Abstract
Background
The presence and implications of abnormal arterial stiffness, a potential independent predictor of outcomes, in community‐dwelling treated hypertensives is unknown. Furthermore, limited data exist regarding the risk of cardiovascular disease (CVD) associated with arterial stiffness across the entire range of blood pressure.
Methods and Results
We measured carotid‐femoral pulse wave velocity (PWV) and classical CVD risk factors in 2127 community‐dwelling participants (mean age 60 years, 57% women) of The Framingham Offspring Cohort. The participants were divided into 4 groups according to hypertension (yes/no, defined as blood pressure ≥140/90 mm Hg or use of antihypertensive treatment) and PWV status (high/low based on age‐ and sex‐specific median values) and followed up for CVD events (CVD death, myocardial infarction, unstable angina, heart failure, and stroke). Sixty percent (233 of 390) of controlled and 90% (232 of 258) of uncontrolled treated hypertensives had high PWV. The multivariable‐adjusted risk for CVD events (n=248, median follow‐up 12.6 years) rose from normotension with low PWV (reference) to normotension with high PWV (hazard ratio 1.29, 95% CI 0.83–2.00) and from hypertension with low PWV (hazard ratio 1.54, 95% CI 1.01–2.36) to hypertension with high PWV (hazard ratio 2.25, 95% CI 1.54–3.29).
Conclusions
A substantial proportion of treated hypertensives have high arterial stiffness, a finding that may explain some of the notable residual CVD risk associated with even well‐controlled hypertension. High PWV is associated with a trend towards increasing CVD risk in both nonhypertensives and hypertensives, a finding that may support the use of arterial stiffness measurements in both populations.
Keywords: antihypertensive agent, arterial stiffness, cardiovascular disease, epidemiology, hypertension
Subject Categories: Hypertension, High Blood Pressure, Vascular Disease, Cardiovascular Disease, Epidemiology
Introduction
Hypertension remains a major challenge for clinicians and a public health problem because of its high prevalence, poor control rates, and major impact on the global burden of disease.1, 2 Arterial stiffening is one of the hallmarks of aging‐related hypertension, and it is strongly associated with increases in pulse pressure and systolic blood pressure in both cross‐sectional and longitudinal settings.3, 4, 5 However, arterial stiffness, most commonly measured by carotid‐femoral pulse wave velocity (PWV), is not only a correlate of blood pressure but it is also an independent predictor of cardiovascular death, myocardial infarction, and stroke.6, 7, 8, 9 These relations are consistent in both men and women, the middle‐aged and the old, and between countries.10
Although the adverse effects of arterial stiffening have been robustly proven, there are still major gaps in our understanding of the risks associated with arterial stiffening in certain situations. First, a significant proportion of the previous studies that have examined the association between arterial stiffness and outcomes have been performed in cohorts consisting of elderly participants or patients with hypertension or kidney disease instead of in population‐based cohorts.10, 11 Limited data, therefore, exist regarding the absolute and relative risks of cardiovascular disease (CVD) and hypertensive target‐organ damage associated with increased arterial stiffness across the whole spectrum of blood pressure including levels considered normal. Additionally, it is unclear whether hypertension status itself modifies the relationship between arterial stiffness and CVD outcomes. Second, treated hypertension is associated with considerable residual risk as even optimally treated and controlled patients have a 50% higher CVD risk than normotensive individuals without prior high blood pressure.12 Residual arterial stiffness could play an important role in the residual risk in treated hypertension because the absence of a parallel PWV decrease along with treatment‐induced blood pressure decreases has been associated with adverse CVD outcomes.13 However, it is unknown to what extent arterial stiffness remains high in community‐dwelling patients with treated hypertension.
We assessed the proportion of treated hypertensives with high arterial stiffness despite antihypertensive treatment to clarify the extent of residual arterial stiffness in the community. In addition, to elucidate whether measurement of PWV can be used for CVD risk assessment across the blood pressure distribution, we studied whether the high PWV is associated with increased risk of CVD and presence of left ventricular hypertrophy (LVH) in both normo‐ and hypertension.
Methods
Participants
We included participants who attended the seventh examination cycle of the Framingham Offspring cohort (n=3539; 1998–2001) in the present investigation. The characteristics and study protocol for the Framingham offspring cohort have been previously published in detail.14 Tonometry measurements were implemented in 2660 participants during the seventh examination cycle beginning in February 1999 as described previously.15, 16 Participants who had incomplete tonometry data (n=359) or prevalent CVD (n=174) were excluded from the present analysis. Measurements for echocardiographic and electrocardiographic LVH were made during the previous, sixth examination cycle (1995–1998). A subpopulation of 1583 participants with echocardiography and ECG data available from examination cycle 6 was used for analyses concerning LVH. All study protocols were approved by Boston University Medical Center's Institutional Review Board, and participants provided written informed consent.
