Abstract
Background
Visit‐to‐visit variability of systolic blood pressure (SBP) has been shown to contribute to cardiovascular events and all‐cause mortality. However, little is known about its long‐term effect on renal function. We aim to examine the relationship between visit‐to‐visit blood pressure variability (BPV) and decline in renal function in patients with hypertension and to determine the level of systolic BPV that is associated with significant renal function decline.
Methods and Results
This is a 15‐year retrospective cohort study of 825 hypertensive patients. Blood pressure readings every 3 months were retrieved from the 15 years of clinic visits. We used SD and coefficient of variation as a measure of systolic BPV. Serum creatinine was captured and estimated glomerular filtration rate was calculated at baseline, 5, 10, and 15 years. The mean SD of SBP was 14.2±3.1 mm Hg and coefficient of variation of SBP was 10.2±2%. Mean for estimated glomerular filtration rate slope was −1.0±1.5 mL/min per 1.73 m2 per year. There was a significant relationship between BPV and slope of estimated glomerular filtration rate (SD: r=−0.16, P<0.001; coefficient of variation: r=−0.14, P<0.001, Pearson's correlation). BPV of SBP for each individual was significantly associated with slope of estimated glomerular filtration rate after adjustment for mean SBP and other confounders. The cutoff values estimated by the receiver operating characteristic curve for the onset of chronic kidney disease for SD of SBP was 13.5 mm Hg and coefficient of variation of SBP was 9.74%.
Conclusions
Long‐term visit‐to‐visit variability of SBP is an independent determinant of renal deterioration in patients with hypertension. Hence, every effort should be made to reduce BPV in order to slow down the decline of renal function.
Keywords: blood pressure variability, hypertension, long‐term, Malaysia, primary care, renal function decline, visit‐to‐visit
Subject Categories: Hypertension, Nephrology and Kidney
Introduction
Chronic kidney disease (CKD), which is estimated to affect more than 10% of the population worldwide, poses a threat to the current healthcare system in view of its increased risk of cardiovascular morbidities and the necessity for renal replacement therapy in end‐stage renal disease.1 Hypertension is known to be a major factor contributing to the rise in number of patients with CKD and end‐stage renal disease.2, 3 Nephrosclerosis caused by systemic and glomerular hypertension is thought to be the factor in progression to CKD.4 Glycemic control, hypoalbuminemia, and high mean blood pressure (BP) are several other risk factors for CKD in patients with diabetes mellitus (DM). Hypertensive patients who are older, with underlying DM, hyperuricemia, and lower baseline estimated glomerular filtration rate (eGFR) may be more likely to develop CKD.5
Visit‐to‐visit blood pressure variability (BPV) has been shown to be a determinant in contributing to cardiovascular morbidity and mortality.6, 7, 8 Several studies have shown the relationship between visit‐to‐visit BPV and further decline of renal function in patients with established CKD.7, 9 However, little is known about visit‐to‐visit BPV and its effect on renal function in patients with hypertension, particularly those with normal kidney function. Hence, we aimed to determine the relationship of long‐term visit‐to‐visit BPV and decline in renal function in patients with hypertension. In addition, we also evaluated risk factors such as mean systolic blood pressure (SBP), DM, cholesterol, and medications that can significantly affect renal function in a population with hypertension.
Methods
This is a 15‐year retrospective cohort study of patients attending a primary care clinic from 1998 until 2012. This study was conducted at an outpatient primary care clinic at the University Malaya Medical Centre, a teaching hospital in the Klang Valley of Kuala Lumpur, Malaysia. All patients aged more than 30 years at baseline (1998), without any cardiovascular event at baseline, were eligible for the study.10 One thousand four hundred sixty‐four subjects were randomly selected from patients registered with the clinic, using a computer‐generated number based on patients' clinic registration number. We selected patients based on the clinical characteristics at baseline year 1998. Out of these 1464 patients, we identified a total of 923 patients who had hypertension at baseline year 1998 (n=560, 60.7%) and had developed hypertension within the 15‐year period of follow‐up (n=363, 39.3%). We excluded 17 patients who did not have the serial serum creatinine level needed to calculate eGFR slope and 2 patients with less than 7 blood pressure readings in the 15‐years of follow‐up. We further excluded 79 patients who had CKD stage 3A and above at baseline (1998). Hence, a total of 825 patients with hypertension were included in the analysis of this study. There were 52 patients (6.3%) who were lost to follow‐up at the end of 15 years. As this was a retrospective study based on patient records, and as all data entry, analysis, and results output were anonymized, no informed consent, verbal or written was obtained. Ethics approval for this study was obtained from the Ethics Committee of University Malaya Medical Centre (University of Malaya Medical Centre Ethics Committee/IRB Reference Number 691.1).
All sociodemographic data and clinical parameters such as age, weight, presence of DM, dyslipidemia, and smoking were captured from patient records at baseline and at 15 years. Results of laboratory investigation including serum creatinine, hemoglobin A1c (HbA1c), total cholesterol, and low‐density lipoprotein cholesterol level were captured as well. All blood tests were performed by the chemical laboratory in the hospital that is certified by the Royal College of Pathologists of Australasia Standards. Use of antihypertensive agents, diabetic medications, and statins were also captured. DM was defined as documented by the attending doctor or the use of hypoglycemic agents or both. Participants are defined as a smoker if they were still smoking at time of blood pressure examination. Nonsmokers were defined as those who never smoke or who are currently not smoking.
Visit‐to‐Visit SBP Variability
Blood pressure was measured by the attending doctors using mercury sphygmomanometers during routine clinical practice. Hypertension is defined according to the JNC guideline.11 A blood pressure reading per visit for every 3 months (ie, maximum 4 readings per year) were retrieved from the 15 years of clinic visits. We used SD and coefficient of variation (CV) for each individual as a measure of BPV. SD of SBP is a measure of dispersion of SBP readings from its mean SBP. It was calculated as the square root of variance by determining the variation between each SBP reading relative to the mean, using the formula , n is the number of visits, xi is the individual's BP at visit i, and is the individual's mean SBP. CV is the ratio of the SD to the mean, comparing the degree of variation .
To examine the association of BPV and eGFR decline with mean SBP, patients were divided into low or high mean BP using SBP of 140 mm Hg as the cutoff point because it is the recommended target of control by guideline.11 Since there are no data on the recommended target or “normal” visit‐to‐visit BPV, we used the mean SD of SBP 14.2 mm Hg as a cutoff point to define low versus high BPV.
