Introduction
Atrial fibrillation (AF) is a global problem with a significant impact on health outcomes, affecting up to 1% to 2% of the global adult population, and is projected to increase in both developed and developing countries over the coming decades.1 AF is associated with higher mortality and morbidity, as well as decreased quality of life, and increases the risk of thromboembolic events (including stroke), heart failure (HF), myocardial infarction, dementia, and chronic kidney disease. The mainstays of AF treatment are arrhythmia management (with pharmacologic or mechanical rate or rhythm control) and prevention of thromboembolism.2 The field of AF treatment is dynamic, with the recent development of new procedures to restore sinus rhythm (catheter and surgical ablation) and new treatments to reduce thromboembolism (target‐specific oral anticoagulants and catheter‐based exclusion of the left atrial appendage). However, there is significant global variation in use of these therapies, particularly in developing countries, and the global implementation, diffusion, and anticipated real‐world effectiveness of these technologies is uncertain.3 Therefore, a better understanding of global AF treatments, barriers and facilitators to its optimal use, and its subsequent outcomes is needed.
As AF incidence and its arsenal of treatments continue to expand globally, the longitudinal and comprehensive nature of clinical registries can provide important insights into the clinical management of AF. Registries are observational databases of clinical conditions and/or therapies in which there are no mandated approaches to therapy and relatively few inclusion or exclusion criteria.4 In addition, registries can continuously collect longitudinal data on their target population, which allows them to monitor current and emerging clinical practices over time and associate them with clinical outcomes. Accordingly, current AF management practices and their associated outcomes can be measured, benchmarked to current best practices and clinical practice guidelines, and compared across different countries, populations, and healthcare systems. As novel AF treatments emerge, their dissemination globally and effect on outcomes, both intended and unintended, can be assessed.
Currently, multiple AF databases and registries spanning a variety of countries, patients, treatments, and outcomes exist.5 However, coordination among these data sources is minimal. In addition, the variations in patient enrollment, data definitions, and characterization of AF therapies in each of the registries prevent effective comparison between registries and limit their ability to track changes over time. Finally, many of the current registries are sponsored by pharmaceutical or device companies, which can limit their focus on those patients and treatments associated with their products.
The potential value of coordinating these disparate registry efforts is significant. More accurate and complete assessment of the treatment and outcomes of AF in various countries and populations around the world could occur. As the arsenal of AF treatments continues to expand, such a registry would allow for comparative effectiveness studies between treatments. Additionally, a global registry would allow for understanding of the impact of treatments in various patient populations and within the context of their local healthcare environments. Large registries could also utilize their size to detect infrequent, but important, safety signals of both AF and its treatment. Thus, the insights from such a registry effort could allow for richer insights into AF and sharing of best practices for its management. In addition, these insights could inform ongoing research efforts, supporting a “learning healthcare system” iterative cycle of knowledge generation from clinical practice insights.6
To respond to this need, we present the International Collaborative Partnership for the Study of Atrial Fibrillation (INTERAF). This worldwide partnership, with initial participants from the United States, Europe, China, Brazil, South Korea, Taiwan, Singapore, Japan, and the Balkan countries, provides a common platform for investigators and countries to study AF populations, treatments, and outcomes. In order to allow for the integration of registries from a wide variety of countries, INTERAF utilizes a distributed data and analytic platform, rather than a single, combined registry structure. The INTERAF leadership provides general data requirements, standards, and governance policies for existing and future country‐ and region‐specific registries. This design allows for easier integration of pre‐existing registries and greater flexibility in data collection, which can lower barriers to participation, allow for an expansive view of global AF treatment and outcome patterns, and support country‐specific and international efforts to optimize the care for the large and growing population of AF patients.
In this report, we present the current knowledge and gaps in global AF management and outcomes, and discuss how INTERAF can fill these gaps. We also outline the organizing principles and structure of the INTERAF partnership, and introduce its initial research agenda and future directions. Finally, we provide initial descriptive data of the participating registries and their AF populations.
Global AF Prevalence and Projections for Growth
AF is a global problem, with projections for significant growth in the coming years. Although comprehensive data are lacking, the 2010 Global Burden of Disease study provides some insights, suggesting that the total number of AF patients is ≈33 million, with an age‐adjusted prevalence of 596 per 100 000 men and 373 per 100 000 women, with significant regional variation (Figure 1).1, 7
Figure 1.

International age‐adjusted AF prevalence rates (per 100 000 population) in the 21 Global Burden of Disease regions, 2010. Figure reproduced from Chugh et al7 with permission from Wolters Kluwer Health, Inc. AF indicates atrial fibrillation.
Over the coming years, AF prevalence is expected to grow significantly. Current census projections for 2050 suggest that the number of Americans, Europeans, and Japanese with AF will increase by 2‐ to 3‐fold.1 In addition, the number of people in the developing world who are older than 60 years, where AF incidence is concentrated, is projected to double by 2050 (Figure 2).1
Figure 2.
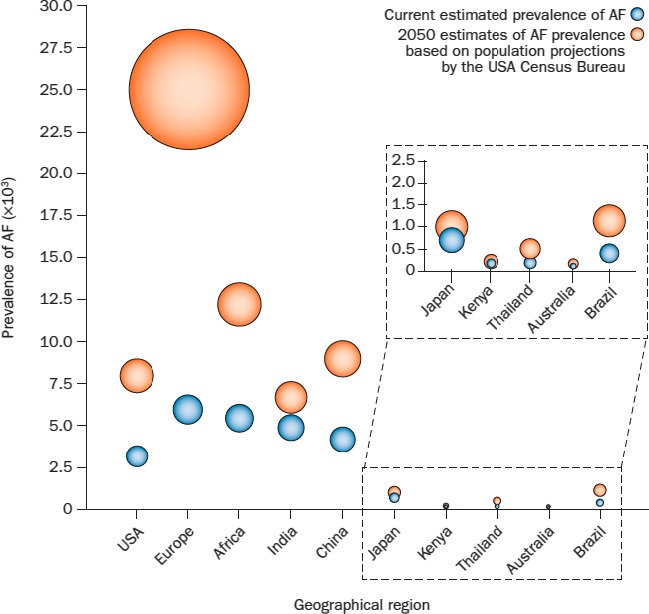
Global AF prevalence and projected increases. Figure reproduced from Rahman et al1 with permission from Nature Publishing Group. AF indicates atrial fibrillation.
Associations Between AF and Mortality, Morbidity, and Quality of Life
AF is associated with increased mortality. As AF prevalence and average lifespan increases over time, this burden will likely increase as well. In adjusted analyses from the Framingham Heart Study in the United States, AF was associated with a 50% higher risk of death among men (odds ratio 1.5, 95% CI 1.2–1.8) and a 90% higher risk of death among women (odds ratio 1.9, 95% CI 1.5–2.2).8 Globally, the age‐adjusted mortality rate, per 100 000 individuals, from AF in 2010 was 1.6 (95% CI 1–2.4) among men and 1.7 (95% CI 1.4–2.2) among women, representing a 2‐fold increase since the previous survey conducted in 1990.7 Perhaps surprisingly, AF mortality rates in the developed world were 3 to 4 times higher than the developing world (Figure 3).
Figure 3.
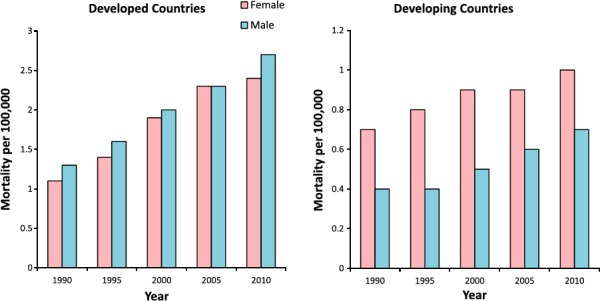
Mortality associated with atrial fibrillation (AF) stratified by sex and type of region (developed vs developing). Figure reproduced from Chugh et al7 with permission from Wolters Kluwer Health, Inc.
