Abstract
Background
This study was aimed to investigate the clinical relevance between glycoprotein Ia (GPIA) rs1126643C/T polymorphism and the outcome of coronary artery disease after coronary artery bypass graft (CABG) surgery and explore the involved potential mechanisms.
Methods and Results
We genotyped GPIA rs1126643 polymorphism of 1592 patients who underwent CABG and followed up for a median period of 72.8 months. Patients who are GPIA rs1126643 T‐allele carriers have a higher major adverse cardiac or cerebrovascular events risk post‐CABG than those who are CC homozygotes (hazard ratio [HR]=1.29; P=0.022). The clinical association between the risk allele (T) carriage and major adverse cardiac or cerebrovascular events was confirmed in another cohort study, which included 646 CABG patients from various health centers across China. Meanwhile, rs1126643 T allele was also linked with increased risk of major adverse cardiac or cerebrovascular events (HR=1.73; P=0.019). To explore the underlying mechanisms, we prospectively recruited 131 coronary artery disease patients, assessed their platelet aggregation function, and focused on detecting their GPIA mRNA level and protein expression. Results showed that patients with rs1126643 T allele have elevated platelet aggregation activity (P=0.029) when protein expression is increased (P<0.001) and not affected by glycoprotein Ia mRNA level.
Conclusions
The synonymous common variant, GPIA rs1126643, increases the long‐term adverse events risk of CABG by augmenting GPIa protein expression and enhancing platelet aggregation function. This finding can serve as the implication of improving secondary prevention of CABG patients.
Keywords: coronary artery bypass grafting, genetic common variant, glycoprotein Ia, long‐term adverse events
Subject Categories: Cardiovascular Surgery
Introduction
As the leading cause of mortality and morbidity worldwide, coronary artery disease (CAD) accounts for 14% of all deaths and is predicted to remain so until 2030.1 Coronary artery bypass graft (CABG) surgery was one of the most important surgical treatments for CAD, especially in patients with diabetes mellitus, left main disease, multivessel disease, or left ventricular dysfunction.2 However, adverse events post‐CABG still concern both patients and clinicians. Several studies have reported that incidence of 5‐year major adverse cardiac and cerebrovascular events (MACCE) post‐CABG is around 11.8% to 31.0%,3, 4, 5 indicating that an intensive secondary prevention is necessary for patients who are prone to MACCE after having CABG.
For patients after having CABG, occurrence of short‐term adverse events mainly depends on factors such as type of surgery, cardiopulmonary bypass time, and cross‐clamp time; long‐term adverse events tend to be related to persistent risk factors, such as diabetes mellitus, and left ventricular function.6 Figuring out the factors that influence the long‐term prognosis of CABG and screening patients who are susceptible to adverse events are pivotal for secondary prevention. Genetic background is a persistent factor that affects the prognosis of CABG patients.7, 8, 9 Previous studies demonstrated that common variants in P‐selectin and C‐reactive protein genes influenced susceptibility to cognitive decline post‐CABG,7 and thrombomodulin polymorphism was associated with long‐term mortality post‐CABG.8
Platelet‐dependent thrombogenesis played an important role on the adverse events post‐CABG10 and was, in part, genetically determined.11 Platelet glycoprotein (GP) Ia‐IIa is one of the major platelet membrane receptors, which could adhere to collagen exposed in subendothelial and, subsequently, activate platelets.12, 13, 14 Defections of GP Ia‐IIa complex would result platelets to be completely unresponsive to collagen.15
Being different from other glycoproteins, GPIa expression levels vary significantly among individuals,16 and this variation results in a significant impact on platelet function.17 Previous studies have demonstrated a silent polymorphism GPIA rs1126643C/T (C807T), and this was associated with the density of GPIa on platelet membrane,18 as well as the risk of CAD.19, 20, 21 As for the prognosis of CAD, a 5‐year follow‐up study showed that rs1126643 T‐allele carriers have higher risk of recurrence after acute coronary syndrome.22
This study was designed to find out whether this synonymous common variant GPIA rs1126643 has any influence on the long‐term prognosis of CABG and explore the potential mechanisms of this influence.
Methods
Study Subjects
The study protocol was approved by the Review Board of Fuwai Hospital, Peking Union Medical College (Beijing, China). We have complied with the World Medical Association Declaration of Helsinki regarding ethical conduct of research involving human subjects and/or animals. All patients were properly informed and have signed all necessary written consent to be involved in the study.