Clinical Evaluation and Definitions
All participants provided a medical history and underwent a physical examination and laboratory assessment of CVD risk factors.14 We assessed the participants for self‐reported cigarette smoking and diabetes mellitus (fasting glucose level of ≥126 mg/dL or the use of hypoglycemic medications). In addition, we measured blood pressure (mean of 2 auscultatory values obtained by a physician using a mercury column sphygmomanometer on the left arm of seated participants using a standardized protocol), body mass index, serum total cholesterol levels, and high‐density lipoprotein cholesterol concentrations. We derived heart rate from a standard 10‐s ECG recording.
Carotid‐Femoral PWV
We evaluated arterial stiffness with carotid‐femoral PWV.17 PWV is inversely related to vascular compliance, and, therefore, a stiffer vessel will conduct the pulse wave faster than a more elastic vessel. Arterial tonometry measures were acquired as previously described after more than 5 minutes of rest in the supine position.15, 16 All recordings were performed on the right side of the body. Arterial tonometry with a simultaneously acquired ECG was obtained for the femoral and carotid arteries. Carotid‐femoral transit distance was estimated by measuring the body surface distance from the suprasternal notch to the carotid and femoral sites and taking the difference to account for parallel transmission along the brachiocephalic and carotid arteries and around the aortic arch. This corrected distance was divided by the carotid‐femoral transit time delay to give PWV.
Left Ventricular Hypertrophy
LVH was defined either as positive LVH by ECG or echocardiography. LVH by ECG was defined according to the Cornell voltage criteria (sum of R‐wave in aVL plus S‐wave in V3 >20 mV in women and >28 mV in men).18 M‐mode and 2‐dimensional echocardiography was performed with a Sonos 1000 Hewlett‐Packard ultrasound device to detect echocardiographic LVH. Digitized images were stored and measured using an off‐line analysis system by certified sonographers or cardiologists. Left ventricular mass was calculated according to the American Society of Echocardiography guidelines.19 Echocardiographic LVH was defined as the ratio of left ventricular mass to height ≥127 g/m in men and ≥100 g/m in women.19
Outcomes
The primary outcome was incidence of a major CVD disease event, a composite outcome of cardiovascular death, fatal or nonfatal myocardial infarction, unstable angina (prolonged ischemic episode with documented reversible ST‐segment changes), heart failure, and stroke. Medical records were obtained for all hospitalizations and physician visits related to CVD disease during follow‐up and were reviewed by an adjudication panel consisting of 3 investigators. Criteria for these CVD events have been described previously.20
Statistical Methods
We divided the participants into 4 groups according to their hypertension status (blood pressure ≥140 mm Hg systolic or ≥90 mm Hg diastolic or use of antihypertensive medication) and presence of high vascular stiffness (PWV at or over age‐ and sex‐specific median). Participants with normal blood pressure and lower vascular stiffness were used as reference in all analyses.
First, we used Fisher's exact test to compare differences in prevalence of high PWV between categories based on hypertension subtypes. Second, we assessed baseline characteristics in groups by hypertension and PWV status. P for trend across categories was assessed with linear or logistic regression by entering the category as a linear term in the models. Third, we studied the associations between the 4 groups defined above and the presence of LVH using multivariable‐adjusted logistic regression models, with the nonhypertensive and lower PWV group serving as referent (with which the other groups were compared). Trend in odds ratios was tested by entering the exposure categories as a linear term in the model. Fourth, we assessed the association between the 4 groups and incident CVD events with Kaplan–Meier plots, log‐rank testing, and multivariable‐adjusted Cox proportional hazard regression models, with the nonhypertensive and lower PWV group serving as referent. Interaction between the exposure categories were tested by entering these variables with interaction terms and P for interaction was obtained. Trend in hazard ratios was tested by entering the exposure categories as a linear term in the model. We assessed proportional hazard assumption using Schoenfeld residuals and found no violation of the proportional hazard assumption. We also performed a subgroup analysis for CVD outcomes in participants with isolated systolic hypertension (systolic blood pressure ≥140 mm Hg and diastolic BP <90 mm Hg irrespective of antihypertensive medication) using high versus low PWV as the exposure variable. All multivariable models were adjusted for age, sex, body mass index, smoking status, diabetes mellitus, heart rate, serum total cholesterol, and high‐density lipoprotein cholesterol. A 2‐sided value of P<0.05 was considered statistically significant. All analyses were performed with Stata software version 13.1 (StataCorp, College Station, TX).
Results
We studied up to 2127 community‐dwelling participants (mean age 60.4 years, 56.6% women). Baseline characteristics in groups according to their PWV and hypertension status are shown in Table 1.
Table 1.
