Abstract
Background
Cyanate has recently gained attention for its role in the pathogenesis of vascular injury. Nonetheless, the effect of cyanate on angiogenesis remains unclear.
Methods and Results
In this study, we demonstrated that oral administration of cyanate impaired blood perfusion recovery in a mouse hind‐limb ischemia model. A reduction in blood perfusion recovery at day 21 was observed in the ischemic tissue of cyanate‐treated mice. Likewise, there were fewer capillaries in the ischemic hind‐limb tissue of cyanate‐exposed mice. Our in vitro study showed that cyanate, together with its carbamylated products, inhibited the migration, proliferation, and tube‐formation abilities of endothelial cells. Further research revealed that cyanate regulated angiogenesis partly by interrupting the vascular endothelial growth factor receptor 2/phosphatidylinositol 3‐kinase/Akt pathway. The serum concentrations of homocitrulline, a marker of cyanate exposure, were determined in 117 patients with stable angina and chronic total occlusion. Consistent with the antiangiogenic role of cyanate, homocitrulline levels were increased in patients with poor coronary collateralization (n=58) compared with those with high collateralization (n=59; 21.09±13.08 versus 15.54±9.02 ng/mL, P=0.009). In addition, elevated homocitrulline concentration was a strong predictor of poor coronary collateral growth.
Conclusions
Impaired angiogenesis induced by cyanate might contribute to poor coronary collateral growth.
Keywords: angiogenesis, carbamylation, chronic total coronary occlusion, collateral circulation, cyanate, endothelial dysfunction
Subject Categories: Angiogenesis, Vascular Biology, Endothelium/Vascular Type/Nitric Oxide, Clinical Studies
Introduction
Angiogenesis, which is described as new capillary formation, is one of the most important mechanisms of coronary collateral development. By providing an alternative route for flow, collateral circulation in the heart can limit ischemic injury and improve myocardial function, thus improving survival of patients with stable angina and chronic total occlusion (CTO).1, 2, 3 Angiogenesis involves several important steps, including activation, migration, and proliferation of differentiated endothelial cells4, 5; however, abnormal accumulation of certain uremic toxins and metabolites has been demonstrated to inhibit these angiogenic processes.6, 7
Cyanate has recently gained attention for its role in the pathogenesis of vascular disease.8 The increasing concentration of cyanate in vivo occurs by 2 pathways. In chronic renal failure, excess cyanate is dissociated mainly from urea and implicated as a uremic toxin.9 The concentration of cyanate can increase to ≈1 mmol/L in end‐stage renal disease, with ≈0.8% of urea converted into cyanate.10 In addition, at sites of inflammation and atherosclerosis, cyanate is generated via myeloperoxidase‐catalyzed oxidation of thiocyanate.11 The role of cyanate in promoting vascular injury is complex. Cyanate is highly reactive, and its exposure to free amino groups on protein residue results in a posttranslational modification termed carbamylation. A principal carbamylation‐derived product is homocitrulline.12 Carbamylation‐derived products facilitate multiple proatherosclerotic effects, especially on endothelial cells. In vitro studies have shown that carbamylated low‐density lipoprotein causes endothelial injury and reactive oxygen species production and promotes adhesion of monocytes to endothelial cells.13, 14, 15 Carbamylated modification of high‐density lipoprotein also impairs its endothelial repair properties.16 In addition to promoting carbamylation, recent studies have demonstrated that cyanate itself can directly induce endothelial dysfunction and adhesion molecule expression17, 18; thus, cyanate could serve as a potential regulator of endothelial function. Nonetheless, the effect of cyanate on endothelial angiogenesis properties remains unclear.
In this study, we tested the hypothesis that administration of cyanate could impair angiogenesis in a mouse hind‐limb ischemia model. Cellular functional tests were carried out in vitro to further explore the potential mechanism. Moreover, the clinical impact of increased cyanate exposure on coronary collateral circulation was evaluated in patients with stable angina and CTO.
Materials and Methods
Ischemic Hind‐Limb Model and Blood Flow Monitoring
The experimental protocol was approved by the Committee on Animal Resources from Shanghai Jiao Tong University. Overall, 20 C57BL/6 mice aged 5 weeks were purchased from the Model Animal Center of Nanjing University and housed in a pathogen‐free isolation facility under a 12/12‐hour light–dark cycle with free access to water and food. The mice were divided into 2 groups: the control group (n=10) with normal drinking water and the cyanate group (n=10) with drinking water containing sodium cyanate (1 mg/mL). Water was renewed 2 times per week for both groups. After 16 weeks of administration, unilateral hind‐limb ischemia was surgically performed by left femoral vessel (artery and vein) removal and excision of femoral bifurcation with all branches, as described previously.19 Hind‐limb blood perfusion was measured with laser Doppler perfusion imaging. The results were expressed as the ratio of perfusion in the ischemic (left) versus nonischemic (right) hind limb. The animal studies were approved by the animal care committee of Shanghai Jiao Tong University.
Tissue Preparation and Immunochemistry
Gastrocnemius tissue from the ischemic hind limb was harvested 21 days after surgery and underwent immediate tissue fixation overnight. For mouse capillary density identification, the tissues were stained with monoclonal CD31 antibodies. For quantification, the capillaries were counted in 5 randomly selected microscopic fields. After the mice had fasted for 4 hours, blood samples were aspirated from the orbital venous plexus, and serum was isolated and stored at −80°C. Serum levels of total cholesterol, triglycerides, low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol, blood urea nitrogen, and creatinine were detected by the Shanghai Fenglin Clinical Laboratory. Serum homocitrulline levels were measured by ELISA (Cell Biolabs Inc) according to the manufacturer's instructions and expressed as homocitrulline (ng/mL). The inter‐ and intra‐assay coefficients of variation were 8% and 4%, respectively.