AF is also associated with significant morbidity. One of the most devastating consequences of AF is arterial thromboembolism. AF is associated with a 4 to 5 times higher risk of stroke, even after accounting for standard stroke risk factors (eg, hypertension, congestive HF, etc).9, 10 However, this risk of thromboembolism and its subsequent outcomes vary significantly among ethnic groups and geographies. For example, Americans of either Hispanic ethnicity or African‐American race appear to have higher stroke risks, despite a lower prevalence of AF relative to white individuals.8, 11 Asians with AF may have particularly high risks for stroke, potentially due to variations in both anticoagulant prescribing practices and variable genetic‐based responses to anticoagulation with warfarin.12, 13, 14, 15 Similar variation in thromboembolism risk and outcomes may also occur in other regions, but comprehensive global assessments of thromboembolism outcomes, particularly in developing countries, are lacking.
AF is also associated with other causes of morbidity, including coronary artery disease, dementia, chronic kidney disease, and HF. Coronary artery disease has been associated with AF across a wide variety of ethnicities and countries.3, 10, 16, 17, 18 A US‐based study found that AF was associated with a 70% increased risk of incident myocardial infarction (hazard ratio, 1.96; 95% CI, 1.52, 2.52), with higher risks in women and blacks.19 AF has also been associated with cognitive decline and dementia. A meta‐analysis of 21 studies found decreased cognitive scores associated with AF in different populations.11 In Brazil, AF patients were 2.8 times more likely to have dementia, relative to those without AF.14 AF also is associated with chronic kidney disease. A US‐based study noted that the development of new‐onset AF in chronic kidney disease patients was associated with a 1.7 times increased risk of developing end‐stage renal disease, relative to those without AF.15 Similarly, a Japanese study found the incidence of kidney dysfunction was 18.2 per 1000 person‐years in AF patients, compared to 6.8 per 1000 person‐years in patients without AF.20 Finally, HF among AF patients is common, occurring in ≈40% of patients, and associated with increased morbidity and mortality.12 These associations between AF and HF in the developed world have also been demonstrated in the developing world. In particular, African studies have documented a 10% to 20% prevalence of AF among patients admitted to hospitals with HF.13
These associations between AF, mortality, and morbidity impair overall quality of life. This effect can be estimated by a disability‐adjusted life years calculation, which adds up the years lost due to the condition with the years lived with the condition, weighted for its impact on quality of life.9 As illustrated in Figure 4, AF‐associated DALYs have consistently increased over the past 2 decades and continued increases are projected for the coming year.7
Figure 4.
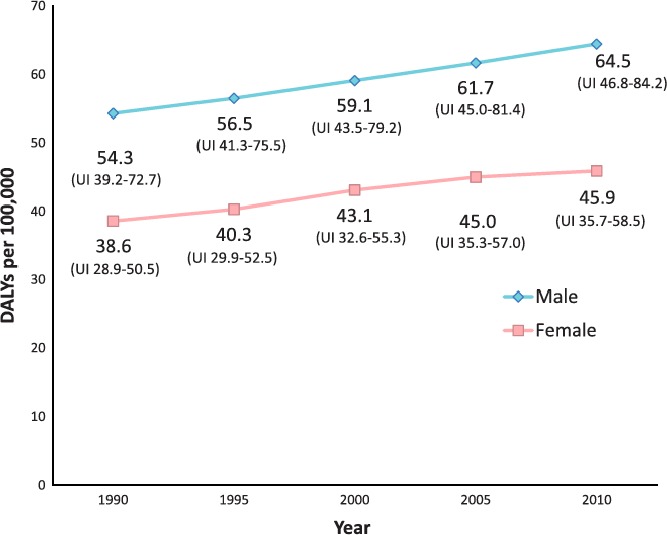
Disability‐adjusted life years (DALYs) related to AF. Estimated global age‐adjusted DALYs (per 100 000) related to atrial fibrillation: 1990 to 2010. UI indicates uncertainty index. Figure reproduced from Chugh et al7 with permission from Wolters Kluwer Health, Inc.
Although these data underscore the impact that AF has on mortality, morbidity, and quality of life, a full assessment of its worldwide impact is lacking. The INTERAF initiative can address these gaps, and uncover additional insights into the impact of AF globally.
Global Trends and Differences in AF Treatments
There are notable variations in AF treatment and outcomes between different patient and country populations. Some of this variation is due to the availability of medications and interventions that, in turn, are dependent on resource availability and differences in healthcare systems (eg, national healthcare systems, private health insurance, or a mixture of both). In general, AF treatments are directed towards symptom reduction, typically via heart rate or rhythm control, and the prevention of complications, such as arterial thromboembolism. Rate and/or rhythm‐control treatments include heart rate controlling medications, antiarrhythmic medications, electrical cardioversion, or AF ablation. Thromboembolism prevention treatments include antithrombotic medications and mechanical left atrial appendage occlusion devices.
The use of rate and rhythm‐control treatments varies globally, both in the developed and developing world. In the Euro Heart Survey, 65% of AF patients received rate‐control medication, 40% received antiarrhythmic medication, and 12% received neither.21 In contrast, a German AF registry—the Central Registry of German Competence Network on Atrial Fibrillation (AFNET)—demonstrated that only 21.3% of patients received antiarrhythmic medication, but 53.4% received electrical or pharmacologic cardioversion and 5% underwent AF ablation.22 Chinese data demonstrated variable use of rate‐control treatments, partly dependent on the AF phenotype.23, 24 Among those with paroxysmal AF, 44% of patients received rate‐control treatment, compared to 83% of patients with permanent AF. These differences likely result from a variety of factors, including availability of more advanced and technically complex treatments, such as AF ablation.25 In the developing world, rate‐control treatments, particularly digoxin, dominate the use of antiarrhythmic medications, given their low cost and wide availability. For example, recent surveys of AF treatments in Cameroon found that 83% of patients were being treated with rate‐control medications, with over 60% receiving digoxin.26 Similarly, over 50% of Kenyan patients on rate‐control therapy were receiving digoxin.27 However, better characterization of rate‐control treatments, especially in developing countries, is needed. In addition, as newer technologies such as AF ablation gain traction in these countries, registries will be needed to guide their safe and effective deployment.