Baseline Characteristics by Hypertension and PWV Status
| Characteristic | All | Normotension With Low PWV | Normotension With High PWV | Hypertension With Low PWV | Hypertension With High PWV | P for Trend |
|---|---|---|---|---|---|---|
| N | 2127 | 715 | 505 | 342 | 565 | |
| Age, y | 60.4±9.5 | 58.2±8.9 | 56.6±8.9 | 65.7±8.4 | 63.5±9.0 | <0.001 |
| Women, n | 1203 (56.6%) | 431 (60.3%) | 293 (58.0%) | 172 (50.3%) | 307 (54.3%) | 0.008 |
| BMI, kg/m2 | 27.3±4.6 | 25.8±3.9 | 27.3±4.8 | 27.6±4.4 | 29.2±4.7 | <0.001 |
| Diabetes mellitus, n | 187 (8.8%) | 18 (2.5%) | 34 (6.7%) | 38 (11.1%) | 97 (17.2%) | <0.001 |
| Current smoker, n | 287 (13.5%) | 117 (16.4%) | 86 (17.0%) | 26 (7.6%) | 58 (10.3%) | <0.001 |
| Cholesterol, mmol/L | 5.2±0.9 | 5.2±0.9 | 5.3±1.0 | 5.2±0.9 | 5.2±1.00 | 0.71 |
| HDL cholesterol, mmol/L | 1.4±0.5 | 1.5±0.5 | 1.4±0.5 | 1.4±0.5 | 1.4±0.4 | <0.001 |
| Systolic BP, mm Hg | 127±19 | 115±12 | 120±11 | 133±17 | 144±19 | <0.001 |
| Diastolic BP, mm Hg | 74±10 | 70±8 | 74±8 | 75±10 | 80±11 | <0.001 |
| Heart rate, bpm | 64.8±10.7 | 62.3±9.3 | 66.9±9.9 | 62.4±10.8 | 67.5±11.7 | <0.001 |
| HTN treatment, n | 648 (30.5%) | 0 (0%) | 0 (0%) | 257 (75.2%) | 391 (69.2%) | <0.001 |
| PWV, m/s | 9.9±3.4 | 7.9±1.4 | 10.1±2.6 | 9.2±1.7 | 13.0±4.1 | <0.001 |
Values are mean±SD for continuous variables or n (%) for categorical variables. P for trend across categories was assessed with linear or logistic regression by entering the category as a linear term in the models. BMI indicates body mass index; BP, elevated blood pressure; HDL, high‐density lipoprotein; HTN, hypertension; PWV, pulse wave velocity.
Risk of CVD Events in Groups by PWV and Hypertension Status
During a median follow‐up of 12.6 years, 248 CVD events occurred. The unadjusted Kaplan–Meier curves in Figure 1 illustrate the cumulative incidence of CVD events in groups by PWV and hypertension status. Again, as for LVH, the incidence rates and both unadjusted and adjusted hazards ratios increased across the groups (Table 2, Figure 2). Risk of CVD events was not significantly increased in participants who were normotensive and had high PWV (hazard ratio 1.33, 95% CI 0.86–2.05). No interaction was found between PWV and hypertension status on CVD risk in the multivariable model (P=0.68). We also performed a subgroup analysis for CVD outcomes in participants with isolated systolic hypertension (n=369) with high versus low PWV as the exposure variable. Among these individuals, high PWV (n=250 with 64 events, hazard ratio 3.06, 95% CI 1.59–5.89) was a particularly potent predictor of CVD events when compared with individuals with low PWV (n=119 with 11 events, hazard ratio 1.00).
Figure 1.
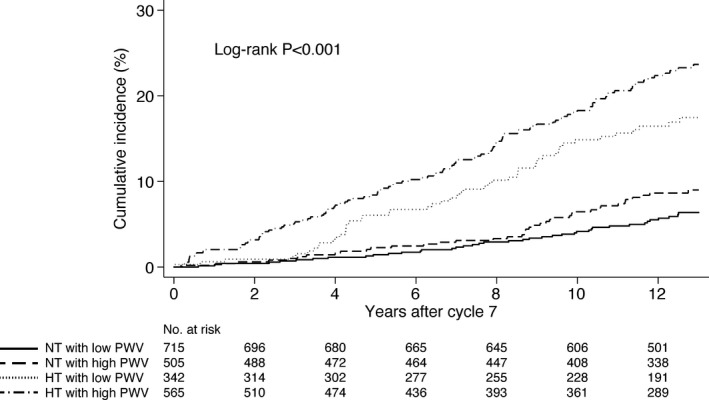
Cumulative incidence of cardiovascular events in groups by hypertension and pulse wave velocity status (truncated at 13 years after baseline). HT indicates hypertension; NT, normotension; PWV, pulse wave velocity.
Table 2.
Risk of Cardiovascular Events in Groups by Hypertension and PWV Status (n=2127)
| Normotension With Low PWV | Normotension With High PWV | Hypertension With Low PWV | Hypertension With High PWV | P (Trend) | |
|---|---|---|---|---|---|
| Number of CVD events (%) | 42 (5.9) | 41 (8.1) | 51 (14.9) | 114 (20.2) | |
| Events per 1000 person‐years | 5.0 (3.7–6.7) | 7.0 (5.2–9.5) | 14.4 (11.0–19.0) | 20.7 (17.2–24.9) | |
| Hazard ratio (95% CI) for CVD events | |||||
| Unadjusted model | 1.00 (reference) | 1.41 (0.92–2.17) | 2.96 (1.97–4.45) | 4.24 (2.98–6.05) | <0.0001 |
| Multivariable‐adjusted model | 1.00 (reference) | 1.29 (0.83–2.00) | 1.54 (1.01–2.36) | 2.25 (1.54–3.29) | <0.0001 |
Multivariable model adjusted for age, sex, body mass index, smoking status, diabetes mellitus, heart rate, total cholesterol, and HDL cholesterol. Trend in hazard ratios was tested by entering the exposure categories as a linear term in the model. CVD indicates cardiovascular disease; PWV, pulse wave velocity.