Cell Culture
We obtained human arterial endothelial cells (HAECs) from Cascade Biologics (Portland, OR). The cells were maintained in Medium 200 supplemented with growth factors and cultured in a humidified atmosphere of 5% CO2 at 37°C.
Carbamylation of Cell Culture Medium
Cell culture medium containing 10% FBS was carbamylated with different concentrations of cyanate (1, 5, and 10 mmol/L) for 72 hours at 37°C. The residual cyanate in the carbamylated cell culture medium was then removed by excessive dialysis at 4°C against fresh cell culture medium under sterile conditions 4 times over 36 hours.
Wound‐Healing Assay
For the carbamylated medium–treated group, HAECs were plated on 6‐well plates and incubated with carbamylated or serum media (control) for 24 hours. HAECs were serum deprived and incubated with 1, 5, or 10 mmol/L cyanate for the cyanate‐treated group or sodium chloride (control) for 24 hours on 6‐well plates. The HAECs were then wounded with a sterile pipette tip, and the width of each wound line was photographed (Olympus) at 0 and 24 hours. Cell motility velocity was quantified using Image‐Pro Plus version 6.2 (Media Cybernetics).
Cell Migration Assay
Cell migration assays were performed using a Boyden chamber (Millipore). The HAECs were either incubated with carbamylated medium or serum deprived and treated with the aforementioned concentrations of cyanate for 24 hours. Cells (0.5×106 cells per mL) were then harvested and placed in the upper chamber, with the lower chamber filled with 500 μL of cell culture medium containing 10% FBS. After 8 hours of incubation, the nonmigrated HAECs on the upper side of the membrane surface were removed by wiping with a cotton swab. The migratory cells were then fixed and stained with crystal violet solution and quantified by optical density (560 nm) measurement.
Tube Formation Assay
Matrigel (BD Bioscience) was added to 96‐well plates, with 70 μL in each well. After treatment with carbamylated medium or cyanate for 24 hours, HAECs were harvested and added to the 96‐well plates. Cells plated on Growth Factor‐Reduced Matrigel (BD Bioscience) served as the negative control. Cells were examined in 4 random microscopic fields, and total length of the capillary structures and branch point numbers were measured by Image‐Pro Plus version 6.2 (Media Cybernetics).
Cell Proliferation Assay
Cell proliferation was measured with BrdU (5‐bromo‐2′‐deoxyuridine) following the manufacturer's instruction. Briefly, after incubation with carbamylated medium or cyanate for 24 hours, cells at a density of 5×103 cells per well were cultured on 96‐well plates. At 24 hours after seeding, cells were incubated with 10 μmol/L BrdU for an additional 4 hours. The cells were fixed and incubated with anti‐BrdU antibody and then underwent tetramethylbenzidine substrate reaction for 15 minutes. Cell proliferation ability was quantified by an ELISA colorimetric kit (Roche Diagnostics).
Western Blot
Proteins from cell lysates at equal loadings were subjected to 7.5% to 12.5% SDS‐PAGE and then transferred to polyvinylidene difluoride membranes. Membranes were further blocked in 5% nonfat milk, followed by incubation with the corresponding primary antibodies overnight at 4°C and with horseradish peroxidase–conjugated secondary antibodies for 1 hour at room temperature. Blots were detected using an electrochemiluminescence system (GE Healthcare Biosciences) and qualified with Quantity One (BioRad) software. We also detected β‐actin as the protein loading control.
Reagents and Antibodies
Antibodies for detection of vascular endothelial growth factor receptor 2 (VEGFR2), phosphatidylinositol 3‐kinase (PI3K), Akt, phosphorylated Akt, and β‐actin were obtained from Abcam. CD31 mouse antibody was purchased from Santa Cruz Biotechnology. We obtained phosphorylated VEGFR2 and secondary antibodies from Cell Signaling Technology. The transwell assay was from Millipore. The BrdU cell proliferation assay kit was obtained from Calbiochem (Merck). Sodium cyanate was purchased from Sigma‐Aldrich.
Study Population
Between September 2012 and December 2015, 117 patients undergoing coronary angiography at Shanghai Ruijin Hospital with stable angina and CTO of at least 1 major epicardial coronary artery were enrolled in this study. The exclusion criteria included acute coronary syndrome, history of coronary artery bypass grafting surgery, pulmonary heart disease or chronic heart failure, and immune system disorder or tumor.
The study protocol was approved by the Hospital Research Ethics Board and was in accordance with the Code of Ethics of the World Medical Association. After a full explanation of the study, written informed consent was obtained from each participant.
Coronary Angiography and Collateral Grading
We performed coronary angiography through radial or femoral access with 6F diagnostic catheters. Collateral circulation was graded according to the Rentrop scoring system by 2 interventional cardiologists blinded to the study protocol: 0=none; 1=filling of side branches of the artery; 2=partial filling of the epicardial segment; 3=complete filling of the epicardial segment of the artery.20 The vessel with the highest collateral grade was chosen for analysis in participants with >1 occluded vessel. Patients were then classified into either the poor (Rentrop score 0–1) or good (Rentrop score 2–3) coronary collateralization group.
Biochemical Measurement in Patients
We obtained blood samples from all patients after overnight fasting. Biochemical assessments were analyzed with standard laboratory protocols on a Hitachi 912 Analyzer (Roche Diagnostics) prior to coronary angiography. We used the Chronic Kidney Disease Epidemiology Collaboration equation to estimate the glomerular filtration rate. Serum homocitrulline levels were determined using the commercially available ELISA kit mentioned earlier.