Thromboembolic events related to AF are significantly reduced with the use of anticoagulation therapies. However, large variations occur in its provision and effect among various populations. For example, despite the recommendation that patients at moderate to high risk for thromboembolism receive anticoagulation, large numbers of patients, across multiple countries, do not.3, 21, 28 Furthermore, even among those patients receiving anticoagulation with warfarin, its use is often suboptimal, with several studies demonstrating significant rates of subtherapeutic warfarin levels globally, particularly in developing countries.29, 30 Finally, certain populations appear to have higher risks of significant bleeding with anticoagulation, thus diminishing its benefit. In particular, Asian, Hispanic, and black populations all appear to have higher rates of intracranial hemorrhage on oral anticoagulation (OAC), relative to white populations.31, 32
In recent years, 2 new modalities for thromboembolism prevention have emerged: direct oral anticoagulants (DOACs) and left atrial appendage occlusion devices. There is a need to understand how these new therapies will diffuse into global practice, and their impact on both thromboembolic event prevention and bleeding outcomes. DOACs have similar or better efficacy than warfarin, and have a dose‐dependent, predictable anticoagulant effect, thus enabling fixed dosing that does not require laboratory monitoring of anticoagulation intensity. In addition, DOACs have a favorable safety profile, particularly for intracranial hemorrhage. All of these characteristics are potentially attractive to regions with relatively underdeveloped healthcare systems. However, DOACs are more expensive than warfarin and, with the exception of dabigatran, cannot be easily reversed.33 These factors may limit their dissemination to the developing world. Left atrial appendage occlusion devices are an option for those patients who have absolute contraindications to OAC or completely refuse OAC, and appear to have similar efficacy to OAC in thromboembolism prevention.34 However, the safety of left atrial appendage occlusion device implantation is largely operator dependent, with better outcomes in those who perform a high number of procedures. Thus, technical complexity, as well as cost, will likely slow its dissemination to the developing world. Registries to monitor its dissemination and safety of these new therapies are needed.35
The International Collaborative Partnership for the Study of Atrial Fibrillation (INTERAF)
In response to this need for a more coordinated approach to AF registries, the International Collaborative Partnership for the Study of Atrial Fibrillation (INTERAF) was established. Organized by an international group of AF and clinical registry experts, INTERAF is a collaborative, international consortium of AF registries to characterize worldwide AF care and establish a foundation for ongoing efforts to optimize AF care. The goals of INTERAF are 4‐fold:
Build the foundation for a sustained international collaboration to identify and address meaningful questions related to AF management and improve AF patient outcomes.
Explore ways to harmonize existing and future AF registries in order to compare AF care across countries, identify gaps, and to influence and change global practice to close those gaps.
Prioritize research projects focused on international quality improvement.
Develop a roadmap for global AF quality improvement and educational initiatives, informed by best practices.
The initial partners of INTERAF include representatives from the United States, Europe, China, Brazil, South Korea, Taiwan, Singapore, Japan, and the Balkan countries (Figure 5). Each of the partners oversees country‐ or region‐specific AF registries, and can thus inform efforts to unify their respective registries. In addition, the countries represent a variety of patient populations and healthcare systems, which can inform the technical challenges inherent in creating a unified, global AF registry. Table 1 lists the participating registries and their various characteristics.
Figure 5.
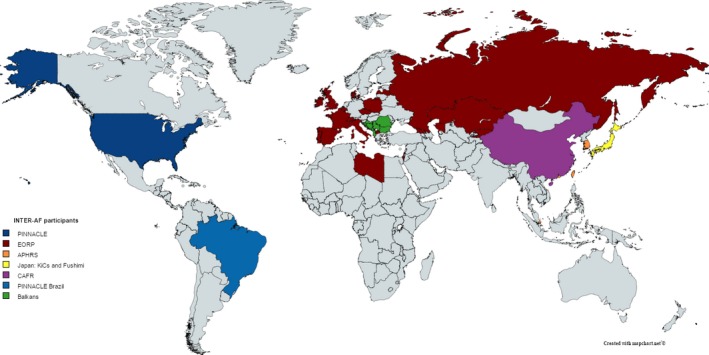
Initial INTERAF partners, by country. APHRS indicates Asia‐Pacific Heart Rhythm Society; CAFR, Chinese Atrial Fibrillation Registry; EORP, EURObservational Research Programme, INTERAF, International Collaborative Partnership for the Study of Atrial Fibrillation; KiCs, Keio Interhospital Cardiovascular Studies; PINNACLE, Practice Innovation and Clinical Excellence.
Table 1.
Characteristics of the Current INTERAF Registries, as of December 31, 2015
| United States NCDR PINNACLE AF Registry | European Society of Cardiology EURObservational Research Program (EORP) AF General Registry | Chinese AF Registry (CAFR) | Brazil PINNACLE AF Registry | Asia Pacific Heart Rhythm Society | Japanese Fushimi AF Registry | Japanese Keio Interhospital Cardiovascular Studies (KiCS) AF Registry | Balkan AF Registry | |
|---|---|---|---|---|---|---|---|---|
| Patient setting | Outpatients | Outpatients | Inpatients and outpatients | Inpatients and outpatients | Inpatients and outpatients | Inpatients and outpatients | Outpatients | Inpatients and outpatients |
| Geographic location | United States | Albania, Belgium, Czech Republic, Denmark, France, Georgia, Ireland, Israel, Italy, Kyrgystan, Kazakhstan, Lativa, Libya, Macedonia, Malta, Poland, Portugal, Romania, Russia, Serbia, Spain, Switzerland, UK | China | Brazil | Hong Kong, South Korea, Singapore, Japan, Taiwan | Japan | Japan | Albania, Bosnia & Herzegovina, Bulgaria, Croatia, Montenegro, Romania and Serbia |
| Number of patients, providers, and healthcare settings | 927 511 patients at 1400 practice locations | 10 000 patients (Target enrollment: 23 000 patients) | 15 000 patients at 31 hospitals (Target enrollment: 20 000 patients) | Target enrollment 10 000 patients (to begin enrolling in late 2015) | Target enrollment 5000 patients with at least 5 centers in each country (to begin enrolling in early 2016) | 4426 patients at 79 participating institutions (2 cardiovascular centers, 10 rehab hospitals, 67 private clinics) | 1284 patients at 10 cardiovascular centers | 2712 patients in 49 centers (university and non‐university hospitals and health centers) |
| Registry dates of collection | 2008–present | 2014–2016 | 2011–present | 2015–2017 | 2016–2017 | 2011–present | 2012–present | December 2015–February 2015 |
| Data collection method | Automated EHR data collection | Paper‐based case report form | EHR and paper data collection | EHR and paper data collection | EHR and paper data collection | EHR and paper data collection | EHR and paper data collection | Electronic case report form, backed up with paper data source |
| Data elements and quality | Complete data on comorbidities, medications, vital signs, and labs; partial data on event history, events between visits; CHADS2 and CHA2DS2‐VASc score calcuation | Complete data on comorbidities, medications, vital signs, CHADS2 and CHA2DS2‐VASc score calculations; partial data on events at 1 year | Data elements similar to US PINNACLE | Data elements similar to US PINNACLE | Complete data on comorbidities, medications, vital signs, CHADS2 and CHA2DS2‐VASc score calculations; partial data on events at 1 year | Complete data on comorbidities, medications, vital signs, CHADS2 and CHA2DS2‐VASc score calculations | Complete data on comorbidities, medications, vital signs, CHADS2 score, CHA2DS2‐VASc score, treatment strategy, and baseline QoL data; partial data embolic events, bleeding events, and QoL at 1 and 2 years | Complete data on patient characteristics, presentation, healthcare setting, AF management strategies, and diagnostic procedures |
| Availability and characteristics of longitudinal data | Linked to longitudinal US Medicare claims data | Annual follow‐up assessment over 3 years | Longitudinal follow‐up assessment every 6 months | Planned longitudinal follow‐up assessment | Annual follow‐up over 2 years | Annual follow‐up assessment | Annual follow‐up over 2 years | None |
| OAC assessment and quality | Assessment of OAC use, including both VKA and DOACs | Assessment of OAC use | Assessment of OAC use, including both VKA and DOACs | Assessment of OAC use | Assessment of OAC use | Assessment of OAC use, including both VKA and DOACs | Assessment of OAC use, including both VKA and DOACs | Assessment of OAC use, including both VKA and DOACs |
| IRB approvala | National IRB waiver; no individual patient consent | Patients individually consented | Patients individually consented | National IRB waiver; some individual patient consent | Patients individually consented | Institutional IRB waiver; no individual patient consent | Patients individually consented | Patients individually consented |
| Feedback to registry participants | Feedback given to participating practices via performance reports | Feedback and some data monitoring | Feedback to hospitals twice a year | Feedback given to participating practices via performance reports | Feedback and some data monitoring | Feedback and some data monitoring | Feedback provided to participating hospitals twice a year | Regular feedback to participating sites |
| Participation in prospective research studies | Sites can participate in cohort studies, but consent needed | Sites can participate in cohort studies, but consent needed | Sites can participate in cohort studies, but consent needed | Sites can participate in cohort studies, but consent needed | Sites can participate in cohort studies, but consent needed | Sites can participate in cohort studies, but consent needed | Sites can participate in cohort studies, but consent needed | Sites can participate in cohort studies, but consent needed |
| Incentives for registry participation | Receipt of feedback reports and automatic submission to Medicare for quality bonus payments | None | CAFR funding to hospitals | Receipt of feedback reports and quality of care certification via the American College of Cardiology and São Paulo Society of Cardiology | None | None | Feedback reports and research projects | None |
AF indicates atrial fibrillation; DOACs, direct oral anticoagulants; EHR, electronic health record; INTERAF, International Collaborative Partnership for the Study of Atrial Fibrillation; IRB, institutional review board; OAC, oral anticoagulant; PINNACLE, Practice Innovation and Clinical Excellence; QoL, quality of life; VKA, vitamin K antagonist.
