Abstract
Background
Variations in distal coronary pressure (Pd)/aortic pressure (Pa) ratio during steady‐state hyperemia with standard (140 μg/kg per minute) adenosine dose may hamper accurate fractional flow reserve assessment. This study investigated to what extent an increased adenosine dose can overcome Pd/Pa variation.
Methods and Results
In a prospective, single‐arm study, out of 95 prospectively screened patients, 38 (40.0%) exhibited significant (≥0.05 difference of max Pd/Pa minus min Pd/Pa) variations in Pd/Pa from 15 s post Pd/Pa dip and until the end of a 3‐minute adenosine (140 μg/kg per minute) infusion. Thirty patients agreed to participate in a post 5‐minute repeat fractional flow reserve assessment using 200 μg/kg per minute 3‐minute adenosine infusion. The study's co‐primary end point of Pd/Pa coefficient of dispersion was lower for the high versus standard adenosine dose: 1.31 (1.13–2.72) versus 2.76 (2.38–5.60), P=0.002. The study's co‐primary end point of ΔPd/Pa was also lower for the high versus standard adenosine dose: 0.065 (0.038–0.10) versus 0.08 (0.06–0.11), P=0.002. This difference was mainly driven by the lowering effect of the high adenosine dose on the maximum Pd/Pa compared to the standard dose: 0.84 (0.81–0.93) versus 0.90 (0.83–0.95), P=0.007, while minimum Pd/Pa remained unaffected. High adenosine dose was adequately tolerated by all patients, without requiring infusion discontinuation in any case.
Conclusions
Pd/Pa variability is frequently observed during standard adenosine infusion and is significantly decreased following a high (200 μg/kg per minute) adenosine dose. This is achieved without a significant difference in the minimum Pd/Pa.
Clinical Trial Registration
URL: https://www.clinicaltrials.gov. Unique identifier: NCT02350439.
Keywords: adenosine, fractional flow reserve, hemodynamics
Subject Categories: Coronary Circulation, Hemodynamics, Diagnostic Testing
Introduction
Fractional flow reserve (FFR) measurement is an established invasive method for assessing the physiological significance of coronary artery stenosis using the distal coronary pressure (Pd)/aortic pressure (Pa) ratio during maximal hyperemia.1, 2, 3 However, variations in Pd/Pa ratio have been observed even during the expected “steady‐state” hyperemia phase with infusion of standard (140 μg/kg per minute) adenosine dose.4, 5 Pd/Pa variations could potentially affect FFR definition (lowest observed versus “steady‐state” value) or hamper the accurate FFR assessment in diffuse or multiple lesions during the pressure pullback curve recording, with final impact on clinical decisions for revascularization.4, 6, 7
The issue of intrinsic adenosine inability to induce a true maximal hyperemic state in the coronary circulation in all cases was raised in an early study.8 Nevertheless, the infusion of a 140 μg/kg per minute adenosine dose through a central vein has been subsequently extensively considered as the “gold standard” to obtain maximum coronary hyperemia.9, 10 Failure to achieve maximal hyperemia with the standard dose was reemphasized and implicated—at least partially—for the reported Pd/Pa variations in recent studies.5, 7
In the present study, we assessed the prospectively identified Pd/Pa variations observed during standard‐dose adenosine infusion and analyzed the impact on them of a higher, 200 μg/kg per minute adenosine dose infusion.
Methods
In a prospective, single‐arm study performed between January 2015 and October 2015, we assessed Pd/Pa variations using a standardized protocol of a 3‐minute adenosine infusion at 140 μg/kg per minute, through the femoral vein in all cases.11 Consecutive patients with stable coronary artery disease or acute coronary syndrome were considered as candidates if they presented with an angiographically significant de novo stenosis (>50% and <90% diameter stenosis by visual assessment) in at least 1 major epicardial coronary artery amenable to percutaneous coronary intervention. We excluded patients >80 years old, with a history of coronary artery bypass surgery, acute myocardial infarction within the previous 5 days, hemodialysis or creatinine clearance <30 mL/min, allergy or contraindication to adenosine, left main disease, anatomy suitable for coronary artery bypass surgery, angiographically visible thrombus at a target lesion, left ventricular ejection fraction <40%, previous q‐wave myocardial infarction in the area of target vessel or presence of collaterals, primary myocardial or valvular heart disease, severe left ventricular hypertrophy, hypotension, significant arrhythmia (eg, excessive premature ventricular contractions or atrial fibrillation), bradycardia (<50 beats/min), or increased risk for bradycardia and caffeine consumption or smoking within the previous 24 hours. All patients were on aspirin (100 mg/day) before FFR measurements.
For each patient, only 1 lesion was assessed by FFR. Intracoronary nitroglycerin (0.3 mg) was administered before coronary angiography and before each FFR measurement, except in the case of systolic blood pressure <100 mm Hg. A 0.014‐inch high‐fidelity pressure‐recording guidewire (PressureWire, St Jude Medical, St Paul, MN) was introduced through a 6‐F end‐hole guiding catheter into the coronary artery. The guidewire was externally zeroed and equalized between the pressure recorded through the catheter and the pressure wire. Careful attention was paid to avoid arterial pressure dampening or variation of the measured coronary catheter pressure. The pressure wire was advanced into the coronary artery with the pressure sensor placed beyond the lesion site under study. Infusion of adenosine was given using a rate‐controlled infusion pump, with continuous pressure signals recording for 30 s prior to and throughout the infusion. Following adenosine discontinuation, a pullback recording was performed to exclude wire drift.
By offline visual assessment and consensus of 2 experienced interventional cardiologists, the onset of Pd/Pa lowering, the subsequent dip in Pd/Pa (onset of hyperemia), and time point of 15 s post Pd/Pa dip were assessed. Postdip period (PDP) was defined as the interval between 15 s post Pd/Pa dip and the end of adenosine infusion. Patients with significant variation in Pd/Pa ratio during PDP, defined as ≥0.05 difference of max Pd/Pa minus min Pd/Pa, were asked to consent to a repeat FFR assessment of the same lesion 5 minutes later using a 3‐minute infusion of high‐dose adenosine (200 μg/kg per minute).4
Following completion of study protocol, patients received ad hoc percutaneous coronary intervention, medical therapy, or coronary artery bypass surgery on the basis of the overall findings. A study flow chart is shown in Figure 1.
Figure 1.
