Abstract
Background
Coronary vasospasm is an important pathogenesis of acute coronary syndrome (ACS). However, the clinical features and prognosis of vasospastic angina (VA) patients presenting with ACS (VAACS) are still unclear. We aimed to evaluate the clinical characteristics and long‐term outcomes of VAACS patients without significant coronary artery stenosis.
Methods and Results
A total of 986 VA patients confirmed by ergonovine provocation test were analyzed. VAACS was defined as VA patients visiting the emergency room with documented electrocardiographic changes, significant arrhythmias, or elevated cardiac biomarkers. VA patients with elevated cardiac biomarkers were further considered to have myocardial infarction (MI; VAMI). During 4.4 years of median follow‐up, we investigated major adverse cardiac events including cardiac death, MI, revascularization, and rehospitalization because of recurrent angina. The VAACS group consisted of 149 patients (15.1%), and VAMI occurred in 81 patients (8.2%). VAACS patients were younger and had a higher prevalence of diabetes mellitus, MI history, and higher levels of inflammatory markers compared with non‐VAACS patients. In multivariable Cox regression analyses, VAACS patients were associated with an increased risk of major adverse cardiac events (hazard ratio, 1.65; 95% CI 1.14–2.37; P=0.007) and recurrent MI hazard ratio, 2.57; 95% CI, 1.35–4.87; P=0.004). In addition, VAMI patients had an increased risk of major adverse cardiac events (hazard ratio, 1.75; 95% CI, 1.11–2.76; P=0.016) and recurrent MI (hazard ratio, 2.43; 95% CI, 1.09–5.40; P=0.03).
Conclusion
VAACS patients showed worse clinical outcomes, driven mainly by recurrent MI. Therefore, intensive medical treatment might be required in VAACS patients.
Keywords: acute coronary syndrome, myocardial infarction, variant angina, vasospasm
Subject Categories: Coronary Artery Disease
Introduction
Coronary vasospastic angina (VA) is relatively common in East Asia.1 Transient myocardial ischemia caused by epicardial coronary artery spasm is the main pathogenesis of VA2; however, initial manifestations are associated with a wide variety of clinical settings, including stable angina, acute coronary syndrome (ACS), life‐threatening arrhythmia, and sudden cardiac death.3 Although VA patients responding to calcium channel blockers have a good prognosis, their clinical risk varies.4 The Japanese Coronary Spasm Association risk score system analyzes VA severity based on clinical presentations including out‐of‐hospital cardiac arrest, angina at rest alone, and ST‐segment elevation.4 However, there are no data on the clinical manifestations of acute myocardial ischemia. Therefore, we distinguished VA patients presenting with ACS (VAACS) who demonstrated documented electrocardiographic changes (ST‐segment elevation, ST‐segment depression, or T‐wave inversion in at least 2 contiguous leads), significant arrhythmia (ventricular tachycardia/fibrillation, sinus pause/arrest, junctional bradycardia, or second‐ or third‐degree atrioventricular block), or elevation of cardiac biomarkers with at least 1 value above the 99th percentile upper‐reference limit. The aim of the present study was to evaluate the clinical characteristics and long‐term outcomes of VAACS patients without significant coronary artery stenosis.
Methods
Study Population
An intracoronary ergonovine provocation test was performed in 3595 patients from Samsung Medical Center between January 2003 and December 2014. A total of 2333 patients showed the negative result of intracoronary ergonovine provocation test, and we enrolled 1198 patients with a positive intracoronary ergonovine provocation test (Figure 1). We excluded 173 patients with coronary artery stenosis greater than 70% and 39 patients who refused follow‐up. Patients were divided into VAACS (n=149) and non‐VAACS (n=837) groups. The Samsung Medical Center Institutional Review Board approved this study and waived the requirement for written informed consent for access to an institutional registry.
Figure 1.
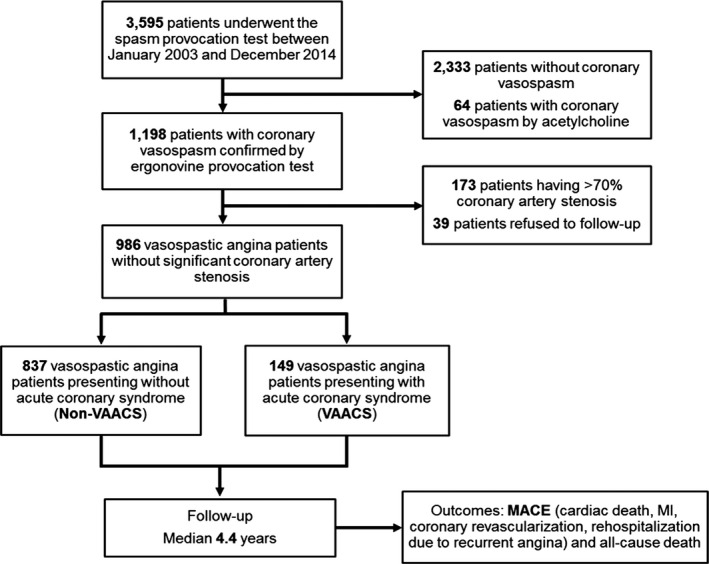
Study design and population. MACE indicates major adverse cardiac events; MI, myocardial infarction; VAACS, vasospastic angina patients presenting with acute coronary syndrome.
