Abstract
Background
Differences in prognosis and baseline clinical presentation have been documented among patient with acute coronary syndrome and coronary artery disease with obstructive (ObCAD) or nonobstructive arteries (NObCAD), but the rates of events largely varied across single studies. We carried out a meta‐analysis to compare the clinical presentation and prognosis of NObCAD versus ObCAD acute coronary syndrome patients, as well as of the subjects with zero versus mild occlusion.
Methods and Results
Searches were made in MedLine, EMBASE, Cochrane databases, and proceedings of international meetings up to June 30, 2015. We compared the risk of events of NObCAD versus ObCAD patients using random‐effect meta‐analyses. We also performed meta‐analyses to estimate the yearly or monthly outcome rates in each single group. In NObCAD and ObCAD patients, respectively, the combined yearly rates were as follows: 2.4% versus 10.1% (all‐cause mortality); 1.2% versus 6.0% (myocardial infarction), 4.0% versus 12.8% (all‐cause mortality plus myocardial infarction), 1.4% versus 5.9% (cardiac death), and 9.2% versus 16.8% (major cardiovascular events). In the studies directly comparing NObCAD versus ObCAD, all of the above outcomes were significantly less frequent in NObCAD subjects (with risk ratios ranging from 0.33 to 0.66). No differences in any outcome rate were observed between mild occlusion (1–49% stenosis) and zero occlusion patients.
Conclusions
NObCAD in patients with acute coronary syndrome has a significantly lower cardiovascular risk at baseline and a subsequent lower likelihood of death or main cardiovascular events. However, these subjects are still at high risk for cardiovascular mortality and morbidity, suggesting potential undertreatment and calling for specific management.
Keywords: acute coronary syndrome, acute myocardial infarction, angina pectoris, coronary artery disease, epicardial vessel stenosis, meta‐analysis, microcirculation, nonobstructive coronary artery disease, obstructive coronary artery disease, prognosis
Subject Categories: Meta Analysis, Mortality/Survival, Acute Coronary Syndromes
Introduction
Coronary artery disease (CAD) is the leading cause of death, morbidity, and disability in Western countries.1 Among CAD patients, acute coronary syndrome (ACS) represents a serious concern because of the major adverse cardiac events (MACE) during follow‐up.2
ACS may develop from the erosion or rupture of obstructive (due to thrombus formation) or nonobstructive coronary atherosclerotic plaques.3, 4 The latter condition, commonly defined as nonobstructive CAD (NObCAD), is less common than obstructive CAD (ObCAD), with a prevalence ranging from 5% to 25%,5, 6 and it has been associated with lower rates of clinical outcomes in several studies.7, 8, 9, 10
A recent systematic review compared the death rates of patients with myocardial infarction and nonobstructive versus obstructive coronary arteries.6 However, no meta‐analyses directly compared the rates of other outcomes including re‐infarction, cardiac death, and MACE in NObCAD versus ObCAD ACS patients.
We carried out a meta‐analysis to compare the likelihood of several clinical outcomes in NObCAD and ObCAD ACS patients, to estimate the rates of events, and to investigate other hypotheses including the potential differences in the prognosis of NObCAD subjects with zero or mild occlusion (0% versus 1–50% stenosis), and the differences in baseline presentation between NObCAD and ObCAD subjects.
Methods
Search, Study Inclusion Criteria, and Quality Assessment
Study inclusion criteria were as follows: (1) inclusion of patients with obstructive or nonobstructive coronary lesion ACS at baseline; (2) prospective or retrospective assessment of ≥1 of the following outcomes: all‐cause mortality, myocardial infarction, all‐cause mortality plus myocardial infarction, cardiac death, and MACE. The search was initially made online in MedLine, Scopus, EMBASE, and the Cochrane Controlled Clinical Trial Register (up to June 2015, with no language restriction). The bibliographies of all relevant articles including reviews were reviewed. When it was not possible to extract any safety or efficacy outcome from a potentially eligible study, attempts to contact the corresponding author were made. The search string was adjusted for each database while maintaining a common overall architecture. We used various combinations of the following terms related to 2 main domains: “death” OR “all‐cause death” OR “all‐cause mortality” OR “mortality” OR “cardiac death” OR “death for cardiovascular disease” OR “myocardial infarction” OR “re‐infarction” OR “MACE” OR “major adverse cardiovascular events” OR “coronary heart disease” (title/abstract) AND “coronary heart disease” OR “heart disease” OR “cardiovascular disease” OR “acute myocardial infarction” OR “angina*” OR “acute coronary syndrome” OR “unstable angina” OR “non‐ST segment elevation myocardial infarction (NSTE‐ACS)” OR “ST‐elevation myocardial infarction (STE‐ACS)” OR “coronary angiograms” OR “normal coronary angiograms,” OR “near‐normal coronary angiograms” OR “non‐obstructive coronary atherosclerosis” and “obstructive coronary atherosclerosis” OR “insignificant coronary artery disease” OR “significant coronary artery disease” OR “mild coronary artery disease”.
We excluded the studies that reported data only on particular subtypes of subjects, eg, ACS due to spontaneous coronary dissection, takotsubo cardiomyopathy, or myocarditis, as well as studies in which coronary angiography in the acute phase of ACS was not performed. When both ACS and stable CAD patients were included in a study, we included the study only if data on ACS subjects could be extracted separately.
Because all the retrieved studies were observational or observational subgroup analyses of randomized trials, we assessed the aspects of the reported methodological quality using an adapted version of the Newcastle Ottawa Quality Assessment Scale, evaluating the comparability across groups at baseline for confounding factors (and examining whether analyses were adjusted adequately for confounders), the appropriateness of outcome assessment, length of follow‐up, and missing data handling and reporting.11
Data Extraction
Using a standardized data extraction form, 2 independent investigators (C.P. and G.M.C.) extracted and tabulated all data. These investigations were not blinded to authors or to institutions. Discrepancies were resolved through revision of the original articles and group discussions. The extracted information included the following: editorial information (lead author, publication year, study size, study design, duration of follow‐up, type and source of financial support, and publication status), clinical presentation of ACS (ST‐elevation acute myocardial infarction, non‐ST‐elevation acute myocardial infarction, and unstable angina), study population information (number of patients for each study, percentage of male population, age, percentage of patients presenting with obstructive and nonobstructive coronary artery disease), coronary risk factors such as smoking, hypertension, dyslipidemia, diabetes mellitus, and findings of coronary angiograms. If the results were presented for more than 1 time‐point, the last available results were extracted.
Outcomes and Data Analysis
NObCAD was defined as no epicardial vessel with a stenosis ≥50% by quantitative coronary angiography. Nonobstructive lesions were additionally grouped as normal coronary vessels (0% lumen stenosis in all vessels) and mild coronary stenosis (1–49% lumen stenosis in at least 1 vessel).
The main outcome was all‐cause mortality during follow‐up; secondary outcomes were myocardial infarction, all‐cause mortality plus myocardial infarction, cardiac death and MACE, as defined by the authors. The definitions of cardiovascular disease for each included study7, 8, 9, 10, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44 are shown in Table 1, together with study characteristics. We extracted both adjusted or propensity score matched estimations and raw data to build 2×2 tables, at any time‐point. However, adjusted or propensity score matched estimates were available from 3 studies only,7, 8, 32 and we thus performed all analyses using raw data.
Table 1.