Patients from discovery cohort were recruited from Fuwai Hospital (Beijing, China) between December 2007 and December 2011. The inclusive criteria of the cohort are (1) aged over 18 years; (2) Chinese patients; (3) scheduled for CABG surgery; (4) and consent to involve in the study. The exclusive criteria are (1) emergent surgery and (2) CABG with other concurrent surgery. Data of this cohort were collected by trained clinical research staffs and were subsequently inputted into the computer database by 2 independent investigators. All aspects of in‐hospital management were done similarly according to standard protocols in this institute. As one of the standard procedures of Fuwai Hospital, all patients were required to return for a routine outpatient follow‐up at 1 month, 6 months, and each year postdischarge. For patients who did not attend the routine outpatient follow‐up, a group of research nurses would make telephone calls to patients or contact the communities in which patients live to get follow‐up information. Patients were asked to fill a predefined follow‐up information collection form at every visit. The medical records of those who reported any adverse events postdischarge were reviewed for further confirmation. For the records of major adverse events reported by other hospitals, patients were requested to mail a copy of all relevant medical information for further confirmation. We recruited 1592 CABG patients with a median follow‐up time of 72.8 months. And, among these patients, 48 (3.0%) were lost during follow‐up.
In the replication cohort, 646 CABG patients were chosen from a multicenter study named HPS2‐THRIVE (Heart Protection Study 2, Treatment of HDL to Reduce the Incidence of Vascular Events; NCT00461630), and it was an institutional review board–approved prospective, randomized, placebo‐controlled trial. The inclusive and exclusive criteria of HPS2‐THRIVE have been described elsewhere.23 The original study of replication cohort, HPS2‐THRIVE aimed to assess the effect of extended‐release niacin on CAD patients, and patients treated by CABG were also included. The criteria to enter our replication cohort include Chinese patients who received isolated CABG surgery after January 2005. Patients who had incomplete information on CABG surgery were excluded from enrollment. Follow‐up visits were conducted at 3 and 6 months following randomization and then every 6 months. All serious adverse events were recorded.23
For functional study, we assumed the mean platelet aggregation rate of patient carrying T allele was 70%, whereas that of CC patient was 60%; the estimated SD was 20%; the power and type I error rate were set to be 0.80 and 0.05, respectively; sample ratio of CC patients versus T carriers was assumed to be 0.8. Then, we calculated the anticipated sample size of the functional study to be 130 patients.24, 25 We recruited CAD patients who were aged over 18 years and scheduled for isolated CABG, and those who had any medical history of bleeding or coagulation disorders were excluded from enrollment. Finally, 131 patients were prospectively recruited from Fuwai Hospital, Peking Union Medical College, between December 2014 and June 2015. Blood samples from these patients were acquired 1 hour before CABG surgery, which was conducted at least 7 days after the stop of antiplatelet regimen.
Primary Endpoints
The primary clinical endpoint was a composite of MACCE, that is, nonfatal stroke, nonfatal myocardial infarction (MI), repeat revascularization, or death from any cause.26 A detailed definition of MACCE is available in Data S1.27
DNA Isolation and Genotyping Analysis
Blood samples for genotyping were collected using vacutainers and transferred to test tubes containing EDTA. Genomic DNA was isolated from whole blood using the Wizard Genomic DNA Purification Kit (Promega, Fitchburg, MA). DNA quality and quantity were assessed by a UV spectrophotometer at 260/280 nm. DNA samples were visualized in gel array and the quality of more than 99.5% DNA samples was high. Genotyping was done by MALDI‐TOF MS using the MassARRAY system (Sequenom, San Diego, CA) as previously described.28 Completed genotyping reactions were spotted using a MassARRAY Nanodispenser (Sequenom) and analyzed by matrix‐assisted laser desorption/ionization time‐of‐flight mass‐spectrometry. Genotype calling was done with MassARRAY RT software (version 3.1; Sequenom) and analyzed using MassARRAY Typer software (version 4.0; Sequenom).
Platelet Aggregation Measurements
Samples from the 131‐patient cohort underwent platelet aggregation tests. Blood was collected in vacutainer tubes containing 3.2% sodium citrate and inverted 3 to 5 times with gentle mixing before use. Platelet aggregation was assessed within 2 hours after blood sampling. We prepared 500 μL of platelet‐rich plasma (PRP) and platelet‐poor plasma (PPP) by centrifugation at 88 g or 1237 g for 10 minutes; 1 μL of collagen (2 μL/mL; Chrono‐Log Corporation, Havertown, PA) was used to induce aggregation. The experiment was carried out using the turbidimetric method in a Chrono‐Log model 700 aggregometer (Chrono‐Log Corporation). Light transmission was adjusted to 0% for PRP and to 100% for PPP in each measurement. Curves were recorded for 8 minutes, and platelet aggregation was determined as the maximal percent change in light transmittance from baseline using PPP as a reference.