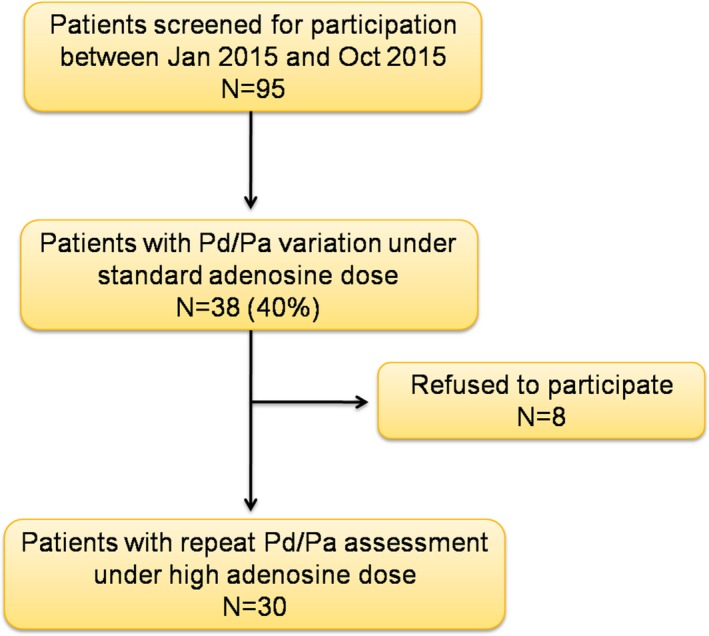
Study flow chart. Pa indicates aortic pressure; Pd, distal coronary pressure.
Measurements
Offline analysis was performed to calculate Pa and Pd at baseline (prior to transition to hyperemia), peak hyperemia, and PDP. The minimum Pd/Pa ratio per 3 beats from the onset of Pd/Pa lowering until the end of the infusion was assessed.4 sFFR was defined as the minimum Pd/Pa during PDP.6, 12 Coefficient of dispersion (COD), expressed as percentage, during PDP was assessed as the ratio of Pd/Pa interquartile range divided by median. Similarly CODs were calculated for Pd and Pa separately. Lowest FFR (lFFR) was defined as the value provided by the automated FFR console during adenosine infusion. Quantitative coronary angiography (PIE Medical's CAAS 2000; Philips, Eindhoven, the Netherlands) of all studied lesions was performed by 2 experienced interventional cardiologists.
End Points
The study's co‐primary end points were the COD of Pd/Pa during PDP and the difference between the maximum and minimum value of Pd/Pa ratio (ΔPd/Pa) during PDP between the 2 groups. Secondary end points were the time onset of Pd/Pa lowering and PDP, lFFR, maximum and minimum Pd/Pa (sFFR) during PDP, COD of Pa, and COD of Pd between groups.
Statistical Analysis
Categorical data are presented as frequencies and group percentages. Continuous data with normal distribution are presented as means±SD or medians (first to third quartile) in case of abnormal distribution. Paired sample t test and Wilcoxon signed rank test were used for comparison of continuous data with and without normal distribution, respectively. McNemar test was used for comparison of categorical data. COD is defined as interquartile range divided by the median and expressed as a percentage. We used Bland–Altman analysis (mean difference or bias, SD of bias and 95% limits of agreement) to assess agreement and Spearman's rank correlation coefficient (r 2) to evaluate associations between (1) sFFR and (2) lFFR values observed with the standard and high adenosine doses. All tests were 2‐tailed and statistical significance was considered for P<0.05. Analyses were performed using SPSS for Windows (version 16.0 SPSS Inc, Chicago, IL) and GraphPad Prism v.5 (GraphPad Software, Inc, San Diego, CA, USA).
Sample Size Calculation
On the basis of pilot data analysis, we hypothesized that high adenosine dose would produce a decrease in COD of Pd/Pa compared to standard dose, with a mean of COD of Pd/Pa paired differences (COD of Pd/Pa under high minus COD of Pd/Pa under standard adenosine dose) of −1.6±2.8. Choosing a power of 80% and using a 2‐sided Wilcoxon signed‐rank test at an α‐level of 0.05, at least 29 pairs would be required to reach statistical significance based on the above assumptions. Similarly, we hypothesized that high adenosine dose would produce a decrease in ΔPd/Pa compared to standard dose, with a mean of ΔPd/Pa paired differences (ΔPd/Pa under high minus ΔPd/Pa under standard adenosine dose) of −0.02±0.035. Choosing a power of 80% and using a 2‐sided Wilcoxon signed‐rank test at an α‐level of 0.05, at least 29 pairs would be required to reach statistical significance based on the above assumptions.
The institutional review board approved the study protocol and written informed consent was obtained from all patients before they entered the study.
Results
Out of 95 screened patients, 38 (40.0%) exhibited variation in Pd/Pa during PDP and 30 agreed to participate. Patients' demographic, clinical, and procedural characteristics are presented in Tables 1 and 2, respectively. Onset of Pd/Pa lowering occurred at a shorter median time with the high versus standard adenosine dose: 19.5 (15.8–30.3) s versus 38.0 (28.0–54.3) s from onset of adenosine infusion, P<0.001 (Figure 2). Onset of PDP occurred at a shorter median time with the high versus standard adenosine dose: 54.0 (44.8–74.0) s versus 68.0 (58.8–86.5) s from onset of adenosine infusion, P=0.001. The interval between onset of Pd/Pa lowering and dip in Pd/Pa did not differ between high versus standard adenosine group: 17.0 (11.0–26.5) s versus 15.0 (12.0–21.8) s, P=0.5. Detailed blood pressure and heart rate data during adenosine infusion are depicted in Figure 3 and in Table 3.
Table 1.
Demographic and Clinical Characteristics of Patients
| N=30 | |
|---|---|
| Male sex | 29 (96.7) |
| Age, y | 62.8±8.5 |
| Weight, kg | 80.1±9.8 |
| Body mass index, kg/m² | 28.0±3.1 |
| Hyperlipidemia | 11 (36.7) |
| Hypertension | 12 (40.0) |
| Diabetes mellitus | 12 (40.0) |
| Current smoker | 23 (76.7) |
| Prior myocardial infarction | 10 (33.3) |
| Prior PCI | 9 (30.0) |
| Clinical presentation | |
| STEMI | 9 (30.0) |
| NSTE‐ACS | 10 (33.3) |
| Stable angina/ischemia in provocative test | 11 (36.7) |
| Medication | |
| Aspirin | 24 (80.0) |
| P2Y12 receptor antagonist | 11 (36.7) |
| Statin | 22 (73.3) |
| Calcium channel blocker | 4 (13.3) |
| Nitrate | 4 (13.3) |
| β‐Blocker | 21 (70.0) |
| Proton‐pump inhibitors | 15 (50.0) |
| Angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker | 10 (33.3) |
| Insulin | 2 (6.7) |
| Oral hypoglycemic agent | 8 (26.7) |
| Laboratory evaluation | |
| Hematocrit, % | 41.4±3.9 |
| Platelets, ×1000/mm³ | 225.6±86.5 |
| Creatinine clearance, mL/min (Cockroft–Gault formula) | 91.8±25.4 |
Values are expressed as means±SD or n (%). NSTE‐ACS indicates non ST elevation acute coronary syndrome; PCI, percutaneous coronary intervention; STEMI, ST‐segment elevation myocardial infarction.