An institutional waiver of consent has been obtained for collective analyses as all data are de‐identified and of minimal risk to any patient.
A challenge for all clinical registries, particularly those that are global in scope, is organizing data collection and analytic efforts to gain meaningful insights. Simply establishing a single registry that collects the same data elements in every participating country is not feasible, given the enormous costs and logistical difficulties associated with such an effort. Instead, INTERAF will be organized as a distributed research network, similar to the National Heart, Lung, and Blood Institute–supported Cardiovascular Research Network and the Patient‐Centered Outcomes Research Institute (PCORI)‐supported PCORnet.36, 37 A distributed research network allows for data collection and local analyses to be conducted within each participating partner's infrastructure. These data are then aggregated and analyzed across partner registries. This distributed structure thus avoids the difficulties with data security, patient privacy, and governance inherent in a single registry structure. At the same time, the structure allows for greater size and power of the overall registry, an ability to assess and compare partner practice patterns, greater diversity in studied patient populations, and an opportunity to assess the heterogeneity of treatment effect in various real‐world settings. It also lowers barriers to participation in INTERAF and engages with local AF experts to best identify partner‐specific characteristics and challenges in AF management for analysis.
In order to effectively manage this distributed research network, a governance structure will be established to determine the minimal data elements and standards needed for partner participation in INTERAF and the technical specifications necessary to integrate registries. Examples of necessary data elements include patient and clinical demographics, AF phenotype (eg, paroxysmal, persistent, permanent) characteristics, relevant clinical comorbidities, stroke and bleeding risk scores, AF therapies (eg, rate control, rhythm control, anticoagulation), and relevant concurrent medications. In addition, efforts to link to other longitudinal outcomes data will occur to help in understanding the impact of AF on mortality, quality of life, healthcare utilization, and costs. Once the individual registry analyses are completed, a dedicated analytic center will utilize informatics tools to merge individual analyses into an aggregated result.
Ensuring effective data harmonization and quality across the partner registries will require significant and ongoing effort. Participating registries in INTERAF will have differences in data elements and enrollment criteria (Table 1), which will require coordination in order to allow for inter‐registry comparisons. For example, the Chinese Atrial Fibrillation Registry (CAFR) is largely an AF ablation registry, the European EurObservational Research Programme (EORP) AF registry enrolls all ambulatory AF patients seen by European cardiologists, and the US Practice Innovation and Clinical Excellence (PINNACLE) registry enrolls all AF patients seen in participating cardiovascular practices. As such, an initial task of the INTERAF consortium is to harmonize the data elements between these registries.
Finally, the success of INTERAF will hinge on effective leadership to organize and accommodate the variety of research, organizational, and cultural needs anticipated in a global partnership. In addition, effective ethical and regulatory oversight will be critical, especially in light of the different countries, healthcare systems, and research standards expected to participate in INTERAF. For example, institutional review board and data privacy standards for each participating registry will be obtained and unified across participants to ensure that appropriate patient protection and privacy occurs.
Once these analytic and organizational priorities are established, then inter‐registries research into AF characteristics, treatments, and outcomes can begin. Table 2 lists the following areas that the INTERAF partners have identified as priorities for inter‐registry research. These priority areas for research were generated from INTERAF partner meetings, after discussion on important areas of focus regarding global AF care.
Table 2.
INTERAF Research Priorities
| 1. | Global and regional comparisons of overall age, sex, and racial/ethnic characteristics of AF populations |
| 2. | Global and regional comparisons of AF management, including both pharmacologic and nonpharmacologic approaches to heart rate and rhythm control |
| 3. | Global and regional comparisons of pharmacologic and nonpharmacologic approaches to thromboembolism prevention |
| 4. | Global and regional determinants of antithrombotic therapy and effective anticoagulation control |
| 5. | Global and regional outcomes associated with AF management practices |
| 6. | Comparison and predictors of embolic and bleeding events associated with AF and its management |
| 7. | Time trends in use of various treatment strategies in different healthcare systems |
| 8. | Impact of local health systems on AF care |
| 9. | Resource utilization for AF care |
| 10. | Patterns of AF care as a function of national and international guideline recommendations |
| 11. | Comparison of “real world” AF populations to those studied in AF clinical trials |
| 12. | Global and regional predictors of high‐quality AF management |
| 13. | Quality improvement initiatives for AF care and outcomes, with a focus on those elements that can be successfully translated across countries and geographic regions |
AF indicates atrial fibrillation; INTERAF, International Collaborative Partnership for the Study of Atrial Fibrillation.
Patient Descriptives
The patient characteristics of the initial AF registries participating in the INTERAF partnership are listed in Table 3 and include all patient information collected through the end of 2015. Of the 8 initial registries participating in the partnership, 7 had begun collecting data by the end of 2015 and the eighth, the Asia Pacific Heart Rhythm Society registry, will begin doing so in 2016. The largest patient cohort is the US‐based NCDR registry, followed by the Chinese AF registry. In general, the majority of patients in all registries were older than 65 years, with the exception of the Chinese registry, which is split between those older and younger than 65. The registries were also generally balanced in patient sex, with the exception of the majority male (72%) KiCS registry in Japan. Given the preponderance of registries in the United States, northern Europe, and Asia, the majority of registry participants were either white or Asian race. Hypertension was a common comorbidity in all registries. Coronary artery disease was much more common in the US PINNACLE registry population, while HF dominated the European and Balkan registries.
Table 3.
