Abstract
Background
During myocardial ischemia/reperfusion (I/R), a large amount of reactive oxygen species (ROS) is produced. In particular, overproduction of hydrogen peroxide (H2O2) is considered to be a main cause of I/R‐mediated tissue damage. We generated novel H2O2‐responsive antioxidant polymer nanoparticles (PVAX and HPOX) that are able to target the site of ROS overproduction and attenuate the oxidative stress‐associated diseases. In this study, nanoparticles were examined for their therapeutic effect on myocardial I/R injury.
Methods and Results
The therapeutic effect of nanoparticles during cardiac I/R was evaluated in mice. A single dose of PVAX (3 mg/kg) showed a significant improvement in both cardiac output and fraction shortening compared with poly(lactic‐coglycolic acid) (PLGA) particle, a non‐H2O2‐activatable nanoparticle. PVAX also significantly reduced the myocardial infarction/area compared with PLGA (48.7±4.2 vs 14.5±2.1). In addition, PVAX effectively reduced caspase‐3 activation and TUNEL‐positive cells compared with PLGA. Furthermore, PVAX significantly decreased TNF‐α and MCP‐1 mRNA levels. To explore the antioxidant effect of PVAX by scavenging ROS, dihydroethidium staining was used as an indicator of ROS generation. PVAX effectively suppressed the generation of ROS caused by I/R, whereas a number of dihydroethidium‐positive cells were observed in a group with PLGA I/R. In addition, PVAX significantly reduced the level of NADPH oxidase (NOX) 2 and 4 expression, which favors the reduction in ROS generation after I/R.
Conclusions
Taken together, these results suggest that H2O2‐responsive antioxidant PVAX has tremendous potential as a therapeutic agent for myocardial I/R injury.
Keywords: antioxidant, inflammation, ischemia reperfusion injury, nanotechnology
Subject Categories: Oxidant Stress, Ischemia, Heart Failure
Introduction
Reactive oxygen species (ROS), such as superoxide anion, hydrogen peroxide (H2O2), and hydroxyl radical, are chemically reactive compounds that oxidize proteins, nucleic acids, and lipids.1 ROS are derived from molecular oxygen and formed as a natural by‐product of aerobic metabolism. Under normal circumstances, ROS are important secondary messengers involved in multiple cellular signaling. The levels of ROS are controlled by antioxidants, which keep them in the picomolar range.2 However, accumulation of ROS over extended periods of time causes oxidative stress, which triggers cell death and irreversible tissue damage. Many diseases are associated with ROS‐mediated oxidative stress, including cancer, neurodegenerative diseases, and I/R injury. In particular, ROS overproduction is one of the central pathological mechanisms in cardiac I/R injury.3 It is well accepted that a large amount of ROS is generated immediately after reperfusion and may elicit cell death, such as apoptosis.4, 5
Among various ROS, superoxide anion and H2O2 are particularly important in cardiomyocytes. Superoxide anion is short‐lived and is rapidly reduced to H2O2 spontaneously or by superoxide dismutase. In contrast, H2O2 has a longer biological life span and is easily diffusible within and between cells.6 A massive accumulation of H2O2 is known to be the critical pathogenic mechanism of I/R‐induced tissue damage by releasing proinflammatory cytokines and triggering apoptosis.7 In this context, targeting the site of H2O2 overproduction and rapid reduction of excessive ROS are promising strategies to alleviate myocardial I/R injury.4, 8 Numerous antioxidants have been explored as therapeutic agents for I/R injuries.8 Various formulations of nanoparticles have also been developed as drug carriers that could release the drug payloads in spatial and temporal‐specific manners to enhance their therapeutic efficacy.9, 10
Recently, we have developed H2O2‐responsive antioxidant copolyoxalates containing hydroxybenzyl alcohol (HBA) (HPOX) and vanillyl alcohol (VA) (PVAX) that could serve as therapeutic agents for ROS‐associated diseases.11, 12 PVAX or HPOX was synthesized using naturally occurring compounds with intrinsic antioxidant and anti‐inflammatory activities, VA and HBA, respectively. These polymers were chemically engineered to possess H2O2‐responsive peroxalate ester linkages and therapeutic agents in their backbone. These antioxidant polymeric prodrug nanoparticles have a mean diameter of ~400 nm. They could be rapidly activated by H2O2 at the site of ROS generation. They prevent the I/R‐induced injuries by exerting antioxidant, anti‐inflammatory, and antiapoptotic effects.11, 12 Their therapeutic effects were explained by the synergistic effects of H2O2‐scavening peroxalate ester bonds with antioxidant and anti‐inflammatory effects of therapeutic agents (VA or HBA).11, 12 However, direct comparison between HPOX and PVAX has not been made, and their potential as therapeutic agents for myocardial I/R injury has not been evaluated. The objective of the present work is to evaluate the potential of antioxidant copolyoxalates as therapeutic agents for myocardial I/R injury. We first used a mouse model of hind limb I/R to directly compare their therapeutic effects on I/R injury. The direct comparison studies revealed that PVAX has superior therapeutic and protective effects against I/R compared to HPOX. We then utilized a mouse cardiac I/R injury model to determine the potential of PVAX as a therapeutic agent.
Methods
Synthesis of PVAX and HPOX
PVAX was synthesized using 1,4‐cyclohexanedimethanol (21.96 mmol), 4‐vanillyl alcohol (VA, 5.49 mmol), and oxalyl chloride (27.45 mmol). HPOX was synthesized using 1,4‐cyclohexanedimethanol (21.96 mmol), and 4‐hydroxybenzyl alcohol (HBA, 5.49 mmol), and oxalyl chloride (27.45 mmol) in dry dichloromethane.