Table 2.
Procedural Characteristics of Patients
| N=30 | |
|---|---|
| Extent of coronary artery disease | |
| 1 vessel | 12 (40.0) |
| 2 vessel | 12 (40.0) |
| 3 vessel | 6 (20.0) |
| FFR vessel | |
| LAD | 18 (60.0) |
| LCX | 7 (23.3) |
| RCA | 5 (16.7) |
| FFR lesion location | |
| Proximal | 8 (26.7) |
| Middle | 21 (70.0) |
| Distal | 5 (16.7) |
| FFR lesion stenosis (%, visual assessment) | 65.2±11.3 |
| Lesion quantitative coronary angiography | |
| Reference diameter, mm | 2.8±0.6 |
| Minimum lumen diameter, mm | 1.4±0.4 |
| Stenosis, % | 50.5±11.8 |
| Lesion length, mm | 8.8±3.4 |
Values are expressed as means±SD or n (%). FFR indicates fractional flow reserve; LAD, left anterior descending artery; LCX, left circumflex artery; RCA, right coronary artery.
Figure 2.
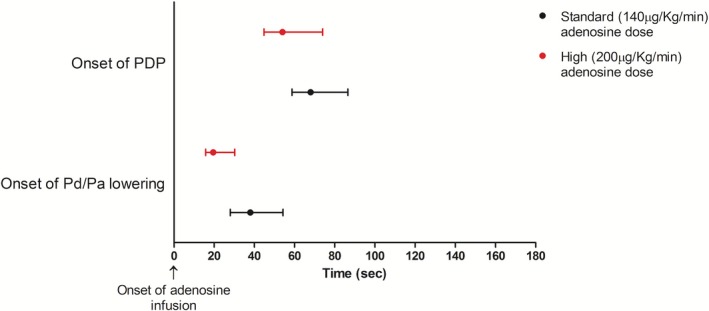
Onset of Pd/Pa lowering and of PDP by adenosine dose. Data are expressed as medians (first to third quartile). Pa indicates aortic pressure; Pd, distal coronary pressure; PDP, postdip period.
Figure 3.
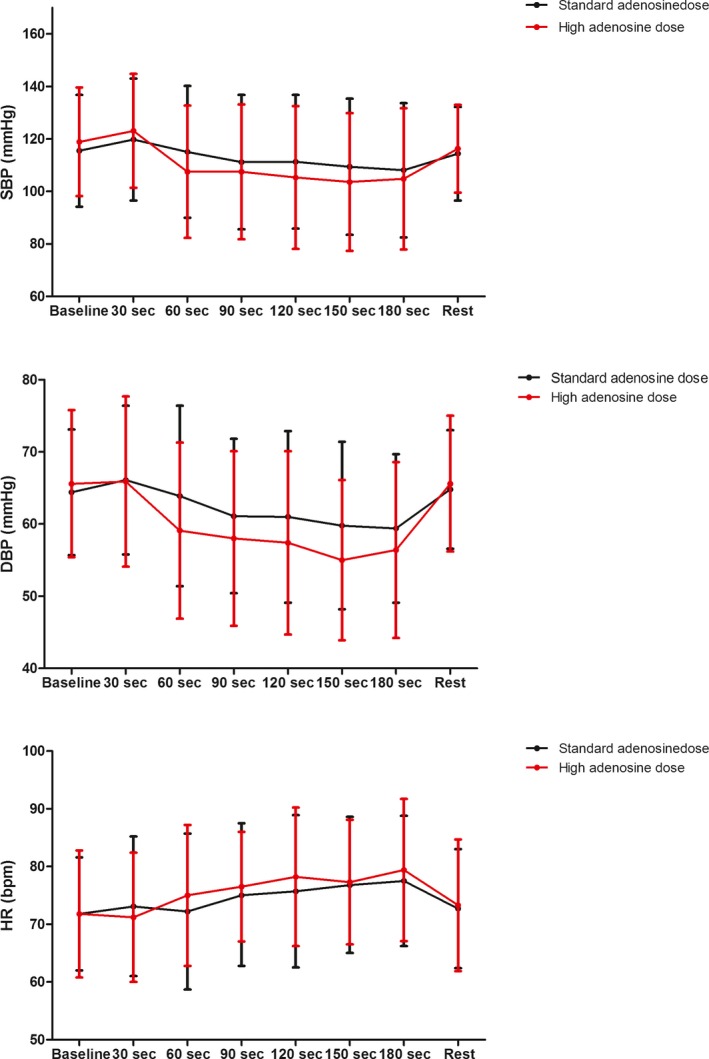
Hemodynamic measurements by adenosine dose. Dots represent means and error bars 1 SD from the mean. bpm indicates beats per min; DBP, diastolic blood pressure; HR, heart rate; SBP, systolic blood pressure.
Table 3.
Hemodynamic Measurements by Adenosine Dose
| Patients With Repeat Pd/Pa Assessment (N=30) | P Valuea | ||
|---|---|---|---|
| Standard (140 μg/kg per minute) Adenosine Dose | High (200 μg/kg per minute) Adenosine Dose | ||
| Baseline | |||
| SBP | 115.5±21.3 | 118.9±20.7 | 0.08 |
| DBP | 64.4±8.7 | 65.6±10.2 | 0.4 |
| HR | 71.8±9.8 | 71.8±11.0 | >0.99 |
| 30‐s infusion | |||
| SBP | 119.8±23.2 | 123.1±21.7 | 0.2 |
| DBP | 66.1±10.3 | 65.9±11.8 | 0.9 |
| HR | 73.1±12.1 | 71.2±11.2 | 0.2 |
| 60‐s infusion | |||
| SBP | 115.1±25.1 | 107.5±25.2 | 0.002 |
| DBP | 63.9±12.5 | 59.1±12.2 | 0.005 |
| HR | 72.2±13.5 | 75.0±12.2 | 0.046 |
| 90‐s infusion | |||
| SBP | 111.2±25.6 | 107.5±25.7 | 0.1 |
| DBP | 61.1±10.7 | 58.0±12.1 | 0.02 |
| HR | 73.1±18.4 | 76.5±9.5 | 0.2 |
| 120‐s infusion | |||
| SBP | 111.3±25.5 | 105.3±27.2 | 0.02 |
| DBP | 61.0±11.9 | 57.4±12.7 | 0.03 |
| HR | 75.7±13.2 | 78.2±12.0 | 0.2 |
| 150‐s infusion | |||
| SBP | 109.4±25.9 | 103.6±26.2 | 0.01 |
| DBP | 59.8±11.6 | 55.0±11.1 | 0.002 |
| HR | 76.8±11.8 | 77.3±10.8 | 0.8 |
| 180‐min infusion | |||
| SBP | 108.1±25.6 | 104.8±26.9 | 0.08 |
| DBP | 59.4±10.3 | 56.4±12.2 | 0.02 |
| HR | 77.5±11.3 | 79.4±12.3 | 0.2 |
| 5‐min postinfusion | |||
| SBP | 114.4±17.8 | 116.3±16.7 | 0.3 |
| DBP | 64.8±8.2 | 65.6±9.4 | 0.5 |
| HR | 72.7±10.3 | 73.3±11.4 | 0.6 |
Data are expressed as means±SD. DBP indicates diastolic blood pressure; HR, heart rate; Pa, aortic pressure; Pd, distal coronary pressure; SBP, systolic blood pressure.