INTERAF Patient Characteristics by Participating Registry
| Patient Characteristics | United States NCDR PINNACLE AF Registry | European Society of Cardiology EURObservational Research Program (EORP) AF General Registry | Chinese AF Registry (CAFR) | Brazil PINNACLE AF Registry | Japanese Fushimi AF Registry | Japanese KiCS AF Registry | Balkan‐AF Registry | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N=969 502 | N=3119 | N=17 467 | N=26 | N=4426 | N=1284 | N=2712 | ||||||||
| Number | Percentage | Number | Percentage | Number | Percentage | Number | Percentage | Number | Percentage | Number | Percentage | Number | Percentage | |
| Demographics | ||||||||||||||
| Age, y | ||||||||||||||
| <65 | 240 778 | 24.8 | 1029 | 33.0 | 8678 | 49.7 | 6 | 23.7 | 779 | 17.6 | 539 | 42.0 | 883 | 32.6 |
| 65 to 74 | 313 479 | 32.3 | 1039 | 33.3 | 5077 | 29.1 | 10 | 38.5 | 1337 | 30.2 | 426 | 33.2 | 882 | 32.5 |
| >75 | 502 917 | 51.9 | 1051 | 33.7 | 3712 | 21.3 | 10 | 38.5 | 2310 | 52.2 | 318 | 24.8 | 947 | 34.9 |
| Male | 547 100 | 56.4 | 1859 | 59.6 | 10 716 | 61.4 | 15 | 57.7 | 2607 | 58.9 | 926 | 72.1 | 1485 | 55.5 |
| Race/ethnicity | ||||||||||||||
| White | 657 162 | 67.8 | NA | 0 | 0.0 | 18 | 69.2 | 0 | 0.0 | 0 | 0.0 | 2494 | 92.0 | |
| Black/African | 32 091 | 3.3 | NA | 0 | 0.0 | 3 | 11.5 | 0 | 0.0 | 0 | 0.0 | 218 | 8.0 | |
| American Indian/Alaska Native | 4591 | 0.5 | NA | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | NA | ||
| Asian | 8135 | 0.8 | NA | 17 467 | 100.0 | 0 | 0.0 | 4426 | 100.0 | 1284 | 100.0 | NA | ||
| Native Hawaiian/Pacific Islander | 2055 | 0.2 | NA | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | NA | ||
| Hispanic/Latino ethnicity | 22 774 | 2.4 | NA | 0 | 0.0 | 5 | 19.2 | 0 | 0.0 | 0 | 0.0 | NA | ||
| South Asian (India/Pakistan/Bangladesh) | NA | NA | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | NA | |||
| AF characteristics | ||||||||||||||
| AF type | ||||||||||||||
| Nonvalvular | 961 593 | 99.2 | 1138 | 36.5 | 5766 | 96.4 | 18 | 69.2 | 4218 | 95.3 | 1278 | 99.5 | 2712 | 100.0 |
| Valvular | 7909 | 0.8 | 1981 | 63.5 | 216 | 3.6 | 5 | 19.2 | 208 | 4.7 | 4 | 0.3 | 0 | 0.0 |
| AF duration | ||||||||||||||
| First detected | 47 383 | 4.89 | 945 | 30.3 | 1270 | 7.3 | 1 | 3.8 | NA | 68 | 5.3 | 632 | 23.3 | |
| Paroxysmal | 246 202 | 25.4 | 827 | 26.5 | 9768 | 56.3 | 6 | 23.1 | 1859 | 42.0 | 663 | 51.6 | 556 | 20.5 |
| Persistent | 48 084 | 5.0 | 811 | 26.0 | 6302 | 36.3 | 1 | 3.8 | 420 | 9.5 | 354 | 27.6 | 383 | 14.1 |
| Permanent | NA | 540 | 17.3 | NA | 14 | 53.8 | 2147 | 48.5 | 186 | 14.5 | 1088 | 40.1 | ||
| Unknown | 627 833 | 64.8 | NA | NA | 4 | 15.4 | NA | 13 | 1.0 | 53 | 2.0 | |||
| AF treatment | ||||||||||||||
| Rhythm control | ||||||||||||||
| Antiarrhythmic drug | 366 832 | 37.8 | 1123 | 36.0 | 5854 | 41.3 | 4 | 15.4 | 854 | 19.3 | 728a | 56.7 | 889 | 32.8 |
| Ablation | 40 912 | 4.2 | 237 | 7.6 | 7162 | 41.6 | 0 | 0.0 | 266 | 6.0 | 95 | 3.5 | ||
| Rate control | 849 645 | 87.6 | 1213 | 38.9 | 8202 | 7.8 | 10 | 38.5 | 1987 | 44.9 | 556 | 43.3 | 1622 | 59.8 |
| Stroke risk and prevention | ||||||||||||||
| CHADS2 score, median (IQR) | 2.0 (1.0–3.0) | 2.0 (1.0–3.0) | 2.0 (2.0–3.0) | 3.0 (2.0–3.0) | 2.0 (1.0–3.0) | 1.0 (0.0–2.0) | 2.0 (1.0–3.0) | |||||||
| CHA2DS2‐VASc score, median (IQR) | 4.0 (3.5–4.5) | 3.0 (2.0–4.0) | 4.0 (2.0–3.0) | 3.0 (2.0–3.0) | 3.0 (2.0–5.0) | 3.0 (2.0–4.0) | 3.0 (2.0–5.0) | |||||||
| OAC among those with either CHADSs or CHA2S2‐VASc score >1 | ||||||||||||||
| Warfarin | 398 816 | 41.1 | 2233 | 71.6 | 5979 | 54.5 | 5 | 19.2 | 1859 | 43.8 | 220 | 17.1 | 461 | 20.1 |
| Acenocoumarol | NA | NA | NA | NA | NA | NA | 964 | 42.0 | ||||||
| Phenprocoumon | NA | NA | NA | 0 | 0.0 | NA | NA | 1 | 0.0 | |||||
| Dabigatran | 81 556 | 8.4 | 212 | 6.8 | 200 | 93.0 | 6 | 23.1 | 152 | 3.6 | 161 | 12.5 | 142 | 6.2 |
| Rivaroxaban | 111 653 | 11.5 | 50 | 1.6 | 6 | 2.8 | 5 | 19.2 | 137 | 3.2 | 266 | 20.7 | 95 | 4.1 |
| Apixaban | 79 742 | 8.2 | 0 | 0.0 | NA | 2 | 7.7 | 130 | 3.1 | 256 | 19.9 | 43 | 1.9 | |
| Edoxaban | 2 349 | 0.24 | NA | NA | 0 | 0.0 | 31 | 0.7 | NA | 0 | 0.0 | |||
| Other | NA | NA | NA | NA | NA | 6 | 0.5 | NA | ||||||
| Other medical conditions | ||||||||||||||
| Coronary artery disease | 479 031 | 49.4 | 1135 | 36.4 | 2647 | 15.2 | 3 | 11.5 | 646 | 14.6 | 101 | 7.9 | 821 | 30.3 |
| Diabetes mellitus | 245 461 | 25.3 | 643 | 20.6 | 3233 | 18.7 | 4 | 15.4 | 1005 | 22.7 | 200 | 15.6 | 668 | 24.6 |
| Hypertension | 770 352 | 79.5 | 2211 | 70.9 | 10 011 | 57.8 | 20 | 76.9 | 2762 | 62.4 | 713 | 55.5 | 2121 | 78.2 |
| Current smoker | 182 998 | 18.9 | 352 | 11.3 | 2075 | 12.0 | 2 | 7.7 | 391 | 8.8 | 217 | 16.9 | 340 | 12.5 |
| Peripheral arterial disease | 102 279 | 10.6 | 349 | 11.2 | 107 | 1.6 | 2 | 7.7 | 186 | 4.2 | 42 | 3.3 | 122 | 4.5 |
| Prior TIA/stroke | 146 132 | 15.1 | 324 | 10.4 | 2509 | 14.5 | 1 | 3.8 | 823 | 18.6 | 103 | 8.0 | 364 | 13.5 |
| Congestive heart failure | 276 642 | 28.5 | 1482 | 47.5 | 1397 | 8.1 | 7 | 26.9 | 1235 | 27.9 | 193 | 15.0 | 1161 | 42.8 |
| Chronic kidney disease | ||||||||||||||
| Stage IIIa (GFR 45–59) | 1117 | 0.1 | NA | 382 | 2.7 | NA | 1226 | 27.7 | 315 | 24.5 | NA | |||
| Stage IIIb (GFR 30–44) | 1222 | 0.1 | NA | 146 | 1.0 | NA | 606 | 13.7 | 169 | 13.2 | NA | |||
| Stage IV (GFR 15–29) | 755 | 0.1 | NA | 60 | 0.4 | NA | 230 | 5.2 | 42 | 3.3 | NA | |||
| Stage V (GFR <15) or HD | 297 | 0.0 | NA | 32 | 0.2 | NA | 124 | 2.8 | 13 | 1.0 | NA | |||
The Brazil PINNACLE registry began enrolling patients in late 2015; The Asia Pacific HRS registry will begin enrolling patients in 2016. AF indicates atrial fibrillation; GFR, glomerular filtration rate; HD, hemodialysis; INTERAF, International Collaborative Partnership for the Study of Atrial Fibrillation; IQR, interquartile range; NA, not available/applicable; NDCR, National Cardiovascular Data Registry; PINNACLE, Practice Innovation and Clinical Excellence; OAC, oral anticoagulant; TIA, transient ischemic attack.