Coronary Angiography and Provocation Test
We retrospectively analyzed an observational registry of patients with VA based on the Guidelines for Diagnosis and Treatment of Patients with VA.5 An intracoronary ergonovine provocation test was performed according to the clinician's decision in patients who had suspicious symptoms and underwent coronary angiography. Baseline coronary angiography was performed for the right and left coronary arteries. Intracoronary ergonovine was infused for the provocation test. Incremental doses of 20, 40, and 80 μg were injected into the left coronary artery. If coronary spasm was not provoked in the left coronary artery, incremental doses of 10 and 20 μg were injected into the right coronary artery. Once spasm was provoked, intracoronary nitroglycerin was infused. Vasoactive drugs were stopped at least 48 hours before coronary angiography. We classified the definite and intermediate VA according to ergonovine provocation test and defined these as a positive test. Total or subtotal (>90% luminal diameter narrowing) occlusion accompanied by chest pain or discomfort and/or ECG changes or spontaneous total or subtotal spasm on baseline coronary angiography resolved by intracoronary nitroglycerin was defined as a positive result. Additionally, we defined intermediate constriction as 50% to 90% luminal narrowing with or without ischemic symptoms and/or ECG changes were also defined as a positive result. A negative result was <50% luminal narrowing without chest pain or discomfort or ECG change. An ECG change during the provocation test was defined as an ST‐segment elevation or depression in at least 2 contiguous leads. A multivessel spasm was defined as a positive spasm in more than 2 major (≥2.5 mm) epicardial coronary arteries.6 The types of spasm were classified as focal or diffuse. The focal type was defined as a discrete spasm localized in 1 coronary segment, whereas spasm observed continuously from the proximal to the distal segments was regarded as diffuse type.7, 8
Clinical Outcomes
The primary outcome was major adverse cardiac events (MACE), defined as a composite of cardiac death, myocardial infarction (MI), revascularization, and rehospitalization because of recurrent angina. All‐cause death and individual outcomes of MACE were assessed for important secondary outcomes. All deaths were considered cardiac unless a definite noncardiac cause could be established. MI was defined as detection of increase and/or decrease of cardiac biomarkers with at least 1 value above the 99th percentile upper‐reference limit and with at least 1 of the following: ischemia symptoms, new significant ST‐T changes, new left bundle branch block, development of pathological Q waves, imaging evidence of new loss of viable myocardium or regional wall motion abnormality, or identification of a thrombus by coronary angiography.9 Revascularization was defined as any revascularization of the epicardial coronary arteries by percutaneous coronary intervention or bypass graft surgery. Rehospitalization was defined as any hospitalization after index hospital discharge because of recurrent angina symptoms: typical chest pain, atypical chest pain, dyspnea, syncope or dizziness, palpitation, or aborted cardiac arrest.
Statistical Analysis
Categorical variables were expressed as percentage and were compared using χ2 test or Fisher's exact test. Continuous variables were presented as mean±SD and were compared using t test or Wilcoxon rank sum test. Survival curves were constructed using Kaplan–Meier estimates and were compared using the log‐rank test. Differences in adjusted risk were evaluated using multivariable Cox regression analysis. Proportional hazard assumptions were confirmed by Schoenfeld's test, and no relevant violation was found. The clinically relevant variables included in the multivariable models were age, sex, diabetes mellitus, hypertension, current smoker, dyslipidemia, and history of MI. Multivariable logistic regression analysis was performed to determine the clinical and angiographic factors associated with VAACS and vasospastic angina presenting with myocardial infarction (VAMI), starting with a model of all clinically relevant factors at the same time: age, sex, body mass index, typical morning angina, diabetes mellitus, hypertension, current smoker, history of MI, white blood cell (WBC) count, total cholesterol, creatinine, high‐sensitivity C‐reactive protein (Hs‐CRP), left ventricular ejection fraction, and left main or left anterior descending coronary artery spasm during provocation test. All P‐values are 2‐tailed, and P<0.05 was considered to be statistically significant. SPSS 20.0 (IBM, Armonk, NY) was used for statistical analyses.
Results
Clinical, Laboratory, and Angiographic Characteristics
A total of 986 VA patients were analyzed. Among them, 149 patients (15.1%) presented with VAACS (Tables 1 and 2). Compared with non‐VAACS patients, VAACS patients were younger, had a higher prevalence of diabetes mellitus, elevated WBC and Hs‐CRP levels, and an increased history of MI; however, they had a lower left ventricular ejection fraction. VAACS patients were more frequently treated with statins (57.0%) and angiotensin‐converting enzyme inhibitors or angiotensin II receptor blockers (25.5%). In angiographic characteristics, left main or left anterior descending spasm during the provocation test frequently occurred in VAACS patients.
Table 1.
