Abstract
Background
The application of transcatheter aortic valve implantation (TAVI) to intermediate‐risk patients is a controversial issue. Of concern, neurological injury in this group remains poorly defined. Among high‐risk and inoperable patients, subclinical injury is reported on average in 75% undergoing the procedure. Although this attendant risk may be acceptable in higher‐risk patients, it may not be so in those of lower risk.
Methods and Results
Forty patients undergoing TAVI with the Edwards SAPIEN‐XT ™ prosthesis were prospectively studied. Patients were of intermediate surgical risk, with a mean±standard deviation Society of Thoracic Surgeons score of 5.1±2.5% and a EuroSCORE II of 4.8±2.4%; participant age was 82±7 years. Clinically apparent injury was assessed by serial National Institutes of Health Stroke Scale assessments, Montreal Cognitive Assessments (MoCA), and with the Confusion Assessment Method. These identified 1 (2.5%) minor stroke, 1 (2.5%) episode of postoperative delirium, and 2 patients (5%) with significant postoperative cognitive dysfunction. Subclinical neurological injury was assessed using brain magnetic resonance imaging, including diffusion‐weighted imaging (DWI) sequences preprocedure and at 3±1 days postprocedure. This identified 68 new DWI lesions present in 60% of participants, with a median±interquartile range of 1±3 lesions/patient and volumes of infarction of 24±19 μL/lesion and 89±218 μL/patient. DWI lesions were associated with a statistically significant reduction in early cognition (mean ΔMoCA −3.5±1.7) without effect on cognition, quality of life, or functional capacity at 6 months.
Conclusions
Objectively measured subclinical neurological injuries remain a concern in intermediate‐risk patients undergoing TAVI and are likely to manifest with early neurocognitive changes.
Clinical Trial Registration
URL: http://www.anzctr.org.au. Australian & New Zealand Clinical Trials Registry: ACTRN12613000083796.
Keywords: aortic stenosis, cerebrovascular disease/stroke, cognitive impairment, transcatheter aortic valve implantation
Subject Categories: Aortic Valve Replacement/Transcather Aortic Valve Implantation, Cerebrovascular Disease/Stroke, Cognitive Impairment
Introduction
Transcatheter aortic valve implantation (TAVI) has substantially expanded the therapeutic options available to patients with severe aortic stenosis. Provided that appropriate selection criteria are applied, TAVI has been established as superior to medical management among inoperable patients1, 2, 3, 4 and at least not inferior to the “gold‐standard” management of open‐heart surgical aortic valve replacement (SAVR) among high‐risk patients with severe aortic stenosis.3, 4, 5, 6 Success in this setting, evolution of the technology, and the prospect of a less‐invasive technique than SAVR has fueled enthusiasm for extending the indications into lower‐risk patient cohorts. This has resulted in a global trend toward the application of TAVI in lower‐risk patients.7
Recently, the landmark Placement of Aortic Transcatheter Valves (PARTNER) 2 study demonstrated noninferiority of TAVI to SAVR when intermediate‐risk patients were randomized to either intervention.8 Another large observational study, using propensity score analysis methods to compare an intermediate‐risk TAVI cohort with patients treated in the SAVR cohort of the PARTNER 2 study, concluded that TAVI was the superior management option.9
However, the risk‐benefit profile in these patients requires clarification. In particular, the incidence of subclinical neurological injury, previously identified as a concern in high‐risk and inoperable patients,10 requires characterization in the intermediate‐risk cohort.
This study was specifically designed to objectively assess the full spectrum of neurological injury in these intermediate‐risk patients. By doing this, we hoped to also identify predictive factors associated with the occurrence of such adverse events and to determine the impact of subclinical injury on subsequent functional status.
Methods
Study Design and Participants
This was a prospective observational study of patients with severe aortic stenosis, at intermediate surgical risk, undergoing isolated TAVI with the SAPIEN‐XT™ (Edwards Lifesciences, Irvine, CA) valve under general anesthesia.
Patients were consecutively screened for inclusion. Suitability for TAVI was determined by a multidisciplinary “heart team” (including general and interventional cardiologists, a cardiothoracic surgeon, a cardiac anesthetist, and nursing staff), in accordance with international guidelines.11 Patients were included in this analysis if they were of intermediate perioperative risk, based on clinical assessment and surgical risk scoring (requiring both EuroSCORE II ≤10% and Society of Thoracic Surgeons [STS] ≤8%).
Key exclusion criteria included a lack of capacity or willingness to consent, previous aortic valve replacement or significant active coronary artery disease requiring revascularization, a preexisting neurological impairment with a modified Rankin score ≥3 (ie, moderate disability, requiring some assistance, but able to mobilize independently), non‐ or poor English‐speaking ability (as the objective assessment tools are of unknown validity in such a population), and a contraindication for magnetic resonance imaging (MRI) (including inability to lie flat, incompatible metallic prosthesis or foreign body, or claustrophobia requiring sedation).
Ethical approval was obtained from the Human Research Ethics Committee of The Prince Charles Hospital, Brisbane, Australia (HREC/12/QPCH/291), and written informed consent was obtained from all participants prior to enrollment. The authors had full access to all the data in the study and take responsibility for its integrity and the data analysis.
TAVI Procedure
The Edwards SAPIEN‐XT™ valve is a trileaflet bovine pericardial balloon‐expandable heart valve and has been previously described.12 The valve was available in 23‐, 26‐, or 29‐mm diameters with stent heights of 14.3, 17.2, and 19.1 mm, respectively. The valves were implanted using the transfemoral, transapical, or transaortic approach, according to previously described techniques,13, 14, 15 with initial preference for the transfemoral route. Transfemoral delivery utilized the NovaFlex+ (18 Fr) system. The Ascendra+ delivery system was used for transapical and transaortic access. Transesophageal echocardiography and intraoperative angiography guided the procedure.
Patients received intravenous heparin intraoperatively, targeting an activated clotting time (ACT) of ≥300 seconds prior to delivery catheter insertion. In addition, patients were typically started on dual antiplatelet therapy (aspirin and clopidogrel), with a loading dose administered to naive patients prior to the procedure, and this medication was continued for 6 months, followed by lifelong aspirin. Those taking preoperative anticoagulation were typically bridged with intravenous heparin. In such cases, a single antiplatelet agent was introduced preoperatively, and the anticoagulant was reintroduced when the risk of bleeding was considered acceptable. The combination of anticoagulant and antiplatelet was continued for at least 6 months following the procedure.
Anesthetic Technique
General anesthesia was performed by 1 of 3 experienced cardiac anesthetists assigned to the TAVI procedures. Anesthesia was typically induced and maintained with use of anesthetic agent (propofol and sevoflurane), muscle relaxant (cisatracurium or rocuronium), and opioid (remifentanil or fentanyl), aiming for hemodynamic stability and guided by depth of anesthesia monitoring with Bispectral Analysis (BIS™, Covidien, Dublin, Ireland). This facilitated tracheal intubation, mechanical ventilation, neuromuscular paralysis, and passage of the transesophageal echocardiography probe. Tracheal extubation was performed at the end of the procedure following a transfemoral approach or after transfer to the intensive care unit following a transapical or transaortic approach.
Assessments and Data Collection
Cerebral MRI
MRI examinations were performed at baseline (within 24 hours prior to procedure) and at 3±1 days postprocedure using a 1.5‐Tesla system (MAGNETOM Aera, Siemens Healthcare, Erlanger, Germany). The baseline imaging protocol was comprehensive, including time‐of‐flight angiography of the circle of Willis, T2 fluid‐attenuated inversion recovery (FLAIR), standard fast‐spin echo, susceptibility‐weighted imaging (SWI), and diffusion‐weighted imaging (DWI) sequences. The more limited follow‐up scan consisted of DWI, SWI, T2, T1, and FLAIR. DWI is recognized as the most sensitive imaging technique for the detection of acute cerebral infarction, with hyperintensity caused by the restricted diffusion of water, occurring reliably within 4 hours and persisting for up to 10 days in the setting of cytotoxic edema.16 This, concurrent with reciprocal low apparent diffusion coefficient hypointensity, was considered indicative of infarction. Manual contouring was performed to determine the planimetry of each lesion, with automated volume calculation using DICOM image‐processing software (OsiriX, Geneva, Switzerland). Where a lesion was present on a single slice, the volume was determined by the product of the surface area and the slice thickness.