P‐value is from paired‐samples t test.
The study's co‐primary end point of COD of Pd/Pa during PDP was lower for the high versus standard adenosine dose: 1.31 (1.13–2.72) versus 2.76 (2.38–5.60), P=0.002. The study's co‐primary end point of ΔPd/Pa during PDP was also lower for the high versus standard adenosine dose: 0.065 (0.038–0.10) versus 0.08 (0.06–0.11), P=0.002 (Table 4, Figure 4). This latter difference was mainly driven by the lowering effect of the high adenosine dose on the maximum Pd/Pa compared to the standard dose: 0.84 (0.81–0.93) versus 0.90 (0.83–0.95), P=0.007, while the minimum Pd/Pa (sFFR) value remained unaffected (Table 4). Further analysis revealed a significant reduction in COD of Pd and no change in COD of Pa following the high dose (Table 4). Examples of Pd/Pa variations with standard and high adenosine dose are depicted in Figure 5. Hemodynamic measurements by adenosine dose in the quartiles of patients with the greatest and lowest reduction in Pd/Pa variation are shown in Figure 6. Moreover, measurements of sFFR with the standard and the high adenosine dose demonstrated very good repeatability (bias=−0.002, SD 0.029, 95% limits of agreement −0.059 to 0.055) and high association between them (r 2=0.91), Figure 7.
Table 4.
Measurements During PDP
| Patients With Repeat Pd/Pa Assessment (N=30) | P Valuea | ||
|---|---|---|---|
| Standard (140 μg/kg per minute) Adenosine Dose | High (200 μg/kg per minute) Adenosine Dose | ||
| COD of Pd/Pa (%) | 2.76 (2.38–5.60) | 1.31 (1.13–2.72) | 0.002 |
| ΔPd/Pa | 0.08 (0.06–0.11) | 0.065 (0.038–0.10) | 0.002 |
| Maximum Pd/Pa | 0.90 (0.83–0.95) | 0.84 (0.81–0.93) | 0.007 |
| Minimum Pd/Pa | 0.79 (0.75–0.86) | 0.78 (0.75–0.86) | 0.7 |
| COD of Pd (%) | 8.47 (6.50–15.34) | 6.82 (4.72–11.08) | 0.015 |
| COD of Pa (%) | 8.20 (5.98–10.15) | 6.94 (4.80–11.32) | 0.3 |
Data are expressed as medians (first to third quartile). COD indicates coefficient of dispersion; Pa, aortic pressure; Pd, distal coronary pressure; PDP, post Pd/Pa dip period; ΔPd/Pa, maximum Pd/Pa minus minimum Pd/Pa.
P value is from Wilcoxon signed‐rank test.
Figure 4.
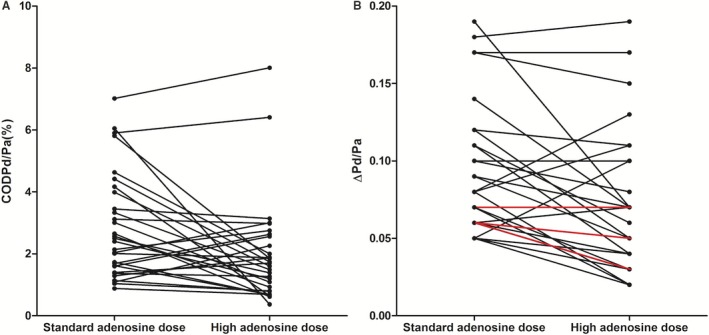
COD of Pd/Pa (A) and ΔPd/Pa (B) under standard and high adenosine dose. Lines in red represent double cases. Compared to the standard and post high adenosine dose, 8 (26.7%) patients presented with increase in COD of Pd/Pa, 5 (16.7%) had up to 25% decrease, and 17 (56.6%) presented with >25% decrease in COD of Pd/Pa. Moreover, 5 (16.7%) patients presented with increase in ΔPd/Pa, 11 (36.7%) had up to 25% decrease, and 14 (46.6%) presented with >25% decrease in ΔPd/Pa. COD indicates coefficient of dispersion; Pa, aortic pressure; Pd, distal coronary pressure; ΔPd/Pa, maximum Pd/Pa minus minimum Pd/Pa.
Figure 5.
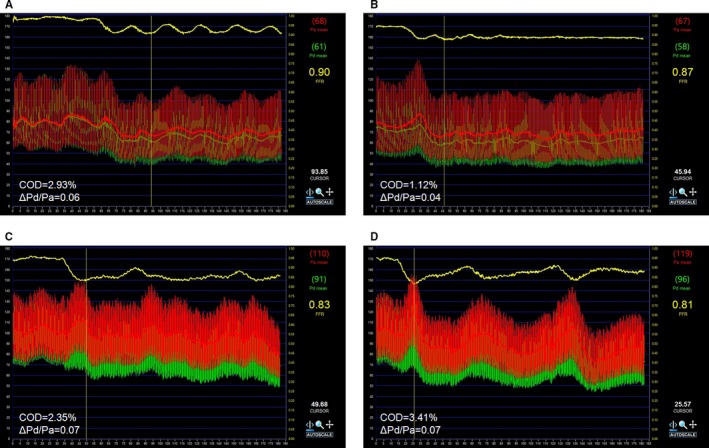
Examples of Pd/Pa variations with standard and high adenosine dose. Pd/Pa tracing of a patient exhibiting the lowering effect in Pd/Pa variation of high (B) compared with standard (A) adenosine dose infusion. Pd/Pa tracing of another patient who did not exhibit such effect under standard (C) and high (D) adenosine dose infusion. The horizontal yellow line represents the beat‐to‐beat Pd/Pa ratio. Pa indicates aortic pressure; Pd, distal coronary pressure.