Measurement of Renal Function Outcomes
Serum creatinine was captured at baseline, 5, 10, and 15 years, and eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration Equation (CKD‐epi).12 Rate of renal function decline was estimated by fitting a linear regression line through the eGFR measurements for each individual patient and expressed as eGFR slope (mL/min per 1.73 m2 per year). This method has been used in other studies as well because eGFR at a point of time may not truly be reflective of change in kidney function due to its variability.13, 14, 15, 16 eGFR was categorized according to the KDIGO 2012 guideline (Table S1). Onset of CKD is defined as eGFR <60 mL/min per 1.73 m2.17
Statistical Analysis
All statistical analysis was carried out using Statistical Package for Social Sciences (IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY). All continuous data are described as mean and SD. Relationship between SD, CV, mean SBP, and slope of eGFR were analyzed using simple linear regression since all the variables are normally distributed. Univariate correlations between slope of renal decline and other continuous variables (age, weight, mean SBP, SD of SBP, CV of SBP, HbA1c, total cholesterol, and low‐density lipoprotein cholesterol) were assessed using Pearson's correlation. Comparisons of mean of eGFR slope between categorical variables (sex, DM, treatment of hypertension, angiotensin‐converting enzyme inhibitor, angiotensin‐receptor blockers [ARB], β‐blocker, calcium channel blockers, diuretics, α‐blocker, and lipid‐lowering medication) were done using independent t test. Comparisons of eGFR slope between groups for mean SBP and SD of SBP and comparisons of eGFR slope between CKD categories were done using 1‐way ANOVA. Multiple linear regression analyses on the slope of eGFR were performed to adjust for the possible confounders (age, sex, weight, SD, CV of SBP, mean SBP, treatment of hypertension, total cholesterol, low‐density lipoprotein cholesterol, angiotensin‐converting enzyme inhibitor, ARB, β‐blocker, calcium channel blockers, diuretics, α‐blockers, lipid‐lowering medication, DM, and HbA1c). Receiver operating characteristics curve was used to examine how well the visit‐to‐visit SBP variability can predict CKD. Youden index was used to determine the cutoff values of SD and CV of SBP.9
Results
Table 1 shows the changes in sociodemographic and clinical characteristics for patients with hypertension from 1998 to 2012. The mean age at baseline was 55.5±9.4 years and consisted of 67.8% females. The number of patients with DM increased from 43.3% in 1998 to 65.3% in 2012. The use of lipid‐lowering medications was significantly increased from 8.2% in 1998 to 84.1% in 2012, which contributed to the decrease in both total cholesterol and low‐density lipoprotein levels.18, 19 In our study, the mean number of SBP readings over the 15 years of follow‐up was 46±7.6, ranging from 9 to 60 readings. There was improvement of both mean SBP and diastolic blood pressure from 1998 to 2012. The reduction in diastolic blood pressure is greater than SBP at the end of 15 years compared to baseline (3.5±23.2 mm Hg for SBP and 8.6±13 mm Hg for diastolic blood pressure, P<0.001). This could be the result of aging and treatment effect. The mean SD of SBP was 14.2±3.1 mm Hg and CV of SBP was 10.2±2%. The mean eGFR slope for the study population was −1.0 mL/min per 1.73 m2 per year. For those who were missing on follow‐up (n=52, 6.3%), there was no statistically significant difference in age, visit‐to‐visit BPV, and eGFR decline slope compared to the rest of subjects who completed the 15‐year follow‐up in this cohort study. We hypothesize that this may not contribute to significant changes to our findings.
Table 1.
Comparison of Sociodemographic and Clinical Characteristics for Patients With Hypertension From 1998 to 2012 (n=825)
| 1998 | 2002 | 2007 | 2012 | |
|---|---|---|---|---|
| Age, y (mean±SD) | 55.5±9.4 | 60.5±9.4 | 65.5±9.4 | 70.5±9.4 |
| Female, n (%) | 559 (67.8) | 559 (67.8) | 559 (67.8) | 559 (67.8) |
| Race, n (%) | ||||
| Malay | 196 (23.8) | 196 (23.8) | 196 (23.8) | 196 (23.8) |
| Chinese | 370 (44.8) | 370 (44.8) | 370 (44.8) | 370 (44.8) |
| Indian | 248 (30.1) | 248 (30.1) | 248 (30.1) | 248 (30.1) |
| Others | 11 (1.3) | 11 (1.3) | 11 (1.3) | 11 (1.3) |
| Weight, kg (mean±SD) | 66.0±13.2 | 66.2±13 | 65.1±13.7 | 64.8±14.6 |
| Systolic BP, mm Hg (mean±SD) | 140.2±18.5 | 139.6±17 | 135±15.9 | 136.7±16.4 |
| Diastolic BP, mm Hg (mean±SD) | 85.1±10 | 83.3±8.3 | 79.1±8.0 | 76.5±9.2 |
| Treatment of hypertension, n (%) | 493 (59.8) | 628 (76.1) | 735 (89.1) | 794 (96.2) |
| Types of antihypertensive agents used, n (%) | ||||
| ACE‐i | 41 (5.5) | 177 (21.9) | 267 (32.4) | 361 (43.8) |
| ARB | 1 (0.1) | 14 (1.7) | 121 (14.7) | 201 (24.4) |
| ß‐Blocker | 271 (32.8) | 321 (38.9) | 321 (38.9) | 299 (36.2) |
| CCB | 193 (23.4) | 271 (33.1) | 375 (45.5) | 509 (61.7) |
| Diuretic | 60 (7.2) | 175 (21.2) | 245 (29.7) | 330 (40) |
| α‐Blocker | 34 (4.1) | 28 (3.4) | 26 (3.2) | 50 (6.1) |
| SD of SBP, mm Hg (mean±SD) | — | — | — | 14.2±3.1 |
| CV of SBP, % (mean±SD) | — | — | — | 10.2±2.0 |
| Diabetes mellitus, n (%) | 357 (43.3) | 438 (53.1) | 501 (60.7) | 539 (65.3) |
| Hemoglobin A1c, % (mean±SD) | 7.8±1.8 | 8.1±2.0 | 7.6±1.7 | 7.6±1.8 |
| Types of diabetic medications used, n (%) | ||||
| Metformin | 190 (23) | 309 (37.5) | 390 (47.3) | 420 (50.9) |
| Sulphonylurea | 283 (34.3) | 326 (39.5) | 352 (42.7) | 321 (38.9) |
| Insulin | 3 (0.4) | 19 (2.3) | 95 (11.5) | 170 (20.6) |
| Use of lipid‐lowering medication, n (%) | 68 (8.2) | 216 (26.2) | 554 (67.2) | 694 (84.1) |
| Total cholesterol, mmol/L (mean±SD) | 6.0±1.1 | 5.5±0.9 | 4.8±1.0 | 4.5±1.0 |
| LDL cholesterol, mmol/L (mean±SD) | 3.7±1.1 | 3.4±0.9 | 2.9±0.8 | 2.5±0.8 |
| CKD Stage 3A and above, n (%) | 0 | 92 (11.2) | 160 (19.4) | 226 (27.4) |
| Serum creatinine, μmol/L (mean±SD) | 74.6±17.7 | 80.7±23.3 | 78.9±30.1 | 92.5±64.8 |
| eGFR by CKD‐epi, mL/min per 1.73 m2 (mean±SD) | 86.3±15.4 | 78.3±16.2 | 78.5±19.2 | 69.6±21.6 |
| Slope of eGFR decline, mL/min per 1.73 m2 per year (mean±SD) | — | — | — | −1.0±1.5 |
ACE‐i indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; CCB, calcium channel blocker; CKD, chronic kidney disease; CV, coefficient of variation; eGFR, estimated glomerular filtration rate; LDL, low‐density lipoprotein; SBP, systolic blood pressure.