Quantitative Real‐Time Polymerase Chain Reaction and Western Blot Analysis
Blood from 20 patients (8 for CC, 8 for CT, and 4 for TT) randomly chosen from the 131‐patient cohort were prepared for quantitative real‐time polymerase chain reaction (qRT‐PCR) and western blot analysis. Blood for qRT‐PCR analysis was collected by Tempus RNA tube, and total RNA was isolated from blood using the Tempus spin RNA isolation kit (Thermo Fisher Scientific, Waltham, MA). Eluted RNA was used immediately in the next reverse‐transcription polymerase chain reaction using the PrimeScript 1st strand cDNA synthesis kit (TaKaRa, Dalian, China). GAPDH was selected as the reference gene in this experiment. Details of the primer information are shown in Table S1. For western blot analysis, the platelet clot was collected from the PRP by centrifugation at 3000g for 15 minutes and clots were lysed by cell lysis buffer (Cell Signaling Technology, Danvers, MA) with 1% proteinase inhibitors (Cell Signaling Technology). The BCA Protein assay kit (Thermo Fisher Scientific) was used to quantify total protein. We loaded 40 μg of total protein, which was separated by 4% to 12% NuPAGE Bis‐Tris gels (Thermo Fisher Scientific) and transferred onto a PVDF membrane. Protein expression was detected using primary antibodies, Anti‐Integrin alpha 2 antibody (1:1000; Abcam, Cambridge, MA), followed by HRP‐conjugated secondary antibody (1:10 000; ZsBio, Beijing, China). Signal was detected with the SuperSignal West Pico Chemiluminescent kit (Thermo Fisher Scientific). Image band densitometry was analyzed with ImageJ software (NIH, Bethesda, MD).
Statistical Analysis
We used the Student t test for continuous variables or chi‐squared test for discrete variables to analyze baseline variables. GPIA rs1126643 polymorphism was classified into 2 genotypic groups, distinguished by the absence or presence of at least 1 copy of the minor allele (homozygote major vs homozygote minor and heterozygote). Associations between overall MACCE and single‐nucleotide polymorphisms were estimated using the Kaplan–Meier method and log‐rank test. Cox proportional hazard analysis was used for multivariable analysis on the outcome. Differences among 3 groups were compared using 1‐way ANOVA. Nonparametric methods Kruskal–Wallis (3 groups) and Mann–Whitney (2 groups) and Bonferroni correction were used in mRNA and protein expression analyses when the number of analytic units per group was quite small. A P<0.05 was considered as statistically significant. All the 3‐group analyses (among CC, CT, and TT groups) are presented in Figures S1 through S3. All statistical analyses were done with SAS software (version 9.3; SAS Institute Inc., Cary, NC) and SPSS software (19.0 for Windows; SPSS, Inc., Chicago, IL).
Results
Clinical Characteristics
Clinical characteristics of CAD cases with or without MACCE are shown in Tables 1 and 2. In the discovery cohort of 1544 cases with an average follow‐up of 72.8 months, there were no significant differences in sex distribution between patients with or without MACCE. Average age and hypertension rate of MACCE cases were significantly higher than those without MACCE (P=0.002 and 0.004). In addition, calcium‐channel blocker users were significantly higher in MACCE cases as compared to patients without MACCE (P=0.036). In the replication cohort with 646 cases with an average follow‐up of 46.5 months, rates of aspirin and statins usage in patients with MACCE were significantly lower than those patients without MACCE (P=0.023 and 0.003). In addition, diuretics users were significantly more in MACCE cases as compared with patients without MACCE (P=0.018).
Table 1.