Preparation and Administration of PVAX and HPOX Nanoparticles
HPOX or PVAX nanoparticles were generated using an emulsion/solvent evaporation method as previously reported.11, 12 In brief, HPOX or PVAX dissolved in DCM was added to 5 mL of 10% (w/v) polyvinyl alcohol (PVA) solution. The mixtures were sonicated and then homogenized to form fine oil/water emulsions. The emulsion was transferred to a 20‐mL PVA (1% w/v) solution and homogenized for another 1 minute. The remaining solvent was removed using a rotary evaporator. The particles were then centrifuged and washed with deionized water 3 times to remove the residual PVA. The suspension was then frozen in liquid nitrogen and lyophilized to produce free‐flowing particles. One milligram of HPOX or PVAX was dissolved in 1 mL of phosphate‐buffered saline (PBS). PVAX or HPOX was administered into the gastrocnemius muscles in hind limb I/R injury model. PVAX was administered intraperitoneally in the cardiac I/R injury model.
Sensitivity of PVAX and HPOX to H2O2
For the evaluation of H2O2 scavenging ability, 1 mg of PVAX or HPOX nanoparticles was added in 1 mL of 10 μmol/L H2O2 solution. One minute after the addition, the concentration of H2O2 solution was determined using the Amplex Red assay kit (Invitrogen, Carlsbad, CA). For the H2O2 sensitivity assay, rubrene‐loaded PVAX or HPOX nanoparticles were prepared as previously reported.11, 12 The chemiluminescent nanoparticles were added in H2O2 solutions with various concentrations. The chemiluminescent intensity was measured using a luminometer (Femtomaster FB12, Zylux Corp, Huntsville, AL).
Flow Cytometry
RAW264.7 cells were cultured for 24 hours in a 24‐well culture plate prior to the experiments. Cells were treated with 100 μg of PVAX or HPOX nanoparticles for 6 hours, and then intracellular ROS generation was induced by treatment with 1 μg of phorbol‐12‐myristate‐13‐acetate (PMA). For the detection of ROS, the cell suspensions were transferred to a 5‐mL culture tube and stained with 5 μmol/L dichlorofluorescein diacetate (DCFH‐DA), followed by gentle mixing. Apoptotic cell death was induced by the addition of 300 μmol/L of H2O2. For the analysis of apoptosis, the cell suspensions were treated with Annexin V‐FITC. The stained cells were analyzed by flow cytometry (FACS Calibur, Becton Dickinson, San Jose, CA). A total of 1.0×104 events were counted for each sample.
Animal Surgeries
Animal surgeries were performed in 12‐ to 13‐week‐old male C57/B mice (Charles River Laboratory, Wilmington, MA). For hind limb I/R surgery, a femoral artery was identified and tied around a specialized 30G catheter with a 7‐0 silk suture after mice were anesthetized with inhalant isoflurane (2%). Full anesthesia was verified with toe pinching. The animal remained under anesthesia for a specified duration of ischemia. Reperfusion was achieved by cutting the suture and reestablishing arterial blood flow. Sham‐operated mice underwent the same procedure without the femoral artery occlusion/reperfusion. For cardiac I/R surgery, mice were anaesthetized, intubated, and placed on a rodent ventilator (model 687, Harvard Respirator). After thoracotomy, the left anterior descending artery (LAD) artery was identified and ligated with a 7‐0 silk suture tied around a specialized 30G catheter. The animal remained under anesthesia and ventilation for 45 minutes of ischemia. Reperfusion was achieved by cutting the suture and reestablishing arterial perfusion. Sham‐operated mice underwent the same procedure without LAD occlusion and reperfusion. Mice were sacrificed by heart extraction after anesthetizing with inhalant isoflurane (2%). Animal experiments performed conform to the NIH guidelines (Guide for the Care and Use of Laboratory Animals) on the protection of animals used for scientific purposes. All experimental procedures were approved by the Institutional Animal Care and Use Committee of Beth Israel Deaconess Medical Center.
Cardiac Functional Analysis
Cardiac function was measured in mice using the left ventricle (LV) pressure‐volume loop and echocardiography 2 weeks after I/R surgery or sham control. After 2% isoflurane inhalant anesthesia, the pressure‐volume parameters were measured using a 1.4Fr microtip pressure‐volume catheter (ScisenseInc, Ontario, Canada). The catheter was first inserted into the right common carotid artery and then gently advanced into the LV to obtain LV hemodynamic parameters. Data were recorded using a Powerlab system (ADInstruments, Colorado Springs, CO). Beat‐by‐beat pressure‐volume parameters including heart rate (HR), stroke volume (SV), stroke work (SW), and cardiac output (CO) were measured and analyzed using CardioSoft Pro software (CardioSoft, Houston, TX). Transthoracic echocardiography was performed using a Vevo2100 echocardiogram (VisualSonics, Toronto, Canada) with a MS400 (18–38 MHz) transducer at baseline and 2 weeks after I/R. Two‐dimensional guided M‐mode images were recorded.
Infarct Size Determination
Infarct size was measured 24 hours after I/R surgery as described previously.13 After thoracotomy, the LAD was religated, and 50 μL green fluorescent FluoSpheres (Molecular Probe, Carlsbad, CA) was injected into the LV of the heart to delineate the area at risk (FluoSpheres‐negative area). The heart was then excised, fixed, and sectioned into 1‐mm perpendicular sections. Sections were stained with 2,3,5‐triphenyltetrazolium chloride (TTC) (Sigma Chemical, St. Louis, MO) solution to determine the infarcted myocardium (TTC‐negative area). The infarction area (IA) and the area at risk (AAR) were determined for each slice using a computer planimetry and NIH Image software.
Reverse Transcriptase‐Polymerase Chain Reaction Analysis for mRNA Expression
Heart tissues were collected for molecular analysis after I/R. Reverse transcriptase‐polymerase chain reaction (RT‐PCR) was performed as described previously.11, 12 The mRNA expression levels were analyzed by RT‐PCR using specific primers. Ribosomal 18S primers acted as internal controls, and all RT‐PCR signals were normalized to the 18S signal of the corresponding RT product to eliminate the measurement error from uneven sample loading and to provide a semiquantitative measure of the relative changes in gene expression. The PCR primers used in this study are listed below: sense TNF‐α, 5‐CCTCAG CCTCTTCTCCTTCCT‐3; antisense TNF‐α, 5‐GGTGTGGGTGAGGAGCA‐3; sense MCP‐1, 5‐CCCCACTCACCTGCTGCTACT‐3; antisense MCP‐1, 5‐GCATCACAGTCCGAGTCACA ‐3; sense 18S, 5‐GTTATGGTTCCTTTGTCGCTCGCTC‐3, anti‐sense 18S, 5‐TCGGCCCGAGGTTATCTAGAGTCAC‐3.