Figure 2.
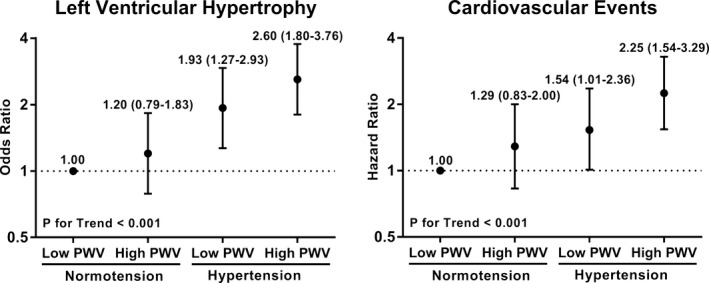
Risk of left ventricular hypertrophy and cardiovascular events in groups by hypertension and pulse wave velocity status. Odds/hazard ratios (95% CIs) are adjusted for age, sex, body mass index, smoking status, diabetes mellitus, total cholesterol, and HDL cholesterol. Trend in odds and hazard ratios was tested by entering the exposure categories as a linear term in the model. HDL indicates high‐density lipoprotein; PWV, pulse wave velocity.
Presence of LVH in Groups by PWV and Hypertension Status
In a subgroup of 1583 participants who had LVH data available (mean age 60.0±9.5, 59.8% women), the prevalence of LVH in groups by PWV and hypertension status are reported in Table 3 and Figure 2. The prevalence rates and both unadjusted and adjusted odds ratios were higher across the groups. LVH rates were not significantly higher in participants who were nonhypertensive and had high PWV (odds ratio 1.20, 95% CI 0.79–1.83). No interaction was found for the effects of PWV and hypertension status on LVH in the multivariable model (P=0.77).
Table 3.
Odds Ratios for Echo‐ or Electrocardiographic LVH in Groups by Hypertension and PWV Status (n=1583)
| Group | n | Participants With LVH (%) | Unadjusted OR (95% CI) | P Value | Multivariable OR (95% CI) | P Value |
|---|---|---|---|---|---|---|
| Normotension with low PWV | 562 | 62 (11.0) | 1.00 (reference) | 1.00 (reference) | ||
| Normotension with high PWV | 376 | 49 (13.0) | 1.21 (0.81–1.80) | 0.35 | 1.20 (0.79–1.83) | 0.40 |
| Hypertension with low PWV | 243 | 66 (27.2) | 3.01 (2.04–4.43) | <0.0001 | 1.93 (1.27–2.93) | 0.002 |
| Hypertension with high PWV | 402 | 143 (35.6) | 4.45 (3.19–6.22) | <0.0001 | 2.60 (1.80–3.76) | <0.0001 |
Multivariable model is adjusted for age, sex, body mass index, smoking status, diabetes mellitus, heart rate, total cholesterol, and HDL cholesterol. Left ventricular hypertrophy was defined as presence of Cornell voltage >20 mV in women and >28 mV in men (n=30), the ratio of echocardiographic left ventricular mass to height in the sex‐specific top quintile (n=278) or both (n=12). P for difference in prevalence of left ventricular hypertrophy across categories is <0.001 (Fisher's exact test). P value for trend in odds ratios across categories was <0.001 in both models (tested by entering the exposure categories as a linear term in the model). HDL indicates high‐density lipoprotein; LVH, left ventricular hypertrophy; OR, odds ratio; PWV, pulse wave velocity.
Prevalence of High PWV by Hypertension Subtype
To assess to what extent PWV remains high in treated hypertension (Figure 3), we also categorized the participants according to their hypertension status: nonhypertensive (n=1220, blood pressure <140/90 mm Hg without antihypertensive medication), controlled treated hypertension (n=390, blood pressure <140/90 mm Hg with antihypertensive medication), uncontrolled treated hypertension (n=258, blood pressure ≥140/90 mm Hg with antihypertensive medication), and untreated hypertension (n=259, blood pressure ≥140/90 mm Hg without antihypertensive medication). Altogether, 71.8% of treated hypertensives had high PWV. Participants with controlled hypertension had a greater prevalence of high PWV (59.7%) than nonhypertensive participants (33.9%, P for difference<0.0001) but a lower prevalence of high PWV than participants with uncontrolled (89.9%, P for difference<0.0001) or untreated hypertension (72.2%, P for difference=0.001).