Baseline Information of Discovery Cohort
| Variable | All Patients (n=1544) | MACCE (n=328) | Without MACCE (n=1216) | P Value |
|---|---|---|---|---|
| Demographics | ||||
| Age, y | 61.32 (±8.61) | 62.54 (±8.08) | 60.99 (±8.71) | 0.002a |
| Female sex | 307 (19.9) | 58 (17.7) | 249 (20.5) | 0.261 |
| BMI, kg/m2 | 25.75 (±4.89) | 25.67 (±2.98) | 25.78 (±5.29) | 0.716 |
| Medical history | ||||
| Smokers | 784 (50.8) | 179 (54.6) | 605 (49.8) | 0.121 |
| Hypertension | 1026 (66.5) | 240 (73.2) | 786 (64.6) | 0.004a |
| Hyperlipidemia | 1042 (67.5) | 221 (67.4) | 821 (67.5) | 0.962 |
| Diabetes mellitus | 514 (33.3) | 116 (35.4) | 398 (32.7) | 0.369 |
| Insulin‐treated diabetes mellitus | 114 (7.4) | 30 (9.1) | 84 (6.9) | 0.169 |
| Renal dysfunction | 11 (0.7) | 4 (1.2) | 7 (0.6) | 0.261 |
| COPD | 7 (0.5) | 3 (0.9) | 4 (0.3) | 0.170 |
| Peripheral arterial disease | 34 (2.2) | 8 (2.4) | 26 (2.1) | 0.742 |
| Previous MI | 586 (32.0) | 128 (39.0) | 458 (37.7) | 0.652 |
| Previous PCI | 150 (9.7) | 30 (9.1) | 120 (9.9) | 0.695 |
| LVEF (%) | 59.64 (±8.64) | 59.26 (±9.06) | 59.74 (±8.52) | 0.501 |
| No. of diseased vessels | ||||
| 2 | 148 (9.6) | 26 (7.9) | 122 (10.0) | 0.250 |
| 3 | 1351 (87.5) | 293 (89.3) | 1058 (87.0) | 0.259 |
| Left main coronary artery | 488 (31.6) | 104 (31.7) | 384 (31.6) | 0.965 |
| Biochemical characteristics | ||||
| LDL cholesterol, mmol/L | 2.56 (±0.70) | 2.49 (±0.60) | 2.58 (±0.73) | 0.037a |
| HDL cholesterol, mmol/L | 1.01 (±0.19) | 1.02 (±0.19) | 1.01 (±0.19) | 0.690 |
| Platelet count, 109/L | 195 (±59) | 197 (±62) | 194 (±58) | 0.447 |
| Procedural characteristics | ||||
| Grafts per patient | 3.35 (±0.80) | 3.31 (±0.80) | 3.36 (±0.80) | 0.332 |
| Off‐pump procedure | 812 (52.6) | 179 (54.6) | 633 (52.1) | 0.418 |
| Complete revascularization | 1451 (94.0) | 305 (93.0) | 1146 (94.2) | 0.396 |
| Blood transfusion | 1078 (69.8) | 236 (72.0) | 842 (69.3) | 0.524 |
| Secondary prevention medication | ||||
| Aspirin | 1439 (93.2) | 307 (93.6) | 1132 (93.1) | 0.747 |
| ACE inhibitor | 493 (31.9) | 102 (31.1) | 391 (32.2) | 0.716 |
| β‐blocker | 963 (62.4) | 210 (64.0) | 753 (61.9) | 0.486 |
| Diuretics | 83 (5.4) | 17 (5.2) | 66 (5.4) | 0.862 |
| Calcium‐channel blocker | 437 (28.3) | 108 (32.9) | 329 (27.1) | 0.036a |
| Statins | 1104 (71.5) | 243 (74.1) | 861 (70.8) | 0.243 |
Values are presented as numbers of patients or means±SD. ACE indicates angiotensin‐converting enzyme; BMI, body mass index; COPD, chronic obstructive pulmonary disease; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; LVEF, left ventricular ejection fraction; MACCE, major adverse cardiac and cerebrovascular events; MI, myocardial infarction; PCI, percutaneous coronary intervention.
Statistically significant (P<0.05).
Table 2.
Baseline Information of Replication Cohort
| Variable | All Patients (n=646) | With MACCE (n=80) | Without MACCE (n=566) | P Value |
|---|---|---|---|---|
| Age, y | 62.54 (±6.85) | 63.73 (±7.12) | 62.38 (±6.80) | 0.099 |
| Female sex | 79 (12.2) | 11 (13.8) | 68 (12.0) | 0.657 |
| BMI, kg/m2 | 26.83 (±3.21) | 27.32 (±3.46) | 26.76 (±3.17) | 0.144 |
| Smokers | 447 (69.2) | 56 (70.0) | 391 (69.1) | 0.868 |
| Hypertension | 430 (66.6) | 51 (63.7) | 379 (67.0) | 0.569 |
| Diabetes mellitus | 327 (50.6) | 36 (45.0) | 291 (51.4) | 0.283 |
| Insulin‐treated diabetes mellitus | 34 (5.3) | 4 (5.0) | 30 (5.3) | 1 |
| Renal dysfunction | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 |
| COPD | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 |
| Peripheral arterial disease | 29 (4.5) | 5 (6.3) | 24 (4.2) | 0.416 |
| Previous MI | 512 (79.3) | 67 (83.8) | 445 (78.6) | 0.29 |
| Previous PCI | 125 (19.3) | 16 (20.0) | 109 (19.3) | 0.875 |
| Aspirin | 609 (94.3) | 71 (88.8) | 538 (95.1) | 0.023a |
| ACE inhibitor | 162 (25.1) | 23 (28.7) | 139 (24.6) | 0.418 |
| β‐blocker | 470 (72.8) | 56 (70.0) | 414 (73.1) | 0.554 |
| Diuretics | 71 (11.0) | 15 (18.8) | 56 (9.9) | 0.018a |
| Calcium‐channel blocker | 210 (32.5) | 26 (32.5) | 184 (32.5) | 0.999 |
| Statins | 433 (67.0) | 42 (52.5) | 391 (69.1) | 0.003a |
Values are presented as numbers of patients or means±SD. ACE indicates angiotensin‐converting enzyme; BMI, body mass index; COPD, chronic obstructive pulmonary disease; LVEF, left ventricular ejection fraction; MACCE, major adverse cardiac and cerebrovascular events.