Baseline Clinical Characteristic of VAACS and Non‐VAACS Patients
| Variable | Non‐VAACS (n=837) | VAACS (n=149) | P Value |
|---|---|---|---|
| Age, y | 56.8±9.2 | 54.5±9.7 | 0.01 |
| Male | 712 (85.1) | 127 (85.2) | 1.00 |
| BMI, kg/m2 | 24.4±2.7 | 24.1±3.1 | 0.23 |
| Typical morning angina | 618 (73.8) | 96 (64.4) | 0.022 |
| Medical history | |||
| Diabetes mellitus | 170 (20.3) | 60 (40.3) | <0.001 |
| Hypertension | 315 (37.6) | 68 (45.6) | 0.07 |
| Current smoking | 229 (27.4) | 50 (33.6) | 0.14 |
| Dyslipidemia | 182 (21.7) | 21 (14.1) | 0.04 |
| History of PCI | 64 (7.6) | 14 (9.4) | 0.51 |
| History of MI | 14 (1.7) | 18 (12.1) | <0.001 |
| History of stroke | 11 (1.3) | 4 (2.7) | 0.26 |
| Laboratory findings | |||
| Hemoglobin, g/dL | 14.5±1.4 | 14.2±1.8 | 0.042 |
| White blood cells, ×103/μL | 6.9±1.9 | 8.5±3.7 | <0.001 |
| Glucose, mg/dL | 114±34 | 134±53 | <0.001 |
| BUN, mg/dL | 15.0±4.2 | 14.9±5.1 | 0.80 |
| Creatinine, mg/dL | 0.92±0.18 | 0.87±0.22 | 0.004 |
| Total cholesterol, mg/dL | 173±36 | 161±36 | <0.001 |
| Triglycerides, mg/dL | 162±114 | 156±118 | 0.61 |
| HDL, mg/dL | 48±12 | 45±12 | 0.013 |
| LDL, mg/dL | 106±31 | 97±31 | 0.002 |
| Hs‐CRP, mg/dL | 0.31±1.18 | 1.07±3.17 | <0.001 |
| LVEF, % | 64.3±6.5 | 61.8±8.8 | 0.001 |
| Medical treatments at discharge | |||
| Aspirin | 532 (63.8) | 93 (62.4) | 0.78 |
| Statin | 372 (44.6) | 85 (57.0) | 0.006 |
| Calcium channel blocker | 798 (95.7) | 142 (95.3) | 0.83 |
| Nitrate | 310 (37.2) | 78 (52.3) | 0.001 |
| Nicorandil | 270 (32.4) | 59 (39.6) | 0.09 |
| ACEI or ARB | 159 (19.1) | 38 (25.5) | 0.08 |
Values are mean±SD or n (%). ACEI indicates angiotensin‐converting enzyme inhibitors; ARB, angiotensin II receptor blockers; BMI, body mass index; BUN, blood nitrogen urea; HDL, high‐density lipoprotein; Hs‐CRP, high‐sensitivity C‐reactive protein; LDL, low‐density lipoprotein; LVEF, left ventricular ejection fraction; MI, myocardial infarction; PCI, percutaneous coronary intervention; VAACS, vasospastic angina presenting with acute coronary syndrome.
Table 2.
Angiographic Characteristics of VAACS and Non‐VAACS Patients
| Variable | Non‐VAACS (n=837) | VAACS (n=149) | P Value |
|---|---|---|---|
| Spasm‐positive vessel | |||
| LM or LAD | 310 (37.0) | 78 (52.3) | 0.001 |
| LCx | 168 (20.1) | 21 (14.1) | 0.09 |
| RCA | 417 (49.8) | 63 (42.3) | 0.09 |
| Type of spasm | 0.12 | ||
| Diffuse | 251 (30.0) | 35 (23.5) | |
| Focal | 586 (70.0) | 114 (76.5) | |
| Spontaneous spasm | 18 (2.2) | 5 (3.4) | 0.37 |
| Myocardial bridging | 15 (1.8) | 3 (2.0) | 0.75 |
| ST‐segment change | 0.28 | ||
| No change | 288 (34.4) | 46 (30.9) | |
| ST‐segment elevation | 452 (54.0) | 92 (61.7) | |
| ST‐segment depression | 72 (8.6) | 8 (5.4) | |
| T wave inversion | 25 (3.0) | 3 (2.0) | |
| TIMI flow | 0.69 | ||
| TIMI 0 to 1 | 609 (72.8) | 111 (74.5) | |
| TIMI 2 to 3 | 228 (27.2) | 38 (25.5) | |
| Fixed coronary stenosis on spasm‐positive vessel | 0.28 | ||
| 0% to 30% | 613 (73.2) | 107 (71.8) | |
| 30% to 50% | 166 (19.8) | 29 (19.5) | |
| 50% to 70% | 58 (6.9) | 13 (8.7) | |
Values are n (%). LAD indicates left anterior descending; LCx, left circumflex; LM, left main; RCA, right coronary artery; TIMI, Thrombolysis in Myocardial Infarction; VAACS, vasospastic angina presenting with acute coronary syndrome.
