Abstract
Background
Bicycling to work may be a viable approach for achieving physical activity that provides cardiovascular health benefits. In this study we investigated the relationship of bicycling to work with incidence of obesity, hypertension, hypertriglyceridemia, and impaired glucose tolerance across a decade of follow‐up in middle‐aged men and women.
Methods and Results
We followed 23 732 Swedish men and women with a mean age of 43.5 years at baseline who attended a health examination twice during a 10‐year period (1990–2011). In multivariable adjusted models we calculated the odds of incident obesity, hypertension, hypertriglyceridemia, and impaired glucose tolerance, comparing individuals who commuted to work by bicycle with those who used passive modes of transportation. We also examined the relationship of change in commuting mode with incidence of these clinical risk factors. Cycling to work at baseline was associated with lower odds of incident obesity (odds ratio [OR]=0.85, 95% CI 0.73–0.99), hypertension (OR=0.87, 95% CI 0.79–0.95), hypertriglyceridemia (OR=0.85, 95% CI 0.76–0.94), and impaired glucose tolerance (OR=0.88, 95% CI 0.80–0.96) compared with passive travel after adjusting for putative confounding factors. Participants who maintained or began bicycling to work during follow‐up had lower odds of obesity (OR=0.61, 95% CI 0.50–0.73), hypertension (OR=0.89, 95% CI 0.80–0.98), hypertriglyceridemia (OR=0.80, 95% CI 0.70–0.90), and impaired glucose tolerance (OR=0.82, 95% CI 0.74–0.91) compared with participants not cycling to work at both times points or who switched from cycling to other modes of transport during follow‐up.
Conclusions
These data suggest that commuting by bicycle to work is an important strategy for primordial prevention of clinical cardiovascular risk factors among middle‐aged men and women.
Keywords: cardiovascular disease prevention, cycling, hypertension, impaired glucose tolerance, obesity, physical exercise, type 2 diabetes mellitus
Subject Categories: Cardiovascular Disease, Epidemiology, Exercise, Lifestyle, Obesity
Introduction
Encouraging population‐wide engagement in physical activity is a key priority for most established health agencies. Active transportation (or active commuting) by means of walking or bicycling to and from work and for other purposes (eg, grocery shopping and transporting children) is a type of physical activity that can be built into everyday life; for many, it constitutes a substantial proportion of the total daily health‐enhancing physical activity and provides cost‐ and time‐effective alternatives to commuting by car or public transport. Besides raising physical activity levels, active transportation may also reduce traffic congestion and air and noise pollution1 that plague many cities.
Whereas active transportation and walking have previously been associated with lower risk of cardiovascular disease and premature mortality in prospective studies in different populations around the world,2, 3 only a few large‐scale prospective studies have specifically assessed the cardiovascular health benefits associated with commuting by bicycle, and no study has assessed the impact of changing commuting mode to work. Estimating the magnitude and population impact of the cardiovascular benefits of commuter cycling is important because this would help inform health policy decision making and facilitate the prioritization of public health resources. Previous studies have estimated that physical inactivity is responsible for a substantial economic burden on healthcare systems.4, 5
In this study, we examined the prospective relationships of cycling to work at baseline with change in commuter cycling with 10‐year incidence of obesity, hypertension, hypertriglyceridemia, and impaired glucose tolerance (IGT) in a large population‐based cohort of men and women from Northern Sweden. We also assessed whether genetic and other candidate factors modified the relationships and examined the percentage of these incident clinical risk factors for cardiovascular disease that could be prevented in this cohort if all participants remained cycling or switched to commuting by bicycle to work during a 10‐year follow‐up period.
Methods
Study Population and Study Design
The Västerbottens Health Survey, also called the Västerbottens Intervention Program, is a population‐based health investigation where risk factors and outcomes are monitored and participants are given rudimentary advice on healthy lifestyle behaviors. This public health strategy, which was implemented to reduce premature cardiovascular disease, started recruiting participants in 1990. Persons living within the county of Västerbottens are invited to participate during the year of their 40th, 50th, and 60th birthdays. The study includes an individual counseling session and health examination at a local primary care center. A detailed description of the study can be found elsewhere.6 The participation rate so far has been 58% among men and 65% among women. Participants with a second health examination available up until January 2011 were eligible for the present study. A total of 32 728 people participated twice in the period from 1990 to 2011. Among these we excluded participants if they reported being unemployed or retired at baseline (n=1782) and if they had missing information on commuting mode, relevant covariates, and outcomes (cardiovascular risk factors), respectively (n=7214), leaving up to 23 732 participants with data for analyses (n=22 072 for 2‐hour postprandial glucose and n=19 751 for triglyceride [TG]). The study was approved by the Regional Ethical Committee at Umeå University (DNR 07‐142Ö) and all participants gave informed consent.
Commuting Mode, Leisure Time Exercise, and Occupational Physical Activity
Information on leisure time exercise, occupational physical activity, and work commuting mode were obtained by self‐report questionnaire. Work commute mode was queried separately by season (winter, spring, summer, fall) with response options by car, bus, walking, and bicycling. Participants traveling by car and bus were collapsed to a passive commuting mode group. The predominant mode of travel during the 4 seasons (at least 3 out of 4 seasons) among participants was used to classify the most typical transport mode. Participants with irregular travel mode to work were grouped together (up to 2 seasons). Thus, we categorized transport mode in 4 categories: passive travel, irregular travel mode, walking, and cycling. Commuting distance was also asked about in an open‐ended question. Leisure time exercise was estimated by asking how often the participant had exercised in training clothes for the past 3 months with response options of never, rarely, once per week, 2–3 times per week, or more than 3 times per week. Participants were also asked about their occupational physical activity with the following response options: seated or standing, light, light and active, sometimes heavy, heavy most of the time.