Statistically significant (P<0.05).
GPIA rs1126643 Genotype Information
Genotype and allele frequency information in both cohorts is displayed in Table S2. The observed genotype distributions of rs1126643 in both cohorts were similar and in Hardy–Weinberg equilibrium (P>0.01 in both cohorts).
Correlation Between GPIA rs1126643 Polymorphism and Clinical Outcomes
In the discovery cohort, MACCE incidence during follow‐up period was 18.8% in the CC homozygotes group, whereas it was significantly higher in the T‐allele carriers group with 23.4% (P=0.028). GPIA rs1126643 was associated with increase of MACCE using univariate Cox‐regression analysis (hazard ratio [HR]=1.292; P=0.022); when adjusted by risk factors (listed in Table S3), this correlation of rs1126643 and MACCE still exist (HR=1.272; P=0.033). Then, this association was validated in the independent replication cohort. MACCE incidence was 9.2% in the CC homozygotes group and increased to 15.3% in the T‐allele carriers group (P=0.019). Univariate (HR=1.739; P=0.019) and multivariate (HR=1.741; P=0.020) Cox regression analyses also suggested that rs1126643 T‐allele carriers have increased MACCE risk in the replication cohort (Table 3). From the Kaplan–Meier survival curves shown in Figure 1, we have noticed that the different MACCE occurrence between 2 groups manifested since the fourth year in the discovery cohort and the third year in the replication cohort, respectively, indicating that rs1126643 T allele is associated only with long‐term MACCE.
Table 3.
Associations between GPIA rs1126643 and MACCE in Discovery Cohort and Replication Cohort
| GPIA rs1126643 | Discovery Cohort | Replication Cohort | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Frequency, % | HR | P Value | Adjusted HR | Adjusted P Value | Frequency, % | HR | P Value | Adjusted HR | Adjusted P Value | |||
| Without MACCE | With MACCE | Without MACCE | With MACCE | |||||||||
| CC | 81.2 | 18.8 | 90.8 | 9.2 | ||||||||
| CT+TT | 76.6 | 23.4 | 84.7 | 15.3 | ||||||||
| 1.292 (1.037–1.609) | 0.022a | 1.272 (1.020–1.587) | 0.033a | 1.739 (1.094–2.765) | 0.019a | 1.741 (1.092–2.773) | 0.020a | |||||
Adjusted risk factors are reported in Table S3. GPIA indicates glycoprotein Ia; HR, hazard ratio; MACCE, major adverse cardiac and cerebrovascular events.
Statistically significant (P<0.05).
Figure 1.

Kaplan–Meier survival curves of GPIA rs1126643 for discovery cohort and replication cohort. A, Survival curve of GPIA rs1126643 for discovery cohort (unadjusted). Four years after CABG in the discovery cohort, rs1126643 T carriers manifested significant low MACCE‐free survival rate. B, Survival curve of GPIA rs1126643 for replication cohort (unadjusted). Three years after CABG in the replication cohort, rs1126643 T‐allele carriers exhibited significant low MACCE‐free survival rate. CABG indicates coronary artery bypass graft; GPIA, glycoprotein Ia; MACCE, major adverse cardiac and cerebrovascular events.
Platelet Counts and Functions Between CC Homozygotes and T‐Allele Carriers Groups
To investigate the influence of GPIA rs1126643 on platelet function, we recruited 131 CAD patients to test their platelet aggregation rate. The baseline information is presented in Table S4. Genotype distributions of this cohort were similar to the discovery and replication cohorts (GPIA rs1126643 CC: n=61, 46.5%; CT: n=63, 48.1%; TT: n=7, 5.3%; Hardy–Weinberg equilibrium, P>0.01). As shown in Figure 2, we found that T‐allele carriers have a higher platelet aggregation rate (CC homozygotes: 58.8±26.9%; T‐allele carriers: 68.3±22.1%; P=0.029) whereas the platelet counts show no between‐group differences.