Visit‐to‐Visit Variability, Mean SBP, and Slope of eGFR
Figure 1 shows the relationships of the SD, CV, and mean of SBP with the slope of eGFR in patients with hypertension over 15 years of follow‐up. SD, CV, and mean SBP were associated significantly with the slope of eGFR, although the associations were weak (SD: r=−0.16, P<0.001, CV: r=−0.14, P=0.001; mean SBP: r=−0.13, P<0.001). The mean SD of SBP was also positively correlated with mean SBP (r=0.55, P<0.001).
Figure 1.
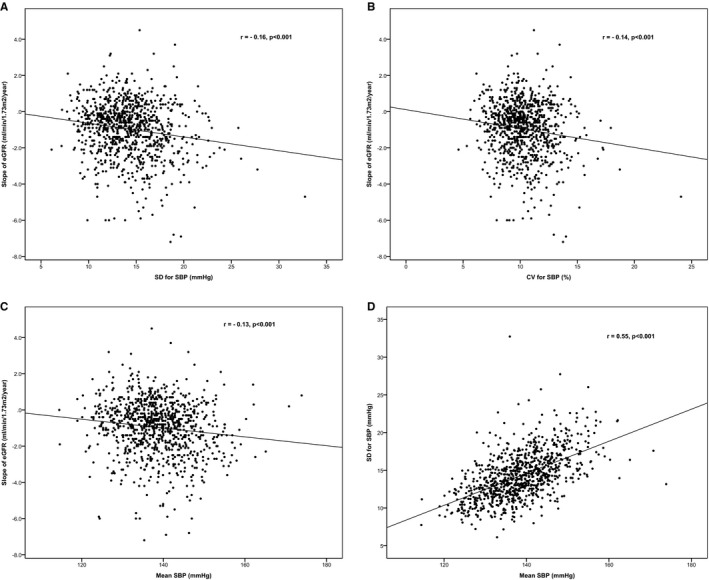
Relationship between the slope of eGFR, SD, CV, and mean SBP. A, Correlation between slope of eGFR and SD of SBP. B, Correlation between slope of eGFR with CV of SBP. C, Correlation between slope of eGFR with mean SBP. D, Correlation between SD of SBP and mean SBP in patients with hypertension. CV indicates coefficient of variation; eGFR, estimated glomerular filtration rate; SBP, systolic blood pressure.
Determinants of eGFR Decline
Table 2 shows the univariate relationship of clinical parameters with the slope of eGFR. Being female, DM, higher HbA1c level, and treatment of hypertension were significantly associated with slope of eGFR. The majority of patients in this cohort study (96.2%) were on more than 1 type of antihypertensive medication. The use of ARB, diuretics, and α‐blockers was associated with greater renal function decline. There was no significant difference in the rate of decline in renal function for angiotensin‐converting enzyme inhibitors, ß‐blockers, and calcium channel blockers. Higher renal function decline was also seen among those on lipid‐lowering medication.
Table 2.
Univariate Relationship of Clinical Parameters With the Slope of the Decline of eGFR
| Continuous Variables | r | P Value |
|---|---|---|
| Age at baseline, y | 0.002 | 0.96 |
| Weight, kg | −0.04 | 0.3 |
| Mean SBP, mm Hg | −0.13 | <0.001 |
| SD of SBP, mm Hg | −0.16 | <0.001 |
| CV of SBP, % | −0.14 | <0.001 |
| HbA1c, % | −0.15 | <0.001 |
| Total cholesterol, mmol/L | 0.03 | 0.41 |
| LDL cholesterol, mmol/L | 0.05 | 0.22 |
| Categorical Variables | Mean of eGFR Slope | P Value |
|---|---|---|
| Sex | ||
| Male (n=266) | −0.83 | 0.01 |
| Female (n=559) | −1.1 | |
| DM | ||
| Yes (n=539) | −1.26 | <0.001 |
| No (n=285) | −0.56 | |
| Treatment of hypertension | ||
| Yes (n=794) | −0.46 | 0.04 |
| No (n=31) | −1.04 | |
| ACE‐i | ||
| Yes (n=361) | −1.0 | 0.59 |
| No (n=464 | −1.0 | |
| ARB | ||
| Yes (n=201) | −1.36 | <0.001 |
| No (n=624) | −0.91 | |
| β‐Blocker | ||
| Yes (n=299) | −1.03 | 0.83 |
| No (n=526) | −1.00 | |
| CCB | ||
| Yes (n=509 | −1.03 | 0.76 |
| No (n=316) | −1.0 | |
| Diuretics | ||
| Yes (n=330) | −1.25 | 0.001 |
| No (n=495) | 0.86 | |
| α‐Blockers | ||
| Yes (n=50) | −1.57 | 0.02 |
| No (n=775) | −0.98 | |
| Lipid‐lowering medication | ||
| Yes (n=694) | −1.08 | 0.008 |
| No (n=131) | −0.70 | |
ACE‐i indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; CCB, calcium channel blocker; CV, coefficient of variation; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1c; LDL, low‐density lipoprotein; SBP, systolic blood pressure.