Statistical Analysis
Statistical analyses were performed with SPSS software (version 20.0; IBM Corp) and GraphPad Prism 5 (GraphPad Software). Continuous variables were expressed as mean±SD. For normally distributed continuous variables, we use unpaired Student t tests to assess differences; otherwise, Mann–Whitney U tests were performed. One‐way ANOVA was used for multiple comparisons. Differences in proportions were analyzed by chi‐square tests. The relationship between serum homocitrulline levels and Rentrop scores was evaluated by the Spearman test. Logistic regression analysis was used to detect whether increasing homocitrulline levels were an independent predictor of poor collateral growth after adjusting for age, sex, body mass index, and conventional risk factors of coronary artery disease, including smoking, hypertension, diabetes mellitus, hyperlipidemia, lipoprotein(a), and reduced glomerular filtration rate. The diagnostic values of the serum homocitrulline levels in the assessment of poor collateral growth in different subgroups were calculated by receiver operating characteristic curves. A P value of <0.05 was considered statistically significant.
Results
Cyanate Depressed Blood Perfusion Recovery In Vivo
To assess the in vivo effect of cyanate on angiogenesis, we examined whether oral administration of cyanate impaired blood perfusion recovery in a mouse hind‐limb ischemia model. The characteristics of the mice receiving cyanate for 16 weeks are summarized in Table 1. As a marker of cyanate exposure and carbamylation level, homocitrulline levels were significantly increased in the cyanate‐treated mice compared with the control mice (28.39±15.89 versus 3.52±1.7 ng/mL, P=0.008). Lipid profiles (triglycerides, total cholesterol, high‐density lipoprotein, low‐density lipoprotein) and renal function (blood urea nitrogen, creatinine) did not differ between the 2 groups. Hind‐limb ischemia was surgically induced in both cyanate‐treated and control mice. After surgery (day 0), the laser Doppler perfusion imaging results showed that surgical induction of hind‐limb ischemia reduced blood perfusion equally in both groups. Blood flow recovery, however, was significantly attenuated in mice treated with cyanate compared with control mice at day 21 (0.69±0.07 versus 0.89±0.04, P<0.05) after surgery (Figure 1A and 1B). At the microvascular level, capillary densities in tissue from the ischemic hind limb were examined at day 21 after surgery. Cyanate treatment was associated with a significant decrease in capillary density in ischemic hind‐limb mice (P<0.05) (Figure 1C).
Table 1.
Characteristics of Control Mice and Mice Receiving Cyanate for 16 Weeks
| Variable | Control | Cyanate | P Value |
|---|---|---|---|
| Triglycerides, mmol/L | 0.67±0.07 | 0.75±0.06 | NS |
| Total cholesterol, mmol/L | 1.12±0.09 | 1.21±0.1 | NS |
| LDL cholesterol, mmol/L | 0.07±0.02 | 0.08±0.01 | NS |
| HDL cholesterol, mmol/L | 0.54±0.05 | 0.49±0.07 | NS |
| BUN, mmol/L | 9.4±1.0 | 9.3±0.77 | NS |
| Creatinine, μmol/L | 7.0±1.0 | 7.7±0.6 | NS |
| Hcit, ng/mL | 3.52±1.7 | 28.39±15.89 | 0.008 |
BUN indicates blood urea nitrogen; Hcit, homocitrulline; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; NS, not significant.
Figure 1.
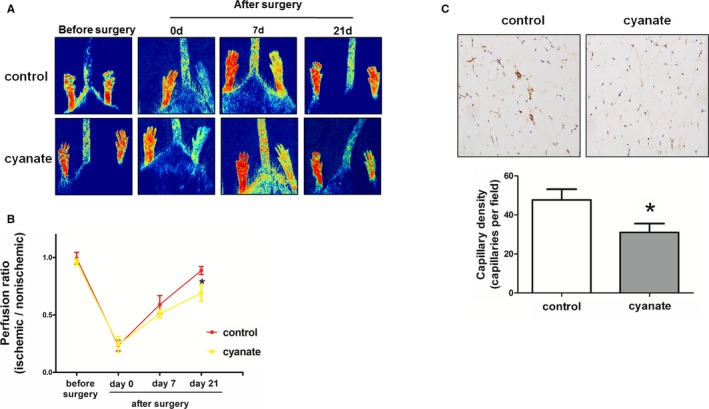
Effect of cyanate on blood flow recovery and new capillary formation following hind‐limb ischemia in control and cyanate‐treated mice. A, Representative laser Doppler measurement at days 0 and 21 after surgery. B, Quantification of laser Doppler perfusion ratio (ischemic/nonischemic) in control and cyanate‐treated mice over time. C, CD31 immunostaining and quantification of capillary density in ischemic hind limb at day 21 after surgery in control and cyanate‐treated mice. *P<0.05 vs control.
Carbamylated Proteins Inhibited Angiogenesis and the VEGFR2 Pathway
Because the in vivo studies could not determine explicitly whether cyanate or its carbamylation adduct inhibited the endothelial angiogenesis process, in vitro studies were divided into 2 parts.
To determine the role of cyanate‐induced carbamylation, endothelial cells were incubated with carbamylated cell culture medium, which underwent extensive dialysis to remove residual cyanate. Compared with the serum medium, carbamylated cell culture medium significantly inhibited endothelial migration ability in a modification‐dependent manner, as observed from the transwell and wound‐healing results (Figure 2A). Furthermore, endothelial cells stimulated with carbamylated medium also showed a reduced capacity to form capillary structures, with tube lengths and branch point numbers both decreased (Figure 2B). Carbamylated medium treatment also led to modification‐dependent inhibition of endothelial cell proliferation (Figure 2C).