Characteristics of the Included Studies
| Study | Year | Design | N | Non Obstr. | Obstr. | Follow‐up | Extracted Outcomes | Study Years | ACS Type | CAD Stratification | ACS Definition/Cardiac Enzyme | Outcome Ascertainment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Studies included in all meta‐analyses | ||||||||||||
| Raymond37 | 1988 | Observ. | 148 | 74 | 74 | 126 months | Death, Cardiac deatha, MIa | 1968–1985 | STE‐ACS, NSTE‐ACS | >50%; ≤50% | Study protocol (Protocol) | Visit |
| Roe39 | 2000 | RCT | 5767 | 696 | 5071 | 1, 6 months | Death, MI | 1995–1997 | NSTE‐ACS, UA | >50%; 0% to 50%; 0% | Protocol, CK | Events reporting records |
| Da Costa18 | 2001 | Observ. | 176 | 88 | 88 | 34 months | MACE, Cardiac death, MI | 1994–1999 | STE‐ACS, NSTE‐ACS | >50%; ≤50% | Protocol | Phone contacts, visit |
| Dokainish7 | 2005 | RCT (sub‐set) | 895 | 107 | 788 | 6 months | Death, MI, ACS | 1997–1999 | STE‐ACS NSTE‐ACS, UA | >50%; ≤50% | Protocol, Troponin | RCT, events reporting records |
| Larsen28 | 2005 | Observ. | 9797 | 726 | 9071 | 12 months | Death | 1995–2000 | STE‐ACS, NSTE‐ACS | >50%; 0% to 50%; 0% | ICD codes | ICD codes |
| Pinheiro34 | 2005 | Observ. | 1351 | 220 | 1131 | In hospital | Death | 1996–2002 | STE‐ACS, NSTE‐ACS | ≥50%; <50% | Protocol, CK | Visit |
| Patel10 | 2006 | Observ. | 38 301 | 3306 | 34 995 | In hospital | Death, MI | 2001–2006 | NSTE‐ACS | >50%; ≤50% | Protocol, Troponin, CK | Visit |
| Larson29 | 2007 | Observ. | 1325 | 187 | 1138 | 12 months | Death | 2003–2006 | STE‐ACS | >50%; ≤50% | Protocol, Troponin | Events reporting records |
| Terefe43 | 2007 | Observ. | 112 | 56 | 56 | 39 months | Cardiac death, MI | 2000–2006 | STE‐ACS, NSTE‐ACS, | >50%; ≤50% | Protocol, Troponin, CK | Not reported |
| Dey19 | 2009 | Observ. | 20 692 | 1560 | 19 132 | 6 months | Death, MI | 1999–2006 | STE‐ACS, NSTE‐ACS, UA | >50%; ≤50% | Protocol, Troponin, CK | Events reporting records |
| Dwyer20 | 2008 | RCT | 180 | 29 | 151 | 12 months | Death+MI | 2003–2004 | UA, MI | >50%; ≤50% | ICD codes | Medical Records, visit |
| Von Korn44 | 2008 | Observ. | 636 | 127 | 509 | 12 months | Death, Cardiac death, MI | 2002–2005 | STE‐ACS, NSTE‐ACS | >50%; ≤50% | Protocol, Troponin, CK | Phone contacts, visit |
| Cortell17 | 2009 | Observ. | 504 | 64 | 440 | 36 months | Death, MI | 2001–2008 | NSTE‐ACS | >50%; ≤50% | Protocol, Troponin | Not reported |
| Kang27 | 2011 | Observ. | 3056 | 126 | 2930 | 6, 12 months | MACE, Death, Cardiac death, MI | 2005–2006 | STE‐ACS, NSTE‐ACS | >50%; ≤50% | Protocol, Troponin, CK | Phone contact, visit |
| Ramanath36 | 2010 | Observ. | 2264 | 123 | 2141 | 6 months | MACE, Death, MI | 1999–2004 | STE‐ACS, NSTE‐ACS, UA | >50%; ≤50% | Protocol, Troponin, CK | Phone contact |
| Hamdan22 | 2012 | Observ. | 124 | 11 | 113 | In hospital | Death | 2008–2009 | STE‐ACS, NSTE‐ACS | ≥50%; <50% | Protocol, Troponin | Visit |
| Rhew38 | 2012 | Observ. | 1220 | 100 | 1120 | 1, 12 months | MACE, Death, Cardiac death, MI | 2006–2008 | STE‐ACS, NSTE‐ACS, | >50%; ≤50% | Protocol, Troponin, CK | Phone contacts, Hospital database |
| Sun42 | 2012 | Observ. | 695 | 49 | 646 | 36 months | MACE, Death, Cardiac death, MI | 2007–2008 | STE‐ACS, NSTE‐ACS, UA | >50%; ≤50% | Protocol, Troponin, CK | Medical records, GPs, death certificates |
| Rossini40 | 2013 | Observ. | 1206 | 888 | 318 | 26 months | MACE, Death, MI | 2009 | STE‐ACS, NSTE‐ACS, UA | >50%; 0% to 50%; 0% | Protocol, Troponin | Events reporting records |
| Manfrini30 | 2014 | Observ. | 1602 | 350 | 1252 | 6 months | Cardiac death | 2003 | STE‐ACS, NSTE‐ACS, UA | >50%; ≤50% | Protocol, Troponin | Events reporting records |
| Minha31 | 2014 | Observ. | 3686 | 163 | 3523 | 1, 12 months | MACE (30 days), Death | 2004–2010 | STE‐ACS, NSTE‐ACS, UA | >50%; 0% to 50%; 0% | Protocol, Troponin, CK | Phone contacts, visit |
| Planer35 | 2014 | RCT (subset) | 2442 | 197 | 2245 | 1, 12 months | Death, Cardiac death, MI | 2003–2005 | NSTE‐ACS, UA | >50%; ≤50% | Protocol, Troponin | Events reporting records |
| Studies included only in the meta‐analyses of event rates by single group | ||||||||||||
| Harris9 | 1980 | Observ. | 1183 | 0 | 1183 | 6, 12 months | Death, Cardiac death, MI | 1973–1978 | STE‐ACS | >50% | Protocol | Phone contacts, visit |
| Hung25 | 2003 | Observ. | 19 | 19 | 0 | 24 months | Death, Cardiac death | 1998–2000 | STE‐ACS, NSTE‐ACS | >50% | Protocol, CK | Phone contacts, visit |
| Golzio8 | 2004 | Observ. | 53 | 53 | 0 | 125 months | Death, Cardiac death | 1985–1990 | STE‐ACS, NSTE‐ACS | ≤50%; 0% | Protocol, CK | Phone contacts, visit |
| Bugiardini14 | 2006 | 3 RCT (subset) | 701 | 701 | 0 | 12 months | MACE, Death, MI | 1996–2001 | NSTE‐ACS, UA | <50%; 0% | Protocol, CK | Medical records, visit |
| Shishehbor41 | 2007 | Observ. | 1240 | 0 | 1240 | 28 months | Death, MI | 1995–2005 | NSTE‐ACS, UA | ≥50% | Protocol, Troponin, CK | Medical records, phone contacts |
| Chan15 | 2008 | Observ. | 8225 | 0 | 8225 | 6, 12 months | Death, MI (6 months) | 2001–2003 | NSTE‐ACS, UA | >50% | Protocol, Troponin, CK | Medical records, phone contacts, visit |
| Hansen23 | 2012 | Observ. | 1595 | 1595 | 0 | 36 months | Death, MI | 2005–2007 | STE‐ACS, NSTE‐ACS | ≤50% | Protocol, CK | ICD codes |
| Abid12 | 2012 | Observ. | 21 | 21 | 0 | 24 months | Death, MI | 2006–2011 | STE‐ACS | >50% | Protocol, CK | Medical records, phone contacts |
| Aldous13 | 2015 | Observ. | 351 | 351 | 0 | 24 months | Death, Cardiac death, MI | 2007–2011 | STE‐ACS, NSTE‐ACS | <50% | Protocol, Troponin | Medical records |
| Johnston26 | 2015 | Observ. | 10 588 | 10 588 | 0 | 31 months | Death, MI | 2005–2010 | STE‐ACS, NSTE‐ACS | <50% | Protocol, Troponin | National registry, Medical records |
| Ohlow32 | 2015 | Observ. | 393 | 204 | 189 | 27, 17 months | Death, Cardiac death, MI | 2002–2011 | STE‐ACS, NSTE‐ACS | >50%; 0% to 50%; 0% | Protocol, Troponin | Medical records, GPs, phone contacts, visit |
| Studies included in meta‐analyses of single groups on the baseline proportion of STE‐ACS only | ||||||||||||
| Hochman24 | 1999 | RCT | 6406 | 737 | 5669 | 1 month | Baseline % of STE‐ACS | 1994–1996 | STE‐ACS, NSTE‐ACS, UA | >50%; ≤50% | Protocol, CK | Events reporting records |
| Germing21 | 2005 | Observ. | 897 | 76 | 821 | 26 months | Baseline % of STE‐ACS | 1996–2000 | STE‐ACS, UA | >50%; ≤50% | Protocol, Troponin, CK | Questionnaire, Phone contact, Medical records |
| Ong33 | 2008 | Observ. | 488 | 138 | 350 | 0 months | Baseline % of STE‐ACS | 2006 | STE‐ACS, NSTE‐ACS, UA | >50%; ≤50% | Protocol, Troponin, CK | — |
| Chokshi16 | 2010 | Observ. | 518 | 106 | 412 | 0 months | Baseline % of STE‐ACS | 2006 | STE‐ACS, NSTE‐ACS, UA | >50%; ≤50% | Protocol, not reported | — |
ACS indicates acute coronary syndrome; CAD, coronary artery disease; CK, creatine kinase; GPs, general practitioners; ICD, International Classification of Diseases; MACE, major adverse cardiac events; MI, myocardial infarction; N, Number of subjects for whom data were extracted and used in the analyses; NSTE, non‐ST segment elevation; Observ., observational; Obstr., obstructive; RCT, randomized controlled trial; UA, unstable angina.