Clinical Outcomes
During the follow‐up period (median 4.4 years), 42 patients (28.2%) in the VAACS group and 161 patients (19.2%) in the non‐VAACS group had MACE. Recurrent MI occurred in 15 (10.1%) VAACS patients and in 38 (4.5%) non‐VAACS patients (Table 3). The Kaplan–Meier curves for MACE, recurrent MI, and all‐cause death in VAACS and non‐VAACS patients are shown in Figure 2. In multivariable Cox regression analyses, VAACS patients had significantly increased risk of MACE (hazard ratio [HR], 1.65; 95% CI, 1.14–2.37; P=0.007), recurrent MI (HR, 2.57; 95% CI, 1.35–4.87; P=0.004), and all‐cause death (HR, 3.08; 95% CI, 1.33–7.14; P=0.009) compared with non‐VAACS patients (Table 3).
Table 3.
Clinical Outcomes of VAACS and Non‐VAACS Patients
| Variable | Non‐VAACS (n=837) | VAACS (n=149) | Unadjusted HR (95% CI) | P Value | Adjusted HRa (95% CI) | P Value |
|---|---|---|---|---|---|---|
| Major adverse cardiac eventsb | 161 (19.2) | 42 (28.2) | 1.66 (1.18–2.33) | 0.004 | 1.65 (1.14–2.37) | 0.007 |
| Cardiac death | 9 (1.1) | 4 (2.7) | 2.62 (0.81–8.52) | 0.11 | 1.68 (0.45–6.30) | 0.45 |
| Myocardial infarction | 38 (4.5) | 15 (10.1) | 2.45 (1.35–4.46) | 0.003 | 2.57 (1.35–4.87) | 0.004 |
| Revascularization | 23 (2.7) | 4 (2.7) | 1.06 (0.37–3.07) | 0.92 | 1.26 (0.42–3.83) | 0.68 |
| Rehospitalization because of recurrent angina | 132 (15.8) | 33 (22.1) | 1.57 (1.07–2.30) | 0.021 | 1.52 (1.01–2.29) | 0.044 |
| All‐cause death | 21 (2.5) | 10 (6.7) | 2.84 (1.34–6.02) | 0.007 | 3.08 (1.33–7.14) | 0.009 |
HR indicates hazard ratio; VAACS, vasospastic angina presenting with acute coronary syndrome.
Adjusted covariates included age, sex, diabetes mellitus, hypertension, current smoker, dyslipidemia, and history of myocardial infarction (MI).
Defined as a composite of cardiac death, MI, revascularization, or rehospitalization because of recurrent angina during follow‐up.
Figure 2.

Kaplan–Meier curves for clinical outcomes including (A) MACE, (B) MI, and (C) all‐cause death in non‐VAACS and VAACS patients. MACE indicates major adverse cardiac events; MI, myocardial infarction; VAACS, vasospastic angina patients presenting with acute coronary syndrome.
VA Patients Presenting With MI Caused by Coronary Vasospasm
The present study included 81 VA patients (8.2%) presenting with MI (VAMI). VAMI patients had higher prevalence of diabetes mellitus, hypertension, current smoking, and history of MI, as well as high WBC and Hs‐CRP levels, but lower left ventricular ejection fraction (Tables 4 and 5). Left main or left anterior descending spasm during the provocation test frequently occurred in VAMI patients. The Kaplan–Meier curves for MACE, recurrent MI, and all‐cause death in VAMI patients are shown in Figure 3. In multivariable Cox regression analyses, VAMI patients had significantly increased risk of MACE (HR, 1.75; 95% CI, 1.11–2.76; P=0.016) and recurrent MI (HR, 2.43; 95% CI, 1.09–5.40; P=0.03; Table 6).
Table 4.
Baseline Clinical Characteristics of VAMI and Non‐VAMI Patients
| Variable | Non‐VAMI (n=905) | VAMI (n=81) | P Value |
|---|---|---|---|
| Age, y | 56.6±9.3 | 54.4±10.0 | 0.06 |
| Male | 768 (84.9) | 71 (87.7) | 0.63 |
| BMI, kg/m2 | 24.4±2.2 | 24.3±3.0 | 0.69 |
| Typical morning angina | 664 (73.4) | 50 (61.7) | 0.028 |
| Medical history | |||
| Diabetes mellitus | 194 (21.4) | 36 (44.4) | <0.001 |
| Hypertension | 343 (37.9) | 40 (49.4) | 0.044 |
| Current smoking | 247 (27.3) | 32 (39.5) | 0.028 |
| Dyslipidemia | 191 (21.1) | 12 (14.8) | 0.20 |
| History of PCI | 71 (7.8) | 7 (8.6) | 0.83 |
| History of MI | 18 (2.0) | 14 (17.3) | <0.001 |
| History of CVA | 12 (1.3) | 3 (3.7) | 0.12 |
| Laboratory findings | |||
| Hemoglobin, g/dL | 14.5±1.5 | 14.4±1.7 | 0.66 |
| White blood cells, ×103/μL | 7.0±2.1 | 8.8±3.6 | <0.001 |