Includes both antiarrhythmic drug and AF ablation.
The majority of patients in the registries had nonvalvular AF, with a mixture of paroxysmal and permanent AF. AF treatment consisted of a mixture of rate and rhythm control. Thromboembolism risk among registry participants was relatively high, with median CHADS2 and CHA2DS2‐VASc scores indicating a moderate‐to‐high risk for stroke. Strategies to reduce this risk were variable, with warfarin use more common in Europe and the Balkan countries, and DOACs more common in the Japanese KiCS and Brazilian PINNACLE registries.
INTERAF Differences From Previous International Registries
Previously developed AF registries have mostly been derived from single nation databases, with varied areas of focus including thromboprophylaxis, rhythm control, and procedural therapies such as ablation and left atrial appendage closure.5 For example, the Euro Heart Survey involved 35 countries and 3890 patients between 2003 and 2004 to evaluate management of oral anticoagulation in AF patients against European guidelines, but only involved European countries.38 Two of the largest multinational registries have aimed to enroll >55 000 patients each, and include the Global Anticoagulant Registry in the Field (GARFIELD) registry evaluating 50 countries worldwide,39 and the Global Registry on Long‐Term Oral Antithrombotic Treatment in Patients with Atrial Fibrillation (GLORIA‐AF) registry involving 62 centers globally.40 The focus of both of these registries has mainly been thromboprophylaxis prescription in AF. The Real‐life global survey evaluating patients with atrial fibrillation (REALISE‐AF) enrolled >10 000 patients in 26 countries between 2009 and 2010,41 and the Registry on Cardiac Rhythm Disorders (RECORDAF) evaluated >5000 patients from 21 countries worldwide between 2007 and 2008.42 Both REALISE‐AF and RECORDAF were 1‐year studies that reported on rhythm‐control therapies in AF patients specifically. The AF ablation Long‐term Registry of EURObservational Research Programme has been enrolling patients undergoing AF ablation in 54 countries since 2010 for long‐term outcomes after ablation.43 The registry's main focus is procedural outcomes after AF ablation.
In contrast to these other registries, INTERAF will provide insight into international AF care across a truly global spectrum of countries, analyzing a wide variety of treatment options, with the potential to adapt to temporal changes in treatment strategies. The global spread of countries represented by INTERAF is broad, with large representation in the United States, South America, Europe, and Asia. While the GARFIELD, GLORIA‐AF, and REALISE‐AF registries had a similar multinational representation, INTERAF has the advantage of larger numbers of AF patients from North America and Asia, providing power for some of the largest global analyses performed regarding treatment of AF patients. As a contemporary global registry that will continue to collect data longitudinally, INTERAF will be able to capture all types of oral anticoagulation strategies (warfarin versus DOACs), and rhythm‐control strategies (antiarrhythmic drug medications and ablation) to allow for a broad spectrum of treatment analyses; most other global registries have been devised to focus only on a specific treatment protocol. Another advantage of INTERAF compared to other global registries will be planned ongoing shared data collection and analyses, which will not be limited by a specific end date, to allow for evolution of the global registry, including the countries that participate in sharing data. By using a distributive analytic method to allow for data collection and analyses, and expanding information collected to several different aspects of AF care, INTERAF would be poised to provide longitudinal information on practice patterns, and track changes over time as a comprehensive global AF registry.
Limitations
The proposed project should be viewed in light of the following limitations. First, each of the multinational registries included in INTERAF have previously identified important covariates to prospectively collect, which may not be completely duplicative. However, we specifically have included and will analyze universally important characteristics and comorbidities that have a common interest related to AF. Although this may seem to produce a challenge with data organization, we have specifically chosen a distributed analytic method by which local analyses may be conducted by each participating registry, and then analyzed across all partner registries. Second, differences in data element definition or inclusion criteria for each country's specific registry may lead to heterogeneity in the overall patient population studied. For example, the percentage of valvular versus nonvalvular as well as paroxysmal versus persistent AF differed across each specific registry, and AF patients from specific countries appeared to have more frequent comorbidities, potentially due to differences in data element definitions. Finally, each registry within INTERAF will have differences in the type of outcomes collected, as well as frequency and timeframe of patient follow‐up. While this may limit the types of specific treatment strategies able to be compared, INTERAF will focus on those outcomes and treatment strategies that have universal availability of data across specific registries to allow for analytic integrity.
Future Directions
The initial organization and goals of INTERAF, as outlined above, are only the beginning of efforts to better characterize and improve global AF care. Future directions for the initiative will include continued data harmonization across country partners and improvements in data collection. Moreover, attempts to validate country‐specific observations, risk stratification schemes, and outcomes may be attempted, in order to identify generalizability of previously established research findings to a multinational AF population, as has been previously performed examining acute kidney injury, bleeding, and mortality in Japanese patients undergoing percutaneous coronary intervention.44, 45 Another important effort will involve INTERAF expansion to include a wide variety of clinical registries and participating countries, with special emphasis on registries in the developing world, where little information about AF care and outcomes currently exists. Efforts to expand the partnership are under way, and we anticipate partners from areas such as the Middle East, Africa, Central America, and Australia.
Conclusions
We present the INTERAF, a worldwide collaboration for observational study of AF. This project can provide a global platform for investigators and countries to study AF populations, treatments, and outcomes and support country‐specific and international efforts to optimize global care for the large and growing population of AF patients. As insights from the consortium accumulate, INTERAF will develop mechanisms to understand regional variation in practice for AF management, with a particular focus on understanding those practices, which can be shared globally.
Disclosures
None of the authors have material conflicts of interest. All of the authors participated in either the drafting or critical review of the manuscript. The International Collaborative Partnership for the Study of Atrial Fibrillation project is supported by an unrestricted award from Bristol‐Myers Squibb and Pfizer, Inc. to the American College of Cardiology. The funders have no role in project governance, management, or research activities.
J Am Heart Assoc. 2016;5:e004037 doi: 10.1161/JAHA.116.004037.