Only for the meta‐analyses of event rates by group: no data were provided on the nonobstructive coronary artery disease group.
The primary, prespecified hypothesis of the study was that clinical outcomes were significantly less frequent in NObCAD than ObCAD patients. This hypothesis was evaluated through random‐effect head‐to‐head meta‐analyses, which included only the studies reporting data on both ObCAD and NObCAD subjects.45 All analyses were stratified by follow‐up duration (1–6 months, ≥12 months). The results were expressed with risk ratio (RR) and 95% CI, and the statistical heterogeneity was quantified using the I2 metric.46
In order to provide some estimates of the incidence rates for each selected outcome, we also performed meta‐analyses of event rates (sometimes defined as “proportion meta‐analysis”) combining the data of NObCAD and ObCAD patients separately.47 Thus, in such analyses we could also include the studies reporting data on NObCAD subjects only (or data on ObCAD patients only), and study crude rates were divided by the number of months of follow‐up to estimate the monthly and yearly rates for each outcome.
Two secondary hypotheses were also investigated: (1) among NObCAD subjects, some clinical outcomes may be less frequent in patients with normal artery CAD (0% stenosis) versus mildly obstructive CAD (1–50% stenosis); (2) NObCAD patients, as compared to ObCAD subjects, may have less cardiovascular risk factors at baseline (including higher age, male sex, diabetes mellitus, hypertension, dyslipidemia, current cigarette smoking), and they may less frequently present with STE‐ACS at hospital discharge and be treated with cardiovascular drugs such as angiotensin‐converting enzyme inhibitors, β‐blockers, statins, aspirin, or P2Y12 inhibitors. As for the primary hypothesis, we used random‐effect meta‐analyses comparing the 2 groups directly, and we estimated the crude outcome rates (or baseline proportions) in both groups through meta‐analyses of the event rates. A random‐effect generic inverse variance approach was used to estimate the mean age at baseline within single groups.
Potential publication bias was assessed using funnel plots (displaying RRs from individual studies versus their precision [1/SE]), and formally tested through the Egger regression asymmetry test.
We used StatsDirect 2.7.8 (StatsDirect Ltd, Altrincham, UK, 2010) and RevMan 5.3 (The Cochrane Collaboration, 2014), to perform, respectively, the meta‐analyses of the event rates and the meta‐analyses comparing directly NObCAD versus ObCAD patients.
Results
Search Results and Overall Study Characteristics
Of the 984 papers initially retrieved (Figure), we identified 33 studies (including a total of 120 548 participants) that evaluated the selected cardiovascular outcomes in NObCAD and/or ObCAD patients.1 Of those, 11 studies (24 369 participants) did not compare directly NObCAD versus ObCAD patients, and thus could be included only in the meta‐analyses estimating the rates of each selected outcome by single group.2 Four other studies (8309 participants) were included only in the meta‐analyses evaluating the baseline proportion of STE‐ACS patients by single group.16, 21, 24, 33
Figure 1.
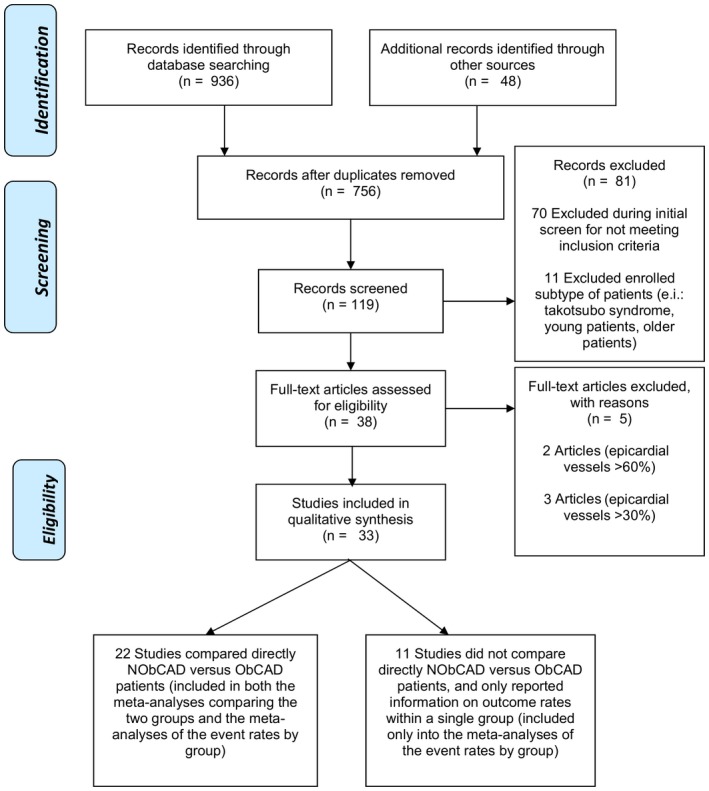
Flowchart of the studies. NObCAD, nonobstructive coronary artery disease; ObCAD, obstructive coronary artery disease.
As reported in Table 1, 7 of the 33 included studies were carried out in the United States, 10 in Europe, 6 studies were international, and the remaining 10 took place in other countries. Three studies were re‐analyses of randomized controlled trials,7, 14, 35 3 studies were randomized controlled trials,20, 24, 39 and all the others had an observational design. Seventeen studies had a sample size >1000; 12 were published after 2010; 25 had a follow‐up ≥12 months. Eight studies further categorized NObCAD patients in mildly versus zero obstructive CAD, and could thus be included into a dedicated meta‐analysis. In 15 studies the outcomes were ascertained through medical visits. The included studies differed widely in the proportion of NObCAD patients and in several baseline patient's characteristics, including the mean age, the percentage of males, diabetics, hypertensive, dyslipidemic, smokers, and subjects with STE‐ACS, unstable angina, and non‐ST elevation myocardial infarction–ACS (Table S1).
Also, because of the large time span of the studies included, and sometimes to their long follow‐up, the definition of ACS has been quite heterogeneous both within and across the studies. Before the Myocardial Universal Definition of 2007, ACS was defined on the basis of symptoms, ECG abnormalities, and cardiac enzymes (mainly creatine kinase MB fraction).48 After 2007, the measurement of cardiac troponin T or I has been preferred over the measurement of creatine kinase MB fraction or other biomarkers for ACS diagnosis. Of the 33 included studies, 12 were published before 2007, and only 1 of these dosed serum troponin and gave results differentiating unstable angina from non‐ST‐segment elevation myocardial infarction.7 After 2007, only 10 studies3 considered patients with unstable angina, and none reported outcomes stratified by type of ACS.
Methodological Quality
The methodological characteristics of the included studies, as measured by the Newcastle Ottawa Quality Assessment Scale,11 are summarized in Table S2. Almost all studies adequately selected the cohort of patients and ascertained the exposure (selection category items); 22 of the 33 studies adequately addressed at least 2 of the 3 items referred to outcome assessment and follow‐up (length and missing data). Among the 22 studies included in head‐to‐head meta‐analyses, the comparability of NObCAD versus ObCAD subjects was not addressed in 14 studies, and only 8 studies reported some form of adjustment for potential confounders.
Differences in the Baseline Characteristics of NObCAD Versus ObCAD Patients
As compared with ObCAD subjects, NObCAD patients were significantly younger (−6.2 years on average), less likely to be male (RR=0.77), diabetic (RR=0.57), hypertensive (RR=0.87), dyslipidemic (RR=0.75), and to be treated with angiotensin‐converting enzyme inhibitors (RR=0.86; 47.0% versus 53.7% among ObCAD patients), β‐blockers (RR=0.83; 70.0% versus 79.4%), statins (RR=0.82; 52.1% versus 64.2%), and P2Y12 inhibitors (RR=0.46; 29.2% versus 63.7%) (all P<0.01; Table 2, Figures S1 through S11). The rate of aspirin treatment was not significantly different (RR=0.94; 81.7% versus 86.8% among ObCAD).
Table 2.
