐times higher risk of VTE compared with participants without stroke. The risk was particularly high during the first month and the subsequent 2 months with a 20‐ and 11‐fold higher risk of VTE, respectively, and declined rapidly thereafter. The explanations for the observed association between ischemic stroke and future risk of VTE are yet unknown, but may include shared risk factors, indirect factors, or a direct relationship.22
Several lines of evidence argue against shared risk factors explaining the association between ischemic stroke and VTE. First, shared risk factors are expected to induce a permanent, and not a transient VTE risk as observed in our study. Second, adjustments for potentially shared cardiovascular risk factors would presumably attenuate the VTE risk by ischemic stroke. In our study, adjustments for cardiovascular risk factors had marginal impact on the risk estimates for the association between ischemic stroke and VTE. Third, ischemic stroke and VTE patients did not share the same risk profile. Cause‐specific analyses of cardiovascular risk factors in the Physician's Health Study revealed that only age and obesity were shared risk factors for ischemic stroke and VTE.11 Our findings do not exclude, however, the possibility of joint effects between shared environmental23, 24, 25 and inherited26 prothrombotic risk factors that would augment the VTE risk under conditions of high thrombosis risk related to the ischemic stroke itself (eg, hospitalization, immobilization, and secondary acute infections).2, 16, 17
Several findings from our study support the concept that mechanism(s) or conditions related to the ischemic stroke itself partly explain the association between stroke and VTE. First, we observed a transient and short‐term risk of VTE after ischemic stroke. Patients with ischemic stroke are hospitalized and medical complications occur frequently (eg, respiratory‐ and urinary tract infections).2, 16, 17 These medical complications may contribute to the increased VTE risk either by themselves or via prolongation of the hospital stay.13 Second, stratified analyses displayed a higher risk of provoked than unprovoked VTE by ischemic stroke, with a particular preponderance of immobilization and acute medical conditions as predisposing factors for VTE among patients with ischemic stroke. Similarly, data from the Worcester VTE study displayed a higher frequency of comorbid conditions and immobilization in patients with stroke‐related VTE compared to VTE patients without stroke.27 Patients with ischemic stroke are often temporarily immobilized due to bed‐rest or neurological deficits of affected limbs, and are therefore more susceptible for thrombus formation secondary to venous stasis.28 Activation of the coagulation system during the acute phase of ischemic stroke or secondary to medical complications may also contribute to the VTE risk.29, 30 Therefore, our findings suggest that transient indirect risk factors, occurring in relation to the ischemic stroke, possibly together with enhanced activity in the coagulation system, are important contributors to the transient risk of VTE after ischemic stroke.
For prevention of VTE, current guidelines recommend initiation of subcutaneous anticoagulation with low‐molecular‐weight heparin or unfractionated heparin within 48 hours after ischemic stroke with duration of treatment throughout the hospital stay or until the patient regains mobility.31 Unfortunately, we do not have information on the use of preventive anticoagulant treatment during the rather long study period (1995–2010). However, it is likely that the risk estimates in our study are an underestimation of the real VTE risk (ie, risk in the absence of thromboprophylaxis), as a proportion of the stroke patients presumably have received thromboprophylaxis. Despite this potential underestimation of the VTE risk in stroke patients, we observed an absolute risk increase of 48.1 per 1000 patients for DVT and 32.0 for PE during the first month after the ischemic stroke (compared to subjects without ischemic stroke). Although some of the preventive effect may already be incorporated in our results, a recent meta‐analysis of randomized clinical trials implies that preventive treatment with low‐molecular‐weight heparin or unfractionated heparin had the potential to reduce the incidence of symptomatic DVT by 70% and the incidence of fatal and nonfatal PE by 30%.31 On the other hand, improved awareness and adherence to current guidelines for medical thromboprophylaxis in stroke patients may have lowered VTE rates during the last years.
Major strengths of our study include the prospective design, the large number of participants recruited from a general population, the long‐term follow‐up, the wide age distribution, and validated events of ischemic stroke and VTE. As many cardiovascular risk factors are modifiable, the participants' individual risk profile may change during follow‐up, leading to regression dilution bias and potentially underestimation of the associations. However, an advantage of our study is the repeated measurements of subject characteristics during follow‐up. Because of this, we may to a greater extent account for changes in risk factor and confounders during follow‐up, resulting in more reliable risk estimates than in a traditional cohort study. Still, some potential limitations merit attention. In a cohort study, some groups are less likely to participate and nonresponse bias is therefore possible. Our estimated incidences of stroke and VTE may therefore be lower than the true incidences. Furthermore, the low number of both exposure and outcome events limits the statistical power in subgroup analyses.