Clinical Neurological and Cognitive Assessment
Clinical neurological status was objectively examined using the National Institutes of Health Stroke Scale (NIHSS) and the modified Rankin score (mRS), with the change in score from baseline allowing categorization of stroke severity (none, minor, moderate, moderate to severe, and severe). Cognitive function was assessed using the Montreal Cognitive Assessment (MoCA) tool, which is a battery of assessments of multiple cognitive domains including executive functions, language, attention/concentration, memory, visuoconstructional skills, conceptual thinking, calculation, and orientation.17 A global score cutoff of ≤24/30 defined cognitive impairment.18 Postoperative cognitive dysfunction was defined as a decrease in MoCA total score of ≥20% compared with baseline.19 Additionally, standard screening with the Confusion Assessment Method and chart review were used to diagnose delirium.20, 21 These tests were administered within 24 hours before the TAVI procedure and at 3 days, 6 weeks, and 6 months post‐TAVI.
Preoperative Assessment of Potential Sources of Embolism
Baseline risk assessment included carotid duplex ultrasonography to identify significant (≥50%) carotid artery stenosis. Noncontrast axial computed tomography (CT) sequences of the chest were performed and assessed for the presence of “porcelain aorta” using the Valve Academic Research Consortium updated (VARC‐2) definition.11
Health Status Assessment
Disease‐specific health status was assessed using the Kansas City Cardiomyopathy Questionnaire (KCCQ), which has been widely validated as reliable among patients with heart failure, including those with severe symptomatic aortic stenosis.22 The assessment involves a 23‐item questionnaire assessing conceptual domains—physical limitation, symptoms, self‐efficacy, quality of life, and social limitation—which are used to calculate an overall score (OS), from 0 to 100, with higher scores indicating a smaller symptom burden and a better quality of life.23 A decrease in the KCCQ‐OS of ≥5 points corresponds to worse outcome, and increases of 5 to 10, 10 to 20, and >20 points correspond to slightly improved, moderately improved, and substantially improved clinical outcomes, respectively.24
Generic health status was evaluated using the EuroQoL‐5D (EQ‐5D), which measures 5 conceptual domains—mobility, self‐care, usual activities, pain/discomfort, and anxiety/depression. Categorical scoring (no, slight, moderate, severe, and extreme problems) in each domain can be converted to a preference‐weighted health status score, or a “utilities” index, that ranges from 0 to 1, with a higher score representing better health. Patients also scored their overall health from 0 (the worst health imaginable) through 100 (the best health imaginable) using a visual analog scale (VAS).25 Health status assessments were performed at baseline and 6 months postprocedure.
Frailty and Physical Function Assessment
Frailty is a multidimensional risk state that is associated with, but separate from, chronological age and represents a lack of physiological reserve across multiple organ systems.26 Although frailty is an independent predictor of an adverse postoperative outcome and an important contributor to the surgical risk status of a patient, it is not considered in the traditional surgical risk scores.27 Despite its important role in risk, there is no consensus on the ideal assessment and diagnosis of frailty.26 The frailty index, calculated as the proportion of potential deficits that are present in a given individual, is a valid measure of frailtyand was performed in all patients at baseline.26 Higher scores reflected a greater frailty burden with an index of ≥0.25 considered to represent the frailty phenotype and a threshold value above which individuals require day‐to‐day assistance.28, 29
The 5‐m walk time (5MWT) is a measure of gait speed, with slow gait (>6 seconds) providing another important indicator of frailty with strong predictive value for healthcare utilization, disability, morbidity, and mortality.27 The 6‐minute walk distance (6MWD) has been validated as both a functional status measure and correlate for activities of daily living.30 Physical function assessments were performed at baseline and 6 months postprocedure.
Statistical Analysis
The primary neurological endpoint was new DWI‐positive lesions on the day 3±1 MRI scan compared with baseline MRI, quantified by number and volume (in microliters, μL), in accordance with recommendations.16, 31 Secondary endpoints were new clinically apparent neurological injury, measured as stroke (major or minor), transient ischemic attack, postoperative cognitive dysfunction, and postoperative delirium at 3 days and 6 weeks, and 6MWD, 5MWT, EQ‐5D, and KCCQ scores at 6 months compared with baseline measures. Summary statistics are presented as mean±standard deviation for approximately normally (or Gaussian) distributed data and median±interquartile range for non–normally distributed data. For longitudinal assessments, postprocedure scores were compared with baseline values using paired t tests. A priori subgroup analysis of patients stratified according to the presence or absence of DWI lesions and according to access approach was also conducted. For the longitudinal endpoints collected over multiple follow‐up times, we used a mixed‐effects regression model with a random intercept for each patient to adjust for within‐patient dependence. Predictors were chosen as those likely to influence the endpoint but with little missing data in order to avoid power loss.
Logistic regression was used to examine associations between potential predictors and health endpoints. A LASSO method was used to select the best set of predictors from a large set.32 The categorical predictors were sex, current smoking, history of stroke or transient ischemic attack, hypertension, chronic renal impairment, chronic lung disease, myocardial infarction, atrial fibrillation, carotid stenosis, left main coronary artery occlusion >50%, and need for postimplantation maneuvers. The continuous predictors were age, body mass index, STS, EuroSCORE II, frailty index, ejection fraction prior to procedure, and baseline MoCA score. Thus, in total, we assessed 18 predictors for a possible association with the occurrence of MRI‐defined brain infarction.
Results
Baseline Patient Characteristics
Forty patients were enrolled between January 2014 and December 2015. Their baseline characteristics are reported in Table 1. The CONSORT flow of patients through the study is in Figure 1. The intermediate‐risk stratum of the cohort was confirmed by a mean STS‐predicted risk of mortality of 5.1±2.5% and a mean EuroSCORE II of 4.8±2.4%. More specifically, 13 of 40 patients had STS <4; 16 of 40 had a EuroSCORE II <4. In keeping with the surgical risk scores, the frailty burden of the cohort was modest with a mean frailty index of 0.20±0.08.
Table 1.
Baseline Characteristics
| Variable | Measure |
|---|---|
| Patient characteristics (n=40) | |
| Age, y | 81.7±6.9 |
| Male sex | 16 (40) |
| EuroSCORE II, % | 4.8±2.4 |
| <4% | 16 (40) |
| 4% to 10% | 24 (60) |
| STS, % | 5.1±2.5 |
| <4% | 13 (32.5) |
| 4% to 10% | 27 (67.5) |
| Frailty index | 0.2±0.1 |
| Previous mediastinal radiation | 0 (0) |
| Chest deformity | 0 (0) |
| Porcelain aorta | 1 (2.5) |
| Carotid disease >50% | 7 (17.5) |
| Stroke | 6 (15) |
| BMI, kg/m2 | 29.1±6.7 |
| Hypertension | 30 (75) |
| Hyperlipidemia | 34 (85) |
| Diabetes mellitus | 14 (35) |
| Creatinine >150 μmol/L | 6 (15) |
| Chronic liver failure or cirrhosis | 0 (0) |
| Significant (>5 pack years) smoking | 15 (37.5) |
| NYHA III or IV | 32 (80) |
| LVEF, % | 59.3±9.6 |
| LVEF ≤50% | 4 (10) |
| LVEF ≤35% | 0 (0) |
| Aortic valve area, cm2 | 0.8±0.2 |
| Mean AV gradient, mmHg | 46.1±10.6 |
| Peak AV jet velocity, m/s | 4.3±0.4 |
| Atrial fibrillation | 11 (27.5) |
| Procedural characteristics (n=40) | |
| Access approach | |
| Transfemoral | 20 (50) |
| Transaortic | 14 (35) |
| Transapical | 6 (15) |
| Device success | 38 (95) |
| Average procedure time, minutes | 71.4±18.4 |
| Fluoroscopic time, minutes | 14.3±8.5 |
| Fluoroscopy contrast volume, mL | 148.9±46.2 |
| Rapid ventricular pacing duration, seconds | 30.3±15.9 |
| ACT at deployment, seconds | 333±49.5 |
Values are expressed as mean±standard deviation or as n (%). ACT indicates activated clotting time; AV, aortic valve; BMI, body mass index; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; STS, Society of Thoracic Surgeons.
Figure 1.