Caspase‐3 and PARP Activity Assay
The activity of caspase‐3 was determined with a colorimetric assay kit (R&D Systems, Minneapolis, MN) as described previously.11, 12 Briefly, protein samples were added to the substrates of Acetyl‐Asp‐Glu‐Val‐Asp‐p‐nitroanilide. The enzyme‐catalyzed release of p‐nitroanilide was measured at 405 nm. For PARP activity assay, activity was measured at 450 nm for incorporation of biotinylated poly(ADP‐ribose) onto histone‐coated proteins in a plate using a colorimetric assay kit (R&D Systems, Minneapolis, MN) as described previously.11
Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling Staining and Dihydroethidium Staining
Terminal deoxynucleotidyl transferase dUTP nick‐end labeling (TUNEL) staining was performed using an in situ cell death detection kit, fluorescein (Roche Applied Science, Indianapolis, IN) as described previously.11, 12 To distinguish cardiomyocyte from noncardiomyocyte nuclei, we used triple staining for nuclei (4′,6‐diamidino‐2‐phenylindole [DAPI] staining), apoptotic nuclei (TUNEL staining), and cardiomyocytes (α‐actinin staining) and analyzed the stained sections using confocal microscopy. For dihydroethidium (DHE) staining, tissue sections were incubated with 5 μmol/L of DHE (Sigma‐Aldrich, St. Louis, MO) at 37°C for 30 minutes in a humidified chamber protected from light, and then DAPI was applied. Images were acquired by confocal fluorescent microscope.
Western Immunoblot
NADPH oxidase 2 (NOX2) and NOX4 proteins were probed with an anti‐NOX2 and anti‐NOX4 rabbit monoclonal antibody (Abcam, Cambridge, MA). Glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) was used as an internal control for loading. Resulting bands were quantified as optical density (OD)×band area by ImageJ analysis system and recorded as arbitrary units.
Primary Adult Rat Ventricular Cardiomyocyte Culture
The primary culture of adult rat ventricular cardiomyocytes (ARVC) was prepared from the hearts of female rats as described previously.14 Rat hearts were extracted after anesthesia with inhalant isoflurane (2%). For cell viability, a 3‐(4,5‐dimethylthiazil‐2yl)‐2,5‐diphenyltetrazolium bromide (MTT) assay was performed according to the manufacturer's instructions (Sigma, St. Louis, MO), and as previously reported.14 Briefly, cells were treated with MTT for 3 hours. Formed formazan crystals were dissolved with dimethyl sulfoxide while plates were agitated on a shaker for 30 minutes in the dark. Absorbance was measured at 570 nm with a reference wavelength at 690 nm.
Statistical Analyses
Data were expressed as means±SEM. For continuous data following a normal distribution, differences between two groups were analyzed by an unpaired 2‐tailed Student t test. Differences among more than 2 groups were analyzed by 1‐way ANOVA with Bonferroni post hoc test. For data not following a normal distribution, differences between 2 groups were analyzed by Mann‐Whitney test, and multiple groups by Kruskal‐Wallis test. R2 values provide linear relationship between 2 groups. All the statistics were calculated using GraphPad Prism 5.0 (San Diego, CA). Probability (P) values of <.05 were considered significant.
Results
H2O2‐Responsiveness of PVAX and HPOX
PVAX and HPOX were synthesized as H2O2‐responsive antioxidant polymeric prodrugs of VA and HBA, respectively (Figure 1A). Both polymers had average molecular weights of ~15 000 Da and formed nanoparticles with mean diameters of ~400 nm with smooth surfaces.11, 12 Although there was no change in H2O2 concentration at each time point in the vehicle control, both PVAX and HPOX nanoparticles (1 mg/mL) significantly eliminated H2O2 in a time‐dependent manner (Figure 1B). However, PVAX nanoparticles showed a stronger H2O2‐scavenging ability than HPOX nanoparticles. The stronger H2O2‐scavenging ability of PVAX nanoparticles over HPOX nanoparticles can be explained by the superior H2O2‐scavenging ability of VA, which may be due to the presence of a methoxy group.15 Their sensitivity to H2O2 was also evaluated based on peroxalate chemiluminescence. Rubrene as a fluorophore was encapsulated in the PVAX and HPOX nanoparticles, and the chemiluminescent nanoparticles were added into H2O2 solutions of various concentrations. Both chemiluminescent nanoparticles showed a linear correlation between chemiluminescence intensity and H2O2 concentration (Figure 1C). However, PVAX nanoparticles showed higher emission intensity than HPOX nanoparticles.
Figure 1.
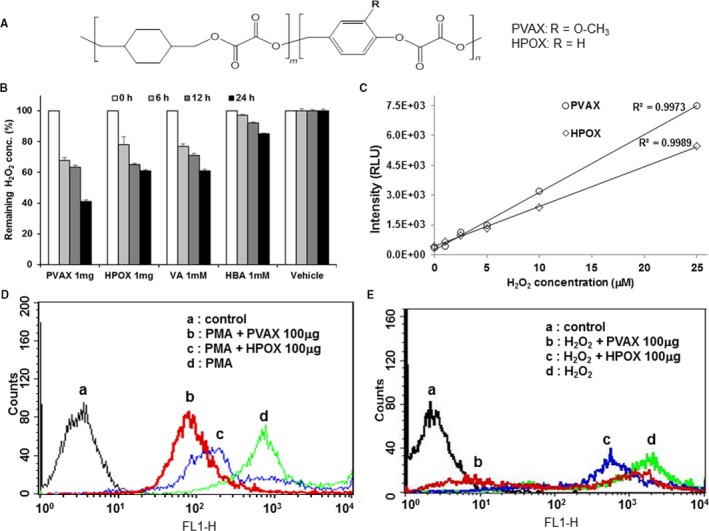
Chemical and biological properties of PVAX and HPOX nanoparticles. A, Chemical structure of antioxidant copolyoxalates, PVAX and HPOX. B, Scavenging of H2O2 by PVAX and HPOX nanoparticles. C, Sensitivity of PVAX and HPOX nanoparticles to H2O2 based on peroxalate chemiluminescence. D, Inhibitory effects of PVAX and HPOX nanoparticles on ROS generation in PMA‐stimulated cells. E, Inhibitory effects of PVAX and HPOX nanoparticles on H2O2‐induced apoptotic cell death. PMA indicates phorbol‐12‐myristate‐13‐acetate; ROS, reactive oxygen species.