Figure 6.
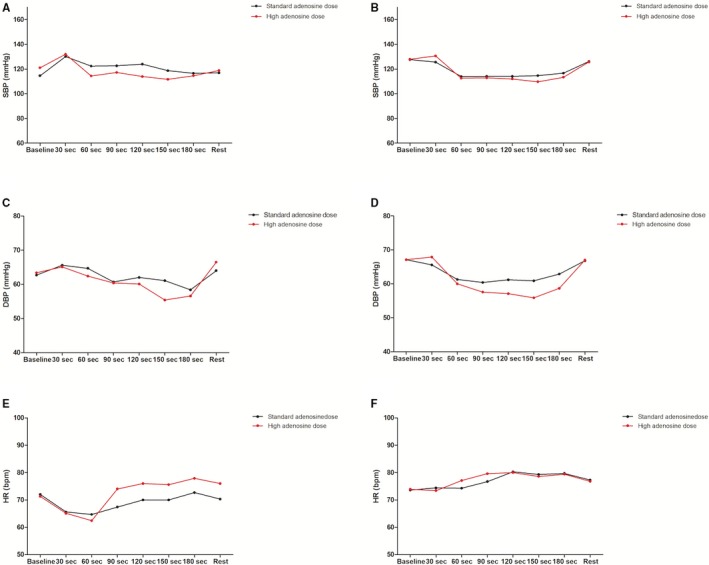
Hemodynamic measurements by adenosine dose in the quartiles of patients with the greatest (A, C, and E) and lowest (B, D, and F) reduction in Pd/Pa variation. Dots represent means. In A, compared with B, a greater decrease in SBP is observed with the high vs the standard adenosine dose. In (E), compared with (F), a greater increase in HR also is observed with the high vs the standard adenosine dose. DBP behavior with high dose was not different among increasers or decreasers of variation, (C) compared with (D). bpm indicates beats per min; DBP, diastolic blood pressure; HR, heart rate; Pa indicates aortic pressure; Pd, distal coronary pressure; SBP, systolic blood pressure.
Figure 7.
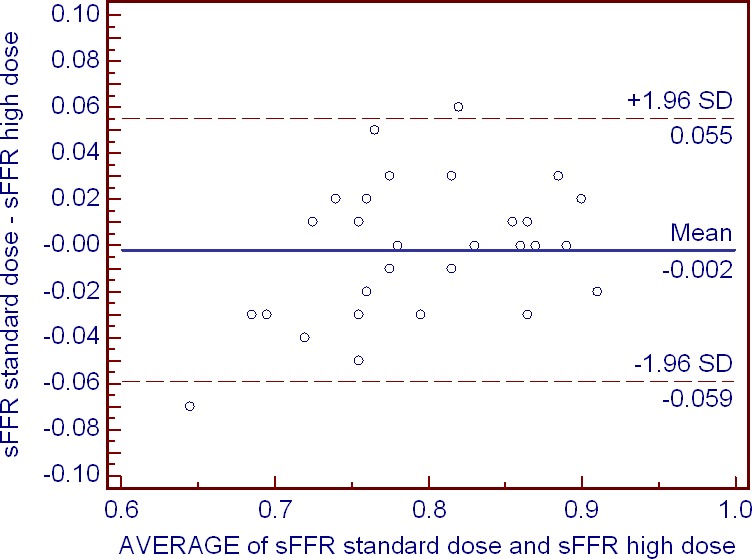
Bland–Altman analysis of sFFR values observed with the standard and high adenosine doses. The effect of adenosine dose is fairly uniform across the studied range of FFR values. sFFR indicates minimum distal coronary pressure/aortic pressure during postdip period.
Although there was a trend towards lower lFFR with the high adenosine dose, no significant difference in lFFR between the high versus standard adenosine dose was observed: 0.77 (0.74–0.85) versus 0.79 (0.75–0.85), P=0.09. Moreover, measurements of lFFR with the standard and the high adenosine dose demonstrated very good repeatability (bias=0.0063, SD 0.021, 95% limits of agreement −0.036 to 0.049) and high association between them (r 2=0.95), Figure 8. Reclassification of treatment decision based on lFFR at the cutoff of ≤0.80 occurred post high adenosine dose in 3 (10.0%) cases (in 2 and 1 cases towards interventional and medical treatment, respectively), Figure 9.
Figure 8.
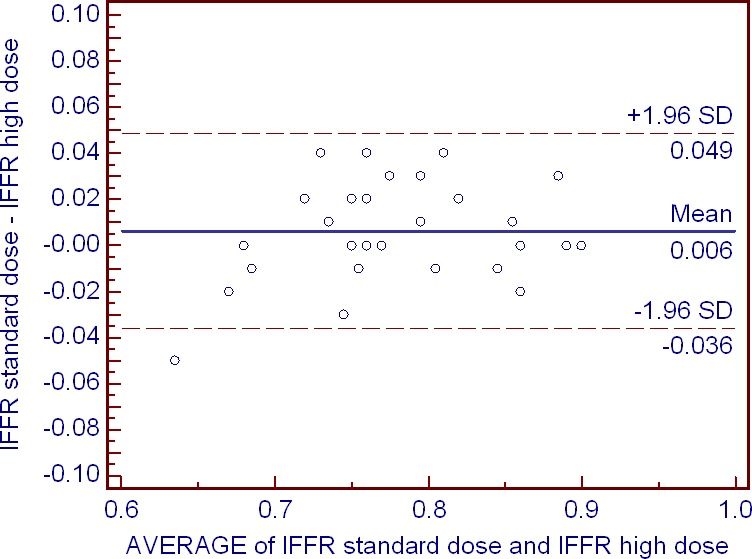
Bland–Altman analysis of lFFR values observed with the standard and high adenosine doses. The effect of adenosine dose is fairly uniform across the studied range of fractional flow reserve values. lFFR indicates lowest fractional flow reserve.
Figure 9.