Figure 2.
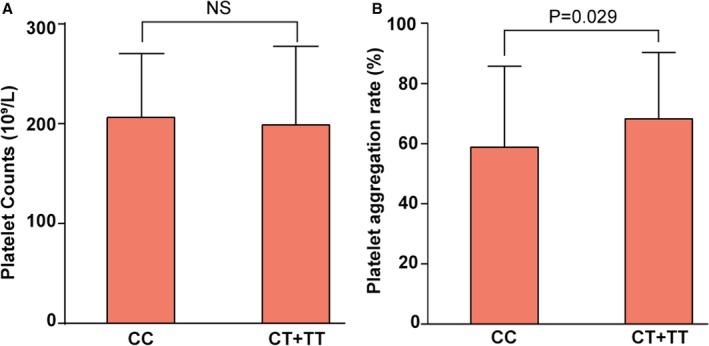
Platelet aggregation rate of CC homozygotes and T‐allele carriers. A, Platelet counts of CC homozygotes (206±64×109/L) and T‐allele carriers (213±68×109/L), P=0.56. B, Platelet aggregation rate of T‐allele carriers (68.3±22.1%) was higher than that of CC homozygotes (58.8±26.9%), P=0.029. NS indicates not significant.
Influences of rs1126643 on GPIA mRNA and Protein Expression
Next, we studied the underlying mechanisms of the influence on platelet function. First, we found that expression levels of GPIA mRNA show no difference between CC homozygotes and T‐allele carriers (Figure 3A). Second, in order to investigate whether T allele would enhance transcription of GPIA, we monitored GPIA protein expression levels among CAD patients. As shown in Figure 3B, T‐allele carriage would increase expression of GPIA protein significantly (P<0.001).
Figure 3.
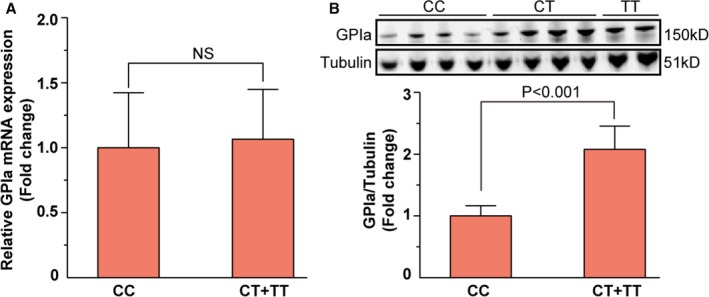
GPIA mRNA and protein expression levels in different genotype groups. A, Relative GPIA mRNA levels of CC homozygotes and T‐allele carriers. GPIA mRNA expression levels have no significant differences between CC homozygotes and T‐allele carriers. B, GPIA protein expression levels of CC homozygotes and T‐allele carriers. GPIA protein expression level of T‐allele carriers was 2.08 times higher than that of CC homozygotes, P<0.001. GPIA, glycoprotein Ia; NS, not significant.
Discussion
This study provides both population‐ and experiment‐based evidence between GPIA rs1126643 and long‐term adverse events post‐CABG. In a population study, we found that GPIA rs1126643 T‐allele carriers have higher MACCE risks 6 years post‐CABG than those patients who do not carry any rs1126643 T allele, and this association was validated in another independent group of CABG patients. In an experimental study, we tested the platelet aggregation rate of a 131‐patient cohort and found that rs1126643 T allele could enhance platelet function. Further study suggested that rs1126643 T allele augmented GPIA protein expression, but not mRNA level. From this evidence, we demonstrated that GPIA rs1126643 polymorphism is associated with long‐term MACCE post‐CABG.
There are several types of glycoprotein on the platelet surface, mediating platelet adhesion, activation, and aggregation, resulting in a sequential of events of thrombus formation.29 Among these glycoproteins, GPIIIa polymorphism PIA2 is associated with higher troponin I concentrations following cardiopulmonary bypass surgery30 and is reported as a hereditary risk factor for graft occlusion, MI, or death in patients post‐CABG.31 Apart from GPIIIa, GP Ia/IIa and GP VI play a vital role to mediate platelet‐collagen interactions.32 Being different from other types of glycoproteins, GP Ia/IIa density varies by up to 10‐fold among normal individuals, which is considered to be caused by genetic variations.33, 34, 35 Moreover, platelet attachment to type I collagen at high shear stress in whole blood is more extensive in patients with high GPIa/IIa density.36 The effect of the extreme case was illustrated in a patient who totally failed to express platelet GPIa, and this patient exhibited complete absence of collagen‐induced aggregation and an excessively prolonged bleeding time.37
Kunicki et al found that individuals who carry the genotype of rs1126643 TT exhibited much more GPIa/IIa expression on platelets than those bearing CC, and the CT heterozygotes had an intermediate level.16 Our findings further indicated that mRNA levels between CC patients and T‐allele carriers are no different, which indicates that the rs1126643 polymorphism does not influence the transcript process or degrade the transcript mRNA.