In our large cohort of subjects recruited from the general population, subjects who developed ischemic stroke had a transiently increased risk of VTE that was independent of traditional cardiovascular risk factors. The transient nature of the VTE risk following an ischemic stroke implies that conditions related to the stroke itself, rather than shared risk factors, are the main contributors to the VTE risk.
Sources of Funding
K.G. Jebsen TREC is supported by an independent grant from the K.G. Jebsen Foundation.
Disclosures
None.
(J Am Heart Assoc. 2016;5:e004311 doi: 10.1161/JAHA.116.004311)
References
- 1. Feigin VL, Lawes CM, Bennett DA, Barker‐Collo SL, Parag V. Worldwide stroke incidence and early case fatality reported in 56 population‐based studies: a systematic review. Lancet Neurol. 2009;8:355–369. [DOI] [PubMed] [Google Scholar]
- 2. Kumar S, Selim MH, Caplan LR. Medical complications after stroke. Lancet Neurol. 2010;9:105–118. [DOI] [PubMed] [Google Scholar]
- 3. Langhorne P, Stott DJ, Robertson L, MacDonald J, Jones L, McAlpine C, Dick F, Taylor GS, Murray G. Medical complications after stroke: a multicenter study. Stroke. 2000;31:1223–1229. [DOI] [PubMed] [Google Scholar]
- 4. International Stroke Trial Collaborative Group . The International Stroke Trial (IST): a randomised trial of aspirin, subcutaneous heparin, both, or neither among 19435 patients with acute ischaemic stroke. Lancet. 1997;349:1569–1581. [PubMed] [Google Scholar]
- 5. Kamphuisen PW, Agnelli G. What is the optimal pharmacological prophylaxis for the prevention of deep‐vein thrombosis and pulmonary embolism in patients with acute ischemic stroke? Thromb Res. 2007;119:265–274. [DOI] [PubMed] [Google Scholar]
- 6. Kelly J, Rudd A, Lewis R, Hunt BJ. Venous thromboembolism after acute stroke. Stroke. 2001;32:262–267. [DOI] [PubMed] [Google Scholar]
- 7. Bounds JV, Wiebers DO, Whisnant JP, Okazaki H. Mechanisms and timing of deaths from cerebral infarction. Stroke. 1981;12:474–477. [DOI] [PubMed] [Google Scholar]
- 8. Wudicks EFM, Scott JP. Pulmonary embolism associated with acute stroke. Mayo Clin Proc. 1997;72:297–300. [DOI] [PubMed] [Google Scholar]
- 9. Heit JA. Venous thromboembolism: disease burden, outcomes and risk factors. J Thromb Haemost. 2005;3:1611–1617. [DOI] [PubMed] [Google Scholar]
- 10. Vazquez SR, Kahn SR. Advances in the diagnosis and management of postthrombotic syndrome. Best Pract Res Clin Haematol. 2012;25:391–402. [DOI] [PubMed] [Google Scholar]
- 11. Glynn RJ, Rosner B. Comparison of risk factors for the competing risks of coronary heart disease, stroke, and venous thromboembolism. Am J Epidemiol. 2005;162:975–982. [DOI] [PubMed] [Google Scholar]
- 12. Rosendaal FR. Venous thrombosis: a multicausal disease. Lancet. 1999;353:1167–1173. [DOI] [PubMed] [Google Scholar]
- 13. Heit JA, Silverstein MD, Mohr DN, Petterson TM, O'Fallon WM, Melton LJ III. Risk factors for deep vein thrombosis and pulmonary embolism: a population‐based case‐control study. Arch Intern Med. 2000;160:809–815. [DOI] [PubMed] [Google Scholar]
- 14. Geerts WH, Bergqvist D, Pineo GF, Heit JA, Samama CM, Lassen MR, Colwell CW; American College of Chest P . Prevention of venous thromboembolism: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133:381S–453S. [DOI] [PubMed] [Google Scholar]
- 15. Beam DM, Courtney DM, Kabrhel C, Moore CL, Richman PB, Kline JA. Risk of thromboembolism varies, depending on category of immobility in outpatients. Ann Emerg Med. 2009;54:147–152. [DOI] [PubMed] [Google Scholar]
- 16. Jorgensen HS, Nakayama H, Raaschou HO, Olsen TS. Recovery of walking function in stroke patients: the Copenhagen Stroke Study. Arch Phys Med Rehabil. 1995;76:27–32. [DOI] [PubMed] [Google Scholar]
- 17. Jorgensen HS, Nakayama H, Raaschou HO, Vive‐Larsen J, Stoier M, Olsen TS. Outcome and time course of recovery in stroke. Part II: time course of recovery. The Copenhagen Stroke Study. Arch Phys Med Rehabil. 1995;76:406–412. [DOI] [PubMed] [Google Scholar]
- 18. Sorensen HT, Horvath‐Puho E, Sogaard KK, Christensen S, Johnsen SP, Thomsen RW, Prandoni P, Baron JA. Arterial cardiovascular events, statins, low‐dose aspirin and subsequent risk of venous thromboembolism: a population‐based case‐control study. J Thromb Haemost. 2009;7:521–528. [DOI] [PubMed] [Google Scholar]
- 19. The Norwegian Institute of Public Health . Overweight and obesity in Norway: fact sheet. Available at: https://www.fhi.no/en/op/public-health-report-2014/risk--protective-factors/overweight-and-obesity-in-norway---/#about-overweight-and-obesity. Accessed February 26, 2015.