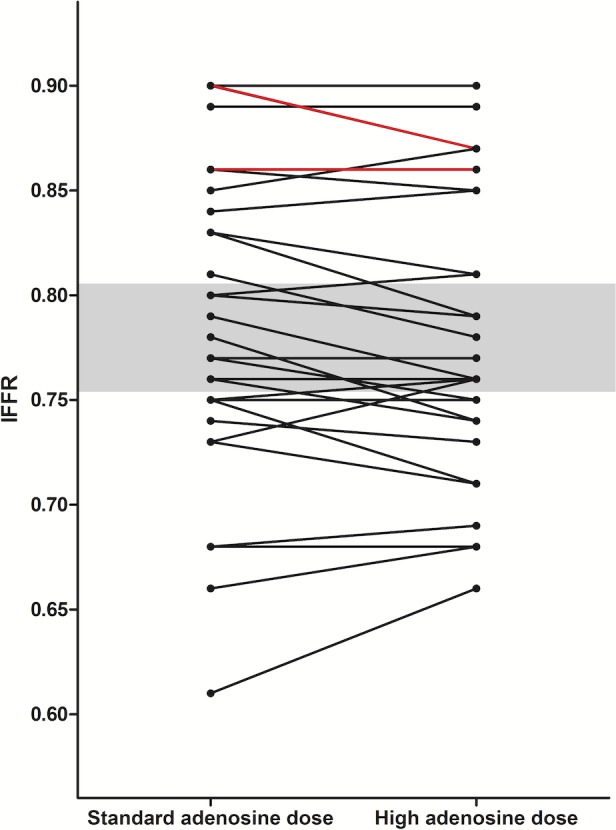
Patients' individual lFFR under standard and high adenosine dose. Lines in red represent double cases. No patient crossed the “gray” zone (lFFR <0.80 and >0.75, shaded area) post high adenosine dose; therefore, the magnitude of any difference in lFFR with standard vs high‐dose IV adenosine was not clinically significant in any of the studied patients. lFFR indicates lowest fractional flow reserve.
High‐dose infusion was overall well tolerated and in no case was discontinuation of adenosine infusion required. The majority of patients reported minor side effects (dyspnea, flushing, and chest discomfort) at an earlier time from the onset of high adenosine infusion compared to the standard dose. Overall, 5 (16.7%) and 7 (23.3%) patients developed transient and asymptomatic second‐ or third‐degree atrioventricular block during standard and high adenosine infusion, respectively (P=0.7). All types of atrioventricular block were of short duration, were well tolerated, and did not require any specific treatment.
Discussion
The current study provides new data that logically builds on the existing literature to answer a clinically important question, namely, the significance of Pd/Pa variations, given the growing uptake of FFR in practice. During standard‐dose, 3‐minute, central vein adenosine infusion, Pd/Pa variability occurs in 40% of the cases and is significantly decreased following a high (200 μg/kg per minute) dose of adenosine infusion. Most importantly, there is no difference in the minimum Pd/Pa—that is relevant for decision making—with the high adenosine dose. Minimum Pd/Pa and lFFR also display excellent repeatability between standard and high adenosine doses. The impact of high adenosine dose on Pd/Pa variation seems to be exerted mainly through a significant decrease in Pd variation. High adenosine dose produces a reduction in max Pd/Pa value observed during PDP compared to the standard dose.
Wilson et al first described Doppler flow velocity cycles during constant adenosine infusion, which were dose related up to the 140 μg/kg per minute adenosine dose.8 The interest in Pd/Pa variations during the infusion of standard adenosine dose was renewed by 2 recent studies, which emphasized the potential impact on FFR measurement accuracy and utility in diffuse or multiple lesions assessment.4, 5 In a retrospective analysis of 51 patients undergoing FFR measurement, Seto et al described Pd/Pa variability in 65% of cases during constant infusion through a peripheral vein. In another retrospective analysis of 196 complete sets of paired data by Johnson et al, 3 general patterns of Pd/Pa response were seen during IV adenosine infusion with a “humped” (sigmoid with superimposed bumps of varying height) pattern in 39% of cases.5 In line with previous studies, we reconfirm in a prospective analysis the common occurrence of Pd/Pa variations following achievement of hyperemia and until the end of 3‐minute, central vein adenosine infusion.
A cyclic variation in coronary conductance resulted from a cyclical variation in adenosine concentration in the blood perfusing the myocardium, as a consequence of cyclic changes in vasodilation and vein blood flow, has been suggested as a mechanism involved in variations in Pd/Pa during constant adenosine infusion.8 More recently, variability of Pd/Pa measurements during presumed steady‐state levels of adenosine were attributed to dynamic physiologic responses to adenosine infusion.4 Factors such as saturation or sensitivity of A2A receptors, exhaustion of cAMP precursors or nitric oxide, recovery of endothelial and smooth muscle vasodilatory mechanisms following exhaustion, and other autoregulatory mechanisms have been implicated. The etiologic impact of adenosine on FFR variations has been additionally supported by observations made with caffeine administration, a blocker of adenosine receptor activity, with more common occurrence of FFR cyclic variation in patients who consumed versus those who refrained from caffeine.13 In our study, we aimed to further elucidate to what extent these variations are adenosine dose–related.
High Adenosine Dose and Pd/Pa Variability
Previous studies with up to 180 to 200 μg/kg per minute adenosine doses failed to elicit any greater vasodilation, difference in minimal Pd/Pa ratio, or decrease in coronary resistance using positron emission tomography.8, 9, 14 Adenosine doses up to 210 μg/kg per minute also could not fully surmount the antagonism provided by caffeine.13
The unchanged sFFR and lFFR with high adenosine dose in our study is in agreement with previous reports. Α distinction between clinical and statistical significance is, however, necessary as FFR differences ≤0.04 between groups (equal to the 95% limits of agreement for repeated FFR measurements of the same lesion made minutes apart) have been considered clinically insignificant.15 Repeatability of sFFR and lFFR between standard and high adenosine doses was also remarkably high and comparable to previous report of FFR measurements 2 minutes apart and using the standard adenosine dose.5 The effect of high adenosine dose was fairly uniform across the studied range of FFR values. However, both the lFFR and sFFR curves were at or outside the ±0.04 limits of agreement inherent to FFR measurement.
High adenosine dose proved overall effective in “suppressing” Pd/Pa variation. Variations in Pd/Pa have been reported to persist if re‐assessed 2 minutes following first assessment.5 In our study, when repeated FFR assessment was performed 5 minutes after the first measurement and with a high adenosine dose on‐board, Pd/Pa variation was significantly reduced in most of our cases. Increases in FFR value during standard dose infusion have been mostly attributed to Pd rising more than Pa following maximal hyperemia.16 High adenosine dose appears to significantly decrease this variation in Pd. Variations of Pd/Pa seems, therefore, to be adenosine dose–dependent, confirming our initial hypothesis. However, this response was not seen in all cases as even an increase in variation was observed in some patients. Although the underlying mechanism of such a response is unclear, milder hemodynamic effects of the high adenosine dose in these cases could be hypothesized.