In addition, we compared the antioxidant and antiapoptotic activities of PVAX and HPOX nanoparticles in vitro. RAW264.7 cells were stimulated with PMA to induce ROS generation (Figure 1D). The generation of ROS in PMA‐stimulated cells was investigated using a ROS probe, DCFH‐DA, which becomes fluorescent on activation by ROS such as H2O2 and hydroxyl radical. Both HPOX and PVAX showed decreases in their fluorescent intensity compared to the PMA alone, but PVAX induced a greater reduction in the fluorescence intensity than HPOX, indicating that PVAX have more inhibitory effects on PMA‐induced ROS generation. Antiapoptotic effects of both nanoparticles on H2O2‐stimulated cells were evaluated by staining with annexin V‐FITC as a marker of apoptosis (Figure 1E). PVAX again exerted stronger antiapoptotic activity in H2O2‐stimulated cells than HPOX.
Comparison of PVAX and HPOX Using a Mouse Model of Hind Limb I/R Injury
To compare antiapoptotic and anti‐inflammatory activities of HPOX and PVAX nanoparticles, a mouse model of hind limb I/R injury was used. I/R injury was induced by tying the femoral artery with a suture for 1 hour, followed by reperfusion. PVAX and HPOX (50 and 100 μg) were directly injected just distal to the ligation site, and their therapeutic effects were compared by measuring the level of anti‐inflammatory and antiapoptotic activities. The activities of caspase‐3 and polyADP ribose polymerase (PARP) were analyzed to measure the level of apoptosis. I/R injury caused significant elevation of caspase‐3 and PARP activities (Figure 2A and 2B). Both PVAX and HPOX nanoparticles significantly decreased the activities of caspase‐3 and PARP in a dose‐dependent manner. However, PVAX showed significantly greater antiapoptotic activity than HPOX. The levels of tumor necrosis factor‐α (TNF‐α) and monocyte chemotactic protein‐1 (MCP‐1) were evaluated as markers of inflammation. Both nanoparticles significantly suppressed I/R‐induced expression of mRNA of TNF‐α and MCP‐1 (Figure 2C through 2E). In particular, 100 μg of PVAX almost completely suppressed the expression of mRNA of TNF‐α and MCP‐1. In addition, H&E staining demonstrated that muscle damage caused by I/R injury was significantly inhibited by PVAX (Figure 2F). Based on these findings, we conclude that PVAX has superior anti‐inflammatory and antiapoptotic activities against ROS‐associated diseases than HPOX. Thus, PVAX was selected as a therapeutic agent for subsequent cardiac I/R injury experiments.
Figure 2.

The therapeutic effects of PVAX and HPOX nanoparticles on hind limb I/R injury. A and B, Quantification of caspase‐3 (A) and PARP activity (B) *P<0.05 vs sham of each group; † P<0.05 vs HPOX I/R. (n=4/group). Mann‐Whitney test was used. C, Expression of inflammatory markers TNF‐α and MCP‐1 in hind limb I/R injury. D and E, Quantification of TNF‐α (D) and MCP‐1 (E) mRNA level, *P<0.05 vs sham of each group; † P<0.05 vs HPOX I/R. (n=4/sham group, n=6/I/Rgroup). ANOVA and Bonferroni test were used. F, Representative images of gastrocnemius muscle tissues stained by hematoxylin and eosin (×200). I/R indicates ischemia/reperfusion; MCP‐1, monocyte chemotactic protein‐1; TNF‐α, tumor necrosis factor‐α.
Therapeutic Effects of PVAX Nanoparticles on H2O2‐Stimulated Adult Rat Cardiomyocytes
First, antioxidant and anti‐inflammatory activities of PVAX nanoparticles were studied using the primary culture of adult rat cardiomyocytes (ARVC). To test protective effect of PVAX against oxidative stress in vitro, we examined H2O2‐induced cell death in ARVC. Compared to the vehicle control, PVAX showed significant protection from H2O2‐induced cell death in a concentration dependent manner (Figure 3A). Treatment with H2O2 (250 μmol/L) significantly increased expression of inflammatory markers, such as TNF‐α, MCP‐1, NOX2, and NOX4 (Figure 3B through 3F). PVAX treatment effectively blocked the H2O2‐induced TNF‐α, MCP‐1, NOX2, and NOX4 activation.
Figure 3.

The therapeutic effect of PVAX on H2O2‐stimulated adult rat cardiomyocytes in vitro. A, Protective effects of PVAX nanoparticles on H2O2‐stimulated cardiomyocytes. (n=3/group). Mann‐Whitney test was used. B, Expression of TNF‐α and MCP‐1. The images are representatives of 4 experiments. C through F, Quantification of TNF‐α (C), MCP‐1 (D), NOX2 (E), and NOX4 (F). *P<0.05 vs sham of each group. (n=4/group). The Mann‐Whitney test was used. MCP‐1 indicates monocyte chemotactic protein‐1; NOX2, NADPH oxidase 2; NOX4, NADPH oxidase 4; TNF‐α, tumor necrosis factor‐α.