Figure 3.
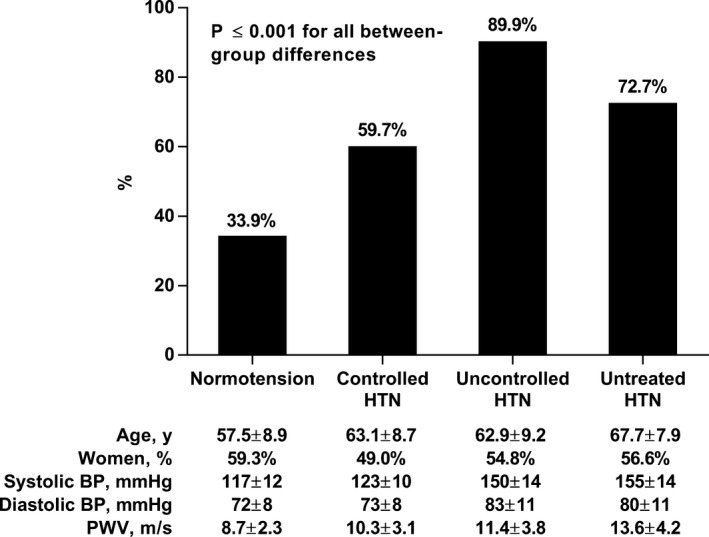
Proportion of participants with increased pulse wave velocity (over age‐ and sex‐specific median) in groups by hypertension subtype. P≤0.001 for all between‐group differences. BP indicates blood pressure; HTN, hypertension; PWV, pulse wave velocity.
Discussion
The results of our study indicate that arterial stiffness was elevated in the majority of treated hypertensives in the community, irrespective of hypertension control. In addition, our results show that there is an increasing stepwise trend for a higher prevalence of LVH and increasing CVD risk in categories cross‐classified by hypertension and arterial stiffness status from nonhypertensive with low PWV to nonhypertensive with high PWV, and from hypertension with low PWV to hypertension with high PWV.
The results of our study demonstrate that approximately only 10% of uncontrolled treated hypertensives and 40% of controlled hypertensives have lower PWV (as defined by the age‐ and sex‐specific median). Many of the standard modifiable risk factors, such as blood pressure and lipids, can be often normalized within a few weeks with aggressive drug therapies that also improve CVD outcomes.21, 22 However, normalizing these risk factors does not necessarily per se immediately lead to improved arterial stiffness, as some of the causes underlying arterial stiffening, such as vascular calcification and fracture of elastin fibers within the arterial media, may be irreversible.23, 24, 25 In addition, whereas agents acting on the renin–angiotensin system and calcium channel blockers are beneficial in reducing arterial stiffness, some β‐blockers may have the opposite effects while still lowering peripheral blood pressure.26, 27, 28 Although it has not yet been unequivocally shown that normalization of arterial stiffness leads to improved outcomes, one study has suggested that changes in PWV in response to decreases in blood pressure favorably influence outcomes independent of the blood pressure changes.13 In that investigation, Guerin et al reported in a cohort of 150 patients that with an absence of PWV decrease they had a 2.4‐fold higher risk for cardiovascular mortality compared to participants in whom PWV decreased. However, as this was a highly selected sample of dialysis patients with end‐stage renal disease, its results may not be generalizable to other populations. Our finding of the high prevalence of increased PWV among treated hypertensives in the community warrants further study because it has been previously shown that patients under antihypertensive medication have a 50% greater residual risk of cardiovascular mortality compared to persons free from hypertension even after adjusting for blood pressure level.12, 29 Difficult‐to‐treat residual arterial stiffness could, therefore, be a key factor in the adverse CVD prognosis in apparently well‐controlled hypertension.