References
- 1. Rahman F, Kwan GF, Benjamin EJ. Global epidemiology of atrial fibrillation. Nat Rev Cardiol. 2014;11:639–654. [DOI] [PubMed] [Google Scholar]
- 2. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary. J Am Coll Cardiol. 2014;64:2246–2280. [DOI] [PubMed] [Google Scholar]
- 3. Oldgren J, Healey JS, Ezekowitz M, Commerford P, Avezum A, Pais P, Zhu J, Jansky P, Sigamani A, Morillo CA, Liu L, Damasceno A, Grinvalds A, Nakamya J, Reilly PA, Keltai K, Van Gelder IC, Yusufali AH, Watanabe E, Wallentin L, Connolly SJ, Yusuf S; on behalf of the RELYAFRI . Variations in cause and management of atrial fibrillation in a prospective registry of 15 400 emergency department patients in 46 countries: the RE‐LY Atrial Fibrillation Registry. Circulation. 2014;129:1568–1576. [DOI] [PubMed] [Google Scholar]
- 4. Bufalino VJ, Masoudi FA, Stranne SK, Horton K, Albert NM, Beam C, Bonow RO, Davenport RL, Girgus M, Fonarow GC, Krumholz HM, Legnini MW, Lewis WR, Nichol G, Peterson ED, Rosamond W, Rumsfeld JS, Schwamm LH, Shahian DM, Spertus JA, Woodard PK, Yancy CW; on behalf of the American Heart Association Advocacy Coordinating C . The American Heart Association's recommendations for expanding the applications of existing and future clinical registries: a policy statement from the American Heart Association. Circulation. 2011;123:2167–2179. [DOI] [PubMed] [Google Scholar]
- 5. Lip GYH, Al‐Khatib SM, Cosio FG, Banerjee A, Savelieva I, Ruskin J, Blendea D, Nattel S, De Bono J, Conroy JM, Hess PL, Guasch E, Halperin JL, Kirchhof P, Cosio MD, Camm AJ. Contemporary management of atrial fibrillation: what can clinical registries tell us about stroke prevention and current therapeutic approaches? J Am Heart Assoc. 2014;3:e001179 doi: 10.1161/JAHA.114.001179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Smith MD; Institute of Medicine . Committee on the Learning Health Care System in A. Best Care at Lower Cost the Path to Continuously Learning Health Care in America. Washington, DC: National Academies Press; 2012. [PubMed] [Google Scholar]
- 7. Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, Gillum RF, Kim YH, McAnulty JH, Zheng ZJ, Forouzanfar MH, Naghavi M, Mensah GA, Ezzati M, Murray CJL. Worldwide epidemiology of atrial fibrillation: a global burden of disease 2010 study. Circulation. 2014;129:837–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–952. [DOI] [PubMed] [Google Scholar]
- 9. Murray CJL, Ezzati M, Flaxman AD, Lim S, Lozano R, Michaud C, Naghavi M, Salomon JA, Shibuya K, Vos T, Wikler D, Lopez AD. GBD 2010: design, definitions, and metrics. Lancet. 2012;380:2063–2066. [DOI] [PubMed] [Google Scholar]
- 10. Krahn AD, Manfreda J, Tate RB, Mathewson FA, Cuddy TE. The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba Follow‐Up Study. Am J Med. 1995;98:476–484. [DOI] [PubMed] [Google Scholar]
- 11. Udompanich S, Lip GYH, Apostolakis S, Lane DA. Atrial fibrillation as a risk factor for cognitive impairment: a semi‐systematic review. QJM. 2013;106:795–802. [DOI] [PubMed] [Google Scholar]
- 12. Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna W, Seward JB, Iwasaka T, Tsang TSM. Incidence and mortality risk of congestive heart failure in atrial fibrillation patients: a community‐based study over two decades. Eur Heart J. 2006;27:936–941. [DOI] [PubMed] [Google Scholar]
- 13. Damasceno A, Mayosi BM, Sani M, Ogah OS, Mondo C, Ojji D, Dzudie A, Kouam CK, Suliman A, Schrueder N, Yonga G, Ba SA, Maru F, Alemayehu B, Edwards C, Davison BA, Cotter G, Sliwa K. The causes, treatment, and outcome of acute heart failure in 1006 Africans from 9 countries. Arch Intern Med. 2012;172:1386–1394. [DOI] [PubMed] [Google Scholar]
- 14. Kawabata‐Yoshihara LA, Scazufca M, Santos Ide S, Whitaker A, Kawabata VS, Benseñor IM, Menezes PR, Lotufo PA. Atrial fibrillation and dementia: results from the Sao Paulo ageing & health study. Arq Bras Cardiol. 2012;99:1108–1114. [DOI] [PubMed] [Google Scholar]
- 15. Bansal N, Fan D, Hsu Cy, Ordonez JD, Marcus GM, Go AS. Incident atrial fibrillation and risk of end‐stage renal disease in adults with chronic kidney disease. Circulation. 2013;127:569–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Uchiyama S, Shibata Y, Hirabayashi T, Mihara B, Hamashige N, Kitagawa K, Goto S, Origasa H, Shimada K, Kobayashi H, Isozaki M, Ikeda Y. Risk factor profiles of stroke, myocardial infarction, and atrial fibrillation: a Japanese Multicenter Cooperative Registry. J Stroke Cerebrovasc Dis. 2010;19:190–197. [DOI] [PubMed] [Google Scholar]
- 17. Goto S, Bhatt DL, Röther J, Alberts M, Hill MD, Ikeda Y, Uchiyama S, D'Agostino R, Ohman EM, Liau C‐S, Hirsch AT, Mas J‐L, Wilson PWF, Corbalán R, Aichner F, Steg PG. Prevalence, clinical profile, and cardiovascular outcomes of atrial fibrillation patients with atherothrombosis. Am Heart J. 2008;156:855–863.e852. [DOI] [PubMed] [Google Scholar]
- 18. Liu Y, Liu H, Dong L, Chen J, Guo J. Prevalence of atrial fibrillation in hospitalized patients over 40 years old: ten‐year data from the People's Hospital of Peking University. Acta Cardiol. 2010;65:221–224. [DOI] [PubMed] [Google Scholar]
- 19. Soliman EZ, Safford MM, Muntner P, Khodneva Y, Dawood FZ, Zakai NA, Thacker EL, Judd S, Howard VJ, Howard G, Herrington DM, Cushman M. Atrial fibrillation and the risk of myocardial infarction. JAMA Intern Med. 2014;174:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Watanabe H, Watanabe T, Sasaki S, Nagai K, Roden DM, Aizawa Y. Close bidirectional relationship between chronic kidney disease and atrial fibrillation: the Niigata preventive medicine study. Am Heart J. 2009;158:629–636. [DOI] [PubMed] [Google Scholar]
- 21. Nieuwlaat R, Capucci A, Camm AJ, Olsson SB, Andresen D, Davies DW, Cobbe S, Breithardt G, Le Heuzey J‐Y, Prins MH, Lévy S, Crijns HJGM; European Heart Survey I . Atrial fibrillation management: a prospective survey in ESC member countries: the Euro Heart Survey on Atrial Fibrillation. Eur Heart J. 2005;26:2422–2434. [DOI] [PubMed] [Google Scholar]
- 22. Nabauer M, Gerth A, Limbourg T, Schneider S, Oeff M, Kirchhof P, Goette A, Lewalter T, Ravens U, Meinertz T, Breithardt G, Steinbeck G. The registry of the German competence network on atrial fibrillation: patient characteristics and initial management. Europace. 2008;11:423–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang S. Atrial fibrillation in mainland China: epidemiology and current management. Heart. 2009;95:1052–1055. [DOI] [PubMed] [Google Scholar]
- 24. Hu D, Sun Y. Epidemiology, risk factors for stroke, and management of atrial fibrillation in China. J Am Coll Cardiol. 2008;52:865–868. [DOI] [PubMed] [Google Scholar]