Cardiovascular Risk Factors
Weight (to the nearest 0.1 kg) and height (to the nearest 1 cm) were measured with participants wearing indoor clothing without shoes, and body mass index (BMI) was calculated. Obesity was defined as a BMI ≥30 kg/m2. Until 2009, systolic and diastolic blood pressure (BP) was measured once after 5 minutes rest with the participant in a supine position and after September 1, 2009 it was measured twice with a mercury sphygmomanometer and the average of these 2 was recorded. Hypertension was defined according to the American Heart Association definition, ie, if the systolic or diastolic BP exceeded 140 and 90 mm Hg and/or use of antihypertensive medications. For participants reporting taking antihypertensive medication, BP values were corrected by adding 15 mm Hg to measured systolic BP and 10 mm Hg to measured diastolic BP.7 A capillary blood sample was obtained after an overnight fast (at least 8 hours of fasting), and a second blood sample was drawn 2 hours after a standard 75‐g oral glucose load following World Health Organization standards in participants who did not have fasting blood glucose values indicative of diabetes mellitus. TG and glucose were measured in plasma by enzymatic methods with a Reflotron benchtop analyzer (Roche Diagnostics Scandinavia AB). We corrected TG levels (+0.207 mmol/L) among participants who reporting taking lipid‐lowering medications according to Wu et al.8 IGT was defined as a 2‐hour glucose level of ≥7.8 to <11.1 mmol/L (≥140–<200 mg/dL).9 Hypertriglyceridemia was defined according to the National Cholesterol Education Program Expert Panel (third report), ≥1.7 mmol/L (≥150 mg/dL).10 Genotyping of the FTO (fat mass and obesity associated) gene variant rs9939609 was done based on genomic DNA extracted from peripheral white blood cells and diluted to 4 ng/μL using OpenArray SNP Genotyping System (BioTrove, Woburn, MA).11 Genotype information for rs9939606 in addition to all other exposures and BMI were available in 3926 participants who were part of the GLACIER Study,12 which is nested within the Northern Sweden Biobank.
Covariates
Information was also gathered by questionnaire on the participants' educational status, employment, smoking habits, alcohol intake, and diet (validated semiquantitative food frequency questionnaire).13 We obtained this information both at baseline and follow‐up. Education was defined according to the International Standard Classification of Education (ISCED), United Nations Educational, Scientific and Cultural Organization (UNESCO) 1997. From the validated food frequency questionnaire, we obtained information on total energy intake (kcal/day), coffee intake (g/day), alcohol intake (g/day), fruit (daily frequency), vegetables (daily frequency), trans fat (g/day), and fiber (g/day) consumption. Participants with calculated total energy intake of <500 kcal/day or >4500 kcal/day were excluded (n=120).
Statistical Analyses
Multivariable‐adjusted effects of commuting mode at baseline with continuous and dichotomous risk factor outcomes at follow‐up were estimated with linear and logistic regression, respectively. Participants with prevalent obesity, hypertension, hypertriglyceridemia, or IGT at baseline were excluded from analyses where these traits were modeled as outcomes. Basic models were adjusted for age at baseline, follow‐up time, baseline levels of risk factor (eg BMI in risk of obesity analyses), and sex. Fully adjusted models additionally included leisure time exercise (categorical variable), occupational physical activity (categorical variable), smoking habits (none/former/current), education (basic education including primary and secondary education, postsecondary nontertiary education, tertiary education according to International Standard Classification of Education level [1997]), alcohol consumption (quintiles), coffee intake (quintiles), total energy intake (continuous), fruit intake (quintiles), vegetable intake (quintiles), intake of trans fat (quintiles), and fiber intake (quintiles).
To estimate the dose‐dependent relationship of cycling to work with incident cardiovascular risk and risk factor levels at follow‐up, we modeled the frequency of bicycling to work by season (0–4 seasons) and the back‐and‐forth distance to work (noncyclists, cycling ≤2 km, cycling >2–8 km, cycling >8 km). Furthermore, we analyzed change in bicycling to work from baseline to follow‐up adjusting for baseline‐ and follow‐up measures of covariates. We did this in several ways; first, we restricted our analysis to participants reporting passive travel to work at baseline and estimated the odds of incident risk factors by categories of commuting mode at follow‐up. Second, we restricted our analysis to participants reporting cycling to work at baseline and estimated the odds of incident risk factors by categories of commuting mode at follow‐up. In these 2 sets of analyses, we combined irregular mode travelers and walkers as the number of participants in these groups was modest. Third, we estimated the odds of incident risk factors comparing participants who maintained or began bicycling to work during follow‐up with participants not cycling to work at both times points or who switched from cycling to other transport modes during follow‐up. Based on this, we calculated the proportion of cases of incident cardiovascular risk factors that could be prevented in the total population if all participants remained or switched to bicycling to work during the 10 years of follow‐up. The population attributable fraction estimates with 95% CI were calculated according to the method described by Greenland and Drescher14 based on calculation of multivariable adjusted odds ratios (ORs) and assuming that these estimates represent unbiased causal relationships. In addition, we evaluated possible heterogeneity of relationships of change in bicycling to work with incident risk factors differing by smoking status, leisure time exercise, sex, educational status, and FTO rs9939609 genotype. In all models of change in commuting mode to work, participants who reported being unemployed or retired at follow‐up were additionally excluded from analysis.
To evaluate the possibility of selection bias due to missing data, we carried out multiple imputation analyses according to Royston15 by comparing estimates of associations in the sample with complete data on covariates and outcome (n=19 751–23 732) with the full sample (n=32 728) with missing values being imputed. A multivariate chained equation imputation approach including all covariates and respective outcomes was used to impute missing values. Regression coefficients and SEs were obtained based on 30 imputed data sets. The imputation analyses were based on the linear regression models (continuous cardiovascular outcomes) as these were less computational.
All analyses were conducted in STATA (StataCorp. 2013. Stata Statistical Software: Release 13. College Station, TX: StataCorp LP).
Results
At baseline, passive travel to work was the most common travel mode (59%) followed by bicycling (24%), walking (11%), and irregular traveling (6%). These percentages were similar at follow‐up (59%, 22%, 14%, and 5%, respectively). The odds of cycling to work at follow‐up was 10.4 (95% CI 9.6–11.3) times higher when comparing noncyclists with cyclists at baseline. During a median of 10 years of follow‐up, the number of incident cases of obesity, hypertension, hypertriglyceridemia, and IGT were 1862 (8.6%), 4718 (25.3%), 2913 (18.4%), and 4230 (21.0%), respectively. Table 1 shows characteristics of the participants by categories of commuting mode at baseline. All baseline characteristics were statistically significantly related to mode of travel to work after adjustment for sex. Overall, based on the distribution of baseline characteristics, participants reporting bicycling to work were healthier, better educated, and more often female compared with passive commuters.
Table 1.