Meta‐Analyses Comparing the Baseline Characteristics of NObCAD Versus ObCAD Patients
| Baseline Characteristics | No. Studies (Total Sample) | n/N | Risk Ratio (95% CI) | P Value | I2, % |
|---|---|---|---|---|---|
| Male sex | 22 (81 586) | 3686/7420 vs 50 870/74 166 | 0.77 (0.72–0.84) | <0.001 | 88 |
| Diabetes mellitus | 22 (81 586) | 1247/7420 vs 22 166/74 166 | 0.57 (0.47–0.78) | <0.001 | 86 |
| Hypertension | 22 (81 586) | 4099/7420 vs 45 511/74 166 | 0.87 (0.81–0.93) | <0.001 | 82 |
| Dyslipidemia | 22 (81 586) | 2680/7420 vs 31 035/74 166 | 0.75 (0.65–0.87) | <0.001 | 93 |
| Current smoking | 22 (81 586) | 2422/7420 vs 29 282/74 166 | 0.94 (0.85–1.03) | 0.2 | 81 |
| STE‐ACS | 11 (21 856) | 365/2229 vs 14 382/19 627 | 0.20 (0.13–0.29) | <0.001 | 93 |
| ACE‐inhibitors use | 8 (76 380) | 3052/6499 vs 37 554/69 881 | 0.86 (0.80–0.92) | <0.001 | 78 |
| β‐Blockers use | 8 (76 380) | 4419/6499 vs 55 486/69 881 | 0.83 (0.75–0.93) | 0.001 | 96 |
| Statins use | 8 (76 380) | 3389/6499 vs 44 892/69 881 | 0.82 (0.70–0.95) | 0.01 | 98 |
| Aspirin use | 8 (76 380) | 5311/6499 vs 60 675/69 881 | 0.94 (0.88–1.01) | 0.08 | 96 |
| P2Y12 inhibitors use | 6 (48 023) | 1197/4118 vs 27 951/43 905 | 0.46 (0.39–0.55) | <0.001 | 85 |
| Baseline Characteristics | No. Studies (Total Sample) | N/N | Mean Difference (95% CI) | P Value | I2, % |
|---|---|---|---|---|---|
| Mean age | 21 (81 438) | 7346/74 092 | −6.16 (−7.85; −4.47) | <0.001 | 94 |
| Mean LVEF | 6 (11 245) | 553/10 692 | 2.44 (0.50; 4.39) | 0.01 | 65 |
| Mean Troponin level, ng/mL | 4 (9822) | 295/9527 | −27.2 (−10.5; −43.8) | <0.001 | 97 |
ACE indicates angiotensin‐converting enzyme; LVEF, left ventricular ejection fraction; n, number of nonobstructive coronary artery disease subjects; N, number of obstructive coronary artery disease participants; NObCAD, nonobstructive coronary artery disease; ObCAD, obstructive coronary artery disease; STE‐ACS, ST‐elevation myocardial infarction.
Although a few studies reported baseline levels of troponin and left ventricular ejection fraction in both groups, the 2 prognostic parameters were significantly better in NObCAD versus ObCAD subjects (+2.44% and −27.2 ng/mL, respectively; both P<0.05) (Figures S12 and S13).
Overall, the likelihood of being diagnosed with STE‐ACS (rather than non‐ST segment elevation–ACS) at baseline was drastically lower among NObCAD versus ObCAD patients (RR=0.20; 95% CI: 0.13–0.29; Table 2, Figure S14).
The estimated rates of the above characteristics at baseline were computed using meta‐analyses by single group and are reported in Table S3 and Figures S15 through S38. Overall, the results were in line with the meta‐analyses comparing the 2 groups directly: among NObCAD and ObCAD patients, respectively, the mean ages were 56.9 and 63.2 years, and the proportions of STE‐ACS were 14.7% and 73.8%.
Crude Rates of Cardiovascular Outcomes in NObCAD and ObCAD Patients
Overall, 33 studies were included in at least 1 meta‐analysis to estimate the rates of cardiovascular outcomes in NObCAD and ObCAD patients (Table 3). In NObCAD patients, the combined yearly rates of death, myocardial infarction, death plus myocardial infarction, cardiac death, and MACE were 2.4%, 1.4%, 4.0%, 1.2%, and 9.2%, respectively (Table 3, Figures S39 through S43). The same rates in ObCAD patients were 10.1%, 5.9%, 12.8%, 6.0%, and 16.8% (Table 3, Figures S44 through S48).
Table 3.
Meta‐Analyses of the Event Rates by Single Group of CAD Patients
| Outcomes | ObCAD (>50% Stenosis) | NObCAD (0–50% Stenosis) | Zero CAD (0% Stenosis) | Mild CAD (1–50% Stenosis) |
|---|---|---|---|---|
| MACE | References 18, 27, 31, 36, 38, 40, 42 | References 14, 18, 27, 31, 36, 38, 40, 42 | References 14, 31, 40 | References 14, 31, 40 |
| No. studies (n/N; total person‐months) | 7 (2039/11 322; 152 890) | 8 (184/1668; 26 842) | — | — |
| Estimated pooled rate (95% CI) | ||||
| Per month | 1.40% (0.61%; 2.50%) | 0.77% (0.43%; 1.21%) | — | — |
| Per year | 16.8% (7.32%; 30.1%) | 9.24% (5.16%; 14.5%) | — | — |
| All deaths | References 7, 9, 10, 15, 17, 19, 22, 27, 28, 29, 31, 32, 34, 35, 36, 37, 38, 39, 40, 41, 42, 44 | References 1, 2, 4, 5, 6, 7, 8, 9, 11, 12, 13, 14, 15, 16, 17, 18, 20, 22, 24, 26, 27, 32 | References 8, 13, 14, 28, 31, 32, 39, 40 | References 8, 13, 14, 28, 31, 32, 39, 40 |
| No. studies (n/N; total person‐months) | 22 (3996/95 895; 657 036) | 26 (1007/21 652; 472 754) | 8 (31/1498; 23 824) | 8 (49/1714; 28 257) |
| Estimated pooled rate (95% CI) | ||||
| Per month | 0.84% (0.53%; 1.23%) | 0.20% (0.14%; 0.27%) | 0.11% (0.05%; 0.19%) | 0.09% (0.04%; 0.15%) |
| Per year | 10.1% (6.36%; 14.8%) | 2.38% (1.69%; 3.18%) | 1.28% (0.59%; 2.24%) | 1.05% (0.48%; 1.85%) |
| Cardiac death | References 9, 18, 27, 30, 32, 35, 38, 42, 43, 44 | References 1, 3, 11, 13, 16, 17, 19, 21, 22, 24, 25, 31 | References 8, 13, 32 | References 8, 13, 32 |
| No. studies (n/N; total person‐months) | 10 (713/10 218; 135 001) | 13 (54/1794; 43 937) | — | — |
| Estimated pooled rate (95% CI) | ||||
| Per month | 0.50% (0.15%; 1.09%) | 0.10% (0.03%; 0.20%) | — | — |
| Per year | 6.00% (1.79%; 13.1%) | 1.22% (0.42%; 2.45%) | — | — |
| MI | References 7, 9, 10, 15, 17, 18, 19, 27, 32, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44 | References 1, 2, 3, 4, 7, 9, 11, 12, 13, 14, 16, 17, 18, 21, 22, 23, 26, 32 | References 13, 14, 32, 39, 40 | References 13, 14, 32, 39, 40 |
| No. studies (n/N; total person‐months) | 18 (3064/81 886; 497 624) | 21 (757/20 451; 458 114) | 5 (10/1124; 17 528) | 5 (16/1146; 17 260) |
| Estimated pooled rate (95% CI) | ||||
| Per month | 0.49% (0.19%; 0.95%) | 0.11% (0.03%; 0.24%) | 0.05% (0.01%; 0.14%) | 0.08% (0.02%; 0.21%) |
| Per year | 5.88% (2.28%; 11.4%) | 1.38% (0.42%; 2.88%) | 0.58% (0.06%; 1.63%) | 1.01% (0.19%; 2.47%) |
| All deaths+MI | References 7, 9, 10, 15, 17, 19, 20, 27, 32, 35, 36, 37, 38, 39, 40, 41, 42, 44 | References 1, 2, 4, 7, 9, 10, 11, 12, 13, 14, 16, 17, 18, 22, 26, 29, 31 | References 14, 32, 39, 40 | References 14, 32, 39, 40 |
| No. studies (n/N; total person‐months) | 17 (7631/81 893; 494 260) | 17 (436/9376; 118 170) | 4 (22/944; 13 208) | 4 (35/975; 13 156) |
| Estimated pooled rate (95% CI) | ||||
| Per month | 1.07% (0.55%; 1.76%) | 0.33% (0.13%; 0.62%) | 0.18% (0.08%; 0.33%) | 0.17% (0.02%; 0.49%) |
| Per year | 12.8% (6.59%; 21.1%) | 3.96% (1.57%; 7.42%) | 2.18% (0.95%; 3.91%) | 2.03% (0.19%; 5.83%) |
CAD indicates coronary artery disease; MACE, major adverse cardiovascular events; MI, myocardial infarction; n, Number of non‐obstructive CAD subjects; N, Number of obstructive CAD participants; NObCAD, nonobstructive coronary artery disease; ObCAD, obstructive coronary artery disease.