Figure 2.
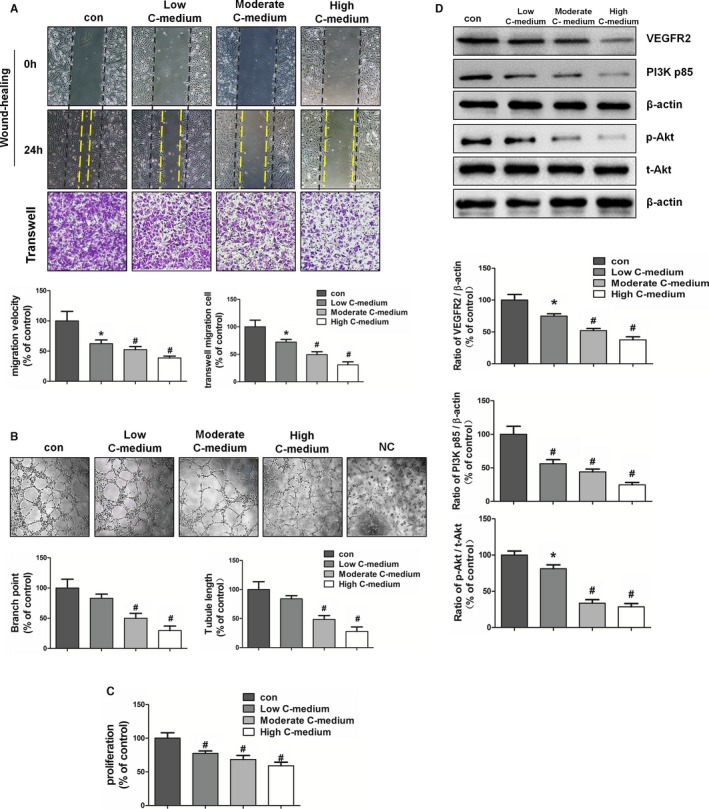
Effect of carbamylated cell culture medium on endothelial angiogenesis and the VEGFR2 pathway. Cell culture medium was carbamylated with different concentrations of cyanate (1, 5, and 10 mmol/L) for 72 hours at 37°C. HAECs were treated with low, moderate, or high carbamylated medium or with serum medium (control). A, Wound healing and transwell assays were done to test the migratory capacity of HAECs. B, Tube formation assay was used to analyze the ability of HAECs to form capillary structures. Representative images were taken, and branch points and tubule lengths were quantified. C, HAEC proliferation was examined by BrdU (5‐bromo‐2′‐deoxyuridine) assay. D, Representative immunoblots and quantitative analysis of the protein levels of VEGFR2 and PI3K and phosphorylation of Akt. *P<0.05 vs control, # P<0.01 vs control. C‐medium indicates carbamylated medium; con, control; HAEC, human arterial endothelial cell; NC, negative control; PI3K, phosphatidylinositol 3‐kinase; VEGFR2, vascular endothelial growth factor receptor 2.
To investigate the molecular mechanism of carbamylated medium–induced inhibition of endothelial angiogenesis ability, we examined the protein expression of VEGFR2 and its downstream signal mediators by Western blot analysis. The treatment of HAECs with carbamylated medium significantly decreased VEGFR2 and downstream PI3K expression as well as Akt phosphorylation in a modification‐dependent manner (Figure 2D).
Cyanate Suppressed Angiogenesis and the VEGFR2 Pathway
To examine the direct effects of cyanate, endothelial cells were incubated with serum‐free medium supplemented with cyanate to avoid protein carbamylation. Cyanate alone also attenuated the ability of endothelial cells to migrate, to proliferate, and to form capillary structures (Figure 3A through 3C). Consistent with endothelial function studies, Western blot results showed that activation of the above VEGFR2 pathway was markedly decreased by cyanate (Figure 3D).
Figure 3.
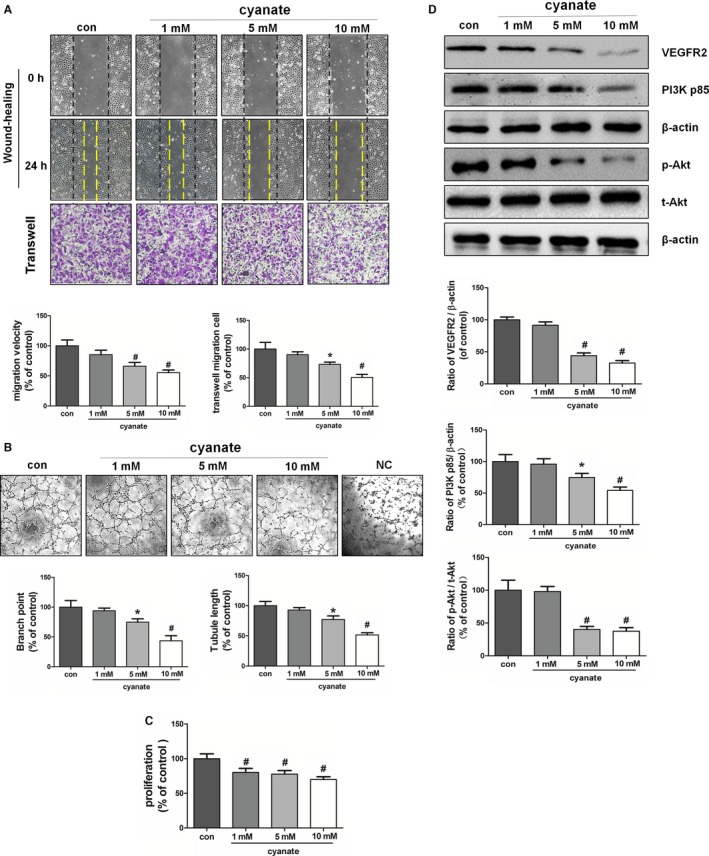
Effect of cyanate on endothelial angiogenesis and the VEGFR2 pathway. HAECs were serum deprived and incubated with 1, 5, or 10 mmol/L cyanate or with sodium chloride (control). A, Migratory abilities of HAECs were determined by wound healing and transwell assays. B, Tube formation assay was used to analyze the in vitro angiogenesis capacity of HAECs. Representative images were taken, and branch points and tubule lengths were quantified. C, Proliferation properties of HAECs were examined by BrdU (5‐bromo‐2′‐deoxyuridine) assay. D, Representative immunoblots and quantitative analysis of the protein levels of VEGFR2 and PI3K and phosphorylation of Akt. *P<0.05 vs control, # P<0.01 vs control.C‐medium indicates carbamylated medium; con, control; HAEC, human arterial endothelial cell; NC, negative control; PI3K, phosphatidylinositol 3‐kinase; VEGFR2, vascular endothelial growth factor receptor 2.