Baseline Characteristics by Commuting Mode to Work Among Working Participants (n=23 732)
| Commuting Mode to Work at Baseline | Bicycling (n=5736) | P a | |||
|---|---|---|---|---|---|
| Passive (n=13 944) | Irregular (n=1451) | Walking (n=2601) | |||
| Sex, % (men) | 55.9 | 37.7 | 34.5 | 35.1 | — |
| Age, y | 43.1 (7.0) | 42.8 (7.1) | 43.7 (7.2) | 43.2 (6.9) | 0.001 |
| Height, cm | 172.8 (9.1) | 170.3 (9.5) | 169.7 (8.9) | 170.3 (9.0) | 0.004 |
| Weight, kg | 76.0 (13.7) | 73.1 (13.0) | 72.7 (13.4) | 71.1 (12.4) | <0.001 |
| BMI, kg/m2 | 25.4 (3.7) | 25.2 (4.0) | 25.2 (3.9) | 24.5 (3.5) | <0.001 |
| Systolic BP, mm Hg | 123.9 (15.4) | 122.8 (15.9) | 123.9 (16.0) | 122.0 (15.2) | <0.001 |
| Diastolic BP, mm Hg | 77.7 (10.6) | 77.1 (10.5) | 77.8 (10.7) | 76.5 (10.1) | <0.001 |
| Triglycerides, mmol/Lb | 1.34 (0.80) | 1.29 (0.89) | 1.26 (0.68) | 1.19 (0.68) | <0.001 |
| 2‐hour glucose, mmol/Lb | 6.37 (1.38) | 6.41 (1.31) | 6.43 (1.35) | 6.37 (1.28) | <0.001 |
| Total energy intake, kcal/day | 1882 (620) | 1814 (630) | 1814 (626) | 1816 (590) | <0.001 |
| Fiber intake, g/day | 18.8 (7.3) | 19.3 (7.6) | 19.6 (7.5) | 19.8 (7.5) | <0.001 |
| Trans fat intake, g/day | 0.8 (0.5) | 0.7 (0.5) | 0.8 (0.6) | 0.7 (0.4) | <0.001 |
| Fruit intake (daily frequency) | 1.4 (1.1) | 1.6 (1.2) | 1.6 (1.2) | 1.7 (1.2) | <0.001 |
| Vegetable intake (daily frequency) | 1.4 (1.1) | 1.6 (1.2) | 1.6 (1.2) | 1.7 (1.2) | <0.001 |
| Coffee intake, g/day | 429 (219) | 407 (210) | 411 (215) | 409 (213) | <0.001 |
| Alcohol intake, g/day | 4.4 (4.5) | 4.0 (4.2) | 3.4 (4.0) | 3.7 (4.1) | <0.001 |
| Smoking status (%) | |||||
| Current | 19.7 | 19.4 | 19.9 | 15.1 | <0.001 |
| Former | 21.3 | 19.0 | 19.6 | 18.8 | |
| Never | 59.0 | 61.6 | 60.5 | 66.1 | |
| Highest education levelc (%) | |||||
| 1 | 21.2 | 19.8 | 26.6 | 18.6 | <0.001 |
| 2 | 54.5 | 50.4 | 48.3 | 45.6 | |
| 3 | 24.3 | 29.8 | 25.1 | 35.8 | |
| Frequency of leisure time exercise (%) | |||||
| Never | 44.0 | 32.8 | 42.6 | 31.2 | <0.001 |
| Rarely | 26.4 | 29.2 | 26.2 | 26.1 | |
| 1 to 3 times/week | 18.4 | 23.9 | 18.1 | 25.3 | |
| >3 times/week | 11.2 | 14.2 | 13.1 | 17.4 | |
| Occupational physical activity (%) | |||||
| Sedentary or standing | 27.7 | 29.3 | 23.1 | 26.7 | <0.001 |
| Light | 19.4 | 17.9 | 14.7 | 18.2 | |
| Light or some physical effort | 21.7 | 22.4 | 22.3 | 22.7 | |
| Heavy work some or most of the time | 31.2 | 30.4 | 39.9 | 32.4 | |
Data are means (SD) or percent. BMI indicates body mass index; BP, blood pressure.
P is sex‐adjusted P‐value for global difference across commuting mode.
The total sample size for triglyceride and 2‐hour glucose was lower than for the other variables (total n=19 912 and n=22 888, respectively).
Based on educational level (International Standard Classification of Education [ISCED] [UNESCO 1997], 1=ISCED level 1 and 2, 2=ISCED level 3 and 4, and 3=ISCED level 5–7.
Table 2 shows the association of commuting mode at baseline and incident cardiovascular risk factors at follow‐up. In the basic models, bicycling to work was associated with lower odds of incident obesity, hypertension, hypertriglyceridemia, and IGT compared with passive travel. Further adjustment for other putative cardiovascular risk factors did not substantially change results; however, all ORs were slightly attenuated. We found similar patterns of associations in the analyses where cardiovascular risk factors at follow‐up were modeled as continuous outcomes (Table S1). In fully adjusted models, bicycling to work was associated with lower levels of BMI, TG, systolic and diastolic BP, and 2‐hour glucose compared with passive travel. Walking to work was unrelated to incident risk factors (Table 2) and risk factor levels at follow‐up (Table S1) in fully adjusted models.
Table 2.