Mechanistically, there are several explanations for the regulation role of rs1126643. First, a codon usage bias may account for it. The rate of translation elongation can be enhanced by usage of common codons for several folds compared with the usage of rare ones.38, 39 In GPIA gene, there are 57 codons translate for phenylalanine. Of these 57 codons, 36 (63.1%) are TTT, which is the common codon in the context of GPIA gene, whereas TTC can be defined as the rare codon in GPIA gene. So, it is reasonable that the rate of translation of GPIA gene is promoted in rs1126643 T‐allele carriers, which would result in an enhancement of GPIa expression. Second, the synonymous variant may alter the microRNA (miRNA) binding site to impede binding of miRNA‐mRNA to promote the translation rate.40 Although most miRNA targets the 3′ untranslated region of the mRNA, increasing evidence produced in recent years suggested that miRNA also binds in the coding region (CDS),41 and the miRNA‐CDS interaction has a negligible impact on mRNA stability, but is potent in inhibiting protein translation.42
To our knowledge, this is the first study to demonstrate that GPIA rs1126643 increases risk of long‐term MACCE in CABG patients. Given that the risk allele T is widely distributed (with a frequency of 0.28 among Eastern Asians and 0.40 among Europeans according to data from the 1000 Genome Project), GPIA rs1126643 would be a powerful prediction factor for the prognosis of CABG patients.
However, several limitations should be noted in this study. First, our study is based on Chinese patients; whether the effects of GPIA rs1126643 still exist in other populations should be validated. Second, because the replication cohort was from HPS2‐THRIVE study, which was not focused on CABG, several factors involved in CABG procedure and perioperative characteristic are not available, which limited further multivariable analyses in the replication cohort.
Conclusions
In conclusion, the present study provided both population‐ and experimental‐based evidence that GPIa rs1126643 T‐allele carriage increases risk of MACCE post‐CABG. The underlying mechanisms involve posttranscription regulation of GPIa expression. These findings suggest that GPIa may be a potential target as improvement in long‐term prognosis post‐CABG. In addition, the genotype of GPIA rs1126643 could be used to recognize patients who are susceptible to MACCE and thus generate an intensive secondary prevention strategy for these patients.
Sources of Funding
The study was funded by the Key Projects in the National Science and Technology Pillar Program during the 12th 5‐year plan period (2011BAI11B02 and 2011BAI11B21) and Innovative Grant of the Chinese Academy of Medical Sciences (2015‐1002‐02‐22).
Disclosures
None.
Supporting information
Data S1. Revascularization procedures.
Table S1. Information of Primers
Table S2. Genotype Information and Hardy–Weinberg Equilibrium Tests for GPIA rs1126643
Table S3. Candidate Risk Factors
Table S4. Baseline Information of Functional Study Cohort
Figure S1. Kaplan–Meier survival curves of GPIA rs1126643 for discovery cohort and replication cohort grouped by CC, CT, and TT genotypes.
Figure S2. Platelet counts and aggregation rate grouped by CC, CT, and TT genotypes.
Figure S3. GPIA mRNA and protein expression levels grouped by CC, CT, and TT genotypes.
Acknowledgments
We thank all the doctors, nurses, and administrative staff members at Fuwai Hospital who assisted with the undertaking of this study.
(J Am Heart Assoc. 2016;5:e004496 doi: 10.1161/JAHA.116.004496)
Contributor Information
Zhou Zhou, Email: zhouzhou@fuwaihospital.org.
Zhe Zheng, Email: zhengzhe@fuwai.com.