Multiple regression analyses were carried out with the slope of eGFR as the dependent variable to determine the association of clinical parameters with slope of eGFR, as shown in Table 3. After adjustment, BPV (SD and CV of SBP), DM, HbA1c, use of ARB, diuretics, α‐blocker, and lipid‐lowering medication remained significantly associated with slope of eGFR decline. The effect of mean SBP on renal function decline was no longer statistically significant after adjusting for BPV and other clinical parameters. At the end of 15 years, 65.3% of patients in our cohort study had DM. It is known that DM is a major factor contributing to decline in renal function.20 We also noticed that DM and HbA1c were the major determinants of renal function decline in this cohort study. Hence, a sensitive analysis was done by dividing the study population into DM and non‐DM to examine the determinants of eGFR decline. BPV still remained significantly associated with renal function decline regardless of the DM status. In patients with both hypertension and DM (n=539), BPV was significantly associated with renal function decline after adjusting for other confounding factors including HbA1c. There is no evidence of association between HbA1c and decline in renal function after adjustment.
Table 3.
Multiple Linear Regression Analyses on the Slope of the Decline of eGFR in Patients With Hypertension Over 15 Years
| Independent Variables | All Subjects (n=825)a | Subjects Without Diabetes Mellitus (n=285)b | Subjects With Diabetes Mellitus (n=539)c | |||
|---|---|---|---|---|---|---|
| β | P Value | β | P Value | Β | P Value | |
| Age, y | 0.01 | 0.79 | −0.11 | 0.14 | 0.08 | 0.97 |
| Sex | −0.07 | 0.13 | 0.03 | 0.70 | −0.08 | 0.13 |
| Weight, kg | −0.01 | 0.88 | 0.01 | 0.89 | −0.01 | 0.86 |
| SD of SBP, mm Hg | −0.10 | 0.03 | −0.21 | 0.001 | −0.13 | 0.01 |
| CV of SBP, % | −0.09 | 0.03 | −0.17 | 0.005 | −0.12 | 0.01 |
| Mean SBP, mm Hg | 0.01 | 0.97 | 0.01 | 0.98 | 0.03 | 0.60 |
| Diabetes mellitus | −0.11 | 0.01 | — | — | — | — |
| HbA1c, % | −0.10 | 0.04 | — | — | −0.04 | 0.40 |
| Treatment of hypertension | −0.02 | 0.62 | −0.02 | 0.79 | −0.04 | 0.46 |
| Total cholesterol, mmol/L | 0.16 | 0.08 | 0.38 | 0.70 | 0.12 | 0.24 |
| LDL cholesterol, mmol/L | −0.04 | 0.67 | −0.04 | 0.79 | −0.06 | 0.55 |
| ACE‐i | −0.03 | 0.62 | −0.09 | 0.21 | −0.01 | 0.91 |
| ARB | −1.0 | 0.02 | −0.17 | 0.01 | −0.07 | 0.25 |
| β‐Blocker | −0.2 | 0.74 | −0.01 | 0.91 | −0.02 | 0.71 |
| CCB | −0.01 | 0.97 | −0.08 | 0.26 | 0.04 | 0.40 |
| Diuretics | −1.0 | 0.02 | −0.03 | 0.67 | −0.13 | 0.01 |
| α‐Blockers | −0.8 | 0.07 | 0.05 | 0.40 | −0.12 | 0.01 |
| Lipid‐lowering medication | −0.11 | 0.01 | −0.08 | 0.22 | −0.12 | 0.01 |
ACE‐i indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; CCB, calcium channel blocker; CV, coefficient of variation; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1c; LDL, low‐density lipoprotein; SBP, systolic blood pressure.
Adjusted for age, sex, weight, SD, CV of SBP, mean SBP, treatment of hypertension, total cholesterol, LDL cholesterol, ACE‐i, ARB, β‐blocker, CCB, diuretics, α‐blockers, lipid‐lowering medication+diabetes and HbA1c.
Adjusted for age, sex, weight, SD, CV of SBP, mean SBP, treatment of hypertension, total cholesterol, LDL cholesterol, ACE‐i, ARB, β‐blocker, CCB, diuretics, α‐blockers, lipid‐lowering medication.
Adjusted for age, sex, weight, SD, CV of SBP, mean SBP, treatment of hypertension, total cholesterol, LDL cholesterol, ACE‐i, ARB, β‐blocker, CCB, diuretics, α‐blockers, lipid‐lowering medication+HbA1c.
Cutoff Values for SD and CV as an Indicator for the Onset of CKD
Figure 2 shows the receiver operating characteristics curve for SD and CV of SBP as indicators for the onset of CKD. The area under the curves for SD and CV of SBP were statistically significant, with the area under the curves for SD being 0.62 and area under the curves for CV being 0.60, indicating moderate discrimination. The cutoff value corresponding to the maximum Youden index [(sensitivity+specificity)−1] for SD of SBP was 13.5 mm Hg (sensitivity 69%, specificity 50%). The cutoff value corresponding to the maximum Youden index for CV of SBP was 9.74% (sensitivity 68%, specificity 48%). In Figure 3, patients with low mean SBP have slower renal function decline compared to those with high mean SBP. In both the low and high mean SBP groups, there was higher eGFR decline in those with higher BPV. However, for those with high mean SBP but low BPV, the rate of renal function decline was similar to those with low mean SBP but high BPV (descriptive statistics in Table S2).
Figure 2.
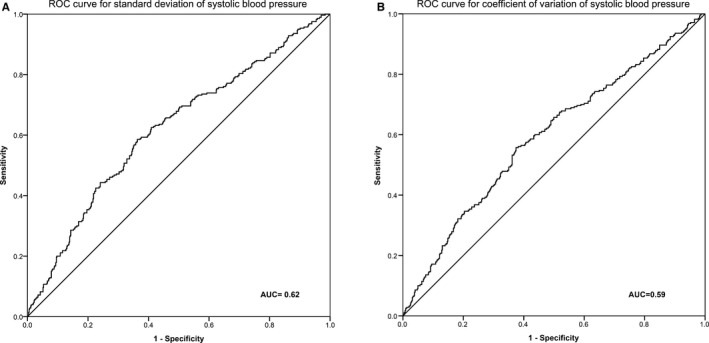
Receiver operating characteristic (ROC) curves of visit‐to‐visit blood pressure variability as indicator for onset of chronic kidney disease in patients with hypertension. A, ROC curve for SD of systolic blood pressure. B, ROC curve for coefficient of variation of systolic blood pressure. AUC indicates area under the curve.
Figure 3.