Combining the few studies that separately considered mildly obstructed CAD patients (1–50% stenosis) and normal artery CAD patients (0% stenosis), the pooled yearly rates of death, myocardial infarction, and death plus myocardial infarction were 1.1%, 1.0%, and 2.0%, respectively, in mildly obstructive CAD patients, and 1.3%, 0.6%, and 2.2% in normal artery CAD patients (Table 3, Figures S49 through S54). No meta‐analysis of event rates was made for cardiac death and MACE because the number of studies with follow‐up longer than 6 months was too limited to allow meaningful analyses.
Clinical Outcomes in Nonobstructive Versus Obstructive CAD
As reported in Table 4, of the 22 studies (including a total of 96 179 participants) that directly compared at least 1 cardiovascular outcome in NObCAD versus ObCAD patients, 18 evaluated all‐cause mortality (n=93 178 participants); 8 cardiac death (n=9939); 14 myocardial infarction (n=77 966); 13 all‐cause mortality or myocardial infarction (n=77 858); and 7 evaluated MACE (n=12 289).
Table 4.
Meta‐Analyses Comparing the Risk of Clinical Outcomes of NObCAD Versus ObCAD Patients
| OutcomesRefs. | No. Studies (No. Patients) | n/N | Risk Ratio | 95% CI | P Value | I2, % |
|---|---|---|---|---|---|---|
| MACE18, 27, 31, 36, 38, 40, 42 | 7 (12 289) | 104/967 vs 2039/11 322 | 0.53 | 0.44 to 0.64 | <0.001 | 0 |
| Stratified by follow‐up durationa | ||||||
| 1 to 6 months7, 31, 36, 38 | 4 (11 943) | 41/584 vs 1574/11 359 | 0.51 | 0.38 to 0.69 | <0.001 | 0 |
| ≥12 months18, 27, 38, 40, 42 | 5 (6353) | 76/681 vs 945/5672 | 0.54 | 0.43 to 0.68 | <0.001 | 0 |
| All deaths7, 10, 17, 19, 22, 27, 28, 29, 31, 34, 35, 36, 37, 38, 39, 40, 42, 44 | 18 (93 178) | 149/8120 vs 3180/85 058 | 0.53 | 0.40 to 0.70 | <0.001 | 57 |
| Stratified by follow‐up durationa | ||||||
| 1 to 6 months7, 10, 19, 22, 27, 31, 34, 35, 36, 38, 39 | 11 (81 515) | 74/6681 vs 2160/74 834 | 0.45 | 0.28 to 0.72 | <0.001 | 66 |
| ≥12 months17, 27, 28, 29, 31, 35, 37, 38, 40, 42, 44 | 11 (23 784) | 87/2097 vs 1323/21 687 | 0.63 | 0.50 to 0.80 | <0.001 | 10 |
| Cardiac death18, 27, 30, 35, 38, 42, 43, 44 | 8 (9939) | 27/1093 vs 523/8846 | 0.44 | 0.19 to 0.98 | 0.05 | 69 |
| Stratified by follow‐up durationa | ||||||
| 1 to 6 months27, 30, 35, 38 | 4 (10 051) | 17/845 vs 438/9206 | 0.33 | 0.12 to 0.91 | 0.03 | 65 |
| ≥12 months18, 27, 35, 38, 42, 43, 44 | 7 (8337) | 16/743 vs 264/7594 | 0.66 | 0.38 to 1.15 | 0.14 | 12 |
| MI7, 10, 17, 18, 19, 27, 35, 36, 37, 38, 39, 40, 42, 43, 44 | 14 (77 966) | 97/6917 vs 2552/71 049 | 0.36 | 0.23 to 0.57 | <0.001 | 66 |
| Stratified by follow‐up durationa | ||||||
| 1 to 6 months7, 10, 19, 27, 35, 36, 38, 39 | 8 (76 368) | 81/6287 vs 2372/70 081 | 0.35 | 0.19 to 0.63 | <0.001 | 73 |
| ≥12 months17, 18, 27, 35, 38, 40, 42, 43, 44 | 9 (10 047) | 21/1125 vs 446/8922 | 0.41 | 0.18 to 0.94 | 0.04 | 67 |
| All deaths+MI7, 10, 17, 19, 20, 27, 35, 36, 37, 38, 39, 40, 42, 44 | 13 (77 858) | 178/6802 vs 6520/71 056 | 0.36 | 0.25 to 0.53 | <0.001 | 79 |
| Stratified by follow‐up durationa | ||||||
| 1 to 6 months7, 10, 19, 27, 35, 36, 38, 39 | 8 (76 368) | 143/6287 vs 6066/70 081 | 0.34 | 0.20 to 0.56 | <0.001 | 85 |
| ≥12 months17, 20, 27, 35, 38, 40, 42, 44 | 8 (9939) | 51/1010 vs 913/8929 | 0.48 | 0.35 to 0.67 | <0.001 | 22 |
MACE indicates major adverse cardiovascular events; MI, myocardial infarction; n, Number of non‐obstructive CAD subjects; N, Number of obstructive CAD participants; NObCAD, nonobstructive coronary artery disease; ObCAD, obstructive coronary artery disease; Refs., references.
As compared with ObCAD patients, NObCAD subjects showed a significantly lower risk of all of the above cardiovascular outcomes (Table 4, Figures S55 through S59): the RR of both all‐cause death and MACE was 0.53; the RR of cardiac death was 0.44; and the RR of both myocardial infarction and death or myocardial infarction was 0.36 (all P<0.05). The results were similar when analyses were stratified by the length of follow‐up, with the sole exception of cardiac death, which was not significant when only studies with follow‐up ≥12 months were included. No study reported a significant higher risk of any outcome for NObCAD versus ObCAD patients. The funnel plots displaying the RRs versus the logarithms of their standard errors appear skewed to the left for studies evaluating all‐cause mortality (Figure S60), but not for studies considering the other outcomes (Figures S61 through S64). The Egger weighted regression method to detect publication bias identified a borderline significant asymmetry for trials considering death (P=0.05).
Among NObCAD patients, the risks of all‐cause mortality, myocardial infarction, or all‐cause mortality plus myocardial infarction did not differ significantly between the subjects with mildly obstructed versus normal artery CAD (Table 5, Figures S65 through S67). However, few events were included in the above meta‐analyses, which lacked statistical power, and no meaningful quantitative analyses could be performed to evaluate cardiac death and MACE.
Table 5.
Meta‐Analyses Comparing the Risk of Clinical Outcomes of Mildly Obstructive Versus Normal Artery CAD Patients
| OutcomesRefs. | No. Studies (No. Patients) | n/N | Risk Ratio | 95% CI | P Value | I2, % |
|---|---|---|---|---|---|---|
| All deaths8, 14, 28, 31, 39, 40 | 6 (2861) | 25/1210 vs 41/1447 | 0.96 | 0.46 to 1.97 | 0.9 | 31 |
| MI14, 39, 40 | 3 (1715) | 7/836 vs 12/879 | 0.63 | 0.25 to 1.58 | 0.3 | 0 |
| All deaths+MI14, 39, 40 | 3 (1715) | 18/836 vs 27/879 | 0.72 | 0.37 to 1.39 | 0.3 | 17 |
CAD indicates coronary artery disease; MI, myocardial infarction; n, Number of non‐obstructive CAD subjects; N, Number of obstructive CAD participants; Refs., references.
Discussion
This meta‐analysis re‐analyzed all the data published regarding the clinical presentation and outcomes of NObCAD and ObCAD patients with ACS, attempting to address several questions and providing quantitative estimates that are difficult to obtain when studies are examined separately. The main findings are the following: (1) when compared to patients with obstructive CAD, the patients with a diagnosis of NObCAD showed a lower baseline cardiovascular risk as they are significantly less likely to be old, male, diabetic, hypertensive, or dyslipidemic; (2) non‐ST‐segment–ACS was the main pattern of presentation among patients with NObACS; (3) as logically follows from the above, NObCAD patients have one third to one half the likelihood of death or a main cardiovascular event than ObCAD subjects; (4) NObCAD subjects, however, are still at high risk for cardiovascular mortality and morbidity, showing yearly rates of death plus myocardial infarction or MACE as large as 4% and 9.2%, respectively. Interestingly, while in the short‐term follow‐up (1–6 months), the cardiac mortality rate was significantly lower in nonobstructive ACS patients, these differences did not persist through the 1‐year follow‐up, making the rates of cardiac death and myocardial infarction comparable between the 2 groups; (5) among NObCAD subjects, having zero stenosis rather than a mildly obstructive stenosis (1–49%) does not seem to be associated with a lower risk of death or cardiovascular outcomes, but these analyses are underpowered and require validation.