The higher compared with the standard adenosine dose resulted in an earlier onset of Pd/Pa lowering (by 50%) and of PDP (by 14 s), without affecting transition time. Although the high adenosine dose applied emerges as the most likely explanation, the contribution of a priming effect on these time intervals certainly contributes, as has been demonstrated earlier.5, 17
Reclassification of treatment decision was uncommon (1 in 10 cases) post high adenosine dose, while no pair (standard and high‐dose measurements) crossed between lFFR <0.75 and lFFR >0.80. This should be seen in the context of a previous evaluation of the effects of FFR measurement variability on FFR‐guided treatment strategy, which described a measurement FFR gray‐zone between 0.75 and 0.85.18 Patients within this zone have increased chance that the FFR‐derived revascularization recommendation would change with repeated measurement. In our study and regarding lFFR during standard adenosine dose, 13/30 patients were in this category. The 10% reclassification rate in the current data is in line with a previous report of 7% to 15% reclassification rate of the FFR index on repeat measurement.19 It is of interest, however, that a very low (2.6%) reclassification rate has been described based on the 2 independent FFR measurements during IV standard‐dose adenosine infusion.20
Tolerability of higher than 140 μg/kg per minute of adenosine dose infusion has been previously questioned.21 In order be clinically useful, it is conceivable that the mediator of hyperemia should be given at a dose that allows for maximal vasodilatation, but does not cause insurmountable adverse effects.22 In our series of selected patients with significant Pd/Pa variations with standard dose, no case had to discontinue the 200 μg/kg per minute adenosine 3‐minute infusion.
Our study prospectively confirms the common occurrence of Pd/Pa variations and provides a clear answer to the questions above: the lowest Pd/Pa is unchanged by increasing the IV adenosine dose, but its stability can be improved. If clinicians are concerned about the variability in FFR, on an individual patient basis, the dose of adenosine can be increased. Several ways have been proposed to overcome the problem of Pd/Pa variations at least in the catheterization laboratory, such as using the lFFR, the “smart” minimum FFR or rapid pullback during the nadir phase. We propose adenosine increase as an effective way for “suppressing” Pd/Pa variations in the majority of cases. This method may help making reliable pullback recordings, so that FFR changes reflect only the vessel and not the hyperemic stimulus. Of note, however, in daily practice, adherence to the adenosine doses that were used to define FFR thresholds for inducible myocardial ischemia is considered necessary, while increasing adenosine doses or using unconventional vasodilatation strategies is discouraged.23 Very recently, intracoronary administration of nicorandil was proposed as a useful tool for confirmation of sufficient maximum hyperemia after IV ATP infusion. Moreover, additional intracoronary nicorandil administration (2 mg/30 s) during ATP infusion resulted in a smaller cyclic change in FFR than in ATP‐alone FFR assessment.24
Limitations
The present study presents several limitations. Adenosine plasma levels were not measured. Simultaneous flow and pressure assessments were not performed either. This extra information could have better elucidated the underlying mechanism for our findings. Repeatability of Pd/Pa variability in 2 measurements 2 minutes apart from each other has been described not in all, but in approximately two thirds of the cases.5 The relative contribution of this “intrinsic” variability to changes observed following the high dose intervention in a post 5‐minute repeat measurement or the effect of priming are not clear. By design, the repeatability of Pd/Pa variability was not assessed in all the recruited patients, but only on those exhibiting ≥0.05 difference of max Pd/Pa minus min Pd/Pa during PDP. It is unclear whether some patients without significant variability during standard‐dose infusion could have presented with significant Pd/Pa variations during a repeat, high‐dose challenge. Both adenosine doses were weight‐ but not unmeasurable distal myocardial mass‐adjusted to achieve stable receptor saturation.4 This may have not been achieved even following the high (200 μg/kg per minute) dose, at least in some cases. The effects of a >200 μg/kg per minute dose cannot be speculated from the present study.
Conclusions
Pd/Pa variability is observed in 40% of the cases during standard dose and is significantly decreased following a high (200 μg/kg per minute) dose of adenosine infusion. This is achieved without a significant difference in the minimum Pd/Pa (sFFR).
Disclosures
Dr Alexopoulos has received speaker honoraria by AstraZeneca and Advisory Board fees by AstraZeneca, Boehringer Ingelheim, Bayer, and the Medicines Company. Other authors have no conflict of interest. All authors have approved the final article.
(J Am Heart Assoc. 2016;5:e004323 doi: 10.1161/JAHA.116.004323)
References
- 1. De Bruyne B, Bartunek J, Sys SU, Heyndrickx GR. Simultaneous coronary pressure and flow velocity measurements in humans: feasibility, reproducibility, and hemodynamic dependence of coronary flow velocity reserve, hyperemic flow versus pressure slope index, and fractional flow reserve. Circulation. 1996;94:1842–1849. [DOI] [PubMed] [Google Scholar]
- 2. Pijls NHJ, van Schaardenburgh P, Manoharan G, Boersma E, Bech JW, van't Veer M, Bär F, Hoorntje J, Koolen J, Wijns W, de Bruyne B. Percutaneous coronary intervention of functionally nonsignificant stenosis: 5‐year follow‐up of the DEFER study. J Am Coll Cardiol. 2007;49:2105–2111. [DOI] [PubMed] [Google Scholar]
- 3. Bech GJ, De Bruyne B, Pijls NH, de Muinck ED, Hoorntje JC, Escaned J, Stella PR, Boersma E, Bartunek J, Koolen JJ, Wijns W. Fractional flow reserve to determine the appropriateness of angioplasty in moderate coronary stenosis: a randomized trial. Circulation. 2001;103:2928–2934. [DOI] [PubMed] [Google Scholar]
- 4. Seto AH, Tehrani DM, Bharmal MI, Kern MJ. Variations of coronary hemodynamic responses to intravenous adenosine infusion: implications for fractional flow reserve measurements. Catheter Cardiovasc Interv. 2014;84:416–425. [DOI] [PubMed] [Google Scholar]