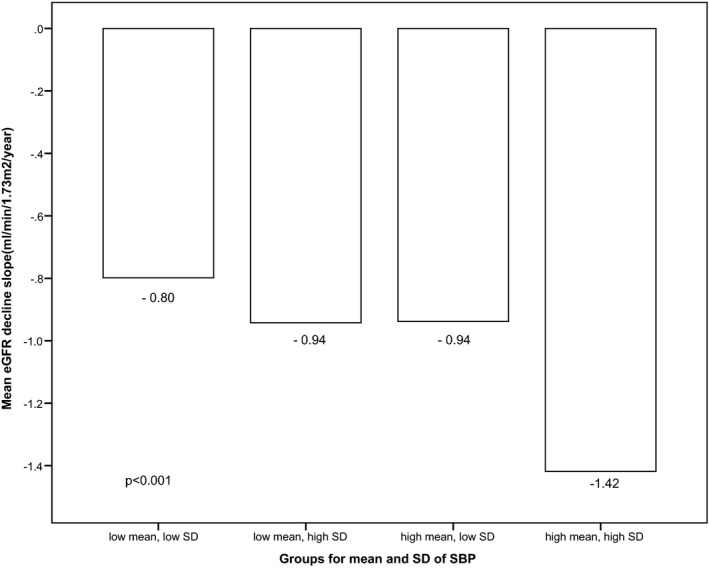
Comparison of the slope of estimated glomerular filtration rate (eGFR) decline among groups of mean and SD of systolic blood pressure (SBP).
CKD and BPV
Patients with CKD in this cohort study (n=226) had higher BPV compared to those without CKD (n=599) (SD of SBP 15.0 mm Hg versus 13.9 mm Hg, P<0.001, respectively). Figure 4 shows a significant difference in BPV and stages of CKD. Higher BPV is related to the higher stages of CKD; 27.9% (n=226) of patients in this study developed CKD at the end of 15 years with 3.3% (n=27) in stage 4 and 1.2% (n=10) in stage 5. To determine whether CKD affects BPV, we examined the mean BPV before and after the onset of CKD for those who developed CKD at year 2002 (n=92) and at year 2007 (n=68). We found that there was no statistical difference in their BPV before and after onset of CKD (onset of CKD at year 2002: 14.4±4.1 mm Hg versus 14.5±3.5 mm Hg, P=0.77, respectively; onset of CKD at year 2007: 14.5±2.9 mm Hg versus 14.3±4.6 mm Hg, P=0.78, respectively).
Figure 4.
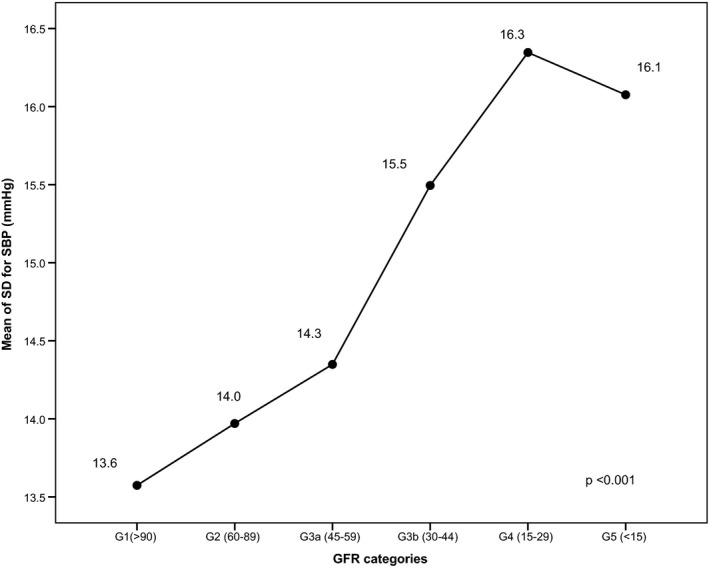
Correlation of mean of SD for systolic blood pressure (SBP) and stages of chronic kidney disease. eGFR indicates glomerular filtration rate.
Discussion
In this study, the rate of the renal function decline in patients with hypertension is −1.0 mL/min per 1.73 m2 per year. This is a surprise finding as the rate of decline in patients with hypertension in our cohort is not higher but similar to that of renal function decline in the general population, which has been reported to be 0.75 to 1.03 mL/min per 1.73 m2 per year.21, 22, 23 We hypothesized that our finding of near normal and not faster decline could be due to the better BP control (mean SBP 136.7 mm Hg). Very few studies have been done that examined visit‐to‐visit BPV, particularly in hypertensive patients with normal renal function (eGFR >60 mL/min per 1.73 m2). In this present study, we found that the long‐term visit‐to‐visit BPV in patients with hypertension is 14.2 mm Hg (SD for SBP) and 10.2% (CV for SBP). The visit‐to‐visit BPV in our cohort is slightly higher than that reported in patients with DM (mean SD 12.4 mm Hg)7 or in patients with CKD (mean SD of 11 mm Hg).24, 25
Our study shows that higher long‐term visit‐to‐visit BPV is significantly associated with greater decline in renal function in hypertensive patients without CKD. In a 3‐year follow‐up study of a large cohort of a general population without DM and CKD in Japan, higher long‐term BPV was found to be associated with risk of onset of CKD.26 Studies in patients with CKD with or without DM also showed that visit‐to‐visit BPV is an independent predictor for eGFR decline9 and progression to end‐stage renal disease.7 In our study, long‐term visit‐to‐visit BPV is significantly associated with eGFR decline in patients with hypertension, although the correlation is weak. This effect is consistent with a study done in diabetic patients that showed a weak association of long‐term BPV and urinary albumin excretion (r=−0.21).27 The weak correlation in our cohort could be because of good control of SBP with mean SBP of 136.7 mm Hg. Of note is that despite mean SBP being a strong determinant of renal function decline, in our cohort where the mean SBP was well controlled, we were still able to show the association of BPV in decline of renal function. Mancia et al showed that long‐term BPV does not affect the serum creatinine in patients with mild‐to‐moderate hypertension.28 Yokota et al failed to showed the relationship between long‐term BPV and renal function decline in DM nephropathy patients because of small sample size (n=69).29 Meanwhile in an elderly population, Di Iorio et al showed that long‐term BPV is not related to progression of CKD.30 These studies have a shorter duration of follow‐up (3–4 years duration), which may not be able to show the effect of BPV on renal function decline. Other studies that examined short‐term 24‐hour BPV also showed that BPV was associated with decline in renal function, suggesting that both short‐ and long‐term BPV are important in determining the progression of kidney function deterioration in patients with hypertension.31, 32
We have found that use of ARBs, diuretics, and α‐blockers was associated with greater renal function decline. The beneficial effect of ARB on eGFR decline may not be seen in our present study because very few patients were on ARB at baseline and even at the end of 10 years (n=1, 0.1% at year 1998; n=14, 1.7% at year 2007, respectively). Few patients were on ARBs because ARBs could only be started when patients developed proteinuria or deterioration of renal function, as this was the practice in our clinical primary care setting because of cost and limited access to these drugs. Diuretics and α‐blockers were usually the subsequent antihypertensive medications added on after the use of calcium channel blockers and angiotensin‐converting enzyme inhibitors in those with uncontrolled hypertension, who because of their uncontrolled hypertension are at higher risk for greater renal function decline. Studies have shown that statin therapy reduces renal function deterioration.33, 34, 35 However, this beneficial effect of statin on eGFR decline was not seen in our study because very few patients were taking statin at baseline (n=68, 8.1%) and most of the patients were started on statins later on. Hence, the full effect of statin was not seen in our cohort study. However, we noticed that BPV is still not well controlled in real‐life clinical practice even in the majority of our patients who are on treatment. Hence, more effort should be made to raise the awareness of clinicians regarding the importance of controlling BPV and to determine the factors causing high BPV.