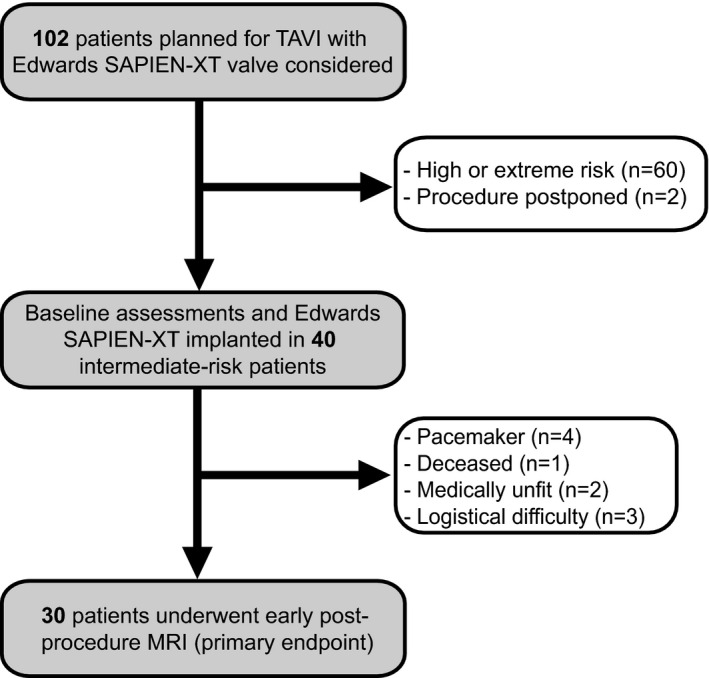
Study flow CONSORT diagram. MRI indicates magnetic resonance imaging; TAVI, transcatheter aortic valve implantation.
Procedural Characteristics
Procedural characteristics of the cohort are presented in Table 1. Device success, using the VARC‐2 definition, was achieved in 38 of 40 patients (95%). Criteria were not met in 2 patients: 1 required valve‐in‐valve implantation, and the other patient died in theater (from annular rupture, cardiac tamponade, and cardiac arrest). Twenty patients (50%) underwent TAVI via transfemoral access, and 20 (50%) via a direct thoracic approach (14 [35%] via transaortic and 6 [15%] via transapical access). Average catheterization time was 71.4±18.4 minutes, fluoroscopy time was 14.3±8.5 minutes, and a mean volume of 148.9±46.2 mL of contrast was administered. The total duration of rapid ventricular pacing averaged only 30.3±15.9 seconds. At the time of device deployment, activated clotting time averaged 333.0±49.5 seconds.
Neurological Outcomes
Cerebral MRI
Baseline MRI scans were performed in all patients (n=40), with the identification of only 1 acute diffusion abnormality on DWI (small subacute periventricular deep white matter infarct) in 1 patient. Thirty of the 40 participants (75%) were then assessed for the primary endpoint (day 3±1 postprocedure MRI) (Figure 1). In 18 of these 30 patients (60%), 68 new DWI‐positive lesions were identified, with a median of 1±3 lesions/patient, and median volumes of infarction of 89±218μL/patient and 24±19 μL/lesion (Table 2 and Figure 2).
Table 2.
Outcome Measures
| Variable | Measure |
|---|---|
| Clinical outcomes (n=40) | |
| Death | 1 (2.5) |
| Myocardial infarction | 0 (0) |
| Life‐threatening bleeding | 1 (2.5) |
| Major bleeding | 0 (0) |
| Pacemaker implantation | 4 (10) |
| Cardiac reintervention | 1 (2.5) |
| Major/disabling stroke | 0 |
| Minor/nondisabling stroke | 1 (2.5) |
| Transient ischemic attack | 0 (0) |
| Postoperative cognitive dysfunction | 1 (2.5) |
| Postoperative delirium | 1 (2.5) |
| Neuroimaging outcomes (n=30) | |
| Patients with new DWI+ lesions | 18/30 (60) |
| Total number of new DWI+ lesions, n | 68 |
| Median±IQR number of DWI+ lesions/patient | 1±2.8 |
| Median±IQR volume, μL/lesion | 24±19 |
| Median±IQR volume, μL/(DWI+ patient) | 89±214 |
Values are expressed as mean±standard deviation or as n (%), unless otherwise indicated. DWI indicates diffusion‐weighted imaging; IQR, interquartile range.
Figure 2.

Distribution of lesions by volume.
Clinical
Although there were no major/disabling strokes, 1 minor/nondisabling stroke was identified, presenting as dysarthria (NIHSS 1, mRS 1) without ongoing residual deficit. Cognitive assessment identified mild cognitive impairment in 14 (35%) of the patients at baseline, and serial postoperative assessments identified early postoperative cognitive dysfunction in 2 (5%) patients; this had resolved in 1 by 6 weeks and in the other by 6 months. Similarly, there was 1 (2.5%) episode of postoperative delirium lasting 48 hours. Overall, there was no statistically significant difference in the cohort means for the MoCA between each time point compared and its baseline (Table 2 and Figures 3 and 4A). Time and STS score were the only 2 potential predictors identified by longitudinal regression, with a mean (95% confidence interval) increase in MoCA score of 0.37 (0.00‐0.77) per STS point and 1.09 (0.28‐1.90) per time point.
Figure 3.
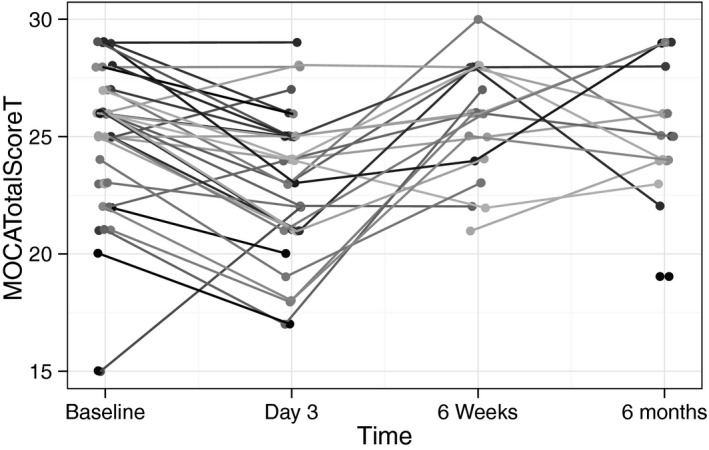
Spaghetti plot of longitudinal changes in individual patients' MoCA scores. MoCA indicates Montreal Cognitive Assessment.
Figure 4.

Box‐and‐whisker plots showing the distribution of MoCA scores at each time point and compared with baseline. A, total study cohort; (B) patients without new DWI changes on postprocedure MRI; (C) patients with new DWI changes on postprocedure MRI. Δ1, 3 days–baseline MoCA score; Δ2, 6 weeks–baseline MoCA score; Δ3, 6 months–baseline MoCA score. DWI indicates diffusion‐weighted imaging; MoCA, Montreal Cognitive Assessment; MRI, magnetic resonance imaging.
When patients were stratified based on the occurrence of DWI lesions (Figure 4B and 4C), those who suffered lesions demonstrated significantly reduced cognitive performance compared with those who did not on the early postoperative MoCA (mean Δ MoCA −3.5±1.7 vs 0.4±3.2; P<0.001). There was a moderate correlation between the degree of early cognitive dysfunction and DWI lesion burden measured either by number (r=0.45) or volume (r=0.40).
Functional Outcomes
Physical Outcomes
There was no statistically significant difference noted between the 6‐month and the baseline assessment results for either the 6MWD (Table 2 and Figure 5) or 5MWT (Table 2 and Figure 6). Longitudinal regression analysis identified time, sex, frailty, and TAVI procedure duration as potential predictors of 6MWD, with a mean (95% confidence interval) increase of 24.7 (11.8‐38.2) m per time point, 57.4 (5.5‐109.2) m for males and decrease of 52.4 (21.8‐83.1) m per 0.1 increase in frailty index and 21.7 (8.0‐35.3) m for each additional 10 minutes of the procedure. Similarly, each 0.1 increase in frailty index and additional 10 minutes of procedure duration were associated with a prolonged 5MWT of 2.1 (1.1‐3.2) and 0.6 (0.1‐1.1) seconds, respectively.
Figure 5.