- 25. Chen Y‐H, Chen H, Wu Y, Hu D. Cardiac electrophysiology in China. Heart Rhythm. 2007;4:862. [DOI] [PubMed] [Google Scholar]
- 26. Dewhurst MJ, Adams PC, Gray WK, Dewhurst F, Orega GP, Chaote P, Walker RW. Strikingly low prevalence of atrial fibrillation in elderly Tanzanians. J Am Geriatr Soc. 2012;60:1135–1140. [DOI] [PubMed] [Google Scholar]
- 27. Shavadia J, Yonga G, Mwanzi S, Jinah A, Moriasi A, Otieno H. Clinical characteristics and outcomes of atrial fibrillation and flutter at the Aga Khan University Hospital, Nairobi: cardiovascular topics. Cardiovasc J Afr. 2013;24:6–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kakkar AK, Mueller I, Bassand J‐P, Fitzmaurice DA, Goldhaber SZ, Goto S, Haas S, Hacke W, Lip GYH, Mantovani LG, Turpie AGG, van Eickels M, Misselwitz F, Rushton‐Smith S, Kayani G, Wilkinson P, Verheugt FWA; for the GRI . Risk profiles and antithrombotic treatment of patients newly diagnosed with atrial fibrillation at risk of stroke: perspectives from the international, observational, prospective GARFIELD registry. PLoS One. 2013;8:e63479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hori M, Connolly SJ, Zhu J, Liu LS, Lau CP, Pais P, Xavier D, Kim SS, Omar R, Dans AL, Tan RS, Chen JH, Tanomsup S, Watanabe M, Koyanagi M, Ezekowitz MD, Reilly PA, Wallentin L, Yusuf S; the RELYI . Dabigatran versus warfarin: effects on ischemic and hemorrhagic strokes and bleeding in Asians and non‐Asians with atrial fibrillation. Stroke. 2013;44:1891–1896. [DOI] [PubMed] [Google Scholar]
- 30. Atarashi H, Inoue H, Okumura K, Yamashita T, Kumagai N, Origasa H; Investigators JRR . Present status of anticoagulation treatment in Japanese patients with atrial fibrillation: a report from the J‐RHYTHM Registry. Circ J. 2011;75:1328–1333. [DOI] [PubMed] [Google Scholar]
- 31. Suzuki S, Yamashita T, Kato T, Fujino T, Sagara K, Sawada H, Aizawa T, Fu L‐T. Incidence of major bleeding complication of warfarin therapy in Japanese patients with atrial fibrillation. Circ J. 2007;71:761–765. [DOI] [PubMed] [Google Scholar]
- 32. Shen AY‐J, Yao JF, Brar SS, Jorgensen MB, Chen W. Racial/ethnic differences in the risk of intracranial hemorrhage among patients with atrial fibrillation. J Am Coll Cardiol. 2007;50:309–315. [DOI] [PubMed] [Google Scholar]
- 33. Pollack CV, Reilly PA, Eikelboom J, Glund S, Verhamme P, Bernstein RA, Dubiel R, Huisman MV, Hylek EM, Kamphuisen PW, Kreuzer J, Jr , Levy JH, Sellke FW, Stangier J, Steiner T, Wang B, Kam C‐W, Weitz JI. Idarucizumab for dabigatran reversal. N Engl J Med. 2015;373:511–520. [DOI] [PubMed] [Google Scholar]
- 34. Holmes DR, Reddy VY, Turi ZG, Doshi SK, Sievert H, Buchbinder M, Mullin CM, Sick P; Investigators PA . Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non‐inferiority trial. Lancet. 2009;374:534–542. [DOI] [PubMed] [Google Scholar]
- 35. Masoudi FA, Calkins H, Kavinsky CJ, Drozda JP, Gainsley P, Slotwiner DJ, Turi ZG. 2015 ACC/HRS/SCAI left atrial appendage occlusion device societal overview. J Am Coll Cardiol. 2015;66:1497–1513. [DOI] [PubMed] [Google Scholar]
- 36. Fleurence RL, Curtis LH, Califf RM, Platt R, Selby JV, Brown JS. Launching PCORnet, a national patient‐centered clinical research network. J Am Med Inform Assoc. 2014;21:578–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Go AS, Magid DJ, Wells B, Sung SH, Cassidy‐Bushrow AE, Greenlee RT, Langer RD, Lieu TA, Margolis KL, Masoudi FA, McNeal CJ, Murata GH, Newton KM, Novotny R, Reynolds K, Roblin DW, Smith DH, Vupputuri S, White RE, Olson J, Rumsfeld JS, Gurwitz JH. The cardiovascular research network: a new paradigm for cardiovascular quality and outcomes research. Circ Cardiovasc Qual Outcomes. 2008;1:138–147. [DOI] [PubMed] [Google Scholar]
- 38. Nieuwlaat R, Capucci A, Lip GY, Olsson SB, Prins MH, Nieman FH, Lopez‐Sendon J, Vardas PE, Aliot E, Santini M, Crijns HJ. Antithrombotic treatment in real‐life atrial fibrillation patients: a report from the Euro Heart Survey on Atrial Fibrillation. Eur Heart J. 2006;27:3018–3026. [DOI] [PubMed] [Google Scholar]
- 39. Kakkar AK, Mueller I, Bassand JP, Fitzmaurice DA, Goldhaber SZ, Goto S, Haas S, Hacke W, Lip GY, Mantovani LG, Verheugt FW, Jamal W, Misselwitz F, Rushton‐Smith S, Turpie AG. International longitudinal registry of patients with atrial fibrillation at risk of stroke: Global Anticoagulant Registry in the FIELD (GARFIELD). Am Heart J. 2012;163:13–19.e11. [DOI] [PubMed] [Google Scholar]
- 40. Huisman MV, Lip GY, Diener HC, Dubner SJ, Halperin JL, Ma CS, Rothman KJ, Teutsch C, Zint K, Ackermann D, Clemens A, Bartels DB. Design and rationale of global registry on long‐term oral antithrombotic treatment in patients with atrial fibrillation: a global registry program on long‐term oral antithrombotic treatment in patients with atrial fibrillation. Am Heart J. 2014;167:329–334. [DOI] [PubMed] [Google Scholar]
- 41. Alam M, Bandeali SJ, Shahzad SA, Lakkis N. Real‐life global survey evaluating patients with atrial fibrillation (REALISE‐AF): results of an international observational registry. Expert Rev Cardiovasc Ther. 2012;10:283–291. [DOI] [PubMed] [Google Scholar]
- 42. Camm AJ, Breithardt G, Crijns H, Dorian P, Kowey P, Le Heuzey JY, Merioua I, Pedrazzini L, Prystowsky EN, Schwartz PJ, Torp‐Pedersen C, Weintraub W. Real‐life observations of clinical outcomes with rhythm‐ and rate‐control therapies for atrial fibrillation RECORDAF (registry on cardiac rhythm disorders assessing the control of atrial fibrillation). J Am Coll Cardiol. 2011;58:493–501. [DOI] [PubMed] [Google Scholar]
- 43. Lip GY, Laroche C, Dan GA, Santini M, Kalarus Z, Rasmussen LH, Ioachim PM, Tica O, Boriani G, Cimaglia P, Diemberger I, Hellum CF, Mortensen B, Maggioni AP. ‘Real‐world’ antithrombotic treatment in atrial fibrillation: the EORP‐AF pilot survey. Am J Med. 2014;127:519–529.e511. [DOI] [PubMed] [Google Scholar]
- 44. Inohara T, Kohsaka S, Miyata H, Ueda I, Maekawa Y, Fukuda K, Cohen DJ, Kennedy KF, Rumsfeld JS, Spertus JA. Performance and validation of the U.S. NCDR acute kidney injury prediction model in Japan. J Am Coll Cardiol. 2016;67:1715–1722. [DOI] [PubMed] [Google Scholar]
- 45. Kohsaka S, Miyata H, Ueda I, Masoudi FA, Peterson ED, Maekawa Y, Kawamura A, Fukuda K, Roe MT, Rumsfeld JS. An international comparison of patients undergoing percutaneous coronary intervention: a collaborative study of the National Cardiovascular Data Registry (NCDR) and Japan Cardiovascular Database‐Keio Interhospital Cardiovascular Studies (JCD‐KICS). Am Heart J. 2015;170:1077–1085. [DOI] [PubMed] [Google Scholar]