Commuting Mode to Work at Baseline and Incident Cardiovascular Risk Factor Outcomes at Follow‐Up
| Commuting Mode to Work at Baseline | ||||
|---|---|---|---|---|
| Passive (Reference) | Irregular | Walking | Bicycling | |
| Incident risk factor | ||||
| Obesity (1882 incident cases) | ||||
| N total/N cases | 12 588/1178 | 1318/110 | 2334/236 | 5379/338 |
| Basic model | 1 | 0.87 (0.69–1.11) | 1.16 (0.97–1.39) | 0.83 (0.71–0.96) |
| Fully adjusted model | 1 | 0.90 (0.70–1.14) | 1.16 (0.97–1.39) | 0.85 (0.73–0.99) |
| Hypertension (4718 incident cases) | ||||
| N total/N cases | 10 836/2891 | 1154/301 | 1983/475 | 4677/1051 |
| Basic model | 1 | 1.04 (0.90–1.21) | 0.85 (0.75–0.96) | 0.81 (0.74–0.89) |
| Fully adjusted model | 1 | 1.10 (0.94–1.28) | 0.88 (0.78–1.00) | 0.87 (0.79–0.95) |
| Hypertriglyceridemia (2913 incident cases) | ||||
| N total/N cases | 9233/1806 | 986/175 | 1908/324 | 4031/608 |
| Basic model | 1 | 1.00 (0.83–1.19) | 0.91 (0.79–1.04) | 0.83 (0.74–0.92) |
| Fully adjusted model | 1 | 1.02 (0.85–1.22) | 0.91 (0.80–1.05) | 0.85 (0.76–0.94) |
| Impaired glucose tolerance (4230 incident cases) | ||||
| N total/N cases | 11 828/2554 | 1219/245 | 2192/484 | 4913/947 |
| Basic model | 1 | 0.91 (0.78–1.06) | 0.96 (0.86–1.08) | 0.84 (0.77–0.92) |
| Fully adjusted model | 1 | 0.94 (0.81–1.09) | 0.96 (0.85–1.08) | 0.88 (0.80–0.96) |
Data are odds ratios (95% CI). Basic model was adjusted for age at baseline, follow‐up time, baseline levels of risk factor (eg body mass index in risk of obesity analyses), and sex. Fully adjusted model was additionally adjusted for leisure time exercise, occupational physical activity, smoking status, educational status, alcohol consumption, and intake of coffee, total energy, fruit, vegetables, trans fat, and fiber.
To evaluate dose‐dependent relationships for bicycling to work and cardiovascular risk factor progression, we then examined the association of frequency of bicycling to work at baseline by season and according to distance back‐and‐forth to work with incident cardiovascular risk and risk factor levels at follow‐up. In multivariable adjusted analyses, we found evidence of dose–response relationships with increasing number of seasons bicycling for all incident factors (Figure 1) and lower risk factor levels (Table S2). The analyses of cycling distance to work also revealed indications of dose‐dependent relationships to all cardiovascular risk factors (Figure 2).
Figure 1.
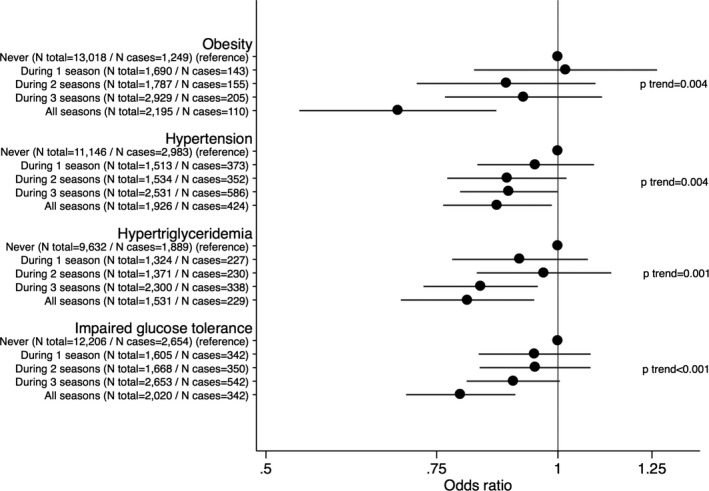
Data are odds ratios (95% CI) according to seasonal frequency of bicycling to work at baseline and incident cardiovascular risk at follow‐up. Models were adjusted for age at baseline, follow‐up time, baseline levels of risk factor (eg BMI in risk of obesity analyses), sex, leisure time exercise, occupational physical activity, smoking status, educational status, alcohol consumption, coffee intake, total energy intake, fruit intake, vegetable intake, intake of trans fat, and fiber intake. BMI indicates body mass index.
Figure 2.
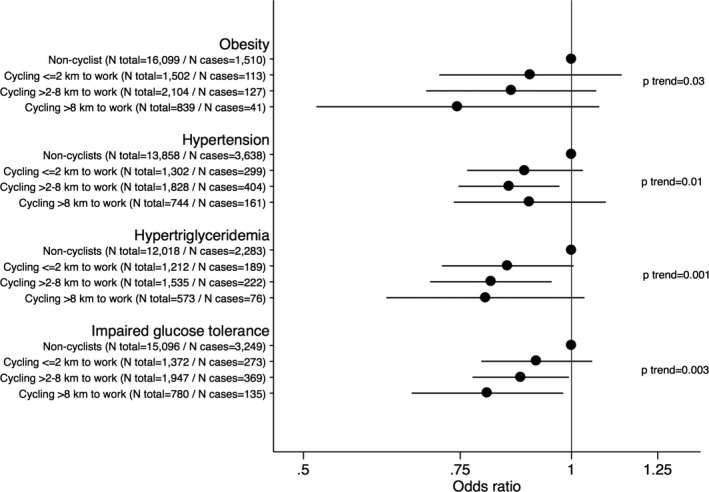
Data are odds ratios (95% CI) according to bicycling to work and the back‐and forth distance at baseline and incident cardiovascular risk at follow‐up. Models were adjusted for age at baseline, follow‐up time, baseline levels of risk factor (eg BMI in risk of obesity analyses), sex, leisure time exercise, occupational physical activity, smoking status, educational status, alcohol consumption, coffee intake, total energy intake, fruit intake, vegetable intake, intake of trans fat, and fiber intake. BMI indicates body mass index.
We then examined the associations of various changes in commuting mode with cardiovascular risk. In analyses restricted to participants reporting passive travel to work at baseline and who reported cycling to work at follow‐up, the odds of incident obesity (OR=0.64, 95% CI 0.49–0.84, P<0.001), hypertension (OR=0.88, 95% CI 0.75–1.03), hypertriglyceridemia (OR=0.76, 95% CI 0.62–0.92, P=0.006), and IGT (OR=0.79, 95% CI 0.68–0.93, P=0.005) were lower compared with participants who remained using passive travel (Figure 3). Furthermore, participants who remained cycling to work had lower odds of incident obesity (OR=0.43, 95% CI 0.30–0.63, P<0.001) compared with participants who switched to passive travel (Figure 4), while relationships to hypertension, hypertriglyceridemia, and IGT were nonsignificant. Generally, these relationships of change in commuting mode with cardiovascular risk factors were supported by similar analyses with continuous risk factor levels in linear regression models, although these analyses showed that participants who remained cycling to work had significantly lower follow‐up levels of BMI, systolic BP, TG, and 2‐hour glucose compared with those who remained using passive travel (Table S3).