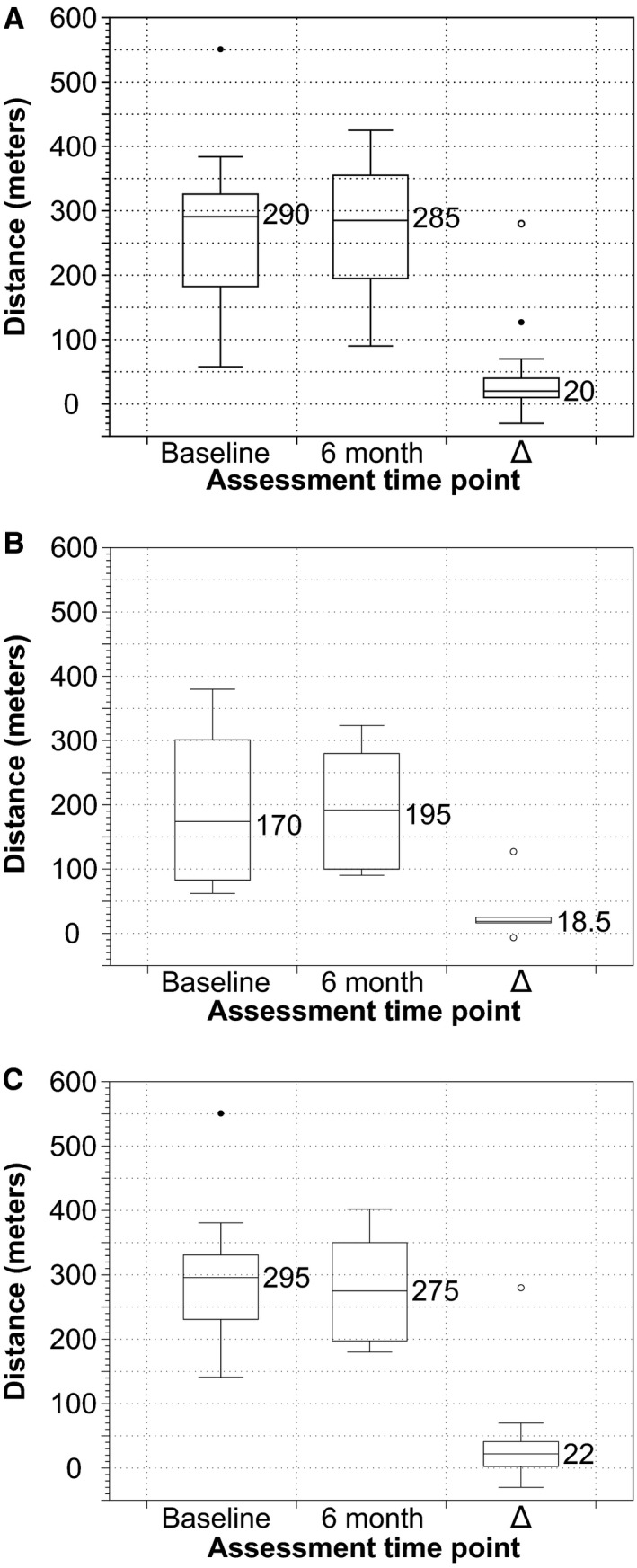
Box‐and‐whisker plots showing the distribution of 6‐minute walk distances at 6 months compared with baseline in (A) total cohort, (B) patients without new DWI changes on postprocedure MRI, and (C) patients with new changes on postprocedure MRI. Δ, 6 month–baseline score/value. DWI indicates diffusion‐weighted imaging; MRI, magnetic resonance imaging.
Figure 6.

Box‐and‐whisker plots showing the distribution of 5‐m walk times at 6 months compared with baseline in (A) total cohort, (B) patients without new DWI changes on postprocedure MRI, and (C) patients with new changes on postprocedure MRI. Δ, 6 month–baseline score/value. DWI indicates diffusion‐weighted imaging; MRI, magnetic resonance imaging.
Health‐Related Quality of Life
Comparison of KCCQ‐OS and EQ‐5D VAS and index values at baseline versus 6 months postprocedure are presented in Table 2 and Figures 7, 8 through 9. A statistically significant improvement was identified in the disease‐specific KCCQ‐OS at 6 months compared with baseline (63±19 vs 50±13; P=0.002). Based on the prespecified categorical levels of change in KCCQ‐OS, 8% had a poor outcome, 17% were unchanged, and 75% showed improvement (21% slight, 29% moderate, and 25% substantial). However, improvement in quality of life was not statistically significant when evaluated with either the EQ‐5D utilities index (0.8±0.2 at 6 months versus 0.7±0.2 at baseline; P=0.1) or the EQ‐5D VAS (72.6±19.9 at 6 months versus 65.9±18.0 at baseline; P=0.2). There was no difference in either assessment measure when stratified according to the presence or absence of new DWI‐positive lesions (panels B and C in Figures 7, 8 through 9).
Figure 7.
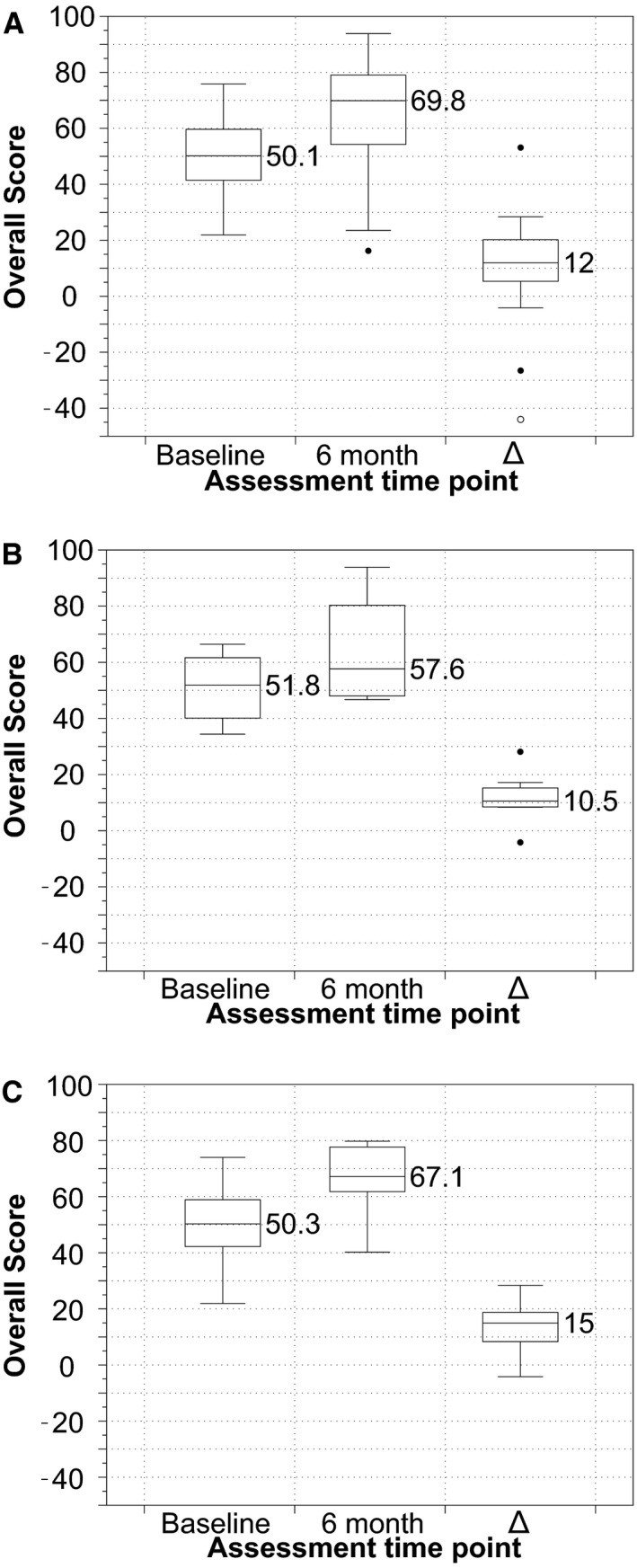
Box‐and‐whisker plots showing the distribution of Kansas City Cardiomyopathy Questionnaire Overall Scores at 6 months compared with baseline in (A) total cohort, (B) patients without new DWI changes on postprocedure MRI, and (C) patients with new changes on postprocedure MRI. Δ, 6 month–baseline score/value. DWI indicates diffusion‐weighted imaging; MRI, magnetic resonance imaging.
Figure 8.