| Glucose, mg/dL | 116±36 | 133±54 | 0.007 |
| BUN, mg/dL | 15.0±4.3 | 14.9±4.7 | 0.89 |
| Creatinine, mg/dL | 0.91±0.19 | 0.88±0.24 | 0.19 |
| Total cholesterol, mg/dL | 172±36 | 166±35 | 0.15 |
| Triglycerides, mg/dL | 160±114 | 166±118 | 0.69 |
| HDL, mg/dL | 47±12 | 46±12 | 0.40 |
| LDL, mg/dL | 105±31 | 98±29 | 0.06 |
| Hs‐CRP, mg/dL | 0.37±1.38 | 1.16±3.61 | <0.001 |
| LVEF, % | 64.1±6.8 | 61.6±8.7 | 0.006 |
| Medical treatments at discharge | |||
| Aspirin | 573 (63.5) | 52 (64.2) | 1.00 |
| Statin | 406 (45.0) | 51 (63.0) | 0.002 |
| Calcium channel blocker | 863 (95.7) | 77 (95.1) | 0.78 |
| Nitrate | 354 (39.2) | 34 (42.0) | 0.64 |
| Nicorandil | 289 (32.0) | 40 (49.4) | 0.002 |
| ACEI or ARB | 175 (19.4) | 22 (27.2) | 0.11 |
Values are mean±SD or n (%). ACEI indicates angiotensin‐converting enzyme inhibitors; ARB, angiotensin II receptor blockers; BMI, body mass index; BUN, blood urea nitrogen; CVA, cerebrovascular accident; HDL, high‐density lipoprotein; Hs‐CRP, high‐sensitivity C‐reactive protein; LDL, low‐density lipoprotein; LVEF, left ventricular ejection fraction; MI, myocardial infarction; PCI, percutaneous coronary intervention; VAMI, vasospastic angina presenting with myocardial infarction.
Table 5.
Angiographic Characteristics in VAMI and Non‐VAMI Patients
| Variable | Non‐VAMI (n=905) | VAMI (n=81) | P Value |
|---|---|---|---|
| Spasm‐positive vessel | |||
| LM or LAD | 345 (38.1) | 43 (53.1) | 0.009 |
| LCx | 178 (19.7) | 11 (13.6) | 0.24 |
| RCA | 446 (49.3) | 34 (42.0) | 0.25 |
| Type of spasm | 0.61 | ||
| Diffuse | 265 (29.3) | 21 (25.9) | |
| Focal | 640 (70.7) | 60 (74.1) | |
| Spontaneous spasm | 21 (2.3) | 2 (2.5) | 0.71 |
| Myocardial bridging | 17 (1.9) | 1 (1.2) | 1.00 |
| ST‐segment change | 0.33 | ||
| No change | 310 (34.3) | 24 (29.6) | |
| ST‐segment elevation | 492 (54.4) | 52 (64.2) | |
| ST‐segment depression | 76 (8.4) | 4 (4.9) | |
| T wave inversion | 27 (3.0) | 1 (1.2) | |
| TIMI flow grade | 0.60 | ||
| TIMI flow grade 0 to 1 | 663 (73.3) | 57 (70.4) | |
| TIMI flow grade 2 to 3 | 242 (26.7) | 24 (29.6) | |
| Fixed coronary stenosis on spasm‐positive vessel | 0.39 | ||
| 0% to 30% | 661 (73.1) | 59 (72.9) | |
| 30% to 50% | 178 (19.7) | 17 (21.0) | |
| 50% to 70% | 66 (7.3) | 5 (6.2) | |
Values are n (%). LAD indicates left anterior descending; LCx, left circumflex; LM, left main; RCA, right coronary artery; TIMI, Thrombolysis in Myocardial Infarction;VAMI, vasospastic angina presenting with myocardial infarction.
Figure 3.
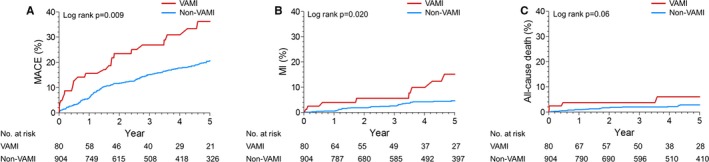
Kaplan–Meier curves for clinical outcomes including (A) MACE, (B) MI, and (C) all‐cause death in non‐VAMI and VAMI patients. MACE indicates major adverse cardiac events; MI, myocardial infarction; VAMI, vasospastic angina presenting with myocardial infarction.
Table 6.
Clinical Outcomes of VAMI and Non‐VAMI Patients
| Variable | Non‐VAMI (n=905) | VAMI (n=81) | Unadjusted HR (95% CI) | P Value | Adjusted HRa (95% CI) | P Value |
|---|---|---|---|---|---|---|
| Major adverse cardiac eventsb | 179 (19.8) | 24 (29.6) | 1.75 (1.15–2.69) | 0.010 | 1.75 (1.11–2.76) | 0.016 |
| Cardiac death | 10 (1.1) | 3 (3.7) | 3.66 (1.01–13.31) | 0.049 | 2.26 (0.51–9.98) | 0.28 |
| Myocardial infarction | 45 (5.0) | 8 (9.9) | 2.39 (1.12–5.08) | 0.024 | 2.43 (1.09–5.40) | 0.030 |
| Revascularization | 25 (2.8) | 2 (2.5) | 1.04 (0.25–4.42) | 0.95 | 1.32 (0.30–5.88) | 0.71 |
| Rehospitalization due to recurrent angina | 147 (16.2) | 18 (22.2) | 1.58 (0.97–2.58) | 0.07 | 1.54 (0.92–2.59) | 0.10 |
| All‐cause death | 26 (2.9) | 5 (6.2) | 2.41 (0.92–6.27) | 0.07 | 2.06 (0.71–6.05) | 0.19 |
Values are n (%). HR indicates hazard ratio; VAMI, vasospastic angina presenting with myocardial infarction.