Figure 3.
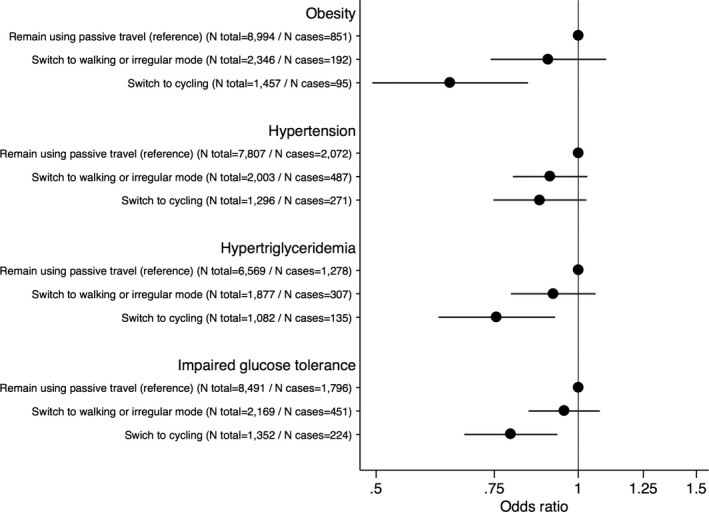
Data are odds ratios (95% CI) of incident cardiovascular risk factors comparing participants using passive travel to work at baseline and remaining passive (reference) or switching to walking or irregular mode, or cycling as transport to work at follow‐up. Models were adjusted for age at baseline, follow‐up time, baseline levels of risk factor (eg BMI in risk of obesity analyses), sex, baseline educational status, and baseline‐ and follow‐up information on leisure time exercise, occupational physical activity, smoking status, alcohol consumption, coffee intake, total energy intake, intake of fruit, intake of vegetables, intake of trans fat, and fiber intake. BMI indicates body mass index.
Figure 4.
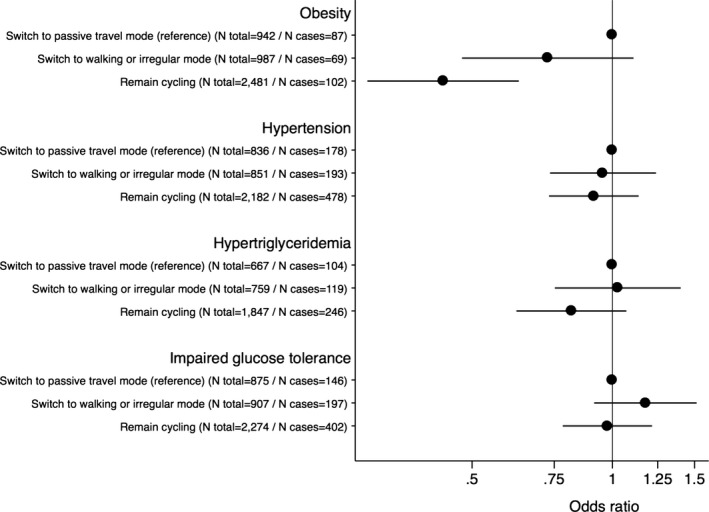
Data are odds ratios (95% CI) of incident cardiovascular risk factors comparing participants cycling to work at baseline and switching to using passive travel (reference), walking or irregular mode, or remain cycling to work at follow‐up. Models were adjusted for age at baseline, follow‐up time, baseline levels of risk factor (eg BMI in risk of obesity analyses), sex, baseline educational status, and baseline‐ and follow‐up information on leisure time exercise, occupational physical activity, smoking status, alcohol consumption, coffee intake, total energy intake, intake of fruit, intake of vegetables, intake of trans fat, and fiber intake. BMI indicates body mass index.
The odds of incident risk factors were significantly lower in participants who maintained or began bicycling to work during follow‐up compared to participants who did not cycle to work at either time point or who switched from cycling to other more passive modes of transport during follow‐up (Table 3). These relationships were also fairly similar between men and women, across categories of educational status, smoking status, and regardless of engagement in leisure‐time exercise (Figure 5). Furthermore, there was no evidence of the FTO rs9939609 variant modifying the relationship of change in bicycling to work with BMI or odds of obesity (P>0.6 for interaction). The estimated percentage of incident cardiovascular risk factor events that could be prevented in the total population if all participants maintained or began to cycle to work during 10 years of follow‐up are also shown in Table 3. The attributable fraction percentage for obesity was 24%, suggesting that approximately a quarter of the new cases of obesity occurring during these 10 years of follow‐up could be prevented in this population if all participants remained‐ or switched to cycling to work. The attributable fraction percentages for hypertension, hypertriglyceridemia, and impaired fasting glucose were 6%, 13%, and 11%, respectively.
Table 3.
Percentage of Cases of Incident Cardiovascular Risk Factors That Could Be Prevented in the Total Population if All Individuals Remained‐ or Switched to Cycling to Work
| Cardiovascular Risk Factor | Remain‐ or Switch to Other Forms of Travel Than Cycling to Work | Remain‐ or Switch to Cycling to Work | OR (95% CI) | PAF (95% CI), % |
|---|---|---|---|---|
| N Total/N Cases | N Total/N Cases | |||
| Obesity | 13 269/1199 | 3938/197 | 0.61 (0.50, 0.73) | 24 (16, 32) |
| Hypertension | 11 497/2930 | 3478/749 | 0.89 (0.80, 0.98) | 6 (1, 11) |
| Hypertriglyceridemia | 9872/1808 | 2929/381 | 0.80 (0.70, 0.90) | 13 (6, 20) |
| Impaired glucose tolerance | 12 442/2590 | 3626/626 | 0.82 (0.74, 0.91) | 11 (5, 16) |
Estimates of OR (odds ratio) and subsequent PAF (population attributable fraction) were from multivariable models including age at baseline, follow‐up time, baseline levels of risk factor (eg body mass index in risk of obesity analyses), sex, baseline educational status, and baseline‐ and follow‐up information on leisure time exercise, occupational physical activity, smoking status, alcohol consumption, coffee intake, total energy intake, intake of fruit, intake of vegetables, intake of trans fat, and fiber intake.
Figure 5.