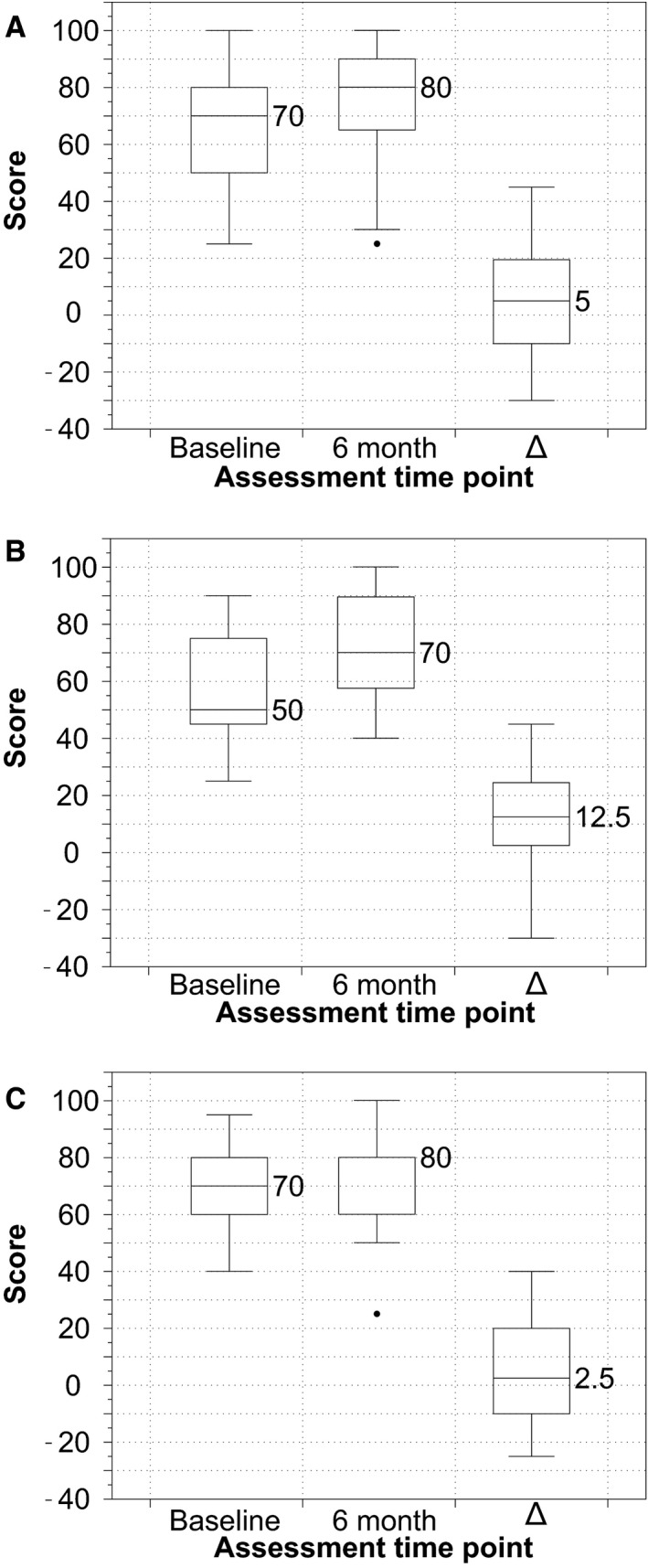
Box‐and‐whisker plots showing the distribution of EQ‐5D Visual Analog Scores at 6 months compared with baseline in (A) total cohort, (B) patients without new DWI changes on postprocedure MRI, and (C) patients with new changes on postprocedure MRI. Δ, 6 month–baseline score/value define. DWI indicates diffusion‐weighted imaging; MRI, magnetic resonance imaging.
Figure 9.

Box‐and‐whisker plots showing the distribution of EQ‐5D Index values at 6 months compared with baseline in (A) total cohort, (B) patients without new DWI changes on postprocedure MRI, and (C) patients with new changes on postprocedure MRI. Δ, 6 month–baseline score/value. DWI indicates diffusion‐weighted imaging; MRI, magnetic resonance imaging.
Multiple Variable Analysis of Demographic and Procedural Risk Factors
Logistic regression failed to identify any significant association between the occurrence of DWI‐positive lesions (by number or volume) and any of the predictors. A post hoc power calculation confirmed that the study was adequately powered to detect important changes (>1.5) in the odds ratio.
Discussion
Incidence
The incidence of clinically apparent stroke after TAVI has been reported by large registries to range between 0% and 10%,10 averaging 3.3% at 30 days according to 1 meta‐analysis,33 and with the most recent “real‐world data” reporting a 4.1% incidence of stroke at 1 year.34 The reported incidence of all stroke in our study of 2.5% is consistent with the most robust data available for intermediate‐risk patients.8, 9 No differences in stroke risk have been identified between TAVI strategies when comparing commonly used devices, access approaches, or anesthetic techniques.35, 36
However, clinically apparent stroke represents only the “tip of the iceberg” of neurological injury, with most events being subclinical.10 DWI is a highly sensitive and specific modality for the early detection of ischemic changes in acute stroke patients and has become a well‐established measure of cerebral embolization.16, 31 A small number of studies have been published using this objective neurological endpoint. These studies have shown a very high (58–91%) incidence of new ischemic lesions after TAVI among high‐risk and inoperable cohorts,37, 38, 39, 40, 41, 42, 43 and the average incidence is considered to be 75%.10
The 60% incidence of lesions and the total lesion volume/patient of 89±218 μL in this cohort are favorable compared to those previously published for high‐risk and inoperable patient cohorts. However, a number of important differences between this and previous studies must be considered when interpreting these results. First, the learning curve effect associated with TAVI is well established.44 At the time recruitment for this study was commenced, the TAVI team performing all procedures had over 7 years' experience and had performed over 300 TAVI procedures. Second, there has been rapid evolution in the prostheses and technique as well as improved patient selection.12, 35 Third, the risk profile was lower in our cohort than in those previously reported.
The timing of MRI is another important consideration. The specific signal intensity changes associated with cerebral infarction—a high signal intensity on DWI and a corresponding low value on the apparent diffusion coefficient map—appear within 4 hours of acute infarction, peak at 40 hours, and persist reliably for only 7 days.45 Competing considerations are the early postoperative changes that occur in all patients and the need for sufficient patient recovery postprocedure to allow an MRI examination to be undertaken safely. For this reason all but 1 study limited the postoperative scan to between 2 and 7 days. The 1 study that published a lower incidence of DWI lesions (45%), and the only other study that was performed in intermediate‐risk patients, permitted scans up to 15 days postprocedure, thus allowing the potential extinction of imaging changes, which would result in an underestimation of the true incidence.46 The importance of the timing of the early postoperative MRI scan cannot be overstated, especially in this setting, where the majority of infarcts are small and the primary endpoint is dichotomous (lesion present or not). This signal loss can result in these lesions easily disappearing, significantly confounding further analysis. Furthermore, in the same study, baseline MRIs were not performed to assess whether DWI lesions were in fact new postprocedure.46
Consequences of Subclinical Cerebral Infarction
In addition to their role as a surrogate marker for clinical cerebrovascular events, subclinical events may involve injury that in itself portends an adverse outcome. To date, the few TAVI studies to assess this issue have failed to identify any consequences from their occurrence. However, in other settings, there is considerable evidence that subclinical brain infarction adversely affects the risk and outcome of subsequent stroke, neurocognitive/neuropsychiatric function, physical activity, quality of life, and risk of mortality.16
The finding of a significant difference between the day‐3 cognitive assessments, when patients were stratified according to the dichotomous presence or absence of MRI‐defined infarction, suggests that these lesions may have early cognitive implications. This is supported by the moderate correlation between early cognitive decline and lesion number and volume. Consequently, as a minimum, longitudinal cognitive assessment in post‐TAVI patients with radiological evidence of subclinical stroke should be considered. Equally, postoperative stroke is likely to manifest with neurocognitive changes (rather than classical stroke symptoms) in the postoperative period. We propose that any cognitive loss in this phase should be screened for cerebrovascular ischemia, even in the absence of confirmatory clinical signs.
In contrast to the early postoperative cognitive decline, the occurrence of MRI‐defined cerebral infarction had no implications for longer‐term follow‐up. In fact, longitudinal regression identified time as a potential predictor for higher MoCA score. Although the possibility of a learning effect from reuse of the same cognitive instruments may have falsely sustained follow‐up scores, the separation in testing and the use of different versions of the MoCA mitigated this effect reasonably and was in line with other studies in this area.