Systolic BP is a known major determinant affecting decline in renal function, especially among patients with established CKD.36, 37, 38 Studies on the general population also show that high SBP is associated with the development of end‐stage renal disease.2, 39 We also found this relationship in our study. We also found that BPV increases proportionally to mean SBP. However, the significance of BPV on renal function decline remained after adjusting for mean SBP, suggesting that BPV is an independent predictor for renal function decline. Furthermore, we have shown that patients with high mean SBP and low BPV have the same rate of renal function decline as those with low mean SBP but high BPV, suggesting that BPV may have a greater impact on decline in renal function. Higher BPV is related to higher average BP, microalbuminuria,40, 41 renal vascular resistance,42 and increased arterial stiffness.43 Persistent BPV fluctuations with increasing hemodynamic load and urinary albumin excretion cause glomerulosclerosis and other renal pathology, leading to significant renal function decline over the long term.44 Increasing BPV may be an important factor in addition to the conventional risk factors in predicting renal dysfunction. Hence, guidelines have recommended that while SBP be the target of BP control, attention should also be paid to BPV. The mechanisms of visit‐to‐visit variability are different from other forms of variability (for example, 24‐hour BP variability). While 24‐hour BPV is due more to influences of sympathetic drive, arterial compliance, effects of humoral and behavioral influences (sleep, physical activity, and postural changes), visit‐to‐visit variability is more associated with adherence to therapy and perhaps to the antihypertensive used.45 Furthermore, while there are no outcome trials to prove that reduction of BPV will improve cardiovascular disease outcome or renal function decline, we suggest that reduction of BPV in particular visit‐to‐visit variability can be achieved by controlling the mean SBP more tightly.
Recently, Chang et al showed the significance of visit‐to‐visit BPV in predicting mortality and hemorrhagic stroke but not progression to end‐stage renal disease in a population of 110 000 with CKD.24 The question of whether BPV causes CKD or CKD worsens BPV has been raised.46 The Jackson Heart Study showed that patients with CKD had higher BPV compared to those without CKD.47 Our study also showed that those with CKD at the end of 15 years have higher BPV compared to those without CKD. However, when comparing the BPV before and after the onset of CKD in our cohort, there was no significant difference in their BPV. Indeed, we have excluded patients with CKD at baseline, and yet we managed to show that higher BPV is associated with higher rate of decline in renal function and development of CKD. This suggests that visit‐to‐visit BPV is a determinant of decline in renal function rather than CKD causing higher BPV.
Up to now, there has been a lack of studies to suggest the cutoff value of visit‐to‐visit BPV that determines the increased risk of CKD and cardiovascular disease events significantly. From our study, we have identified SD of SBP of 13.5 mm Hg and CV of SBP of 9.73% as the cutoff values for the onset of CKD in hypertensive patients. Our finding is similar to Yokota's study where a reported cutoff value of 14.8 mm Hg for SD of visit‐to‐visit SBP predicts renal end points among nondiabetic patients with CKD.9 These cutoff values may be a useful guide for clinicians who are managing patients with hypertension, particularly in those with higher fluctuations of BP.
Strengths and Limitations
First, our study has a relatively large sample size, consisting of 825 hypertensive patients, which is large enough to show the relationship between BPV and renal function decline in hypertensive patients. Second, our study was carried out in a primary care setting where there were no CKD patients at baseline. This population of primary care patients is suitable to be studied as the majority of them have not reached CKD yet and hence present the best opportunity for early primary prevention of CKD. Third, we have a long duration of follow‐up of 15 years, which enables us to capture multiple BP readings (as many as 60 readings) for more accurate BPV estimation. We also have serum creatinine level every 5 years that showed the trend of renal function decline over 15 years. The long observation period in our study enables us to adequately capture any significant renal function decline.
One of the limitations of our study is that we do not have the time to onset of CKD in our cohort to ascertain timing of the renal events end point. As this is a retrospective cohort study, missing data are not unexpected. Of note is that the BP of hypertensive patients in this study is well controlled, and it may not reflect the rate of renal function decline in those with uncontrolled blood pressure. However, we would like to stress that the effect of visit‐to‐visit BPV on renal function decline remained significant even in a population with well‐controlled BP.
Perspectives
This present study reported the significance of long‐term visit‐to‐visit BPV on renal function decline over 15 years in patients with hypertension and normal renal function. Hence, every effort should be made to reduce BPV in order to slow down the decline of renal function in patients with hypertension. More studies are needed to evaluate the factors affecting the visit‐to‐visit BPV.
Sources of Funding
This study was supported by research grants from the University of Malaya (UMRG 116/09HTM) and Malaysian Society of Hypertension (MSH RG/2015/LHM). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosures
Chia has received a speaker honorarium from Pfizer. The remaining authors have no conflicts to disclose.
Supporting information
Table S1. eGFR Categories in Chronic Kidney Disease According to KDIGO 2012
Table S2. Descriptive Statistics for Groups of Mean and SD of Systolic Blood Pressure
Acknowledgments
The authors would like to acknowledge Department of Primary Care at the University of Malaya for providing support during the data collection.