Adjusted covariates included age, sex, diabetes mellitus, hypertension, current smoker, dyslipidemia, and history of myocardial infarction.
Defined as a composite of cardiac death, myocardial infarction, revascularization, or rehospitalization due to recurrent angina during follow‐up.
Multivariable Models for Determining Factors Associated With VAACS and VAMI
Diabetes mellitus, history of MI, and higher WBC (more than 10.0×103/μL) and Hs‐CRP (more than 0.3 mg/dL) levels were factors significantly associated with VAACS and VAMI (Table 7).
Table 7.
Multivariable Models for Determining Factors Associated With VAACS or VAMI
| Variable | VAACS | VAMI | ||
|---|---|---|---|---|
| OR (95% CI)a | P Value | OR (95% CI)a | P Value | |
| Age >60 y | 0.64 (0.37–1.11) | 0.11 | 0.37 (0.18–0.75) | 0.006 |
| Sex | 0.88 (0.40–1.93) | 0.75 | 1.15 (0.47–2.82) | 0.76 |
| BMI >25 kg/m2 | 0.70 (0.40–1.20) | 0.20 | 0.61 (0.32–1.19) | 0.15 |
| Typical morning angina | 1.02 (0.59–1.74) | 0.96 | 0.76 (0.41–1.44) | 0.40 |
| Diabetes mellitus | 2.48 (1.47–4.20) | 0.001 | 2.53 (1.35–4.75) | 0.004 |
| Hypertension | 1.38 (0.82–2.32) | 0.23 | 1.59 (0.85–2.98) | 0.15 |
| Current smoker | 1.30 (0.75–2.25) | 0.35 | 1.53 (0.80–2.93) | 0.20 |
| History of MI | 9.61 (3.08–30.04) | <0.001 | 13.37 (4.39–40.79) | <0.001 |
| WBC >10.0×103/μL | 4.27 (1.88–9.70) | 0.001 | 2.59 (1.06–6.29) | 0.036 |
| Total cholesterol >240 mg/dL | 0.52 (0.13–2.03) | 0.35 | 0.62 (0.13–3.08) | 0.56 |
| Creatinine >1.30 mg/dL | 1.27 (0.25–6.56) | 0.77 | 1.56 (0.27–9.15) | 0.62 |
| Hs‐CRP >0.3 mg/dL | 2.36 (1.29–4.32) | 0.005 | 2.10 (1.04–4.24) | 0.038 |
| LVEF >55% | 0.40 (0.18–0.87) | 0.021 | 0.61 (0.25–1.50) | 0.28 |
| LM or LAD spasm | 1.89 (1.13–3.13) | 0.015 | 1.39 (0.76–2.56) | 0.29 |
BMI indicates body mass index; Hs‐CRP, high‐sensitivity C‐reactive protein; LAD, left anterior descending; LM, left main; LVEF, left ventricular ejection fraction; MI, myocardial infarction; OR, odds ratio; VAACS, vasospastic angina presenting with acute coronary syndrome; VAMI, vasospastic angina presenting with myocardial infarction; WBC, white blood cells.
Multivariable logistic models including all clinically relevant variables at the same time.
Discussion
We found that the prognosis of patients presenting with ACS was worse than that of other VA patients without significant coronary artery stenosis. The main findings of this study are as follows: (1) VAACS patients were associated with increased risk of MACE, recurrent MI, rehospitalization because of recurrent angina, and all‐cause death; (2) VAMI patients more frequently suffered from MACE and recurrent MI than non‐VAMI patients; (3) diabetes mellitus, history of MI, and higher WBC (more than 10.0×103/μL) and Hs‐CRP (more than 0.3 mg/dL) levels were independently associated with VAACS and VAMI.
It has been documented that coronary artery spasm is an important etiology of ACS in patients without obstructive coronary artery disease.10 ACS patients with coronary spasm and nonobstructive coronary artery disease have a favorable prognosis for survival and coronary events.11, 12 In the Coronary Artery Spasm in Patients with Acute Coronary Syndrome study, nonobstructive ACS patients with documented coronary spasm showed no cardiac death or nonfatal MI during a 3‐year follow‐up.11 In a Japanese population, cardiovascular events occurred in 15 (4.7%) patients with coronary artery spasm, including 1 (0.3%) patient with cardiovascular death and 4 (1.3%) patients with MI during a mean follow‐up duration of 20 months.12 Although nonobstructive ACS patients with documented coronary spasm have an excellent prognosis for survival and coronary events compared with patients with obstructive ACS, there are no long‐term follow‐up data. Furthermore, a recent study proposed that acute presentations including out‐of‐hospital cardiac arrest, angina at rest alone, and ST‐segment elevation were significant predictors of adverse outcomes in VA patients.4 However, there were no comparative data of VA patients presenting with or without ACS.