Currently, no cognitive assessment has been validated for the detection of early cognitive decline in patients undergoing TAVI. A formal neuropsychology assessment is generally considered the benchmark; however, this is time consuming and excessively burdensome in the setting of postoperative patients and repeated/longitudinal assessments. The MoCA has emerged as a useful screening tool, and although it is less familiar than the commonly used Folstein mini‐mental state examination (MMSE), it is generally considered more sensitive (94% vs 66%, respectively), but less specific (42% vs 97%) for cognitive impairment.18, 47 The improved sensitivity results, at least partially, from inclusion of a more thorough executive function assessment, which is more sensitive to vascular cognitive impairment. Although limited, results of previous TAVI studies suggest that reduced specificity is of little importance, and the normal cognitive function at baseline exhibited by this group would have performed within the ceiling effect range of the MMSE. Consequently, larger changes in cognition would be required to register a decrement in MMSE testing, which supports our use of the MoCA.
In stark contrast to previous evidence and the improvement seen in the disease‐specific health status (KCCQ‐OS) of the cohort, we were unable to identify significant improvement in the EQ‐5D utilities index or VAS, irrespective of DWI status. This may be due to the high baseline assessment scores and level of function of this intermediate‐risk cohort, decreasing the margin for detecting improvement. Alternatively, this may reflect the effects of aging or a large ceiling effect with EQ‐5D assessment, either of which may have blunted the detection of improvement at the 6‐month postprocedure assessment time point.
Risk Factors for Cerebral Infarction
Patient‐specific risk factors identified to date include age41 and aortic arch atheroma severity (thickness of >5 mm or mobile atheroma in the thoracic aorta and arch).41, 43 Procedural predictors of new cerebral infarction following TAVI include the need for balloon postdilation,48 valvuloplasty balloon size, and fluoroscopy time (a surrogate for procedural complexity and device manipulation).43
Prior to intervention, baseline assessments directed toward the detection of potential sources of embolism were performed. However, the high incidence of DWI lesions made independent predictors of TAVI‐associated embolism hard to detect. Regression analysis was performed primarily to look for significant differences between patients who sustained DWI lesions and those who did not. However, none of the 18 variables considered were found to have predictive value, and this remained the case when ischemic lesion volume was substituted in the model as the dependent variable.
Risk Stratification in TAVI Patients
In clinical practice, experienced surgeons and cardiologists pragmatically and informally estimate individual risk based on their experience, clinical intuition, and judgment.49 However, robust research methodology mandates validated assessments that objectively predict the probability that a patient will develop an adverse outcome, so as to allow for appraisal of procedural efficacy. For this purpose, traditional surgical risk scores (eg, the STS and EuroSCORE II) are the most widely adopted, with the cutoff scores used in this study (STS ≤8 and EuroSCORE II ≤10) consistent with those generally accepted for intermediate risk.
Although the accepted standard, these models of risk prediction are imperfect, particularly in light of questionable calibration to the TAVI population.50 This may be due to the omission of important prognostic variables such as porcelain aorta, liver failure/cirrhosis, and anatomical abnormalities of the chest including deformity and previous mediastinal radiation.50
As previously mentioned, frailty is another important factor not accounted for by the traditional surgical risk scores. To overcome this shortcoming, we considered the frailty index score as previously described, comprising relative age‐related decrements in health status.29 The frailty burden in this cohort was only modest, and care should be taken not to extrapolate these findings to frail patients. For instance, frailty may increase susceptibility to the effects of silent ischemia and forebode other negative consequences such as functional decline, falls, and incontinence.51
Strengths and Limitations
Our study has several important limitations for clinical translation that warrant further discussion. First, although the sample size is consistent with that of previous similar studies performed in high‐risk and inoperable patients, it was a relatively small and nonrandomized patient cohort that lacked a surgical aortic valve replacement group for comparison. Furthermore, although homogeneity was maximized with the use of a single valve type (Edwards SAPIEN‐XT™ valve) in procedures performed by the same operators (D.L.W. and A.J.C.) at a high‐volume TAVI center, heterogeneity was introduced by the inclusion of differing access approaches (including transfemoral, transaortic, and transapical). A subanalysis was not possible in the current study because of the comparatively few transaortic and transapical procedures included. Furthermore, the limited sample size and the high incidence of cerebral infarction also affected the sensitivity for revealing predictors of ischemic brain lesions.
A second limitation is that although the assessment procedures were performed in a comprehensive manner, postoperative assessments could only be performed in those who had sufficiently recovered in a timely fashion and did not have contraindications. Ten patients (25%) were not able to undergo the follow‐up MRI scan because of death, clinical instability, or pacemaker insertion, and this may have introduced attrition bias. Importantly, there were no statistically significant differences in any baseline characteristics between those who underwent the early postoperative MRI and those who did not.
Finally, our study used the Edwards SAPIEN‐XT™ valve in isolation, and the findings and outcomes may be different with other valve prostheses. In particular, the trend toward fully or partially retrievable and repositionable valves (eg, the Boston Scientific Lotus™, Medtronic CoreValve® Evolut™, St. Jude Portico™, Symetis Acurate™, and the JenaValve™) may encourage increased manipulation, resulting in a consequent increase in the incidence of cerebral embolization.
Conclusions
Objectively measured structural and functional neurological injuries remain a frequent occurrence, affecting the majority of intermediate‐risk patients who undergo TAVI. New MRI‐defined ischemic lesions were associated with reduced early cognitive function. These subtle injuries are of increasing significance for lower‐risk patients who have both more time for neurological sequelae to manifest and alternate management options available to them compared with high‐risk and inoperable patients. Thus, enthusiasm for extending TAVI into lower‐risk patients must be tempered until the risk associated with subclinical injury is clarified and optimal neuroprotective strategies can be pursued.
Sources of Funding
Funding for this study was provided by The Prince Charles Hospital Foundation. Fanning is supported by the Royal Australasian College of Physicians (Joseph Thornton Tweddle Scholarship), the National Heart Foundation of Australia (Health Professional Scholarship ref: 101105) and previously by a scholarship from the Cardiac Society of Australia and New Zealand. Fraser is supported by a Queensland Government Office of Health and Medical Research Fellowship.
Disclosures
Walters is a consultant to Boston Scientific and Edwards, investigator for Edwards, Medtronic, Symetis and Boston Scientific clinical studies, and past proctor for Edwards. No other author has any competing interests to declare.
Acknowledgments
We thank Lynnette Munck, Bernadette Madden, Kristin Kirwan, Rhonda Lamb, the Department of Anaesthesia, and members of the TAVI service and the Critical Care Research Group at the Prince Charles Hospital for their assistance with patient assessment and data collection/management. We also thank Cardiovascular Outcomes Inc and ViDEX 2325954 Ontario Inc for their assistance with licensing and use of the KCCQ and online frailty index, respectively.
(J Am Heart Assoc. 2016;5:e004203 doi: 10.1161/JAHA.116.004203)
Presented as an Abstract at the Scientific Sessions of the American Heart Association, from November 12–16, in New Orleans, LA.