- 5. Johnson NP, Johnson DT, Kirkeeide RL, Berry C, De Bruyne B, Fearon WF, Oldroyd KG, Pijls NH, Gould KL. Repeatability of fractional flow reserve despite variations in systemic and coronary hemodynamics. JACC Cardiovasc Interv. 2015;8:1018–1027. [DOI] [PubMed] [Google Scholar]
- 6. Tarkin JM, Nijjer S, Sen S, Petraco R, Echavarria‐Pinto M, Asress KN, Lockie T, Khawaja MZ, Mayet J, Hughes AD, Malik IS, Mikhail GW, Baker CS, Foale RA, Redwood S, Francis DP, Escaned J, Davies JE. Hemodynamic response to intravenous adenosine and its effect on fractional flow reserve assessment: results of the Adenosine for the Functional Evaluation of Coronary Stenosis Severity (AFFECTS) study. Circ Cardiovasc Interv. 2013;6:654–661. [DOI] [PubMed] [Google Scholar]
- 7. Echavarria‐Pinto M, Petraco R, van de Hoef TP, Gonzalo N, Nijjer S, Tarkin JM, Ibanez B, Sen S, Jimenez‐Quevedo P, Nunez‐Gil IJ, Nombela‐Franco L, Alfonso F, Fernandez‐Ortiz A, Macaya C, Piek JJ, Davies J, Escaned J. Fractional flow reserve and minimum Pd/Pa ratio during intravenous adenosine infusion: very similar but not always the same. EuroIntervention. 2016;11:1013–1019. [DOI] [PubMed] [Google Scholar]
- 8. Wilson RF, Wyche K, Christensen BV, Zimmer S, Laxson DD. Effects of adenosine on human coronary arterial circulation. Circulation. 1990;82:1595–1606. [DOI] [PubMed] [Google Scholar]
- 9. De Bruyne B, Pijls NH, Barbato E, Bartunek J, Bech JW, Wijns W, Heyndrickx GR. Intracoronary and intravenous adenosine 5‐triphosphate, adenosine, papaverine, and contrast medium to assess fractional flow reserve in humans. Circulation. 2003;107:1877–1883. [DOI] [PubMed] [Google Scholar]
- 10. Pijls NH, Tonino PA. The crux of maximum hyperemia: the last remaining barrier for routine use of fractional flow reserve. JACC Cardiovasc Interv. 2011;4:1093–1095. [DOI] [PubMed] [Google Scholar]
- 11. Alexopoulos D, Davlouros P, Tsigkas G, Koutsogiannis N, Hassapi A, Scortsanitis C, Pentara I, Vogiatzi C, Xanthopoulou I. Comparison of ticagrelor vs thienopyridine loading effect on fractional flow reserve in patients with coronary artery disease. Am J Cardiol. 2016;117:22–28. [DOI] [PubMed] [Google Scholar]
- 12. Pijls NH, Sels JW. Functional measurement of coronary stenosis. J Am Coll Cardiol. 2012;59:1045–1057. [DOI] [PubMed] [Google Scholar]
- 13. Matsumoto H, Nakatsuma K, Shimada T, Ushimaru S, Mikuri M, Yamazaki T, Matsuda T. Effect of caffeine on intravenous adenosine‐induced hyperemia in fractional flow reserve measurement. J Invasive Cardiol. 2014;26:580–585. [PubMed] [Google Scholar]
- 14. Kaufmann PA, Gnecchi‐Ruscone T, di Terlizzi M, Schäfers KP, Lüscher TF, Camici PG. Coronary heart disease in smokers: vitamin C restores coronary microcirculatory function. Circulation. 2000;102:1233–1238. [DOI] [PubMed] [Google Scholar]
- 15. Berry C, van ‘t Veer M, Witt N, Kala P, Bocek O, Pyxaras SA, McClure JD, Fearon WF, Barbato E, Tonino PA, De Bruyne B, Pijls NH, Oldroyd KG. VERIFY (VERification of Instantaneous Wave‐Free Ratio and Fractional Flow Reserve for the Assessment of Coronary Artery Stenosis Severity in EverydaY Practice): a multicenter study in consecutive patients. J Am Coll Cardiol. 2013;61:1421–1427. [DOI] [PubMed] [Google Scholar]
- 16. Seto AH, Tehrani D, Bharmal M, Kern M. Evaluation of variability of FFR measurements during a single reading period using intravenous adenosine–induced hyperemia. J Am Coll Cardiol. 2013;61(10_S): doi:10.1016/S0735‐1097(13)61762‐3. [Google Scholar]
- 17. Scott P, Sirker A, Dworakowski R, Paul G, Candilio L, Jahagirdar N, Melikian N, Byrne J. Fractional flow reserve in the transradial era: will hand vein adenosine infusion suffice? A comparative study of the extent, rapidity, and stability of hyperemia from hand and femoral venous routes of adenosine administration. JACC Cardiovasc Interv. 2015;8:527–535. [DOI] [PubMed] [Google Scholar]
- 18. Petraco R, Sen S, Nijjer S, Echavarria‐Pinto M, Escaned J, Francis DP, Davies JE. Fractional flow reserve‐guided revascularization: practical implications of a diagnostic gray zone and measurement variability on clinical decisions. JACC Cardiovasc Interv. 2013;6:222–225. [DOI] [PubMed] [Google Scholar]
- 19. Petraco R, Escaned J, Sen S, Nijjer S, Asrress KN, Echavarria‐Pinto M, Lockie T, Khawaja MZ, Cuevas C, Foin N, Broyd C, Foale RA, Hadjiloizou N, Malik IS, Mikhail GW, Sethi A, Kaprielian R, Baker CS, Lefroy D, Bellamy M, Al‐Bustami M, Khan MA, Hughes AD, Francis DP, Mayet J, Di Mario C, Redwood S, Davies JE. Classification performance of instantaneous wave‐free ratio (iFR) and fractional flow reserve in a clinical population of intermediate coronary stenoses: results of the ADVISE registry. EuroIntervention. 2013;9:91–101. [DOI] [PubMed] [Google Scholar]
- 20. Schlundt C, Bietau C, Klinghammer L, Wiedemann R, Rittger H, Ludwig J, Achenbach S. Comparison of intracoronary versus intravenous administration of adenosine for measurement of coronary fractional flow reserve. Circ Cardiovasc Interv. 2015;8:e001781. [DOI] [PubMed] [Google Scholar]
- 21. Uematsu T, Kozawa O, Matsuno H, Niwa M, Yoshikoshi H, Oh‐uchi M, Kohno K, Nagashima S, Kanamaru M. Pharmacokinetics and tolerability of intravenous infusion of adenosine (SUNY4001) in healthy volunteers. Br J Clin Pharmacol. 2000;50:177–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kern MJ, Seto AH. Selecting the right fractional flow reserve in an unsteady state: keep it simple. JACC Cardiovasc Interv. 2015;8:1028–1030. [DOI] [PubMed] [Google Scholar]
- 23. Vranckx P, Cutlip DE, McFadden EP, Kern MJ, Mehran R, Muller O. Coronary pressure‐derived fractional flow reserve measurements: recommendations for standardization, recording, and reporting as a core laboratory technique. Proposals for integration in clinical trials. Circ Cardiovasc Interv. 2012;5:312–317. [DOI] [PubMed] [Google Scholar]
- 24. Takami H, Sonoda S, Muraoka Y, Sanuki Y, Kashiyama K, Fukuda S, Oginosawa Y, Tsuda Y, Araki M, Otsuji Y. Impact of additional intracoronary nicorandil administration during fractional flow reserve measurement with intravenous adenosine 5'‐triphosphate infusion. J Cardiol. 2016; pii: S0914‐5087(16)00029‐0. doi: 10.1016/j.jjcc.2016.01.018. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]