The better baseline CHD risk profile of NObCAD versus ObCAD subjects was already well known and documented in numerous studies, which listed several potential explanations related to the progression of the atherosclerotic plaque and hypothesized a stronger role of nonclassical risk factors (inflammation, insulin resistance, psychosocial factors, physical inactivity) in ACS etiology for NObCAD subjects.6, 10, 28, 39, 49, 50 This meta‐analysis adds quantitative estimates with tight confidence intervals on the distribution of the most common CHD risk factors in ObCAD and NObCAD groups, which can be used either for clinical practice or to support prognostic multivariate modeling.
In all but 627, 35, 36, 38, 42, 44 of the 60 direct comparisons, NObCAD patients showed a better prognosis than ObCAD subjects, with all meta‐analyses reporting significantly lower rates of events, from half to one third of those reported by ObCAD patients. Also, 5 of the 6 comparisons with divergent results were underpowered, including 5 or fewer events in the NObCAD group.27, 36, 38, 42, 44 In addition, a lower mortality rate for patients with myocardial infarction and nonobstructive coronary arteries was also documented in a recent systematic review.6 The most likely potential explanations for these findings include the younger age and the lower rate of diabetes mellitus (both of which are independent predictor of MACE) among NObCAD subjects. Also, given the drastically lower likelihood of baseline presentation with ST‐segment‐elevation–ACS of NObCAD patients, it has been suggested that their average amount of myocardial infarction might be smaller than that of ObCAD subjects.42
It has been suggested that, among NObCAD patients, those with normal coronary arteries may carry a lower CHD risk than the subjects with mildly obstructed CAD, representing a different population of younger patients with a possible tendency for spontaneous thrombosis and other etiologies leading to CAD (eg, takotsubo cardiomyopathy, variant angina pectoris, microvascular dysfunction, and coronary vasospasm).5, 6, 31 Indeed, a meta‐analysis of 18 studies including unselected or stable patients without significant epicardial coronary artery disease reported that coronary events were 6‐fold more frequent in patients with mild (0–20%) stenosis and 15‐fold more frequent in patients with moderate stenosis (20–40%), when compared with patients with smooth and normal arteriograms.48 From the present meta‐analysis, a different picture emerges for ACS. In the noncritical stenosis range, normal (0%) coronary arteries were associated with no better prognosis than a nonobstructive (1–49%) coronary stenosis. Such a discrepancy, however, may be artificial, because (1) our meta‐analyses restricted to NObCAD subjects included a very scarce number of events and were largely underpowered; (2) heterogeneous conditions were included under the umbrella term of NObACS, which may encompass disparate entities, such as epicardial artery coronary vasospasm, takotsubo acute cardiomyopathy, cocaine or other illicit drug abuse, spontaneous coronary dissection, or even acute myocarditis with ACS‐like presentation. In fact, a major concern in the clinical definition of nonobstructive ACS is that the majority of the included studies did not carry out special diagnostic investigations to rule out the potential role of microcirculation as a cause of myocardial acute injury (as shown in Table S4), nor were diagnostic tests carried out to assess the effective vessel atherosclerotic burden that is often undetectable at angiography due to negative atherosclerotic remodeling.49
Although the prognostic profile of NObCAD patients was more favorable than ObCAD subjects, the former showed absolute yearly rates of events as high as 9.2% (MACE) and 4.0% (all deaths plus myocardial infarction), in clear contrast with the common assumption of a good prognosis for NObCADs.40 Given that the underlying mechanisms that lead to clinical presentation of ACS in NObCAD subjects are not well understood,5, 10, 39 and that the appropriate therapeutic approach for such patients is also unknown,31 it has been suggested that their unfavorable outcome might be explained, at least in part, by the lower rate of the prescription of β‐blockers, angiotensin‐converting enzyme inhibitors, statins, and antiplatelet drugs.10, 40 In our meta‐analysis, all of the above drugs were less frequently prescribed to NObCAD patients. Unfortunately, none of the included studies reported data on dual antiplatelet therapy, which is one of the most important disease‐modifying medications, when left ventricular function is preserved. However, while the vast majority of patients in both groups received aspirin (81.7% and 86.8% in NObCAD and ObCAD groups, respectively), P2Y12 inhibitors were administered only to 29.2% and NObCAD patients (and 63.7% ObCAD subjects), and it could thus be hypothesized that only a minority of patients with NObCAD were under dual antiplatelet therapy. This might be due to the fact that the decision to treat patients was driven by angiographic results rather than by clinical presentation, supporting the hypothesis that these patients are often undertreated, probably in the belief that NObCAD represents a benign condition.40 Overall, our findings reinforce the calls for a specific management or an expansion of formal guidelines to include specific recommendations for secondary prevention measures in NObCAD patients.5, 31
Limitations
Several limitations should be considered in the interpretation of our data. First, the heterogeneity across studies was substantial in both the baseline characteristics and the length of follow‐up. While quantifying the differences in baseline characteristics was one of the aims of the analysis, we computed yearly and monthly rates rather than the overall rates of event to reduce the potential bias deriving from varying follow‐ups. In any case, it cannot be excluded that the higher event rate that typically occurs in the first 6 months of follow‐up might have led to an overestimate of the event rates in those comparisons where the number of short‐term follow‐up studies was larger. Second, meta‐regression might have been used to explore both the causes of heterogeneity and the independent contribution of each of the CAD risk factors in determining the excess risk in ObCAD versus NObCAD subjects. However, any meta‐regression model that we fit was at serious risk of bias due to the relatively scarce number of individual studies in each meta‐analysis and, most importantly, due to the serious imbalance in each study sample size between NObCAD and ObCAD subjects (with 1 NObCAD each 6–20 ObCAD subjects for many studies). This implies that the overall value of a determined risk factor (eg, age) of an unbalanced study (eg, Dokainish et al7) is very similar to the mean age value of the ObCAD group, and studies with balanced groups seem to have a lower mean age than those with unbalanced groups, regardless of their relative risk of event. Even exploring other alternative options (eg, age differential between each study group), the bias caused by the scarce number of balanced‐group studies determined a serious lack of reliability for any meta‐regression, the results of which were thus not shown. Third, as for all meta‐analyses based upon published studies, although we made an extensive systematic search, we cannot exclude that additional data exist that were not considered. Fourth, as previously noted, some of the meta‐analyses were populated by a low number of events and certainly require confirmation from additional data.
Conclusions
This meta‐analysis confirms that ACS patients with and without obstructive CAD are significantly different. NObCAD patients have a significantly lower cardiovascular risk at baseline and a subsequent lower rate of death or a main cardiovascular event. However, these subjects are still at high risk for cardiovascular mortality and morbidity, suggesting potential undertreatment and calling for a specific management. Our findings, other than demonstrating a significant medical treatment gap, highlight an important opportunity for improving the quality of care and, in turn, the outcomes of patients being diagnosed with NObACS.50 In the context of NObCAD, no differences in prognosis were noted between zero stenosis versus mildly obstructive stenosis (1–49%), but such analyses require validation.
Author Contributions
All authors participated in the design, analysis, and interpretation of the study. Manzoli, Flacco, and Pizzi were involved in all phases of the study. Xhyheri, Costa, and Pizzi made the bibliographic search. Manzoli and Flacco led the statistical analysis, Gualano and Xhyheri performed the data extraction. Gualano carried out the methodological quality assessment. Manzoli, Flacco, and Pizzi wrote the manuscript, which was revised by Costa and Fragassi. Manzoli had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Disclosures
None.
Supporting information
Appendix S1. Supplemental figures, tables, and references.