References
- 1. Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Brown DL, Block PC, Guyton RA, Pichard AD, Bavaria JE, Herrmann HC, Douglas PS, Petersen JL, Akin JJ, Anderson WN, Wang D, Pocock S. Transcatheter aortic‐valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–1607. [DOI] [PubMed] [Google Scholar]
- 2. Popma JJ, Adams DH, Reardon MJ, Yakubov SJ, Kleiman NS, Heimansohn D, Hermiller J, Hughes GC, Harrison JK, Coselli J, Diez J, Kafi A, Schreiber T, Gleason TG, Conte J, Buchbinder M, Deeb GM, Carabello B, Serruys PW, Chenoweth S, Oh JK. Transcatheter aortic valve replacement using a self‐expanding bioprosthesis in patients with severe aortic stenosis at extreme risk for surgery. J Am Coll Cardiol. 2014;63:1972–1981. [DOI] [PubMed] [Google Scholar]
- 3. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, Guyton RA, O'Gara PT, Ruiz CE, Skubas NJ, Sorajja P, Sundt TM, Thomas JD, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Creager MA, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Stevenson WG, Yancy CW. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:2440–2492. [DOI] [PubMed] [Google Scholar]
- 4. Vahanian A, Alfieri O, Andreotti F, Antunes MJ, Barón‐Esquivias G, Baumgartner H, Borger MA, Carrel TP, De Bonis M, Evangelista A, Falk V, Iung B, Lancellotti P, Pierard L, Price S, Schäfers HJ, Schuler G, Stepinska J, Swedberg K, Takkenberg J, Von Oppell UO, Windecker S, Zamorano JL, Zembala M, Bax JJ, Ceconi C, Dean V, Deaton C, Fagard R, Funck‐Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Popescu BA, Reiner Ž, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Von Segesser L, Badano LP, Bunc M, Claeys MJ, Drinkovic N, Filippatos G, Habib G, Pieter Kappetein A, Kassab R, Lip GYH, Moat N, Nickenig G, Otto CM, Pepper J, Piazza N, Pieper PG, Rosenhek R, Shuka N, Schwammenthal E, Schwitter J, Mas PT, Trindade PT, Walther T. Guidelines on the management of valvular heart disease (version 2012). Eur Heart J. 2012;33:2451–2496. [DOI] [PubMed] [Google Scholar]
- 5. Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Williams M, Dewey T, Kapadia S, Babaliaros V, Thourani VH, Corso P, Pichard AD, Bavaria JE, Herrmann HC, Akin JJ, Anderson WN, Wang D, Pocock SJ. Transcatheter versus surgical aortic‐valve replacement in high‐risk patients. N Engl J Med. 2011;364:2187–2198. [DOI] [PubMed] [Google Scholar]
- 6. Adams DH, Popma JJ, Reardon MJ, Yakubov SJ, Coselli JS, Deeb GM, Gleason TG, Buchbinder M, Hermiller J, Kleiman NS, Chetcuti S, Heiser J, Merhi W, Zorn G, Tadros P, Robinson N, Petrossian G, Hughes GC, Harrison JK, Conte J, Maini B, Mumtaz M, Chenoweth S, Oh JK. Transcatheter aortic‐valve replacement with a self‐expanding prosthesis. N Engl J Med. 2014;370:1790–1798. [DOI] [PubMed] [Google Scholar]
- 7. Fanning JP, Fraser JF. “Indication Creep”—TAVR utilization exceeding current guidelines. N Engl J Med. 2016;374:1690–1692. [Google Scholar]
- 8. Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, Thourani VH, Tuzcu EM, Miller DC, Herrmann HC, Doshi D, Cohen DJ, Pichard AD, Kapadia S, Dewey T, Babaliaros V, Szeto WY, Williams MR, Kereiakes D, Zajarias A, Greason KL, Whisenant BK, Hodson RW, Moses JW, Trento A, Brown DL, Fearon WF, Pibarot P, Hahn RT, Jaber WA, Anderson WN, Alu MC, Webb JG. Transcatheter or surgical aortic‐valve replacement in intermediate‐risk patients. N Engl J Med. 2016;374:1609–1620. [DOI] [PubMed] [Google Scholar]
- 9. Thourani VH, Kodali S, Makkar RR, Herrmann HC, Williams M, Babaliaros V, Smalling R, Lim S, Malaisrie SC, Kapadia S, Szeto WY, Greason KL, Kereiakes D, Ailawadi G, Whisenant BK, Devireddy C, Leipsic J, Hahn RT, Pibarot P, Weissman NJ, Jaber WA, Cohen DJ, Suri R, Tuzcu EM, Svensson LG, Webb JG, Moses JW, Mack MJ, Miller DC, Smith CR, Alu MC, Parvataneni R, D'Agostino RB, Leon MB. Transcatheter aortic valve replacement versus surgical valve replacement in intermediate‐risk patients: a propensity score analysis. Lancet. 2016;387:2218–2225. [DOI] [PubMed] [Google Scholar]
- 10. Fanning JP, Walters DL, Platts DG, Eeles E, Bellapart J, Fraser JF. Characterization of neurological injury in transcatheter aortic valve implantation: how clear is the picture? Circulation. 2014;129:504–515. [DOI] [PubMed] [Google Scholar]
- 11. Kappetein AP, Head SJ, Généreux P, Piazza N, van Mieghem NM, Blackstone EH, Brott TG, Cohen DJ, Cutlip DE, van Es G‐A, Hahn RT, Kirtane AJ, Krucoff MW, Kodali S, Mack MJ, Mehran R, Rodés‐Cabau J, Vranckx P, Webb JG, Windecker S, Serruys PW, Leon MB. Updated standardized endpoint definitions for transcatheter aortic valve implantation. J Am Coll Cardiol. 2012;60:1438–1454. [DOI] [PubMed] [Google Scholar]
- 12. Fanning JP, Platts DG, Walters DL, Fraser JF. Transcatheter aortic valve implantation (TAVI): valve design and evolution. Int J Cardiol. 2013;168:1822–1831. [DOI] [PubMed] [Google Scholar]
- 13. Webb JG, Altwegg L, Masson J‐B, Al Bugami S, Al Ali A, Boone RA. A new transcatheter aortic valve and percutaneous valve delivery system. J Am Coll Cardiol. 2009;53:1855–1858. [DOI] [PubMed] [Google Scholar]
- 14. Walther T, Schuler G, Borger MA, Kempfert J, Seeburger J, Rückert Y, Ender J, Linke A, Scholz M, Falk V, Mohr FW. Transapical aortic valve implantation in 100 consecutive patients: comparison to propensity‐matched conventional aortic valve replacement. Eur Heart J. 2010;31:1398–1403. [DOI] [PubMed] [Google Scholar]
- 15. Bapat V, Thomas M, Hancock J, Wilson K. First successful trans‐catheter aortic valve implantation through ascending aorta using Edwards SAPIEN THV system. Eur J Cardiothorac Surg. 2010;38:811–813. [DOI] [PubMed] [Google Scholar]
- 16. Fanning JP, Wesley AJ, Wong AA, Fraser JF. Emerging spectra of silent brain infarction. Stroke. 2014;45:3461–3471. [DOI] [PubMed] [Google Scholar]
- 17. Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. [DOI] [PubMed] [Google Scholar]
- 18. Godefroy O, Fickl A, Roussel M, Auribault C, Bugnicourt JM, Lamy C, Canaple S, Petitnicolas G. Is the Montreal Cognitive Assessment superior to the Mini‐Mental State Examination to detect poststroke cognitive impairment? A study with neuropsychological evaluation. Stroke. 2011;42:1712–1716. [DOI] [PubMed] [Google Scholar]
- 19. Rudolph JL, Schreiber KA, Culley DJ, McGlinchey RE, Crosby G, Levitsky S, Marcantonio ER. Measurement of post‐operative cognitive dysfunction after cardiac surgery: a systematic review. Acta Anaesthesiol Scand. 2010;54:663–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Inouye SK, Van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method: a new method for detection of delirium. Ann Intern Med. 1990;113:941–948. [DOI] [PubMed] [Google Scholar]
- 21. Inouye SK, Leo‐Summers L, Zhang Y, Bogardus ST, Leslie DL, Agostini JV. A chart‐based method for identification of delirium: validation compared with interviewer ratings using the confusion assessment method. J Am Geriatr Soc. 2005;53:312–318. [DOI] [PubMed] [Google Scholar]
- 22. Arnold SV, Spertus JA, Lei Y, Allen KB, Chhatriwalla AK, Leon MB, Smith CR, Reynolds MR, Webb JG, Svensson LG, Cohen DJ. Use of the Kansas City Cardiomyopathy Questionnaire for monitoring health status in patients with aortic stenosis. Circ Heart Fail. 2013;6:61–67. [DOI] [PubMed] [Google Scholar]
- 23. Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35:1245–1255. [DOI] [PubMed] [Google Scholar]
- 24. Reynolds MR, Magnuson EA, Wang K, Thourani VH, Williams M, Zajarias A, Rihal CS, Brown DL, Smith CR, Leon MB, Cohen DJ. Health‐related quality of life after transcatheter or surgical aortic valve replacement in high‐risk patients with severe aortic stenosis: results from the PARTNER (Placement of AoRTic TraNscathetER Valve) Trial (Cohort A). J Am Coll Cardiol. 2012;60:548–558. [DOI] [PubMed] [Google Scholar]