Therapeutic Effects of PVAX Nanoparticles on Cardiac Functions After I/R Injury
Because reperfusion of the ischemic myocardium triggers further production of ROS causing myocardial dysfunction, we evaluated the beneficial effects of H2O2‐responsive PVAX nanoparticles using a mouse model of cardiac I/R injury. Previous studies have demonstrated that cardiac function is decreased 2 weeks after I/R injury.16, 17, 18 Thus, the cardiac functions were evaluated 2 weeks after cardiac I/R injury using PV loop analysis and echocardiogram. The overproduction of ROS at the time of reperfusion is the critical time when the tissue injuries likely occur, so we initially compared single administration of PVAX (3 mg/kg) 10 minutes before reperfusion with daily injection of PVAX (3 mg/kg per day). Interestingly, single administration of PVAX (3 mg/kg) was comparable to daily injection of PVAX in terms of attenuating cardiac dysfunction (Figure 4A and 4B). Therefore, we chose single‐dose administration of PVAX for our further experiments.
Figure 4.
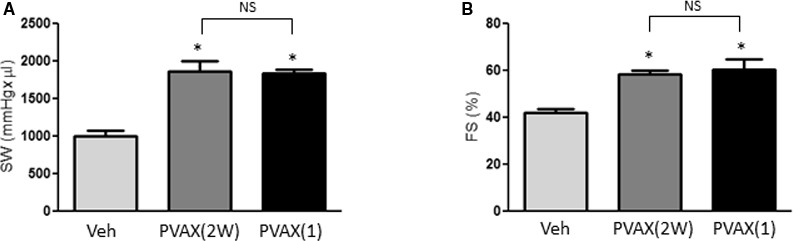
Effect of different PVAX nanoparticle administration methods on I/R‐induced cardiac dysfunction. A and B, Cardiac functions after I/R were evaluated after vehicle (Veh), daily injection of PVAX (3 mg/kg per day for 2 weeks) (2W), or single injection of PVAX (3 mg/kg) (1). Stroke work (SW) (A) was determined using PV loop analysis. Fractional shortening (FS) (B) was measured using echocardiography. *P<0.05 vs Veh. NS=not significant (n=6/veh, n=4/PVAX 2W and 1 group). The Kruskal‐Wallis and Dunn tests were used. I/R indicates ischemia/reperfusion.
Next, the beneficial effects of PVAX after cardiac I/R were compared with poly(lactic‐coglycolic acid) (PLGA) nanoparticles, which have no H2O2 responsivess, as a vehicle comparison. Administration of PLGA nanoparticles before reperfusion did not improve I/R‐induced cardiac dysfunction when evaluated with PV loop and echocardiography analyses (Figure 5A through 5C). There was no mortality after I/R surgery in both PLGA and PVAX I/R groups. However, significant differences of cardiac function parameters were observed between PVAX‐ and PLGA‐treated animals after I/R. In addition, we evaluated infarct size 24 hours after I/R. Consistent with cardiac function evaluation, PVAX nanoparticles significantly reduced the infarct size compared with the PLGA group (48.7±4.2 vs 14.5±2.1, P<0.05) (Figure 5D through 5F).
Figure 5.
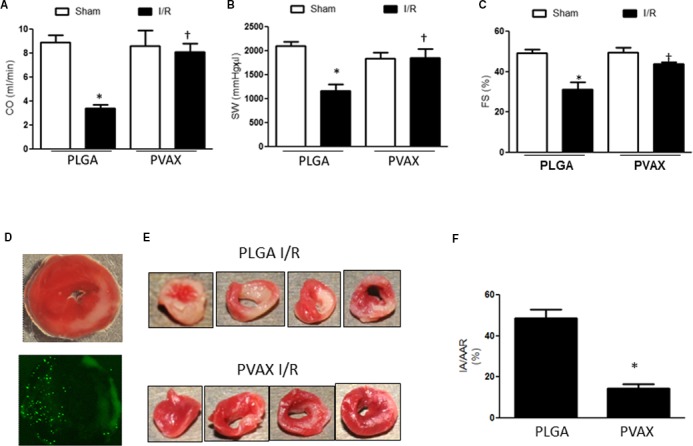
Effect of PVAX and PLGA nanoparticles on I/R‐induced cardiac dysfunction. A, Cardiac output (CO), (B) stroke work (SW), and (C) fractional shortening (FS) 2 weeks after I/R in mice after PLGA or PVAX (3 mg/kg, single injection). *P<0.05 vs sham of each group; † P<0.05 vs PLGA I/R. (n=5/sham group, n=6/IR group). ANOVA and a Bonferroni test were used. D, Representative images of heart tissues after I/R injury after TTC staining (top) or fluorescent microsphere administration (bottom). E, Representative heart tissue sections stained with TTC solution. Red‐colored regions in the TTC‐stained sections indicate nonischemic areas; pale‐colored regions indicate ischemic portions of the heart. F, Quantification of infarction area (IA)/area at risk (AAR) *P<0.05 (n=4/PLGA, n=5/PVAX). Student t test was used. I/R indicates ischemia/reperfusion; TTC, triphenyltetrazolium chloride.
Cell death and inflammation are important mechanisms involved in cardiac I/R injury. To determine whether the beneficial effects of PVAX nanoparticles are associated with their antiapoptotic and anti‐inflammatory activities, we examined the level of apoptosis and inflammation after I/R. PVAX significantly reduced caspase‐3 activation and the TUNEL‐positive cells after I/R compared to the PLGA‐treated group (Figure 6A and 6B). In addition, PVAX significantly decreased TNF‐α and MCP‐1 mRNA expressions compared to I/R in PLGA mice (Figure 6C through 6E). These results indicate that PVAX effectively prevents I/R‐induced damage by inhibiting inflammation and apoptosis.
Figure 6.