Increased Homocitrulline Levels Related to Poor Collateral Development
To test the potential clinical relevance of cyanate to collateral development, we performed an observational study investigating the relationship between homocitrulline levels and poor collateral growth in patients with stable angina and CTO. The clinical and laboratory characteristics of patients are summarized in Table 2. Patients with poor collateral growth (n=58) had a higher incidence of diabetes mellitus (55.2% versus 33.9%, P=0.021), increased serum homocitrulline levels (21.09±13.08 versus 15.54±9.02 ng/mL, P=0.009), and decreased glomerular filtration rates (76.45±18.22 versus 86.63±15.88 mL/min per 1.73 m2, P=0.002) compared with those exhibiting good collateral growth (n=59). Angiographic characteristics of the occlusion vessels did not differ significantly between the 2 groups (Table 3). Homocitrulline levels were inversely related to Rentrop score before (Spearman r=−0.229, P=0.013) and after (Spearman r=−0.211, P=0.028) adjusting for age, sex, body mass index, and conventional risk factors of coronary artery disease, including presence of diabetes mellitus, hyperlipidemia, smoking, hypertension, and reduced glomerular filtration rate. Logistic regression results demonstrated that increased homocitrulline levels (odds ratio 1.043, 95% CI 1.003–1.085, P=0.033) were associated with poor collateral development after adjusting for the noted risk factors (Table 4). In addition, receiver operating characteristic curve analysis indicated the prediction value of serum homocitrulline levels for poor collateral growth in all patients and in the subgroup with diabetes mellitus (Table 5).
Table 2.
Baseline Characteristics in Patients With Poor and Good Collateral Growth
| Poor Collateral Growth (n=58) | Good Collateral Growth (n=59) | P Value | |
|---|---|---|---|
| Age, y | 67.1±8.4 | 65.3±7.2 | NS |
| Male/female | 38/20 | 40/19 | NS |
| BMI | 25.0±3.9 | 25.4±3.4 | NS |
| Hypertension, n (%) | 36 (62.1) | 35 (59.3) | NS |
| Diabetes mellitus, n (%) | 32 (55.2) | 20 (33.9) | 0.021 |
| Smoking, n (%) | 27 (46.6) | 28 (47.5) | NS |
| Dyslipidemia, n (%) | 26 (44.8) | 19 (32.2) | NS |
| Triglycerides, mmol/L | 1.81±0.86 | 1.83±0.82 | NS |
| Total cholesterol, mmol/L | 4.28±1.17 | 4.1±0.97 | NS |
| HDL cholesterol, mmol/L | 1.03±0.22 | 1.04±0.21 | NS |
| LDL cholesterol, mmol/L | 2.77±1.11 | 2.46±1.08 | NS |
| Lipoprotein(a), g/L | 0.25±0.17 | 0.22±0.16 | NS |
| GFR, mL/min/1.73 m2 | 76.45±18.22 | 86.63±15.88 | 0.002 |
| Hcit, ng/mL | 21.09±13.08 | 15.54±9.02 | 0.009 |
| Medical treatment | |||
| ACEI/ARB, n (%) | 31 (53.4) | 27 (45.8) | NS |
| β‐blocker, n (%) | 29 (50.0) | 32 (54.2) | NS |
| Calcium channel blocker, n (%) | 15 (25.9) | 13 (22.0) | NS |
| Statin, n (%) | 42 (72.4) | 50 (84.7) | NS |
ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; GFR, glomerular filtration rate; Hcit, homocitrulline; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; NS, not significant.
Table 3.
Angiographic Characteristics in Patients With Poor and Good Collateral Growth
| Poor Collateral Growth (n=58) | Good Collateral Growth (n=59) | P Value | |
|---|---|---|---|
| Occluded LAD, n (%) | 25 (43.1) | 30 (50.8) | NS |
| Occluded LCX, n (%) | 21 (36.2) | 19 (32.2) | NS |
| Occluded RCA, n (%) | 23 (39.7) | 18 (30.5) | NS |
| Severity of CAD, n (%) | |||
| 1‐vessel disease, n (%) | 15 (25.9) | 10 (16.9) | NS |
| 2‐vessel disease, n (%) | 18 (31.0) | 20 (33.8) | NS |
| 3‐vessel disease, n (%) | 25 (43.1) | 29 (49.2) | NS |
CAD indicates coronary artery disease; LAD, left ascending artery; LCX, left circumflex artery; RCA, right coronary artery.