(J Am Heart Assoc. 2016;5:e004185 doi: 10.1161/JAHA.116.004185)
An accompanying Appendix S1 is available at http://jaha.ahajournals.org/content/5/12/e004185/DC1/embed/inline-supplementary-material-1.pdf
Notes
References
- 1. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER III, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB. Heart Disease and Stroke Statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–e322. [DOI] [PubMed] [Google Scholar]
- 2. Mukherjee D, Fang J, Chetcuti S, Moscucci M, Kline‐Rogers E, Eagle KA. Impact of combination evidence‐based medical therapy on mortality in patients with acute coronary syndromes. Circulation. 2004;109:745–749. [DOI] [PubMed] [Google Scholar]
- 3. Nissen SE, Yock P. Intravascular ultrasound: novel pathophysiological insights and current clinical applications. Circulation. 2001;103:604–616. [DOI] [PubMed] [Google Scholar]
- 4. Rioufol G, Finet G, Ginon I, Andre‐Fouet X, Rossi R, Vialle E, Desjoyaux E, Convert G, Huret JF, Tabib A. Multiple atherosclerotic plaque rupture in acute coronary syndrome: a three‐vessel intravascular ultrasound study. Circulation. 2002;106:804–808. [DOI] [PubMed] [Google Scholar]
- 5. Niccoli G, Scalone G, Crea F. Acute myocardial infarction with no obstructive coronary atherosclerosis: mechanisms and management. Eur Heart J. 2015;36:475–481. [DOI] [PubMed] [Google Scholar]
- 6. Pasupathy S, Air T, Dreyer RP, Tavella R, Beltrame JF. Systematic review of patients presenting with suspected myocardial infarction and nonobstructive coronary arteries. Circulation. 2015;131:861–870. [DOI] [PubMed] [Google Scholar]
- 7. Dokainish H, Pillai M, Murphy SA, DiBattiste PM, Schweiger MJ, Lotfi A, Morrow DA, Cannon CP, Braunwald E, Lakkis N. Prognostic implications of elevated troponin in patients with suspected acute coronary syndrome but no critical epicardial coronary disease: a TACTICS‐TIMI‐18 substudy. J Am Coll Cardiol. 2005;45:19–24. [DOI] [PubMed] [Google Scholar]
- 8. Golzio PG, Orzan F, Ferrero P, Bobbio M, Bergerone S, Di Leo M, Trevi GP. Myocardial infarction with normal coronary arteries: ten‐year followup. Ital Heart J. 2004;5:732–738. [PubMed] [Google Scholar]
- 9. Harris PJ, Behar VS, Conley MJ, Harrell FE Jr, Lee KL, Peter RH, Kong Y, Rosati RA. The prognostic significance of 50% coronary stenosis in medically treated patients with coronary artery disease. Circulation. 1980;62:240–248. [DOI] [PubMed] [Google Scholar]
- 10. Patel MR, Chen AY, Peterson ED, Newby LK, Pollack CV Jr, Brindis RG, Gibson CM, Kleiman NS, Saucedo JF, Bhatt DL, Gibler WB, Ohman EM, Harrington RA, Roe MT. Prevalence, predictors, and outcomes of patients with non‐ST‐segment elevation myocardial infarction and insignificant coronary artery disease: results from the Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA Guidelines (CRUSADE) initiative. Am Heart J. 2006;152:641–647. [DOI] [PubMed] [Google Scholar]
- 11. Wells GA, Shea B, O'Connell D, Petersen J, Welch V, Losos M, Tugwell P. The Newcastle‐Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta‐analyses. Ottawa, ON: Dept of Epidemiology and Community Medicine, University of Ottawa; 2003. [Google Scholar]
- 12. Abid L, Bahloul A, Frikha Z, Mallek S, Abid D, Akrout M, Hentati M, Kammoun S. Myocardial infarction and normal coronary arteries: the experience of the cardiology department of Sfax, Tunisia. Intern Med. 2012;51:1959–1967. [DOI] [PubMed] [Google Scholar]
- 13. Aldous S, Elliott J, McClean D, Puri A, Richards AM. Outcomes in patients presenting with symptoms suggestive of acute coronary syndrome with elevated cardiac troponin but non‐obstructive coronary disease on angiography. Heart Lung Circ. 2015;24:869–878. [DOI] [PubMed] [Google Scholar]
- 14. Bugiardini R, Manfrini O, De Ferrari GM. Unanswered questions for management of acute coronary syndrome: risk stratification of patients with minimal disease or normal findings on coronary angiography. Arch Intern Med. 2006;166:1391–1395. [DOI] [PubMed] [Google Scholar]
- 15. Chan MY, Mahaffey KW, Sun LJ, Pieper KS, White HD, Aylward PE, Ferguson JJ, Califf RM, Roe MT. Prevalence, predictors, and impact of conservative medical management for patients with non‐ST‐segment elevation acute coronary syndromes who have angiographically documented significant coronary disease. JACC Cardiovasc Interv. 2008;1:369–378. [DOI] [PubMed] [Google Scholar]
- 16. Chokshi NP, Iqbal SN, Berger RL, Hochman JS, Feit F, Slater JN, Pena‐Sing I, Yatskar L, Keller NM, Babaev A, Attubato MJ, Reynolds HR. Sex and race are associated with the absence of epicardial coronary artery obstructive disease at angiography in patients with acute coronary syndromes. Clin Cardiol. 2010;33:495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cortell A, Sanchis J, Bodi V, Nunez J, Mainar L, Pellicer M, Minana G, Santas E, Dominguez E, Palau P, Llacer A. Non‐ST‐elevation acute myocardial infarction with normal coronary arteries: predictors and prognosis. Rev Esp Cardiol. 2009;62:1260–1266. [DOI] [PubMed] [Google Scholar]
- 18. Da Costa A, Isaaz K, Faure E, Mourot S, Cerisier A, Lamaud M. Clinical characteristics, aetiological factors and long‐term prognosis of myocardial infarction with an absolutely normal coronary angiogram; a 3‐year follow‐up study of 91 patients. Eur Heart J. 2001;22:1459–1465. [DOI] [PubMed] [Google Scholar]
- 19. Dey S, Flather MD, Devlin G, Brieger D, Gurfinkel EP, Steg PG, Fitzgerald G, Jackson EA, Eagle KA. Sex‐related differences in the presentation, treatment and outcomes among patients with acute coronary syndromes: the Global Registry of Acute Coronary Events. Heart. 2009;95:20–26. [DOI] [PubMed] [Google Scholar]
- 20. Dwyer JP, Redfern J, Freedman SB. Low utilisation of cardiovascular risk reducing therapy in patients with acute coronary syndromes and non‐obstructive coronary artery disease. Int J Cardiol. 2008;129:394–398. [DOI] [PubMed] [Google Scholar]
- 21. Germing A, Lindstaedt M, Ulrich S, Grewe P, Bojara W, Lawo T, von Dryander S, Jager D, Machraoui A, Mugge A, Lemke B. Normal angiogram in acute coronary syndrome‐preangiographic risk stratification, angiographic findings and follow‐up. Int J Cardiol. 2005;99:19–23. [DOI] [PubMed] [Google Scholar]
- 22. Hamdan R, Frangieh A, Zadri Z, Hajje F, Hazar R, Salame E, Jaoude SA, Kassab R, Badaoui G. What do we know about myocardial infarction with normal coronary arteries? Gazz Med Ital Arch Sci Med. 2012;171:7–12. [Google Scholar]
- 23. Hansen KW, Hvelplund A, Abildstrom SZ, Prescott E, Madsen M, Madsen JK, Jensen JS, Thuesen L, Thayssen P, Tilsted HH, Jorgensen E, Galatius S. No gender differences in prognosis and preventive treatment in patients with AMI without significant stenoses. Eur J Prev Cardiol. 2012;19:746–754. [DOI] [PubMed] [Google Scholar]
- 24. Hochman JS, Tamis JE, Thompson TD, Weaver WD, White HD, Van de Werf F, Aylward P, Topol EJ, Califf RM. Sex, clinical presentation, and outcome in patients with acute coronary syndromes. Global Use of Strategies to Open Occluded Coronary Arteries in Acute Coronary Syndromes IIb Investigators. N Engl J Med. 1999;341:226–232. [DOI] [PubMed] [Google Scholar]
- 25. Hung MJ, Cherng WJ, Kuo LT, Wang CH. Acute myocardial infarction in Taiwanese with angiographically normal coronary arteries: role of coronary artery spasm. Acta Cardiol Sin. 2003;19:31–38. [Google Scholar]
- 26. Johnston N, Jonelid B, Christersson C, Kero T, Renlund H, Schenck‐Gustafsson K, Lagerqvist B. Effect of gender on patients with ST‐elevation and non‐ST‐elevation myocardial infarction without obstructive coronary artery disease. Am J Cardiol. 2015;115:1661–1666. [DOI] [PubMed] [Google Scholar]