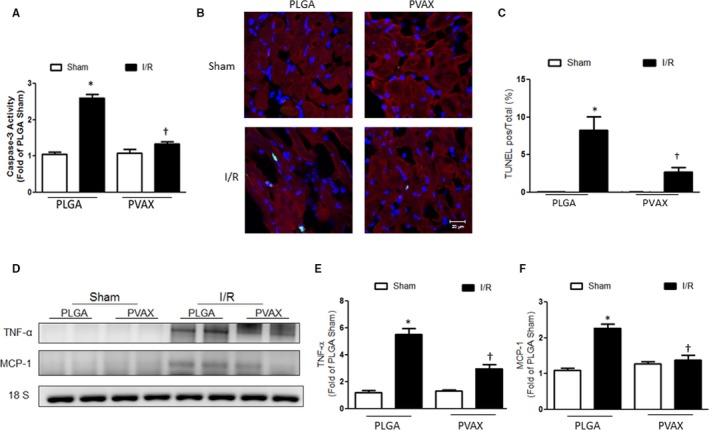
Anti‐inflammatory and antiapoptotic activities of PVAX nanoparticles after cardiac I/R injury. A, Quantification of caspase‐3 activity assay. *P<0.05 vs sham of each group; † P<0.05 vs PLGA I/R (n=4/sham group, n=5 per I/R group). ANOVA and a Bonferroni test were used. B, Representative images of TUNEL staining. TUNEL, green; nuclei, blue; α‐actinin, red. C, Quantification of TUNEL‐positive cells/total cells. *P<0.05 vs sham of each group; † P<0.05 vs PLGA I/R. (n=4/sham group, n=5 per I/R group). ANOVA and a Bonferroni test were used. D, Expression of inflammatory markers TNF‐α and MCP‐1. E and F, Quantification of TNF‐α (E) and MCP‐1 (F) mRNA level. *P<0.05 vs sham of each group; † P<0.05 vs PLGA I/R. (n=4/sham group, n=5 per I/R group). ANOVA and a Bonferroni test were used. I/R indicates ischemia/reperfusion; TUNEL, terminal deoxynucleotidyl transferase dUTP nick‐end labeling; TNF‐α, tumor necrosis factor‐α.
Effects of PVAX on NOX‐Induced ROS Generation After Cardiac I/R
Recent studies show that NOX‐derived ROS plays a pivotal role in cardiac I/R. The increased level of NOX activity/expression and NOX‐dependent ROS production influence several key components of cardiac remodeling, such as myocyte hypertrophy, contractile dysfunction, apoptosis, and fibrosis.19 To investigate antioxidant activities of PVAX, heart tissues were stained with DHE as a fluorescent marker of ROS. A number of DHE‐positive cells were observed in a PLGA I/R group, indicating that I/R injury induced a large generation of ROS. PVAX nanoparticles effectively reduced the generation of ROS caused by I/R (Figure 7A). We also investigated the effects of PVAX nanoparticles on the expression of NOX2 and NOX4 24 hours after reperfusion. The levels of NOX2 and NOX4 were elevated after I/R (Figure 7B through 7D). However, PVAX nanoparticles significantly reduced the levels of NOX2 and NOX4 compared to PLGA nanoparticles. These results suggest that the reduction of ROS production is attributed to the blockade of NOX2 and NOX4 expression.
Figure 7.
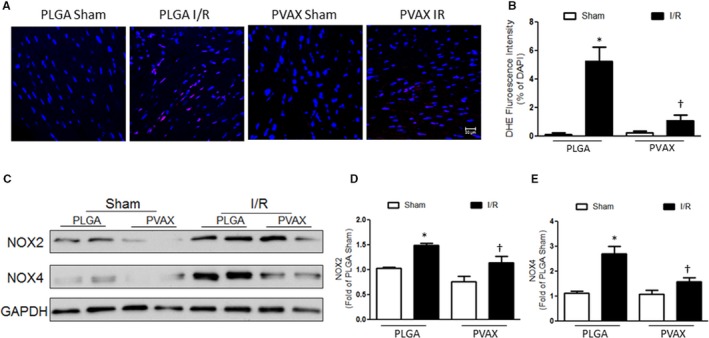
Antioxidant activities of PVAX nanoparticles after cardiac I/R injury. A, Representative image of DHE staining. DHE=red, nuclei=blue. B, Quantification of DHE‐positive nuclei. *P<0.05 vs sham of each group; † P<0.05 vs PLGA I/R (n=4/sham group, n=5 per I/R group). ANOVA and a Bonferroni test were used. C, Representative images of mRNA expression of NOX2 and NOX4. D and E, Quantification of NOX2 (D) and NOX4 (E) expression. *P<0.05 vs sham of each group; † P<0.05 vs PLGA I/R (n=4/sham group, n=5 per I/R group). ANOVA and a Bonferroni test were used. DHE indicates dihydroethidium; I/R, ischemia/reperfusion; NOX2, NADPH oxidase 2.
Discussion
Although mechanisms of cardiac I/R injury are complex, ROS are major initiators of myocardial damage during I/R. With an elevated level of ROS, cells experience oxidative stress, which then could lead to irreversible damages due to proinflammatory cytokine release, apoptosis, and interstitial fibrosis. Among various ROS, H2O2 is the most abundant and critical for redox signaling because of its long half‐life and diffusion distance (~1‐1600 μm).6 In particular, overproduction of H2O2 is an important pathogenic mechanism of I/R‐induced tissue damage through an increase in proinflammatory cytokines such as TNF‐α, through activation of p38 MAPK.20 Excessive ROS after reperfusion also resulted in a marked increase in infarct size, and counteracting the ROS generation reduced the infarct size by up to 50%.6 Thus, great efforts have been made to develop therapeutic strategies to attenuate I/R injury by suppressing ROS generation and inflammation.13, 21, 22, 23 Antioxidant nanoparticles formulated from cerium, yttrium, and platinum have been reported to protect against the progression of cardiac dysfunction and remodeling by attenuation of myocardial oxidative stress and inflammatory processes, although there are concerns regarding their toxicity.24, 25, 26 Nanoparticle‐based drug delivery systems have also been developed for targeted delivery of antioxidant therapeutics to reduce infarct volumes and provide sufficient cardioprotective effects during acute myocardial infarction.10, 27 However, various antioxidant strategies failed to reveal their beneficial effects in the clinical setting, and their therapeutic effects were not clearly elucidated.