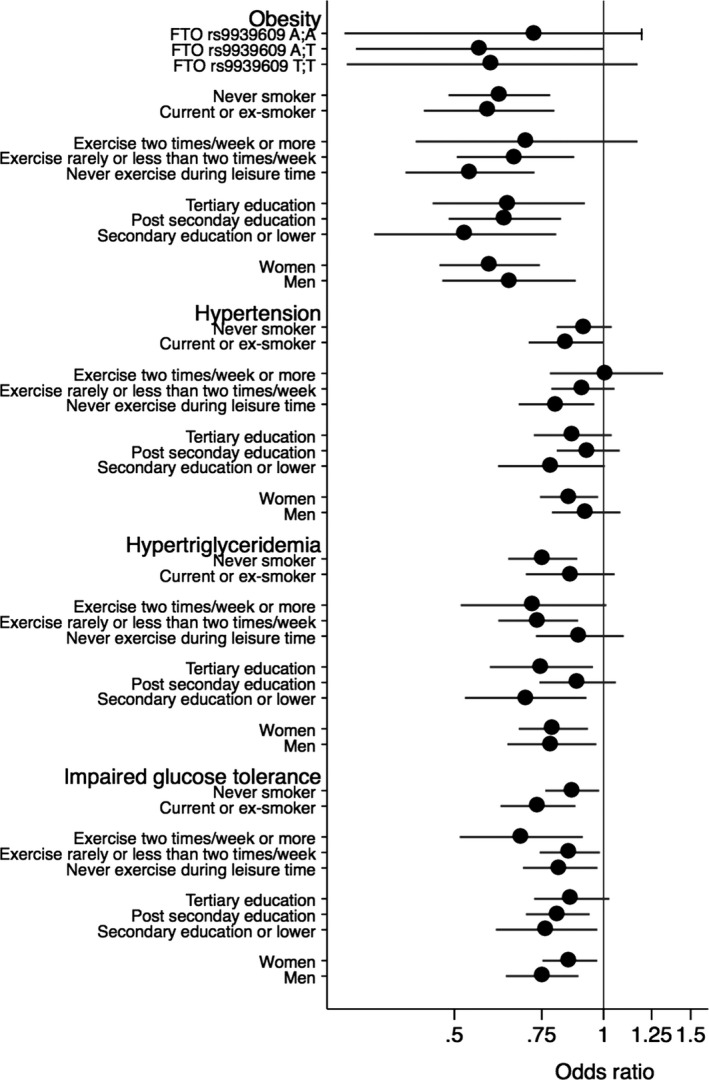
Estimates are multivariable adjusted (as Table 3) odds ratios (95% CI) of incident risk factors comparing participants who remained‐ or switched to bicycling to work with participants not cycling to work at both time points or switched from cycling to other transport modes during follow‐up by smoking status, leisure time exercise, educational level, sex, and FTO rs9939609 genotype.
The analyses imputing missing values among up to 7214 participants in a multivariate approach suggested fairly similar 10‐year follow‐up cardiovascular benefits associated with cycling to work at baseline: BMI −0.16 kg/m2 (95% CI −0.23 to −0.09); systolic BP −1.01 mm Hg (95% CI −1.48 to −0.55); diastolic BP −0.74 mm Hg (95% CI −1.02 to −0.46); triglyceride −0.03 mmol/L (95% CI −0.05 to −0.01); 2‐hour glucose −0.07 (95% CI −0.13 to −0.02) compared with passive travel in fully adjusted models as in Table S1.
Discussion
Findings from this study among a population‐based sample of more than 20 000 Swedish men and women followed for 10 years show that bicycling to work lowers the risk of clinically relevant cardiovascular risk factors (ie, obesity, hypertension, hypertriglyceridemia, and IGT) compared with passive travel. There was some evidence of dose–response relationships of frequency of bicycling to work and commuting distance with these incident cardiovascular risk outcomes, and the associations were independent of major confounding factors. The analyses of 10‐year incident cardiovascular risk were supported by analyses of continuous cardiovascular risk factors; participants reporting bicycling to work at baseline had lower levels of systolic and diastolic BP, BMI, TG, and 2‐hour postprandial glucose at follow‐up compared with participants reporting passive travel. Furthermore, our analyses of change in commuting mode additionally support that remaining cycling to work or switching from passive travel to cycling lowers the risk of these cardiovascular risk outcomes, and there was no evidence that these relationships differed by genetic or other characteristics. If we assume that these associations are causal, we estimate that 24%, 6%, 13%, and 11% of individuals developing obesity, hypertension, hypertriglyceridemia, and IGT, respectively could be prevented if all individuals in this population remained with or switched to bicycling as commuting mode to work. Thus, the public health impact of substituting bicycling to work for other forms of transport or making sure that people remain using their bicycle to commute to work may be substantial.
While a number of cross‐sectional studies on the association of bicycling to work and prevalent cardiovascular risk factors have been carried out,16, 17, 18 we are not aware of any prospective studies examining specific associations for bicycling to work. A report from the Nurses' Health Study II has shown that an increase in time spent recreational bicycling was associated with lower change in weight over 16 years in premenopausal women.19 A cross‐sectional study based on a representative sample of adults from the United Kingdom reports lower odds of prevalent hypertension and diabetes mellitus among individuals bicycling to work compared with individuals using private passive transport mode.17 Similarly, a recent cross‐sectional study among adults residing in India found that individuals reporting bicycling to work were less likely to have hypertension or diabetes mellitus than those reporting traveling to work by private passive transport.16 We are aware of 2 prospective studies examining the association of bicycling to work or for other purposes with the risk of mortality. A study in Chinese women20 and a study conducted among Danish men and women21 each reported bicycling to work and for other reasons being inversely associated with the risk of all‐cause mortality compared with passive travel. Another recent published study of Danish middle‐aged and older adults reports that bicycling to work is related to lower risk of type 2 diabetes mellitus.22 In a Dutch cohort study, engagement in nonspecific bicycling was associated with lower risk of cardiovascular disease.23 Furthermore, in a nested case–control study, we have previously reported that compared with bicycling to work, commuting by car was associated with greater risk of myocardial infarction,24 and this relationship was to a large extent explained by inflammatory and hemostatic biomarkers.25 We extend these findings by providing evidence that commuting to work by bicycle is prospectively associated with lower risk of incident (and more favorable levels of) risk factors for cardiovascular diseases and type 2 diabetes mellitus.