Although the presence of LVH and risk of incident CVD was not statistically higher in nonhypertensive participants with high PWV than in nonhypertensive participants with low PWV, the risk trended to increase across the 4 groups by hypertension and PWV status. Another interesting finding of our study is that although the majority of individuals with isolated systolic hypertension expectedly also had high PWV, individuals with isolated systolic hypertension and low PWV had a 3‐fold lower risk of CVD events than those with high PWV. Furthermore, hypertension status itself did not modify the relationship between PWV and LVH or CVD outcomes, as evidenced by the statistically nonsignificant interaction terms. Previous meta‐analyses have not directly compared the prognostic significance of PWV in hypertensive or nonhypertensive groups, although these studies did test for interaction with between PWV and several other variables.10, 11 In these studies, the results by population type (general versus clinical) were contradictory as a literature‐based meta‐analysis by Vlachopoulos et al concluded that the risk ratios for CVD events associated with higher PWV were significantly higher in high‐risk than in low‐risk populations. However, Ben‐Shlomo et al reported in their individual‐level data meta‐analysis that the increased risk associated with PWV was not modified by population type or antihypertensive medication.10, 11 Although the results from our and previous studies demonstrate that measurement of arterial stiffness improves CVD risk assessment in most subpopulations, the optimal approach for CVD disease prevention using PWV still remains controversial as it has not yet been demonstrated that normalization of arterial stiffness, independent of standard CVD risk factors, improves prognosis. The ongoing SPARTE (Strategy for Preventing Cardiovascular and Renal Events Based on ARTErial Stiffness) trial that aims at comparing the efficacy of a therapeutic strategy targeting normalization of arterial stiffness, instead of blood pressure, for reducing CVD and renal events will hopefully finally provide an answer to this key question.30
Studying the distinctive roles of arterial stiffness and hypertension as predictors of CVD outcomes has certain challenges as PWV represents on one side hypertensive end organ damage and on the other side a cause of hypertension, while both arterial stiffness and hypertension may be elements of a vicious cycle. Despite the strengths of our study, such as a moderate‐sized community‐based sample with long‐term follow‐up, our results must be interpreted with caution. First, our study could have benefited from a larger study sample and greater number of CVD events; we were inherently underpowered to demonstrate a statistically increased CVD risk among normotensive participants with a high PWV. It is also conceivable that elevated PWV alone may be necessary but not sufficient to elevate CVD risk, or that not everyone with elevated PWV may develop high blood pressure during the limited period of observation in our study. Second, an enhanced statistical power would have also enabled us assess the CVD prognosis associated with high versus low PWV in individuals with controlled hypertension, which in turn would have allowed us to elucidate whether arterial stiffness is a key factor in contributing to the residual risk in treated hypertension. Third, PWV is only a measure of large‐artery stiffness, and does not adequately reflect the universal lifetime panarterial damage sustained due to high blood pressure and other risk factors. Fourth, because our study sample consisted mainly of middle‐aged and older white individuals, our results may not be generalizable to other age groups or ethnicities. Fifth, as LVH was ascertained ≈3 years earlier, blood pressure status, PWV status, and LVH status all could have changed in the interval. Sixth, a doctor measured the blood pressure only twice at the clinic and no out‐of‐office BP measurements were available. Seventh, we could not assess the distinctive effects of various drug classes on arterial stiffness in our cross‐sectional study because many of the treated hypertensives in our study were on combination antihypertensive therapy. Furthermore, these analyses would have also been confounded by indication bias as many comorbidities (eg, diabetes mellitus) at the same time affect arterial stiffness and may also guide the selection of antihypertensive therapy.
In conclusion, high PWV is associated with a stepwise trend towards increasing prevalence of LVH cross‐sectionally, and greater CVD risk prospectively regardless of hypertension status (nonhypertensive versus hypertensive). These findings may therefore support the use of arterial stiffness measurements in both populations. Furthermore, the high prevalence of increased PWV in both controlled and uncontrolled treated individuals with hypertension observed in our study warrants further research as this residual increased arterial stiffness may explain some of the notable residual CVD risk associated with even well‐controlled hypertension.
Sources of Funding