Table 4.
Logistic Regression Analysis for the Risk of Poor Coronary Collateral Growth
| Variable | OR (95% CI) | P Value |
|---|---|---|
| Age ≥65 years | 1.605 (0.700–3.676) | NS |
| Sex | 0.699 (0.295–1.657) | NS |
| BMI | 0.997 (0.891–1.116) | NS |
| Hypertension | 0.677 (0.282–1.629) | NS |
| Smoking | 0.942 (0.413–2.148) | NS |
| Diabetes mellitus | 2.326 (1.014–5.339) | 0.046 |
| Dyslipidemia | 1.581 (0.667–3.745) | NS |
| GFR | 0.965 (0.940–0.990) | 0.006 |
| Hcit | 1.043 (1.003–1.085) | 0.033 |
BMI indicates body mass index; GFR, glomerular filtration rate; Hcit, homocitrulline; NS, not significant; OR, odds ratio.
Table 5.
Diagnostic Values of Homocitrulline Levels in the Assessment of Poor Collateral Growth in Different Subgroups
| n | Poor, n (%) | AUC (95% CI) | P Value | |
|---|---|---|---|---|
| Overall | 117 | 58 (49.6) | 0.632 (0.538–0.719) | 0.011 |
| Diabetes mellitus | 52 | 32 (61.5) | 0.674 (0.530–0.798) | 0.021 |
| GFR <90, mL/min/1.73 m2 | 81 | 43 (53.1) | 0.603 (0.489–0.710) | 0.11 |
AUC indicates area under the curve; GFR, glomerular filtration rate.
Discussion
To the best of our knowledge, this report is the first demonstrating cyanate‐impaired angiogenesis in a mouse hind‐limb ischemia model. Cyanate, together with its carbamylated products, inhibited endothelial migration, proliferation, and tube formation partly by interrupting the VEGFR2/PI3K/Akt pathway. Moreover, our observational study indicated that impaired angiogenesis induced by cyanate might contribute to poor coronary collateral growth in patients with stable angina and CTO.
Coronary collateral development is a physiological adaptive response to high‐grade stenosis or occlusion and is capable of supplying perfusion to ischemic tissue distal to an occluded segment.21, 22 However, this protective physiological response displays significant interindividual heterogeneity in patients with CTO, and the underlying mechanism remains uncharacterized. An imbalance between pro‐ and antiangiogenic factors after artery occlusion might lead to poor collateral growth and adverse clinical outcomes in patients with coronary artery disease.23, 24, 25
In this study, we reported for the first time that the concentration of homocitrulline, a marker of cyanate exposure, was increased in patients with poor coronary collateralization compared with those with high collateralization. Furthermore, elevated homocitrulline concentration was associated with poor collateral development in patients with angina and CTO, even after adjusting for other significant risk factors of coronary artery disease. Consequently, our observational study, coupled with the fact that homocitrulline levels are often used as a biomarker of renal impairment and enhanced inflammation,11, 26, 27 indicated that increasing homocitrulline levels might serve as a risk factor for poor collateral growth. Our clinical data also demonstrated a link between impaired coronary collateral development and the antiangiogenic regulation of cyanate.
Cyanate was orally administered in mice to test its antiangiogenic properties in vivo. After hind‐limb ischemia, a reduction in blood flow recuperation at day 21 was observed in the ischemic tissue of cyanate‐treated mice compared with that of the control mice at both the macrovascular and microvascular levels. Consequently, the impaired tissue perfusion induced by cyanate might be mediated in part by the attenuation of endothelial angiogenic function. Similarly, recent studies have demonstrated endothelial dysfunction and reduced endothelial nitric oxide synthase in cyanate‐treated mice.17 Our results, combined with others, suggest that exposure to cyanate in vivo could impair endothelial function.
We also evaluated the antiangiogenic activity of cyanate on HAECs in vitro. Based on our results, the mechanisms by which cyanate could impair angiogenesis are potentially diverse. First, carbamylation mediates protein alteration, which can yield both structural and functional changes in different pathophysiological conditions. Nonetheless, the biological effects of the target carbamylated proteins can differ dramatically, and thus the risks associated with these different carbamylated proteins are distinct. Accumulating evidence from clinical and basic studies has illustrated that carbamylated lipoproteins, like carbamylated low‐ and high‐density lipoproteins, harbor atherogenic properties.28, 29, 30, 31 Interestingly, carbamylation of immunoglobulin participates in the pathogenesis of rheumatoid arthritis32; however, the overall effect of carbamylated circulating proteins on endothelial cells has not been studied. In the present research, we incubated endothelial cells with carbamylated cell culture medium to mimic the overall circulating carbamylated state in vivo. Our results provide evidence that the functional activities of endothelial cells are attenuated following carbamylated medium treatment.
Our evidence also supports cyanate itself having antiangiogenic effects, which are independent of inducing protein carbamylation. In the present study, to avoid the protein carbamylation effect, cyanate was incubated with starved endothelial cells to test its direct antiangiogenic properties. A significant reduction in migration of the cyanate‐treated endothelial cells was observed, as was impairment of both proliferation and tube formation. This result is consistent with recent data showing that cyanate itself amplifies vascular inflammation.17 Our in vitro study suggested that cyanate impaired angiogenesis by at least 2 mechanisms: (1) direct effects on endothelial cells, and (2) indirect effects by promoting protein carbamylation. Our in vivo and in vitro studies together indicated that cyanate limits physiological angiogenesis.