(J Am Heart Assoc. 2016;5:e003825 doi: 10.1161/JAHA.116.003825)
References
- 1. Levey AS, Coresh J. Chronic kidney disease. Lancet. 2012;379:165–180. [DOI] [PubMed] [Google Scholar]
- 2. Klag MJ, Whelton PK, Randall BL, Neaton JD, Brancati FL, Ford CE, Shulman NB, Stamler J. Blood pressure and end‐stage renal disease in men. N Engl J Med. 1996;334:13–18. [DOI] [PubMed] [Google Scholar]
- 3. Hsu C, McCulloch CE, Darbinian J, Go AS, Iribarren C. Elevated blood pressure and risk of end‐stage renal disease in subjects without baseline kidney disease. Arch Intern Med. 2005;165:923–928. [DOI] [PubMed] [Google Scholar]
- 4. Marin R, Gorostidi M, Fernandez‐Vega F, Alvarez‐Navascues R. Systemic and glomerular hypertension and progression of chronic renal disease: the dilemma of nephrosclerosis. Kidney Int. 2005;68:S52–S56. [DOI] [PubMed] [Google Scholar]
- 5. Chia YC, Ching SM. Hypertension and the development of new onset chronic kidney disease over a 10 year period: a retrospective cohort study in a primary care setting in Malaysia. BMC Nephrol. 2012;13:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rothwell PM, Howard SC, Dolan E, O'Brien E, Dobson JE, Dahlöf B, Sever PS, Poulter NR. Prognostic significance of visit‐to‐visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet. 2010;375:895–905. [DOI] [PubMed] [Google Scholar]
- 7. McMullan CJ, Lambers Heerspink HJ, Parving HH, Dwyer JP, Forman JP, de Zeeuw D. Visit‐to‐visit variability in blood pressure and kidney and cardiovascular outcomes in patients with type 2 diabetes and nephropathy: a post hoc analysis from the RENAAL study and the Irbesartan Diabetic Nephropathy Trial. Am J Kidney Dis. 2014;64:714–722. [DOI] [PubMed] [Google Scholar]
- 8. Muntner P, Whittle J, Lynch AI, Colantonio LD, Simpson LM, Einhorn PT, Levitan EB, Whelton PK, Cushman WC, Louis GT, Davis BR, Oparil S. Visit‐to‐visit variability of blood pressure and coronary heart disease, stroke, heart failure, and mortality: a cohort study. Ann Intern Med. 2015;163:329–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yokota K, Fukuda M, Matsui Y, Hoshide S, Shimada K, Kario K. Impact of visit‐to‐visit variability of blood pressure on deterioration of renal function in patients with non‐diabetic chronic kidney disease. Hypertens Res. 2013;36:151–157. [DOI] [PubMed] [Google Scholar]
- 10. Chia YC, Gray SY, Ching SM, Lim HM, Chinna K. Validation of the Framingham general cardiovascular risk score in a multiethnic Asian population: a retrospective cohort study. BMJ Open. 2015;5:e007324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. James PA, Oparil S, Carter BL, Cushman WC, Dennison‐Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegde O, Smith SC Jr, Svetkey LP, Taler SJ, Townsend RR, Wright JT Jr, Narva AS, Ortiz E. 2014 evidence‐based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507–520. [DOI] [PubMed] [Google Scholar]
- 12. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yokoyama H, Kanno S, Takahashi S, Yamada D, Itoh H, Saito K, Sone H, Haneda M. Determinants of decline in glomerular filtration rate in nonproteinuric subjects with or without diabetes and hypertension. Clin J Am Soc Nephrol. 2009;4:1432–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zoppini G, Targher G, Chonchol M, Ortalda V, Negri C, Stoico V, Bonora E. Predictors of estimated GFR decline in patients with type 2 diabetes and preserved kidney function. Clin J Am Soc Nephrol. 2012;7:401–408. [DOI] [PubMed] [Google Scholar]
- 15. Van Pottelbergh G, Den Elzen WP, Degryse J, Gussekloo J. Prediction of mortality and functional decline by changes in eGFR in the very elderly: the Leiden 85‐plus study. BMC Geriatr. 2013;13:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vaes B, Beke E, Truyers C, Elli S, Buntinx F, Verbakel JY, Goderis G, van Pottelbergh G. The correlation between blood pressure and kidney function decline in older people: a registry‐based cohort study. BMJ Open. 2015;5:e007571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO . Clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2012;2013:1–150. [Google Scholar]
- 18. Scandinavian Simvastatin Survival Study Group . Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet. 1994;344:1383–1389. [PubMed] [Google Scholar]
- 19. Cholesterol Treatment Trialists Collaboration . The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta‐analysis of individual data from 27 randomised trials. Lancet. 2012;380:581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. de Boer IH, Rue TC, Hall YN, Heagerty PJ, Weiss NS, Himmelfarb J. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA. 2011;305:2532–2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lindeman RD, Tobin J, Shock NW. Longitudinal studies on the rate of decline in renal function with age. J Am Geriatr Soc. 1985;33:278–285. [DOI] [PubMed] [Google Scholar]
- 22. Eriksen BO, Ingebretsen OC. The progression of chronic kidney disease: a 10‐year population‐based study of the effects of gender and age. Kidney Int. 2006;69:375–382. [DOI] [PubMed] [Google Scholar]
- 23. Glassock RJ, Winearls C. Ageing and the glomerular filtration rate: truths and consequences. Trans Am Clin Climatol Assoc. 2009;120:419–428. [PMC free article] [PubMed] [Google Scholar]
- 24. Chang TI, Tabada GH, Yang J, Tan TC, Go AS. Visit‐to‐visit variability of blood pressure and death, end‐stage renal disease, and cardiovascular events in patients with chronic kidney disease. J Hypertens. 2016;34:244–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mallamaci F, Minutolo R, Leonardis D, D'Arrigo G, Tripepi G, Rapisarda F, Cicchetti T, Maimone I, Enia G, Postorino M, Santoro D, Fuiano G, De Nicola L, Conte G, Zoccali C. Long‐term visit‐to‐visit office blood pressure variability increases the risk of adverse cardiovascular outcomes in patients with chronic kidney disease. Kidney Int. 2013;84:381–389. [DOI] [PubMed] [Google Scholar]