- 20. Investigators WMPP . The World Health Organization MONICA project (monitoring trends and determinants in cardiovascular disease): a major international collaboration. J Clin Epidemiol. 1988;41:105–114. [DOI] [PubMed] [Google Scholar]
- 21. Braekkan SK, Borch KH, Mathiesen EB, Njolstad I, Wilsgaard T, Hansen JB. Body height and risk of venous thromboembolism: the Tromso Study. Am J Epidemiol. 2010;171:1109–1115. [DOI] [PubMed] [Google Scholar]
- 22. Lijfering WM, Flinterman LE, Vandenbroucke JP, Rosendaal FR, Cannegieter SC. Relationship between venous and arterial thrombosis: a review of the literature from a causal perspective. Semin Thromb Hemost. 2011;37:885–896. [DOI] [PubMed] [Google Scholar]
- 23. Andersson HM, Siegerink B, Luken BM, Crawley JT, Algra A, Lane DA, Rosendaal FR. High VWF, low ADAMTS13, and oral contraceptives increase the risk of ischemic stroke and myocardial infarction in young women. Blood. 2012;119:1555–1560. [DOI] [PubMed] [Google Scholar]
- 24. Maino A, Rosendaal FR, Algra A, Peyvandi F, Siegerink B. Hypercoagulability is a stronger risk factor for ischaemic stroke than for myocardial infarction: a systematic review. PLoS One. 2015;10:e0133523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Siegerink B, Rosendaal FR, Algra A. Antigen levels of coagulation factor XII, coagulation factor XI and prekallikrein, and the risk of myocardial infarction and ischemic stroke in young women. J Thromb Haemost. 2014;12:606–613. [DOI] [PubMed] [Google Scholar]
- 26. Casas JP, Hingorani AD, Bautista LE, Sharma P. Meta‐analysis of genetic studies in ischemic stroke: thirty‐two genes involving approximately 18,000 cases and 58,000 controls. Arch Neurol. 2004;61:1652–1661. [DOI] [PubMed] [Google Scholar]
- 27. Piazza G, Goldhaber SZ, Kroll A, Goldberg RJ, Emery C, Spencer FA. Venous thromboembolism in patients with prior stroke. Clin Appl Thromb Hemost. 2014;20:43–49. [DOI] [PubMed] [Google Scholar]
- 28. Yi X, Lin J, Han Z, Zhou X, Wang X, Lin J. The incidence of venous thromboembolism following stroke and its risk factors in eastern China. J Thromb Thrombolysis. 2012;34:269–275. [DOI] [PubMed] [Google Scholar]
- 29. Takano K, Yamaguchi T, Kato H, Omae T. Activation of coagulation in acute cardioembolic stroke. Stroke. 1991;22:12–16. [DOI] [PubMed] [Google Scholar]
- 30. Fisher M, Francis R. Altered coagulation in cerebral ischemia. Platelet, thrombin, and plasmin activity. Arch Neurol. 1990;47:1075–1079. [DOI] [PubMed] [Google Scholar]
- 31. Lansberg MG, O'Donnell MJ, Khatri P, Lang ES, Nguyen‐Huynh MN, Schwartz NE, Sonnenberg FA, Schulman S, Vandvik PO, Spencer FA, Alonso‐Coello P, Guyatt GH, Akl EA; American College of Chest P . Antithrombotic and thrombolytic therapy for ischemic stroke: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141:e601S–e636S. [DOI] [PMC free article] [PubMed] [Google Scholar]