This work was supported by the National Heart, Lung, and Blood Institute's Framingham Heart Study (National Institute of Health [NIH] contracts N01‐HC‐25195 and HHSN268201500001I) and NIH grants HL080124, HL071039, HL077447, HL107385, 1R01HL126136‐01A1, 5R01HL107385‐04, 1R01HL60040, and 1RO1HL70100.
Disclosures
Mitchell is owner of Cardiovascular Engineering, Inc., a company that designs and manufactures devices that measure vascular stiffness. The company uses these devices in clinical trials that evaluate the effects of diseases and interventions on vascular stiffness. The remaining authors report no conflicts.
(J Am Heart Assoc. 2016;5:e004271 doi: 10.1161/JAHA.116.004271)
References
- 1. Chow CK, Teo KK, Rangarajan S, Islam S, Gupta R, Avezum A, Bahonar A, Chifamba J, Dagenais G, Diaz R, Kazmi K, Lanas F, Wei L, Lopez‐Jaramillo P, Fanghong L, Ismail NH, Puoane T, Rosengren A, Szuba A, Temizhan A, Wielgosz A, Yusuf R, Yusufali A, McKee M, Liu L, Mony P, Yusuf S; PURE (Prospective Urban Rural Epidemiology) Study investigators . Prevalence, awareness, treatment, and control of hypertension in rural and urban communities in high‐, middle‐, and low‐income countries. JAMA. 2013;310:959–968. [DOI] [PubMed] [Google Scholar]
- 2. Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair‐Rohani H, Amann M, Anderson HR, Andrews KG, Aryee M, Atkinson C, Bacchus LJ, Bahalim AN, Balakrishnan K, Balmes J, Barker‐Collo S, Baxter A, Bell ML, Blore JD, Blyth F, Bonner C, Borges G, Bourne R, Boussinesq M, Brauer M, Brooks P, Bruce NG, Brunekreef B, Bryan‐Hancock C, Bucello C, Buchbinder R, Bull F, Burnett RT, Byers TE, Calabria B, Carapetis J, Carnahan E, Chafe Z, Charlson F, Chen H, Chen JS, Cheng AT, Child JC, Cohen A, Colson KE, Cowie BC, Darby S, Darling S, Davis A, Degenhardt L, Dentener F, Des Jarlais DC, Devries K, Dherani M, Ding EL, Dorsey ER, Driscoll T, Edmond K, Ali SE, Engell RE, Erwin PJ, Fahimi S, Falder G, Farzadfar F, Ferrari A, Finucane MM, Flaxman S, Fowkes FG, Freedman G, Freeman MK, Gakidou E, Ghosh S, Giovannucci E, Gmel G, Graham K, Grainger R, Grant B, Gunnell D, Gutierrez HR, Hall W, Hoek HW, Hogan A, Hosgood HD, Hoy D, Hu H, Hubbell BJ, Hutchings SJ, Ibeanusi SE, Jacklyn GL, Jasrasaria R, Jonas JB, Kan H, Kanis JA, Kassebaum N, Kawakami N, Khang YH, Khatibzadeh S, Khoo JP, Kok C, Laden F, Lalloo R, Lan Q, Lathlean T, Leasher JL, Leigh J, Li Y, Lin JK, Lipshultz SE, London S, Lozano R, Lu Y, Mak J, Malekzadeh R, Mallinger L, Marcenes W, March L, Marks R, Martin R, McGale P, McGrath J, Mehta S, Mensah GA, Merriman TR, Micha R, Michaud C, Mishra V, Mohd Hanafiah K, Mokdad AA, Morawska L, Mozaffarian D, Murphy T, Naghavi M, Neal B, Nelson PK, Nolla JM, Norman R, Olives C, Omer SB, Orchard J, Osborne R, Ostro B, Page A, Pandey KD, Parry CD, Passmore E, Patra J, Pearce N, Pelizzari PM, Petzold M, Phillips MR, Pope D, Pope CA III, Powles J, Rao M, Razavi H, Rehfuess EA, Rehm JT, Ritz B, Rivara FP, Roberts T, Robinson C, Rodriguez‐Portales JA, Romieu I, Room R, Rosenfeld LC, Roy A, Rushton L, Salomon JA, Sampson U, Sanchez‐Riera L, Sanman E, Sapkota A, Seedat S, Shi P, Shield K, Shivakoti R, Singh GM, Sleet DA, Smith E, Smith KR, Stapelberg NJ, Steenland K, Stockl H, Stovner LJ, Straif K, Straney L, Thurston GD, Tran JH, Van Dingenen R, van Donkelaar A, Veerman JL, Vijayakumar L, Weintraub R, Weissman MM, White RA, Whiteford H, Wiersma ST, Wilkinson JD, Williams HC, Williams W, Wilson N, Woolf AD, Yip P, Zielinski JM, Lopez AD, Murray CJ, Ezzati M, AlMazroa MA, Memish ZA. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. O'Rourke MF, Hashimoto J. Mechanical factors in arterial aging: a clinical perspective. J Am Coll Cardiol. 2007;50:1–13. [DOI] [PubMed] [Google Scholar]
- 4. Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS, Mitchell GF. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA. 2012;308:875–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Safar ME, Toto‐Moukouo JJ, Bouthier JA, Asmar RE, Levenson JA, Simon AC, London GM. Arterial dynamics, cardiac hypertrophy, and antihypertensive treatment. Circulation. 1987;75:I156–I161. [PubMed] [Google Scholar]
- 6. Mattace‐Raso FU, van der Cammen TJ, Hofman A, van Popele NM, Bos ML, Schalekamp MA, Asmar R, Reneman RS, Hoeks AP, Breteler MM, Witteman JC. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation. 2006;113:657–663. [DOI] [PubMed] [Google Scholar]
- 7. Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010;121:505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Willum‐Hansen T, Staessen JA, Torp‐Pedersen C, Rasmussen S, Thijs L, Ibsen H, Jeppesen J. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation. 2006;113:664–670. [DOI] [PubMed] [Google Scholar]
- 9. Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, Ducimetiere P, Benetos A. Aortic stiffness is an independent predictor of all‐cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–1241. [DOI] [PubMed] [Google Scholar]