- 25. Dolan P. Modeling valuations for EuroQol health states. Med Care. 1997;35:1095–1108. [DOI] [PubMed] [Google Scholar]
- 26. Rockwood K. What would make a definition of frailty successful? Age Ageing. 2005;34:432–434. [DOI] [PubMed] [Google Scholar]
- 27. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. [DOI] [PubMed] [Google Scholar]
- 28. Song X, Mitnitski A, Rockwood K. Prevalence and 10‐year outcomes of frailty in older adults in relation to deficit accumulation. J Am Geriatr Soc. 2010;58:681–687. [DOI] [PubMed] [Google Scholar]
- 29. Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. 2007;62:722–727. [DOI] [PubMed] [Google Scholar]
- 30. Mok M, Nombela‐Franco L, Urena M, Delarochellière R, Doyle D, Ribeiro HB, Côté M, Pibarot P, Delarochellière H, Laflamme L, Poirier P, Dumont E, Rodés‐Cabau J. Prognostic value of exercise capacity as evaluated by the 6‐minute walk test in patients undergoing transcatheter aortic valve implantation. J Am Coll Cardiol. 2013;61:897–898. [DOI] [PubMed] [Google Scholar]
- 31. Meller SM, Baumbach A, Voros S, Mullen M, Lansky AJ. Challenges in cardiac device innovation: is neuroimaging an appropriate endpoint? Consensus from the 2013 Yale‐UCL Cardiac Device Innovation Summit. BMC Med. 2013;11:257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Friedman J, Hastie T, Tibshirani R. Regularization paths for generalized linear models via coordinate descent. J Stat Softw. 2010;33:1–22. [PMC free article] [PubMed] [Google Scholar]
- 33. Eggebrecht H, Schmermund A, Voigtländer T, Kahlert P, Erbel R, Mehta RH. Risk of stroke after transcatheter aortic valve implantation (TAVI): a meta‐analysis of 10,037 published patients. EuroIntervention. 2012;8:129–138. [DOI] [PubMed] [Google Scholar]
- 34. Holmes DR, Brennan JM, Rumsfeld JS, Dai D, O'Brien SM, Vemulapalli S, Edwards FH, Carroll J, Shahian D, Grover F, Tuzcu EM, Peterson ED, Brindis RG, Mack MJ. Clinical outcomes at 1 year following transcatheter aortic valve replacement. JAMA. 2015;313:1019. [DOI] [PubMed] [Google Scholar]
- 35. Athappan G, Gajulapalli RD, Sengodan P, Bhardwaj A, Ellis SG, Svensson L, Tuzcu EM, Kapadia SR. Influence of transcatheter aortic valve replacement strategy and valve design on stroke after transcatheter aortic valve replacement: a meta‐analysis and systematic review of literature. J Am Coll Cardiol. 2014;63:2101–2110. [DOI] [PubMed] [Google Scholar]
- 36. Mayr NP, Hapfelmeier A, Martin K, Kurz A, van der Starre P, Babik B, Mazzitelli D, Lange R, Wiesner G, Tassani‐Prell P. Comparison of sedation and general anaesthesia for transcatheter aortic valve implantation on cerebral oxygen saturation and neurocognitive outcome. Br J Anaesth. 2016;116:90–99. [DOI] [PubMed] [Google Scholar]
- 37. Arnold M, Schulz‐Heise S, Achenbach S, Ott S, Dörfler A, Ropers D, Feyrer R, Einhaus F, Loders S, Mahmoud F, Roerick O, Daniel WG, Weyand M, Ensminger SM, Ludwig J. Embolic cerebral insults after transapical aortic valve implantation detected by magnetic resonance imaging. JACC Cardiovasc Interv. 2010;3:1126–1132. [DOI] [PubMed] [Google Scholar]
- 38. Ghanem A, Müller A, Nähle CP, Kocurek J, Werner N, Hammerstingl C, Schild HH, Schwab JO, Mellert F, Fimmers R, Nickenig G, Thomas D. Risk and fate of cerebral embolism after transfemoral aortic valve implantation: a prospective pilot study with diffusion‐weighted magnetic resonance imaging. J Am Coll Cardiol. 2010;55:1427–1432. [DOI] [PubMed] [Google Scholar]
- 39. Kahlert P, Knipp SC, Schlamann M, Thielmann M, Al‐Rashid F, Weber M, Johansson U, Wendt D, Jakob HG, Forsting M, Sack S, Erbel R, Eggebrecht H. Silent and apparent cerebral ischemia after percutaneous transfemoral aortic valve implantation: a diffusion‐weighted magnetic resonance imaging study. Circulation. 2010;121:870–878. [DOI] [PubMed] [Google Scholar]
- 40. Rodés‐Cabau J, Dumont E, Boone RH, Larose E, Bagur R, Gurvitch R, Bédard F, Doyle D, De Larochellière R, Jayasuria C, Villeneuve J, Marrero A, Côté M, Pibarot P, Webb JG. Cerebral embolism following transcatheter aortic valve implantation. J Am Coll Cardiol. 2011;57:18–28. [DOI] [PubMed] [Google Scholar]
- 41. Fairbairn T, Mather A, Bijsterveld P, Worthy G, Currie S, Goddard JP, Blackman DJ, Plein S, Greenwood JP. Diffusion‐weighted MRI determined cerebral embolic infarction following transcatheter aortic valve implantation: assessment of predictive risk factors and the relationship to subsequent health status. Heart. 2012;98:18–23. [DOI] [PubMed] [Google Scholar]
- 42. Astarci P, Glineur D, Kefer J, Hoore WD, Renkin J. Magnetic resonance imaging evaluation of cerebral embolization during percutaneous aortic valve implantation: comparison of transfemoral and trans‐apical approaches using Edwards Sapiens valve. Eur J Cardiothorac Surg. 2011;40:475–479. [DOI] [PubMed] [Google Scholar]
- 43. Uddin A, Fairbairn TA, Djoukhader IK, Igra M, Kidambi A, Motwani M, Herzog B, Ripley DP, Musa TA, Goddard AJP, Blackman DJ, Plein S, Greenwood JP. Consequence of cerebral embolism after transcatheter aortic valve implantation compared with contemporary surgical aortic valve replacement: effect on health‐related quality of life. Circ Cardiovasc Interv. 2015;8:e001913. [DOI] [PubMed] [Google Scholar]
- 44. Alli OO, Booker JD, Lennon RJ, Greason KL, Rihal CS, Holmes DR Jr. Transcatheter aortic valve implantation: assessing the learning curve. JACC Cardiovasc Interv. 2012;5:72–79. [DOI] [PubMed] [Google Scholar]
- 45. Allen LM, Hasso AN, Handwerker J, Farid H. Sequence‐specific MR imaging findings that are useful in dating ischemic stroke. Radiographics. 2012;32:1285–1297. [DOI] [PubMed] [Google Scholar]
- 46. Abdul‐Jawad Altisent O, Ferreira‐Gonzalez I, Marsal JR, Ribera A, Auger C, Ortega G, Cascant P, Urena M, Del Blanco BG, Serra V, Sureda C, Igual A, Rovira A, González‐Alujas MT, Gonzalez A, Puri R, Cuellar H, Tornos P, Rodés‐Cabau J, Garcia‐Dorado D. Neurological damage after transcatheter aortic valve implantation compared with surgical aortic valve replacement in intermediate risk patients. Clin Res Cardiol. 2016;105:508–517. [DOI] [PubMed] [Google Scholar]
- 47. Dong Y, Lee WY, Basri NA, Collinson SL, Merchant RA, Venketasubramanian N, Chen CL. The Montreal Cognitive Assessment is superior to the Mini‐Mental State Examination in detecting patients at higher risk of dementia. Int Psychogeriatr. 2012;24:1749–1755. [DOI] [PubMed] [Google Scholar]
- 48. Nombela‐Franco L, Webb JG, De Jaegere PP, Toggweiler S, Dager AE, Amat‐santos IJ, Cheung A, Ye J, Van Der Boon R, Van Mieghem NM, Benitez LM, Binder RK, Perez S, Lopez J, San Roman A, Doyle D, De Larochelliere R, Leipsic J, Dumont E, Rodés‐Cabau J. Timing, predictive factors and prognostic value of cerebrovascular events in a large cohort of patients undergoing transcatheter aortic valve implantation. J Am Coll Cardiol. 2012;126:3041–3053. [DOI] [PubMed] [Google Scholar]
- 49. Jain R, Duval S, Adabag S. How accurate is the eyeball test? A comparison of physician's subjective assessment versus statistical methods in estimating mortality risk after cardiac surgery. Circ Cardiovasc Qual Outcomes. 2014;7:151–156. [DOI] [PubMed] [Google Scholar]
- 50. Thalji NM, Suri RM, Greason KL, Schaff HV. Risk assessment methods for cardiac surgery and intervention. Nat Rev Cardiol. 2014;11:704–714. [DOI] [PubMed] [Google Scholar]
- 51. Lee JSW, Auyeung TW, Leung J, Kwok T, Woo J. Transitions in frailty states among community‐living older adults and their associated factors. J Am Med Dir Assoc. 2014;15:281–286. [DOI] [PubMed] [Google Scholar]