- 26. Yano Y, Fujimoto S, Kramer H, Sato Y, Konta T, Iseki K, Iseki C, Moriyama T, Yamagata K, Tsuruya K, Narita I, Kondo M, Kimura K, Asahi K, Kurahashi I, Ohashi Y, Watanabe T. Long‐term blood pressure variability, new‐onset diabetes mellitus, and new‐onset chronic kidney disease in the Japanese general population. Hypertension. 2015;66:30–36. [DOI] [PubMed] [Google Scholar]
- 27. Okada H, Fukui M, Tanaka M, Inada S, Mineoka Y, Nakanishi N, Senmaru T, Sakabe K, Ushigome E, Asano M, Yamazaki M, Hasegawa G, Nakamura N. Visit‐to‐visit variability in systolic blood pressure is correlated with diabetic nephropathy and atherosclerosis in patients with type 2 diabetes. Atherosclerosis. 2012;220:155–159. [DOI] [PubMed] [Google Scholar]
- 28. Mancia G, Facchetti R, Parati G, Zanchetti A. Visit‐to‐visit blood pressure variability in the European Lacidipine Study on Atherosclerosis: methodological aspects and effects of antihypertensive treatment. J Hypertens. 2012;30:1241–1251. [DOI] [PubMed] [Google Scholar]
- 29. Yokota K, Fukuda M, Matsui Y, Kario K, Kimura K. Visit‐to‐visit variability of blood pressure and renal function decline in patients with diabetic chronic kidney disease. J Clin Hypertens (Greenwich). 2014;16:362–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Di Iorio B, Pota A, Sirico ML, Torraca S, Di Micco L, Rubino R, Guastaferro P, Bellasi A. Blood pressure variability and outcomes in chronic kidney disease. Nephrol Dial Transplant. 2012;27:4404–4410. [DOI] [PubMed] [Google Scholar]
- 31. Mule G, Calcaterra I, Costanzo M, Geraci G, Guarino L, Foraci AC, Vario MG, Cerasola G, Cottone S. Relationship between short‐term blood pressure variability and subclinical renal damage in essential hypertensive patients. J Clin Hypertens (Greenwich). 2015;17:473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gorostidi M, Sarafidis P, Sierra Ade L, Banegas JR, De La Cruz JJ, Vinyoles E, Segura J, Ruilope LM. 3D.02: blood pressure variability increases with advancing chronic kidney disease stage. A cross‐sectional analysis of 14,382 hypertensive patients from Spain. J Hypertens. 2015;33(suppl 1):e40. [DOI] [PubMed] [Google Scholar]
- 33. Sanguankeo A, Upala S, Cheungpasitporn W, Ungprasert P, Knight EL. Effects of statins on renal outcome in chronic kidney disease patients: a systematic review and meta‐analysis. PLoS One. 2015;10:e0132970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sandhu S, Wiebe N, Fried LF, Tonelli M. Statins for improving renal outcomes: a meta‐analysis. J Am Soc Nephrol. 2006;17:2006–2016. [DOI] [PubMed] [Google Scholar]
- 35. Geng Q, Ren J, Song J, Li S, Chen H. Meta‐analysis of the effect of statins on renal function. Am J Cardiol. 2014;114:562–570. [DOI] [PubMed] [Google Scholar]
- 36. Anderson AH, Yang W, Townsend RR, Pan Q, Chertow GM, Kusek JW, Charleston J, He J, Kallem R, Lash JP, Miller ER III, Rahman M, Steigerwalt S, Weir M, Wright JT Jr, Feldman HI. Time‐updated systolic blood pressure and the progression of chronic kidney disease: a cohort study. Ann Intern Med. 2015;162:258–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. de Goeij MC, Voormolen N, Halbesma N, de Jager DJ, Boeschoten EW, Sijpkens YW, Dekker FW, Grootendorst DC. Association of blood pressure with decline in renal function and time until the start of renal replacement therapy in pre‐dialysis patients: a cohort study. BMC Nephrol. 2011;12:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sarnak MJ, Greene T, Wang X, Beck G, Kusek JW, Collins AJ, Levey AS. The effect of a lower target blood pressure on the progression of kidney disease: long‐term follow‐up of the modification of diet in renal disease study. Ann Intern Med. 2005;142:342–351. [DOI] [PubMed] [Google Scholar]
- 39. Tozawa M, Iseki K, Iseki C, Kinjo K, Ikemiya Y, Takishita S. Blood pressure predicts risk of developing end‐stage renal disease in men and women. Hypertension. 2003;41:1341–1345. [DOI] [PubMed] [Google Scholar]
- 40. Okada H, Fukui M, Tanaka M, Matsumoto S, Mineoka Y, Nakanishi N, Asano M, Yamazaki M, Hasegawa G, Nakamura N. Visit‐to‐visit blood pressure variability is a novel risk factor for the development and progression of diabetic nephropathy in patients with type 2 diabetes. Diabetes Care. 2013;36:1908–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Noshad S, Mousavizadeh M, Mozafari M, Nakhjavani M, Esteghamati A. Visit‐to‐visit blood pressure variability is related to albuminuria variability and progression in patients with type 2 diabetes. J Hum Hypertens. 2014;28:37–43. [DOI] [PubMed] [Google Scholar]
- 42. Kawai T, Ohishi M, Kamide K, Onishi M, Takeya Y, Tatara Y, Oguro R, Yamamoto K, Sugimoto K, Rakugi H. The impact of visit‐to‐visit variability in blood pressure on renal function. Hypertens Res. 2012;35:239–243. [DOI] [PubMed] [Google Scholar]
- 43. Xiong H, Wu D, Tian X, Lin W‐H, Li C, Zhang H, Cai Y, Zhang YT. The relationship between the 24 h blood pressure variability and carotid intima‐media thickness: a compared study. Comput Math Methods Med. 2014;2014:303159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Parati G, Liu X, Ochoa JE. Clinical relevance of visit‐to‐visit blood pressure variability: impact on renal outcomes. J Hum Hypertens. 2014;28:403–409. [DOI] [PubMed] [Google Scholar]
- 45. Parati G, Ochoa JE, Lombardi C, Bilo G. Assessment and management of blood‐pressure variability. Nat Rev Cardiol. 2013;10:143–155. [DOI] [PubMed] [Google Scholar]
- 46. Redon J. The risk of visit‐to‐visit blood pressure variability in chronic kidney disease: cause or consequence. J Hypertens. 2016;34:188–190. [DOI] [PubMed] [Google Scholar]
- 47. Tanner RM, Shimbo D, Dreisbach AW, Carson AP, Fox ER, Muntner P. Association between 24‐hour blood pressure variability and chronic kidney disease: a cross‐sectional analysis of African Americans participating in the Jackson heart study. BMC Nephrol. 2015;16:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. eGFR Categories in Chronic Kidney Disease According to KDIGO 2012
Table S2. Descriptive Statistics for Groups of Mean and SD of Systolic Blood Pressure