We are unaware of any long‐term randomized controlled trial examining the effect of commuter cycling with other forms of transport on cardiovascular risk factors. However, a short‐term (8‐week) randomized trial showed that starting cycling to school significantly lowered a composite cardiovascular risk factor score among 43 12‐year‐old Danish children.26 Another short‐term randomized trial among 48 Danish adults reported that starting cycling to work increased fitness and lowered adiposity during an 8‐week intervention period.27 These modest‐sized short‐term randomized trials support that commuter cycling is an important causal factor that can be used for primordial prevention of cardiovascular risk including maintaining or improving cardiorespiratory fitness. Previous observational studies in adults suggest that improving cardiorespiratory fitness is related to lower risk of hypertension, hypercholesterolemia, type 2 diabetes mellitus, and the metabolic syndrome.28, 29
There are some limitations to the study. We were not able to quantify the contribution of bicycling to work to the total physical activity level, and we did not have detailed information on the intensity of bicycling to work, which would provide additional possibilities to better characterize the possible dose‐dependent relationship with cardiovascular risk. Although we were able to adjust for many important confounding factors including educational status and lifestyle factors such as diet, smoking, alcohol intake, and other physical activity, residual and unknown confounding may still be an issue. We observed little heterogeneity of relationships by categories of major confounders, which reassures us that residual confounding by these measured confounders is less likely. Finally, obesity was defined according to BMI and because bicycling is likely to increase lower extremity muscle mass, the estimates of differences between passive travel and bicycling we report may be underestimated due to differential misclassification. The major strengths of the study were the prospective study design, the long follow‐up period, study populations' wide engagement in cycling to work, the large sample size in conjunction with the availability of a number of important confounding factors at both time points, careful exclusion of participants being unemployed or retired and therefore unable to be exposed, and that we were able to analyze bicycling to work with some information on dose and study change in commuting mode. The combination of these analyses provides us with greater confidence that the estimates of associations and population impact represent causal relationships.
The study participants were middle‐aged adults living in the County of Västerbötten in northern Sweden where cycling is common and its infrastructure politically prioritized. The findings from our study may not be generalizable to populations living in areas with a different infrastructure. Nevertheless, it was a population‐based study and we did not observe evidence that the benefits of cycling to work differed according to educational status or sex, which may suggest wider generalizability of the results.
In conclusion, in a population sample of middle‐aged adults living in northern Sweden, bicycling to work was related to lower risk of incident obesity, hypertension, hypertriglyceridemia, and IGT compared with passive travel consistent with a dose–response relationship. Our estimates of the public health impact of substituting bicycling to work for passive transport suggest significant cardiovascular risk factor improvements in this population. Efforts to encourage population‐wide active commuting by bicycle, in addition to other domains of physical activity, may be an effective strategy for primordial prevention of cardiovascular disease and type 2 diabetes mellitus in the general population.
Sources of Funding
AG was supported by the Lundbeck Foundation (R151‐2013‐14641) and the Danish Council for Independent Research (DFF‐4004‐00111). The work was supported by grants from the Novo Nordisk Foundation, Swedish Research Council, Swedish Heart‐Lung Foundation, and the European Research Council.
Disclosures
None.
Supporting information
Table S1. Commuting Mode to Work at Baseline and Cardiovascular Risk Factor Levels at Follow‐Up
Table S2. Seasonal Frequency of Bicycling to Work at Baseline and Cardiovascular Risk Factor Levels at Follow‐Up
Table S3. Change in Commuting Mode to Work and Cardiovascular Risk Factor Levels at Follow‐Up
(J Am Heart Assoc. 2016;5: e004413 doi: 10.1161/JAHA.116.004413)
Contributor Information
Anders Grøntved, Email: agroentved@health.sdu.dk.
Paul W. Franks, Email: paul.franks@med.lu.se.
References
- 1. de Nazelle A, Nieuwenhuijsen MJ, Antó JM, Brauer M, Briggs D, Braun‐Fahrlander C, Cavill N, Cooper AR, Desqueyroux H, Fruin S, Hoek G, Panis LI, Janssen N, Jerrett M, Joffe M, Andersen ZJ, van Kempen E, Kingham S, Kubesch N, Leyden KM, Marshall JD, Matamala J, Mellios G, Mendez M, Nassif H, Ogilvie D, Peiró R, Pérez K, Rabl A, Ragettli M, Rodríguez D, Rojas D, Ruiz P, Sallis JF, Terwoert J, Toussaint J‐F, Tuomisto J, Zuurbier M, Lebret E. Improving health through policies that promote active travel: a review of evidence to support integrated health impact assessment. Environ Int. 2011;37:766–777. [DOI] [PubMed] [Google Scholar]
- 2. Hamer M, Chida Y. Active commuting and cardiovascular risk: a meta‐analytic review. Prev Med. 2008;46:9–13. [DOI] [PubMed] [Google Scholar]
- 3. Hamer M, Chida Y. Walking and primary prevention: a meta‐analysis of prospective cohort studies. Br J Sports Med. 2008;42:238–243. [DOI] [PubMed] [Google Scholar]
- 4. Ding D, Lawson KD, Kolbe‐Alexander TL, Finkelstein EA, Katzmarzyk PT, van Mechelen W, Pratt M. The economic burden of physical inactivity: a global analysis of major non‐communicable diseases. The Lancet. 2016;pii:S0140‐6736(16)30383‐X. [DOI] [PubMed] [Google Scholar]