In our study, VAACS patients with documented myocardial ischemia had increased risk of MACE, driven mainly by recurrent MI compared with non‐VAACS patients. These findings could possibly be explained by the fact that urgent presentation with documented myocardial ischemia reflects severe stenosis or occlusion of the coronary artery by vasospasm. In these patients, disturbances of the coronary microcirculation result in decreased coronary reserve or microvascular spasm that causes repetitive angina during exercise or at rest.11 In addition, the increased risks of MACE and recurrent MI were consistently observed in VAMI patients. It is likely that prolonged coronary spasm mimicking true MI caused repetitive myocardial damage and inflammation. In our study, VAACS and VAMI patients had higher levels of inflammatory markers such as WBC and Hs‐CRP. A previous study has also reported that laboratory findings including significant elevation of Hs‐CRP and fibrinogen levels were similar to those seen in patients with acute MI with significant stenosis.13 Furthermore, we hypothesized that suboptimal use of medications that improve the prognosis of ACS and MI such as statins or renal angiotensin system blockades might lead to adverse outcomes in VAACS and VAMI patients. In the present study, statins were prescribed in approximately half of VAACS patients, and renal angiotensin system blockades were prescribed in one quarter of VAACS patients. A recent study showed that statin therapy in patients with coronary spasm–induced AMI with nonobstructive coronary artery disease was associated with improved clinical outcomes.14 Previous studies have also demonstrated that the incidence of cardiac death and nonfatal MI was significantly increased in VA patients in whom medications were reduced or discontinued.15, 16, 17 Accordingly, appropriate pharmacological treatment should be considered for VAACS and VAMI patients without obstructive coronary artery disease.
In the present study, clinical factors associated with VAACS and VAMI were diabetes mellitus, history of MI, and higher level of inflammatory markers. Diabetes mellitus is a chronic inflammatory condition, and our and previous data suggest that inflammation might be associated with severe coronary vasospasm.3 Most importantly, our data showed that prior MI in VA patients is a strong associated factor of VAACS and VAMI, and that VAACS and VAMI patients have increased rates of recurrent MI. Therefore, VA patients with diabetes mellitus and prior MI are a high‐risk group, and more intensive management and follow‐up should be required.
This study had several limitations inherent to a retrospective analysis of observational data. First, some of the baseline and angiographic characteristics were unfavorable in the VAACS group compared with the non‐VAACS group, which may have contributed to the differences in adverse outcomes between the 2 groups. Accordingly, it is difficult to assert that the presence of coronary spasm is the direct cause of worse outcomes. However, adjusted Cox regression analysis showed that VAACS patients were at increased risk of adverse outcomes after adjustment for pre‐existing cardiovascular risk factors. Therefore, severe coronary vasospasm causing electrocardiographic changes, significant arrhythmias, or elevation of cardiac biomarkers might be closely associated with worse clinical outcomes. However, we could not elucidate the pathogenesis of severe presentation and higher probability of future MI in VAACS patients in this study. Second, the cause–effect relationship between treatment and patient outcomes could not be established. We are conducting a study on the long‐term follow‐up results of a prospective VA‐KOREA (Vasospastic Angina in Korea) registry to further analyze this relationship.
Conclusion
VAACS patients had worse clinical outcomes, driven mainly by recurrent MI, compared with non‐VAACS patients. These findings were observed consistently in VAMI patients. Therefore, more intensive clinical attention and medical treatment are required in VAACS and VAMI patients without obstructive coronary artery disease.
Disclosures
None.