- 10. Ben‐Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, Boutouyrie P, Cameron J, Chen CH, Cruickshank JK, Hwang SJ, Lakatta EG, Laurent S, Maldonado J, Mitchell GF, Najjar SS, Newman AB, Ohishi M, Pannier B, Pereira T, Vasan RS, Shokawa T, Sutton‐Tyrell K, Verbeke F, Wang KL, Webb DJ, Willum Hansen T, Zoungas S, McEniery CM, Cockcroft JR, Wilkinson IB. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta‐analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol. 2014;63:636–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all‐cause mortality with arterial stiffness: a systematic review and meta‐analysis. J Am Coll Cardiol. 2010;55:1318–1327. [DOI] [PubMed] [Google Scholar]
- 12. Blacher J, Evans A, Arveiler D, Amouyel P, Ferrieres J, Bingham A, Yarnell J, Haas B, Montaye M, Ruidavets JB, Ducimetiere P; PRIME Study Group . Residual cardiovascular risk in treated hypertension and hyperlipidaemia: the PRIME Study. J Hum Hypertens. 2010;24:19–26. [DOI] [PubMed] [Google Scholar]
- 13. Guerin AP, Blacher J, Pannier B, Marchais SJ, Safar ME, London GM. Impact of aortic stiffness attenuation on survival of patients in end‐stage renal failure. Circulation. 2001;103:987–992. [DOI] [PubMed] [Google Scholar]
- 14. Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham Offspring Study. Am J Epidemiol. 1979;110:281–290. [DOI] [PubMed] [Google Scholar]
- 15. Mitchell GF, Guo CY, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, Vasan RS, Levy D. Cross‐sectional correlates of increased aortic stiffness in the community: the Framingham Heart Study. Circulation. 2007;115:2628–2636. [DOI] [PubMed] [Google Scholar]
- 16. Mitchell GF, Parise H, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, Vasan RS, Levy D. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham Heart Study. Hypertension. 2004;43:1239–1245. [DOI] [PubMed] [Google Scholar]
- 17. Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker‐Boudier H; European Network for Non‐invasive Investigation of Large Arteries . Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. [DOI] [PubMed] [Google Scholar]
- 18. Casale PN, Devereux RB, Kligfield P, Eisenberg RR, Miller DH, Chaudhary BS, Phillips MC. Electrocardiographic detection of left ventricular hypertrophy: development and prospective validation of improved criteria. J Am Coll Cardiol. 1985;6:572–580. [DOI] [PubMed] [Google Scholar]
- 19. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. [DOI] [PubMed] [Google Scholar]
- 20. Kannel WB, Wolf PA, Garrison RJ, eds. Section 34: Some Risk Factors Related to the Annual Incidence of Cardiovascular Disease and Death in Pooled Repeated Biennial Measurements. Framingham Heart Study, 30 Year Follow‐Up. Bethesda, MD: US Department of Health and Human Services; 1987. [Google Scholar]
- 21. Taylor F, Huffman MD, Macedo AF, Moore TH, Burke M, Davey Smith G, Ward K, Ebrahim S. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013;1:CD004816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, Chalmers J, Rodgers A, Rahimi K. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta‐analysis. Lancet. 2015;387:957–967. [DOI] [PubMed] [Google Scholar]
- 23. Avolio A, Jones D, Tafazzoli‐Shadpour M. Quantification of alterations in structure and function of elastin in the arterial media. Hypertension. 1998;32:170–175. [DOI] [PubMed] [Google Scholar]
- 24. McEniery CM, McDonnell BJ, So A, Aitken S, Bolton CE, Munnery M, Hickson SS, Yasmin , Maki‐Petaja KM, Cockcroft JR, Dixon AK, Wilkinson IB; Anglo‐Cardiff Collaboration Trial Investigators . Aortic calcification is associated with aortic stiffness and isolated systolic hypertension in healthy individuals. Hypertension. 2009;53:524–531. [DOI] [PubMed] [Google Scholar]
- 25. O'Rourke MF, Nichols WW. Aortic diameter, aortic stiffness, and wave reflection increase with age and isolated systolic hypertension. Hypertension. 2005;45:652–658. [DOI] [PubMed] [Google Scholar]
- 26. Williams B, Lacy PS, Thom SM, Cruickshank K, Stanton A, Collier D, Hughes AD, Thurston H, O'Rourke M; CAFE Investigators, Anglo‐Scandinavian Cardiac Outcomes Trial Investigators, CAFE Steering Committee and Writing Committee . Differential impact of blood pressure‐lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation. 2006;113:1213–1225. [DOI] [PubMed] [Google Scholar]
- 27. Asmar RG, London GM, O'Rourke ME, Safar ME; REASON Project Coordinators and Investigators . Improvement in blood pressure, arterial stiffness and wave reflections with a very‐low‐dose perindopril/indapamide combination in hypertensive patient: a comparison with atenolol. Hypertension. 2001;38:922–926. [DOI] [PubMed] [Google Scholar]
- 28. Dudenbostel T, Glasser SP. Effects of antihypertensive drugs on arterial stiffness. Cardiol Rev. 2012;20:259–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Asayama K, Satoh M, Murakami Y, Ohkubo T, Nagasawa SY, Tsuji I, Nakayama T, Okayama A, Miura K, Imai Y, Ueshima H, Okamura T; Evidence for Cardiovascular Prevention From Observational Cohorts in Japan (EPOCH‐JAPAN) Research Group . Cardiovascular risk with and without antihypertensive drug treatment in the Japanese general population: participant‐level meta‐analysis. Hypertension. 2014;63:1189–1197. [DOI] [PubMed] [Google Scholar]
- 30. Laurent S, Briet M, Boutouyrie P. Arterial stiffness as surrogate end point: needed clinical trials. Hypertension. 2012;60:518–522. [DOI] [PubMed] [Google Scholar]