- 5. Carlson SA, Fulton JE, Pratt M, Yang Z, Adams EK. Inadequate physical activity and health care expenditures in the United States. Prog Cardiovasc Dis. 2015;57:315–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hallmans G, Agren A, Johansson G, Johansson A, Stegmayr B, Jansson JH, Lindahl B, Rolandsson O, Soderberg S, Nilsson M, Johansson I, Weinehall L. Cardiovascular disease and diabetes in the Northern Sweden Health and Disease Study Cohort—evaluation of risk factors and their interactions. Scand J Public Health Suppl. 2003;61:18–24. [DOI] [PubMed] [Google Scholar]
- 7. Tobin MD, Sheehan NA, Scurrah KJ, Burton PR. Adjusting for treatment effects in studies of quantitative traits: antihypertensive therapy and systolic blood pressure. Stat Med. 2005;24:2911–2935. [DOI] [PubMed] [Google Scholar]
- 8. Wu J, Province MA, Coon H, Hunt SC, Eckfeldt JH, Arnett DK, Heiss G, Lewis CE, Ellison RC, Rao DC, Rice T, Kraja AT. An investigation of the effects of lipid‐lowering medications: genome‐wide linkage analysis of lipids in the HyperGEN study. BMC Genet. 2007;8:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. American Diabetes A . Diagnosis and classification of diabetes mellitus. Diabetes Care 2008;31(suppl 1): S55–S60. [DOI] [PubMed] [Google Scholar]
- 10. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation. 2002;106:3143. [PubMed] [Google Scholar]
- 11. Renström F, Shungin D, Johansson I, MAGIC Investigators , Florez JC, Hallmans G, Hu FB, Franks PW. Genetic predisposition to long‐term nondiabetic deteriorations in glucose homeostasis. Diabetes. 2011;60:345–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kurbasic A, Poveda A, Chen Y, Agren A, Engberg E, Hu FB, Johansson I, Barroso I, Brandstrom A, Hallmans G, Renstrom F, Franks PW. Gene‐lifestyle interactions in complex diseases: design and description of the GLACIER and VIKING studies. Curr Nutr Rep. 2014;3:400–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Johansson I, Hallmans G, Wikman A, Biessy C, Riboli E, Kaaks R. Validation and calibration of food‐frequency questionnaire measurements in the Northern Sweden Health and Disease cohort. Public Health Nutr. 2002;5:487–496. [DOI] [PubMed] [Google Scholar]
- 14. Greenland S, Drescher K. Maximum likelihood estimation of the attributable fraction from logistic models. Biometrics. 1993;49:865–872. [PubMed] [Google Scholar]
- 15. Royston P. Multiple imputation of missing values. Stata J. 2004;4:227–241. [Google Scholar]
- 16. Millett C, Agrawal S, Sullivan R, Vaz M, Kurpad A, Bharathi AV, Prabhakaran D, Reddy KS, Kinra S, Smith GD, Ebrahim S; Indian Migration Study Group . Associations between active travel to work and overweight, hypertension, and diabetes in India: a cross‐sectional study. PLoS Med. 2013;10:e1001459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Laverty AA, Mindell JS, Webb EA, Millett C. Active travel to work and cardiovascular risk factors in the United Kingdom. Am J Prev Med. 2013;45:282–288. [DOI] [PubMed] [Google Scholar]
- 18. Hollingworth M, Harper A, Hamer M. Dose–response associations between cycling activity and risk of hypertension in regular cyclists: the UK Cycling for Health study. J Hum Hypertens. 2015;29:219–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lusk AC, Mekary RA, Feskanich D, Willett WC. Bicycle riding, walking, and weight gain in premenopausal women. Arch Intern Med. 2010;170:1050–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Matthews CE, Jurj AL, Shu Xo, Li HL, Yang G, Li Q, Gao YT, Zheng W. Influence of exercise, walking, cycling, and overall nonexercise physical activity on mortality in Chinese women. Am J Epidemiol. 2007;165:1343–1350. [DOI] [PubMed] [Google Scholar]
- 21. Andersen LB, Schnohr P, Schroll M, Hein HO. All‐cause mortality associated with physical activity during leisure time, work, sports, and cycling to work. Arch Intern Med. 2000;160:1621–1628. [DOI] [PubMed] [Google Scholar]
- 22. Rasmussen MG, Grontved A, Blond K, Overvad K, Tjonneland A, Jensen MK, Ostergaard L. Associations between recreational and commuter cycling, changes in cycling, and type 2 diabetes risk: a cohort study of Danish men and women. PLoS Med. 2016;13:e1002076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hoevenaar‐Blom MP, Wendel‐Vos GC, Spijkerman AM, Kromhout D, Verschuren WM. Cycling and sports, but not walking, are associated with 10‐year cardiovascular disease incidence: the MORGEN Study. Eur J Cardiovasc Prev Rehabil. 2011;18:41–47. [DOI] [PubMed] [Google Scholar]
- 24. Wennberg P, Lindahl B, Hallmans G, Messner TR, Weinehall L, Johansson L, Boman K, Jansson JH. The effects of commuting activity and occupational and leisure time physical activity on risk of myocardial infarction. Eur J Cardiovasc Prev Rehabil. 2006;13:924–930. [DOI] [PubMed] [Google Scholar]
- 25. Wennberg P, Wensley F, Johansson L, Boman K, Angelantonio ED, Rumley A, Lowe G, Hallmans G, Jansson JH. Reduced risk of myocardial infarction related to active commuting: inflammatory and haemostatic effects are potential major mediating mechanisms. Eur J Cardiovasc Prev Rehabil. 2010;17:56–62. [DOI] [PubMed] [Google Scholar]
- 26. Ostergaard L, Borrestad LA, Tarp J, Andersen LB. Bicycling to school improves the cardiometabolic risk factor profile: a randomised controlled trial. BMJ Open. 2012;2:e001307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moller NC, Ostergaard L, Gade JR, Nielsen JL, Andersen LB. The effect on cardiorespiratory fitness after an 8‐week period of commuter cycling—a randomized controlled study in adults. Prev Med. 2011;53:172–177. [DOI] [PubMed] [Google Scholar]
- 28. Carnethon MR, Gidding SS, Nehgme R, Sidney S, Jacobs DR Jr, Liu K. Cardiorespiratory fitness in young adulthood and the development of cardiovascular disease risk factors. JAMA. 2003;290:3092–4100. [DOI] [PubMed] [Google Scholar]
- 29. Lee DC, Sui X, Church TS, Lavie CJ, Jackson AS, Blair SN. Changes in fitness and fatness on the development of cardiovascular disease risk factors hypertension, metabolic syndrome, and hypercholesterolemia. J Am Coll Cardiol. 2012;59:665–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Commuting Mode to Work at Baseline and Cardiovascular Risk Factor Levels at Follow‐Up
Table S2. Seasonal Frequency of Bicycling to Work at Baseline and Cardiovascular Risk Factor Levels at Follow‐Up
Table S3. Change in Commuting Mode to Work and Cardiovascular Risk Factor Levels at Follow‐Up