- 27. Kang WY, Jeong MH, Ahn YK, Kim JH, Chae SC, Kim YJ, Hur SH, Seong IW, Hong TJ, Choi DH, Cho MC, Kim CJ, Seung KB, Chung WS, Jang YS, Rha SW, Bae JH, Cho JG, Park SJ. Are patients with angiographically near‐normal coronary arteries who present as acute myocardial infarction actually safe? Int J Cardiol. 2011;146:207–212. [DOI] [PubMed] [Google Scholar]
- 28. Larsen AI, Galbraith PD, Ghali WA, Norris CM, Graham MM, Knudtson ML. Characteristics and outcomes of patients with acute myocardial infarction and angiographically normal coronary arteries. Am J Cardiol. 2005;95:261–263. [DOI] [PubMed] [Google Scholar]
- 29. Larson DM, Menssen KM, Sharkey SW, Duval S, Schwartz RS, Harris J, Meland JT, Unger BT, Henry TD. “False‐positive” cardiac catheterization laboratory activation among patients with suspected ST‐segment elevation myocardial infarction. JAMA. 2007;298:2754–2760. [DOI] [PubMed] [Google Scholar]
- 30. Manfrini O, Morrell C, Das R, Barth JH, Hall AS, Gale CP, Cenko E, Bugiardini R. Effects of angiotensin‐converting enzyme inhibitors and beta blockers on clinical outcomes in patients with and without coronary artery obstructions at angiography (from a Register‐Based Cohort Study on Acute Coronary Syndromes). Am J Cardiol. 2014;113:1628–1633. [DOI] [PubMed] [Google Scholar]
- 31. Minha S, Gottlieb S, Magalhaes MA, Gavrielov‐Yusim N, Krakover R, Goldenberg I, Vered Z, Blatt A. Characteristics and management of patients with acute coronary syndrome and normal or nonsignificant coronary artery disease: results from Acute Coronary Syndrome Israeli Survey (ACSIS) 2004–2010. J Invasive Cardiol. 2014;26:389–393. [PubMed] [Google Scholar]
- 32. Ohlow MA, Wong V, Brunelli M, von Korn H, Farah A, Memisevic N, Richter S, Tukhiashvili K, Lauer B. Acute coronary syndrome without critical epicardial coronary disease: prevalence, characteristics, and outcome. Am J Emerg Med. 2015;33:150–154. [DOI] [PubMed] [Google Scholar]
- 33. Ong P, Athanasiadis A, Hill S, Vogelsberg H, Voehringer M, Sechtem U. Coronary artery spasm as a frequent cause of acute coronary syndrome: the CASPAR (Coronary Artery Spasm in Patients With Acute Coronary Syndrome) Study. J Am Coll Cardiol. 2008;52:523–527. [DOI] [PubMed] [Google Scholar]
- 34. Pinheiro M, Rabelo A Junior, de Jesus RS, Nascimento LC, Costa UM. Acute coronary syndromes in the absence of significant coronary artery disease. Arq Bras Cardiol. 2005;84:24–28. [DOI] [PubMed] [Google Scholar]
- 35. Planer D, Mehran R, Ohman EM, White HD, Newman JD, Xu K, Stone GW. Prognosis of patients with non‐ST‐segment‐elevation myocardial infarction and nonobstructive coronary artery disease: propensity‐matched analysis from the Acute Catheterization and Urgent Intervention Triage Strategy Trial. Circ Cardiovasc Interv. 2014;7:285–293. [DOI] [PubMed] [Google Scholar]
- 36. Ramanath VS, Armstrong DF, Grzybowski M, Rahnama‐Mohagdam S, Tamhane UU, Gordon K, Froehlich JB, Eagle KA, Jackson EA. Receipt of cardiac medications upon discharge among men and women with acute coronary syndrome and nonobstructive coronary artery disease. Clin Cardiol. 2010;33:36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Raymond R, Lynch J, Underwood D, Leatherman J, Razavi M. Myocardial infarction and normal coronary arteriography: a 10 year clinical and risk analysis of 74 patients. J Am Coll Cardiol. 1988;11:471–477. [DOI] [PubMed] [Google Scholar]
- 38. Rhew SH, Ahn Y, Kim MC, Jang SY, Cho KH, Hwang SH, Lee MG, Ko JS, Park KH, Sim DS, Yoon NS, Yoon HJ, Kim KH, Hong YJ, Park HW, Kim JH, Jeong MH, Cho JG, Park JC, Kang JC. Is myocardial infarction in patients without significant stenosis on a coronary angiogram as benign as believed? Chonnam Med J. 2012;48:39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Roe MT, Harrington RA, Prosper DM, Pieper KS, Bhatt DL, Lincoff AM, Simoons ML, Akkerhuis M, Ohman EM, Kitt MM, Vahanian A, Ruzyllo W, Karsch K, Califf RM, Topol EJ. Clinical and therapeutic profile of patients presenting with acute coronary syndromes who do not have significant coronary artery disease. The Platelet Glycoprotein IIb/IIIa in Unstable Angina: Receptor Suppression Using Integrilin Therapy (PURSUIT) Trial Investigators. Circulation. 2000;102:1101–1106. [DOI] [PubMed] [Google Scholar]
- 40. Rossini R, Capodanno D, Lettieri C, Musumeci G, Limbruno U, Molfese M, Spatari V, Calabria P, Romano M, Tarantini G, Gavazzi A, Angiolillo DJ. Long‐term outcomes of patients with acute coronary syndrome and nonobstructive coronary artery disease. Am J Cardiol. 2013;112:150–155. [DOI] [PubMed] [Google Scholar]
- 41. Shishehbor MH, Lauer MS, Singh IM, Chew DP, Karha J, Brener SJ, Moliterno DJ, Ellis SG, Topol EJ, Bhatt DL. In unstable angina or non‐ST‐segment acute coronary syndrome, should patients with multivessel coronary artery disease undergo multivessel or culprit‐only stenting? J Am Coll Cardiol. 2007;49:849–854. [DOI] [PubMed] [Google Scholar]
- 42. Sun J, Zhang W, Zeng Q, Dong S, Sun X. Three‐year follow‐up in patients with acute coronary syndrome and normal coronary angiography. Coron Artery Dis. 2012;23:162–166. [DOI] [PubMed] [Google Scholar]
- 43. Terefe YG, Niraj A, Pradhan J, Kondur A, Afonso L. Myocardial infarction with angiographically normal coronary arteries in the contemporary era. Coron Artery Dis. 2007;18:621–626. [DOI] [PubMed] [Google Scholar]
- 44. von Korn H, Graefe V, Ohlow MA, Yu J, Huegl B, Wagner A, Gruene S, Lauer B. Acute coronary syndrome without significant stenosis on angiography: characteristics and prognosis. Tex Heart Inst J. 2008;35:406–412. [PMC free article] [PubMed] [Google Scholar]
- 45. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials. 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 46. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ. 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Manzoli L, De Vito C, Salanti G, D'Addario M, Villari P, Ioannidis JP. Meta‐analysis of the immunogenicity and tolerability of pandemic influenza A 2009 (H1N1) vaccines. PLoS One. 2011;6:e24384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Thygesen K, Alpert JS, White HD, Jaffe AS, Apple FS, Galvani M, Katus HA, Newby LK, Ravkilde J, Chaitman B, Clemmensen PM, Dellborg M, Hod H, Porela P, Underwood R, Bax JJ, Beller GA, Bonow R, Van der Wall EE, Bassand JP, Wijns W, Ferguson TB, Steg PG, Uretsky BF, Williams DO, Armstrong PW, Antman EM, Fox KA, Hamm CW, Ohman EM, Simoons ML, Poole‐Wilson PA, Gurfinkel EP, Lopez‐Sendon JL, Pais P, Mendis S, Zhu JR, Wallentin LC, Fernandez‐Aviles F, Fox KM, Parkhomenko AN, Priori SG, Tendera M, Voipio‐Pulkki LM, Vahanian A, Camm AJ, De Caterina R, Dean V, Dickstein K, Filippatos G, Funck‐Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Widimsky P, Zamorano JL, Morais J, Brener S, Harrington R, Morrow D, Lim M, Martinez‐Rios MA, Steinhubl S, Levine GN, Gibler WB, Goff D, Tubaro M, Dudek D, Al‐Attar N. Universal definition of myocardial infarction. Circulation. 2007;116:2634–2653. [DOI] [PubMed] [Google Scholar]
- 49. Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J, Lisheng L. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case‐control study. Lancet. 2004;364:937–952. [DOI] [PubMed] [Google Scholar]
- 50. Greenland P, Knoll MD, Stamler J, Neaton JD, Dyer AR, Garside DB, Wilson PW. Major risk factors as antecedents of fatal and nonfatal coronary heart disease events. JAMA. 2003;290:891–897. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supplemental figures, tables, and references.