VEGF is the most potent regulator of angiogenesis. The angiogenic response to VEGF is mainly mediated through activation of VEGFR2 and initiation of its downstream signaling pathway to promote angiogenesis.33, 34 Several lines of investigation suggest that inhibition of VEGFR2 induces impaired angiogenesis.25, 35 Our in vitro study indicated that the observed decrease in angiogenesis ability in cyanate‐treated endothelial cells could be attributed to the attenuation of VEGFR2 expression rather than phosphorylation (Figure S1).
In summary, this report is the first describing cyanate as a negative regulator of angiogenesis and thus a contributor to poor collateral growth. This study could provide a reference for novel risk assessment and therapeutic strategy for patients with angina and CTO.
Author Contributions
Sun, Yang, Shen, Zhang conceived and designed the experiments. Sun, Mao, Q.H. Wu, L.P. Wu, Wang performed the experiments. Yang, Mao, Lu analyzed the data. Sun, Yang, Zhang wrote the manuscript.
Sources of Funding
This work was supported by grants from the Chinese National Nature Science Foundation (81370401, 81470547, 81570316).
Disclosures
None.
Supporting information
Figure S1. Effect of carbamylated medium or cyanate on phosphorylation level of VEGFR2. A, HAECs were treated with low, moderate, or high carbamylated medium or with serum medium (control) for 1 hour to measure phosphorylation of VEGFR2. B, HAECs were serum deprived and incubated with 1, 5, or 10 mmol/L cyanate or with sodium chloride (control) for 1 hour to test the phosphorylation of VEGFR2. C, Band intensity was quantified by scanning densitometry in 3 independent experiments. C‐medium indicates carbamylated medium; HAEC, human arterial endothelial cell; VEGFR2, vascular endothelial growth factor receptor 2
(J Am Heart Assoc. 2016;5:e004700 doi: 10.1161/JAHA.116.004700)
References
- 1. Cochain C, Channon KM, Silvestre JS. Angiogenesis in the infarcted myocardium. Antioxid Redox Signal. 2013;18:1100–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schaper W. Collateral circulation: past and present. Basic Res Cardiol. 2009;104:5–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Meier P, Hemingway H, Lansky AJ, Knapp G, Pitt B, Seiler C. The impact of the coronary collateral circulation on mortality: a meta‐analysis. Eur Heart J. 2012;33:614–621. [DOI] [PubMed] [Google Scholar]
- 4. Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–660. [DOI] [PubMed] [Google Scholar]
- 5. Arderiu G, Pena E, Badimon L. Angiogenic microvascular endothelial cells release microparticles rich in tissue factor that promotes postischemic collateral vessel formation. Arterioscler Thromb Vasc Biol. 2015;35:348–357. [DOI] [PubMed] [Google Scholar]
- 6. Zhu JZ, Zhang J, Yang K, Du R, Jing YJ, Lu L, Zhang RY. P‐cresol, but not p‐cresylsulphate, disrupts endothelial progenitor cell function in vitro. Nephrol Dial Transplant. 2012;27:4323–4330. [DOI] [PubMed] [Google Scholar]
- 7. Zhu J, Yang K, Jing Y, Du R, Zhu Z, Lu L, Zhang R. The effects of low‐dose nepsilon‐(carboxymethyl)lysine (CML) and nepsilon‐(carboxyethyl)lysine (CEL), two main glycation free adducts considered as potential uremic toxins, on endothelial progenitor cell function. Cardiovasc Diabetol. 2012;11:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hawkins CL. Role of cyanate in the induction of vascular dysfunction during uremia: more than protein carbamylation? Kidney Int. 2014;86:875–877. [DOI] [PubMed] [Google Scholar]
- 9. Stark GR. Reactions of cyanate with functional groups of proteins. II. Formation, decomposition, and properties of N‐carbamylimidazole. Biochemistry. 1965;4:588–595. [DOI] [PubMed] [Google Scholar]
- 10. Blackmore DJ, Elder WJ, Bowden CH. Urea distribution in renal failure. J Clin Pathol. 1963;16:235–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang Z, Nicholls SJ, Rodriguez ER, Kummu O, Horkko S, Barnard J, Reynolds WF, Topol EJ, DiDonato JA, Hazen SL. Protein carbamylation links inflammation, smoking, uremia and atherogenesis. Nat Med. 2007;13:1176–1184. [DOI] [PubMed] [Google Scholar]
- 12. Jaisson S, Pietrement C, Gillery P. Carbamylation‐derived products: bioactive compounds and potential biomarkers in chronic renal failure and atherosclerosis. Clin Chem. 2011;57:1499–1505. [DOI] [PubMed] [Google Scholar]
- 13. Ok E, Basnakian AG, Apostolov EO, Barri YM, Shah SV. Carbamylated low‐density lipoprotein induces death of endothelial cells: a link to atherosclerosis in patients with kidney disease. Kidney Int. 2005;68:173–178. [DOI] [PubMed] [Google Scholar]
- 14. Speer T, Owala FO, Holy EW, Zewinger S, Frenzel FL, Stahli BE, Razavi M, Triem S, Cvija H, Rohrer L, Seiler S, Heine GH, Jankowski V, Jankowski J, Camici GG, Akhmedov A, Fliser D, Luscher TF, Tanner FC. Carbamylated low‐density lipoprotein induces endothelial dysfunction. Eur Heart J. 2014;35:3021–3032. [DOI] [PubMed] [Google Scholar]