Ineffectiveness of antioxidant therapy could be explained by various reasons, such as incorrect dosing, dosing frequency, late administration, and inappropriate biomarkers of oxidative damage. However, one of the most likely reasons for the failure of antioxidant strategies for I/R injury could be the lack of interactions between the therapeutic antioxidant and the specific ROS driving the pathological conditions.8 In this context, scavenging of overproduced H2O2 in the site of oxidative stress is a logical strategy for effective treatment of myocardial I/R injury. In this present study we evaluated the potential of PVAX nanoparticles, which are rapidly activated by H2O2, to exert potent antioxidant, anti‐inflammatory, and antiapoptotic activities during myocardial I/R. PVAX nanoparticles dramatically reduced the ROS‐mediated oxidative stress and effectively attenuated I/R injury with reduction of infarct size and improvement of cardiac function.
The present study also demonstrated that PVAX nanoparticles effectively blocked NOX‐derived ROS overproduction. It is well known that NOX2 and NOX4, electron‐transporting membrane enzymes, are major components of the NOX isoforms that produce ROS, particularly superoxide anions and H2O2, in heart.28 NOX2 is involved in cardiac remodeling after myocardial infarction, whereas NOX4 is a critical mediator of mitochondrial oxidative stress and mitochondrial dysfunction during heart failure.29, 30 Previous studies reported that I/R induced significant increases in the levels of NOX2 and NOX4 expression.31 In addition, knockdown of NOX2 or NOX4 led to a significant decrease in the infarct size accompanied with decrease in cardiomyocyte apoptosis after I/R. We found that PVAX nanoparticles significantly reduced the level of NOX2 and NOX4 expression, which favors the reduction in ROS generation after I/R. The down‐regulation of NOX2 and NOX4 may not reflect a true decrease in the activity of the NOXs to generate ROS. However, previous study showed that down‐regulation of a single NOX is sufficient to achieve a >50% reduction of ROS.31 Altogether, blocking the undesirable actions of NOXs with PVAX nanoparticles could be a logical therapeutic strategy for treating oxidative stress‐related pathologies such as I/R injury and neurodegenerative and metabolic diseases.
Sources of Funding
This work was supported in part by the grants from NIH 1 R44 DK103389‐01 (P.M. Kang), Brain Korea 21 Plus program from Ministry of Education, Science and Technology, Korea, (D. Lee, P.M. Kang), Korea Health Technology R&D Project (HI13C1370) (D. Lee), and Handok Pharmaceuticals (P.M. Kang, S.U. Lee). S. Dilmen was supported by a Scientific and Technological Research Council of Turkey (TÜBİTAK) grant.
Disclosures
None.
(J Am Heart Assoc. 2016;5:e003697 doi: 10.1161/JAHA.116.003697)
Contributor Information
Dongwon Lee, Email: dlee@jbnu.ac.kr.
Peter M. Kang, Email: pkang@bidmc.harvard.edu.
References
- 1. Santiago E, Contreras C, Garcia‐Sacristan A, Sanchez A, Rivera L, Climent B, Prieto D. Signaling pathways involved in the H2O2‐induced vasoconstriction of rat coronary arteries. Free Radic Biol Med. 2013;60:136–146. [DOI] [PubMed] [Google Scholar]
- 2. Taverne YJ, Bogers AJ, Duncker DJ, Merkus D. Reactive oxygen species and the cardiovascular system. Oxid Med Cell Longev. 2013;2013:862423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Munzel T, Gori T, Bruno RM, Taddei S. Is oxidative stress a therapeutic target in cardiovascular disease? Eur Heart J. 2010;31:2741–2748. [DOI] [PubMed] [Google Scholar]
- 4. Bolli R, Jeroudi MO, Patel BS, DuBose CM, Lai EK, Roberts R, McCay PB. Direct evidence that oxygen‐derived free radicals contribute to postischemic myocardial dysfunction in the intact dog. Proc Natl Acad Sci USA. 1989;86:4695–4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gottlieb RA, Burleson KO, Kloner RA, Babior BM, Engler RL. Reperfusion injury induces apoptosis in rabbit cardiomyocytes. J Clin Invest. 1994;94:1621–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Slezak J, Tribulova N, Pristacova J, Uhrik B, Thomas T, Khaper N, Kaul N, Singal PK. Hydrogen peroxide changes in ischemic and reperfused heart. Cytochemistry and biochemical and x‐ray microanalysis. Am J Pathol. 1995;147:772–781. [PMC free article] [PubMed] [Google Scholar]
- 7. Lee D, Khaja S, Velasquez‐Castano JC, Dasari M, Sun C, Petros J, Taylor WR, Murthy N. In vivo imaging of hydrogen peroxide with chemiluminescent nanoparticles. Nat Mater. 2007;6:765–769. [DOI] [PubMed] [Google Scholar]
- 8. Rosenbaugh EG, Savalia KK, Manickam DS, Zimmerman MC. Antioxidant‐based therapies for angiotensin II‐associated cardiovascular diseases. Am J Physiol Regul Integr Comp Physiol. 2013;304:R917–R928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kim KS, Khang G, Lee D. Application of nanomedicine in cardiovascular diseases and stroke. Curr Pharm Des. 2011;17:1825–1833. [DOI] [PubMed] [Google Scholar]