(J Am Heart Assoc. 2016;5:e004336 doi: 10.1161/JAHA.116.004336)
References
- 1. Beltrame JF, Sasayama S, Maseri A. Racial heterogeneity in coronary artery vasomotor reactivity: differences between Japanese and Caucasian patients. J Am Coll Cardiol. 1999;33:1442–1452. [DOI] [PubMed] [Google Scholar]
- 2. Lanza GA, Careri G, Crea F. Mechanisms of coronary artery spasm. Circulation. 2011;124:1774–1782. [DOI] [PubMed] [Google Scholar]
- 3. Yasue H, Nakagawa H, Itoh T, Harada E, Mizuno Y. Coronary artery spasm–clinical features, diagnosis, pathogenesis, and treatment. J Cardiol. 2008;51:2–17. [DOI] [PubMed] [Google Scholar]
- 4. Takagi Y, Takahashi J, Yasuda S, Miyata S, Tsunoda R, Ogata Y, Seki A, Sumiyoshi T, Matsui M, Goto T, Tanabe Y, Sueda S, Sato T, Ogawa S, Kubo N, Momomura S, Ogawa H, Shimokawa H; Japanese Coronary Spasm A . Prognostic stratification of patients with vasospastic angina: a comprehensive clinical risk score developed by the Japanese Coronary Spasm Association. J Am Coll Cardiol. 2013;62:1144–1153. [DOI] [PubMed] [Google Scholar]
- 5. Guidelines for diagnosis and treatment of patients with vasospastic angina (Coronary Spastic Angina) (JCS 2013). Circ J. 2014;78:2779–2801. [DOI] [PubMed] [Google Scholar]
- 6. Park YM, Han SH, Ko KP, Koh KK, Kang WC, Lee K, Shin KC, Suh SY, Ahn TH, Choi IS, Shin EK. Diffuse multi‐vessel coronary artery spasm: incidence and clinical prognosis. Int J Cardiol. 2013;167:398–402. [DOI] [PubMed] [Google Scholar]
- 7. Takagi Y, Yasuda S, Takahashi J, Tsunoda R, Ogata Y, Seki A, Sumiyoshi T, Matsui M, Goto T, Tanabe Y, Sueda S, Sato T, Ogawa S, Kubo N, Momomura S, Ogawa H, Shimokawa H; Japanese Coronary Spasm A . Clinical implications of provocation tests for coronary artery spasm: safety, arrhythmic complications, and prognostic impact: multicentre registry study of the Japanese Coronary Spasm Association. Eur Heart J. 2013;34:258–267. [DOI] [PubMed] [Google Scholar]
- 8. Shin DI, Baek SH, Her SH, Han SH, Ahn Y, Park KH, Kim DS, Yang TH, Choi DJ, Suh JW, Kwon HM, Lee BK, Gwon HC, Rha SW, Jo SH. The 24‐month prognosis of patients with positive or intermediate results in the intracoronary ergonovine provocation test. JACC Cardiovasc Interv. 2015;8:914–923. [DOI] [PubMed] [Google Scholar]
- 9. Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD; the Writing Group on behalf of the Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction . Third universal definition of myocardial infarction. J Am Coll Cardiol. 2012;60:1581–1598. [DOI] [PubMed] [Google Scholar]
- 10. Ong P, Athanasiadis A, Hill S, Vogelsberg H, Voehringer M, Sechtem U. Coronary artery spasm as a frequent cause of acute coronary syndrome: the CASPAR (coronary artery spasm in patients with acute coronary syndrome) study. J Am Coll Cardiol. 2008;52:523–527. [DOI] [PubMed] [Google Scholar]
- 11. Ong P, Athanasiadis A, Borgulya G, Voehringer M, Sechtem U. 3‐year follow‐up of patients with coronary artery spasm as cause of acute coronary syndrome: the CASPAR (coronary artery spasm in patients with acute coronary syndrome) study follow‐up. J Am Coll Cardiol. 2011;57:147–152. [DOI] [PubMed] [Google Scholar]
- 12. Nakayama N, Kaikita K, Fukunaga T, Matsuzawa Y, Sato K, Horio E, Yoshimura H, Mizobe M, Takashio S, Tsujita K, Kojima S, Tayama S, Hokimoto S, Sakamoto T, Nakao K, Sugiyama S, Kimura K, Ogawa H. Clinical features and prognosis of patients with coronary spasm‐induced non‐ST‐segment elevation acute coronary syndrome. J Am Heart Assoc. 2014;3:e000795 doi: 10.1161/JAHA.114.000795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim W, Cho JS, Hong YJ, Ahn Y, Jeong MH. Clinical and laboratory characteristics in patients with acute myocardial infarction due to occlusive vasospasm. J Cardiol. 2010;56:320–325. [DOI] [PubMed] [Google Scholar]
- 14. Piao ZH, Jeong MH, Li Y, Jin L, Kim HK, Park KH, Sim DS, Kim KH, Hong YJ, Park H, Kim JH, Ahn Y, Cho JG, Park JC, Kim YJ, Cho MC, Kim CJ, Kim HS; Other Korea Acute Myocardial Infarction Registry I . Benefit of statin therapy in patients with coronary spasm‐induced acute myocardial infarction. J Cardiol. 2016;68:7–12. [DOI] [PubMed] [Google Scholar]
- 15. Lette J, Gagnon RM, Lemire JG, Morissette M. Rebound of vasospastic angina after cessation of long‐term treatment with nifedipine. Can Med Assoc J. 1984;130:1169–1171, 1174. [PMC free article] [PubMed] [Google Scholar]
- 16. Takagi Y, Yasuda S, Tsunoda R, Ogata Y, Seki A, Sumiyoshi T, Matsui M, Goto T, Tanabe Y, Sueda S, Sato T, Ogawa S, Kubo N, Momomura S, Ogawa H, Shimokawa H; Japanese Coronary Spasm A . Clinical characteristics and long‐term prognosis of vasospastic angina patients who survived out‐of‐hospital cardiac arrest: multicenter registry study of the Japanese Coronary Spasm Association. Circ Arrhythm Electrophysiol. 2011;4:295–302. [DOI] [PubMed] [Google Scholar]
- 17. Kim PJ, Seung KB, Kim DB, Her SH, Shin DI, Jang SW, Park CS, Park HJ, Jung HO, Baek SH, Kim JH, Choi KB. Clinical and angiographic characteristics of acute myocardial infarction caused by vasospastic angina without organic coronary heart disease. Circ J. 2007;71:1383–1386. [DOI] [PubMed] [Google Scholar]