- 15. Apostolov EO, Ray D, Savenka AV, Shah SV, Basnakian AG. Chronic uremia stimulates LDL carbamylation and atherosclerosis. J Am Soc Nephrol. 2010;21:1852–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sun JT, Yang K, Lu L, Zhu ZB, Zhu JZ, Ni JW, Han H, Chen N, Zhang RY. Increased carbamylation level of HDL in end‐stage renal disease: carbamylated‐HDL attenuated endothelial cell function. Am J Physiol Renal Physiol. 2016;310:F511–F517. [DOI] [PubMed] [Google Scholar]
- 17. El‐Gamal D, Holzer M, Gauster M, Schicho R, Binder V, Konya V, Wadsack C, Schuligoi R, Heinemann A, Marsche G. Cyanate is a novel inducer of endothelial ICAM‐1 expression. Antioxid Redox Signal. 2012;16:129–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. El‐Gamal D, Rao SP, Holzer M, Hallstrom S, Haybaeck J, Gauster M, Wadsack C, Kozina A, Frank S, Schicho R, Schuligoi R, Heinemann A, Marsche G. The urea decomposition product cyanate promotes endothelial dysfunction. Kidney Int. 2014;86:923–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Couffinhal T, Silver M, Zheng LP, Kearney M, Witzenbichler B, Isner JM. Mouse model of angiogenesis. Am J Pathol. 1998;152:1667–1679. [PMC free article] [PubMed] [Google Scholar]
- 20. Rentrop KP, Cohen M, Blanke H, Phillips RA. Changes in collateral channel filling immediately after controlled coronary artery occlusion by an angioplasty balloon in human subjects. J Am Coll Cardiol. 1985;5:587–592. [DOI] [PubMed] [Google Scholar]
- 21. Traupe T, Gloekler S, de Marchi SF, Werner GS, Seiler C. Assessment of the human coronary collateral circulation. Circulation. 2010;122:1210–1220. [DOI] [PubMed] [Google Scholar]
- 22. Meier P, Schirmer SH, Lansky AJ, Timmis A, Pitt B, Seiler C. The collateral circulation of the heart. BMC Med. 2013;11:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Clayton JA, Chalothorn D, Faber JE. Vascular endothelial growth factor‐a specifies formation of native collaterals and regulates collateral growth in ischemia. Circ Res. 2008;103:1027–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Seiler C, Stoller M, Pitt B, Meier P. The human coronary collateral circulation: development and clinical importance. Eur Heart J. 2013;34:2674–2682. [DOI] [PubMed] [Google Scholar]
- 25. Freedman SB, Isner JM. Therapeutic angiogenesis for ischemic cardiovascular disease. J Mol Cell Cardiol. 2001;33:379–393. [DOI] [PubMed] [Google Scholar]
- 26. Drechsler C, Kalim S, Wenger JB, Suntharalingam P, Hod T, Thadhani RI, Karumanchi SA, Wanner C, Berg AH. Protein carbamylation is associated with heart failure and mortality in diabetic patients with end‐stage renal disease. Kidney Int. 2015;87:1201–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jaisson S, Kazes I, Desmons A, Fadel F, Oudart JB, Santos‐Weiss IC, Millart H, Toure F, Rieu P, Gillery P. Homocitrulline as marker of protein carbamylation in hemodialyzed patients. Clin Chim Acta. 2016;460:5–10. [DOI] [PubMed] [Google Scholar]
- 28. Carracedo J, Merino A, Briceno C, Soriano S, Buendia P, Calleros L, Rodriguez M, Martin‐Malo A, Aljama P, Ramirez R. Carbamylated low‐density lipoprotein induces oxidative stress and accelerated senescence in human endothelial progenitor cells. FASEB J. 2011;25:1314–1322. [DOI] [PubMed] [Google Scholar]
- 29. Holzer M, Gauster M, Pfeifer T, Wadsack C, Fauler G, Stiegler P, Koefeler H, Beubler E, Schuligoi R, Heinemann A, Marsche G. Protein carbamylation renders high‐density lipoprotein dysfunctional. Antioxid Redox Signal. 2011;14:2337–2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shiu SW, Xiao SM, Wong Y, Chow WS, Lam KS, Tan KC. Carbamylation of LDL and its relationship with myeloperoxidase in type 2 diabetes mellitus. Clin Sci (Lond). 2014;126:175–181. [DOI] [PubMed] [Google Scholar]
- 31. Verbrugge FH, Tang WH, Hazen SL. Protein carbamylation and cardiovascular disease. Kidney Int. 2015;88:474–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Koro C, Bielecka E, Dahl‐Knudsen A, Enghild JJ, Scavenius C, Brun JG, Binder V, Hellvard A, Bergum B, Jonsson R, Potempa J, Blom AM, Mydel P. Carbamylation of immunoglobulin abrogates activation of the classical complement pathway. Eur J Immunol. 2014;44:3403–3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Holmes K, Roberts OL, Thomas AM, Cross MJ. Vascular endothelial growth factor receptor‐2: structure, function, intracellular signalling and therapeutic inhibition. Cell Signal. 2007;19:2003–2012. [DOI] [PubMed] [Google Scholar]
- 34. Olsson AK, Dimberg A, Kreuger J, Claesson‐Welsh L. VEGF receptor signalling—in control of vascular function. Nat Rev Mol Cell Biol. 2006;7:359–371. [DOI] [PubMed] [Google Scholar]
- 35. Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Effect of carbamylated medium or cyanate on phosphorylation level of VEGFR2. A, HAECs were treated with low, moderate, or high carbamylated medium or with serum medium (control) for 1 hour to measure phosphorylation of VEGFR2. B, HAECs were serum deprived and incubated with 1, 5, or 10 mmol/L cyanate or with sodium chloride (control) for 1 hour to test the phosphorylation of VEGFR2. C, Band intensity was quantified by scanning densitometry in 3 independent experiments. C‐medium indicates carbamylated medium; HAEC, human arterial endothelial cell; VEGFR2, vascular endothelial growth factor receptor 2