- 10. Yun X, Maximov VD, Yu J, Zhu H, Vertegel AA, Kindy MS. Nanoparticles for targeted delivery of antioxidant enzymes to the brain after cerebral ischemia and reperfusion injury. J Cereb Blood Flow Metab. 2013;33:583–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee D, Bae S, Hong D, Lim H, Yoon JH, Hwang O, Park S, Ke Q, Khang G, Kang PM. H2O2‐responsive molecularly engineered polymer nanoparticles as ischemia/reperfusion‐targeted nanotherapeutic agents. Sci Rep. 2013;3:2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee D, Bae S, Ke Q, Lee J, Song B, Karumanchi SA, Khang G, Choi HS, Kang PM. Hydrogen peroxide‐responsive copolyoxalate nanoparticles for detection and therapy of ischemia‐reperfusion injury. J Control Release. 2013;172:1102–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang J, Marden JJ, Fan C, Sanlioglu S, Weiss RM, Ritchie TC, Davisson RL, Engelhardt JF. Genetic redox preconditioning differentially modulates AP‐1 and NF kappa B responses following cardiac ischemia/reperfusion injury and protects against necrosis and apoptosis. Mol Ther. 2003;7:341–353. [DOI] [PubMed] [Google Scholar]
- 14. Choudhury S, Bae S, Kumar SR, Ke Q, Yalamarti B, Choi JH, Kirshenbaum LA, Kang PM. Role of AIF in cardiac apoptosis in hypertrophic cardiomyocytes from Dahl salt‐sensitive rats. Cardiovasc Res. 2010;85:28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jang YW, Lee JY, Kim CJ. Anti‐asthmatic activity of phenolic compounds from the roots of Gastrodia elata Bl. Int Immunopharmacol. 2010;10:147–154. [DOI] [PubMed] [Google Scholar]
- 16. Choudhury S, Bae S, Ke Q, Lee JY, Kim J, Kang PM. Mitochondria to nucleus translocation of AIF in mice lacking Hsp70 during ischemia/reperfusion. Basic Res Cardiol. 2011;106:397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pawlinski R, Tencati M, Hampton CR, Shishido T, Bullard TA, Casey LM, Andrade‐Gordon P, Kotzsch M, Spring D, Luther T, Abe J, Pohlman TH, Verrier ED, Blaxall BC, Mackman N. Protease‐activated receptor‐1 contributes to cardiac remodeling and hypertrophy. Circulation. 2007;116:2298–2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang JX, Jiao JQ, Li Q, Long B, Wang K, Liu JP, Li YR, Li PF. MIR‐499 regulates mitochondrial dynamics by targeting calcineurin and dynamin‐related protein‐1. Nat Med. 2011;17:71–78. [DOI] [PubMed] [Google Scholar]
- 19. Brandes RP, Weissmann N, Schroder K. Redox‐mediated signal transduction by cardiovascular NOX NADPH oxidases. J Mol Cell Cardiol. 2014;73:70–79. [DOI] [PubMed] [Google Scholar]
- 20. Meldrum DR, Dinarello CA, Cleveland JC Jr, Cain BS, Shames BD, Meng X, Harken AH. Hydrogen peroxide induces tumor necrosis factor alpha‐mediated cardiac injury by a p38 mitogen‐activated protein kinase‐dependent mechanism. Surgery. 1998;124:291–296; discussion 297. [DOI] [PubMed] [Google Scholar]
- 21. Adlam VJ, Harrison JC, Porteous CM, James AM, Smith RA, Murphy MP, Sammut IA. Targeting an antioxidant to mitochondria decreases cardiac ischemia‐reperfusion injury. FASEB J. 2005;19:1088–1095. [DOI] [PubMed] [Google Scholar]
- 22. Yarbrough WM, Mukherjee R, Stroud RE, Meyer EC, Escobar GP, Sample JA, Hendrick JW, Mingoia JT, Spinale FG. Caspase inhibition modulates left ventricular remodeling following myocardial infarction through cellular and extracellular mechanisms. J Cardiovasc Pharmacol. 2010;55:408–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Guo J, Wang SB, Yuan TY, Wu YJ, Yan Y, Li L, Xu XN, Gong LL, Qin HL, Fang LH, Du GH. Coptisine protects rat heart against myocardial ischemia/reperfusion injury by suppressing myocardial apoptosis and inflammation. Atherosclerosis. 2013;231:384–391. [DOI] [PubMed] [Google Scholar]
- 24. Niu J, Azfer A, Rogers LM, Wang X, Kolattukudy PE. Cardioprotective effects of cerium oxide nanoparticles in a transgenic murine model of cardiomyopathy. Cardiovasc Res. 2007;73:549–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yamagishi Y, Watari A, Hayata Y, Li X, Kondoh M, Yoshioka Y, Tsutsumi Y, Yagi K. Acute and chronic nephrotoxicity of platinum nanoparticles in mice. Nanoscale Res Lett. 2013;8:395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schubert D, Dargusch R, Raitano J, Chan SW. Cerium and yttrium oxide nanoparticles are neuroprotective. Biochem Biophys Res Commun. 2006;342:86–91. [DOI] [PubMed] [Google Scholar]
- 27. Chang MY, Yang YJ, Chang CH, Tang AC, Liao WY, Cheng FY, Yeh CS, Lai JJ, Stayton PS, Hsieh PC. Functionalized nanoparticles provide early cardioprotection after acute myocardial infarction. J Control Release. 2013;170:287–294. [DOI] [PubMed] [Google Scholar]
- 28. Joshi‐Barr S, de Gracia Lux C, Mahmoud E, Almutairi A. Exploiting oxidative microenvironments in the body as triggers for drug delivery systems. Antioxid Redox Signal. 2014;21:730–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kuroda J, Ago T, Matsushima S, Zhai P, Schneider MD, Sadoshima J. NADPH oxidase 4 (NOX4) is a major source of oxidative stress in the failing heart. Proc Natl Acad Sci USA. 2010;107:15565–15570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Looi YH, Grieve DJ, Siva A, Walker SJ, Anilkumar N, Cave AC, Marber M, Monaghan MJ, Shah AM. Involvement of NOX2 NADPH oxidase in adverse cardiac remodeling after myocardial infarction. Hypertension. 2008;51:319–325. [DOI] [PubMed] [Google Scholar]
- 31. Matsushima S, Kuroda J, Ago T, Zhai P, Ikeda Y, Oka S, Fong GH, Tian R, Sadoshima J. Broad suppression of NADPH oxidase activity exacerbates ischemia/reperfusion injury through inadvertent downregulation of hypoxia‐inducible factor‐1alpha and upregulation of peroxisome proliferator‐activated receptor‐alpha. Circ Res. 2013;112:1135–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]