References
- 1. Mirzaei M, Truswell AS, Taylor R, Leeder SR. Coronary heart disease epidemics: not all the same. Heart. 2009;95:740–746. [DOI] [PubMed] [Google Scholar]
- 2. Task Force M , Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Budaj A, Bugiardini R, Crea F, Cuisset T, Di Mario C, Ferreira JR, Gersh BJ, Gitt AK, Hulot JS, Marx N, Opie LH, Pfisterer M, Prescott E, Ruschitzka F, Sabate M, Senior R, Taggart DP, van der Wall EE, Vrints CJ; Guidelines ESCCfP , Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S; Document R , Knuuti J, Valgimigli M, Bueno H, Claeys MJ, Donner‐Banzhoff N, Erol C, Frank H, Funck‐Brentano C, Gaemperli O, Gonzalez‐Juanatey JR, Hamilos M, Hasdai D, Husted S, James SK, Kervinen K, Kolh P, Kristensen SD, Lancellotti P, Maggioni AP, Piepoli MF, Pries AR, Romeo F, Ryden L, Simoons ML, Sirnes PA, Steg PG, Timmis A, Wijns W, Windecker S, Yildirir A, Zamorano JL. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34:2949–3003. [DOI] [PubMed] [Google Scholar]
- 3. Farkouh ME, Domanski M, Sleeper LA, Siami FS, Dangas G, Mack M, Yang M, Cohen DJ, Rosenberg Y, Solomon SD, Desai AS, Gersh BJ, Magnuson EA, Lansky A, Boineau R, Weinberger J, Ramanathan K, Sousa JE, Rankin J, Bhargava B, Buse J, Hueb W, Smith CR, Muratov V, Bansilal S, King S III, Bertrand M, Fuster V; Investigators FT . Strategies for multivessel revascularization in patients with diabetes. N Engl J Med. 2012;367:2375–2384. [DOI] [PubMed] [Google Scholar]
- 4. Mohr FW, Morice MC, Kappetein AP, Feldman TE, Stahle E, Colombo A, Mack MJ, Holmes DR Jr, Morel MA, Van Dyck N, Houle VM, Dawkins KD, Serruys PW. Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three‐vessel disease and left main coronary disease: 5‐year follow‐up of the randomised, clinical SYNTAX trial. Lancet. 2013;381:629–638. [DOI] [PubMed] [Google Scholar]
- 5. Kappetein AP, Head SJ, Morice MC, Banning AP, Serruys PW, Mohr FW, Dawkins KD, Mack MJ; Investigators S . Treatment of complex coronary artery disease in patients with diabetes: 5‐year results comparing outcomes of bypass surgery and percutaneous coronary intervention in the SYNTAX trial. Eur J Cardiothorac Surg. 2013;43:1006–1013. [DOI] [PubMed] [Google Scholar]
- 6. Hogue CW Jr, Murphy SF, Schechtman KB, Davila‐Roman VG. Risk factors for early or delayed stroke after cardiac surgery. Circulation. 1999;100:642–647. [DOI] [PubMed] [Google Scholar]
- 7. Mathew JP, Podgoreanu MV, Grocott HP, White WD, Morris RW, Stafford‐Smith M, Mackensen GB, Rinder CS, Blumenthal JA, Schwinn DA, Newman MF; Team PI . Genetic variants in P‐selectin and C‐reactive protein influence susceptibility to cognitive decline after cardiac surgery. J Am Coll Cardiol. 2007;49:1934–1942. [DOI] [PubMed] [Google Scholar]
- 8. Lobato RL, White WD, Mathew JP, Newman MF, Smith PK, McCants CB, Alexander JH, Podgoreanu MV; Duke Perioperative G, Safety Outcomes Investigative T . Thrombomodulin gene variants are associated with increased mortality after coronary artery bypass surgery in replicated analyses. Circulation. 2011;124:S143–S148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Podgoreanu MV, Schwinn DA. New paradigms in cardiovascular medicine: emerging technologies and practices: perioperative genomics. J Am Coll Cardiol. 2005;46:1965–1977. [DOI] [PubMed] [Google Scholar]
- 10. Gao G, Zheng Z, Pi Y, Lu B, Lu J, Hu S. Aspirin plus clopidogrel therapy increases early venous graft patency after coronary artery bypass surgery a single‐center, randomized, controlled trial. J Am Coll Cardiol. 2010;56:1639–1643. [DOI] [PubMed] [Google Scholar]
- 11. Loscalzo J. Functional polymorphisms in a candidate gene for atherothrombosis: unraveling the complex fabric of a polygenic phenotype. J Am Coll Cardiol. 2003;41:946–948. [DOI] [PubMed] [Google Scholar]
- 12. Handa M, Watanabe K, Kawai Y, Kamata T, Koyama T, Nagai H, Ikeda Y. Platelet unresponsiveness to collagen: involvement of glycoprotein Ia‐IIa (alpha 2 beta 1 integrin) deficiency associated with a myeloproliferative disorder. Thromb Haemost. 1995;73:521–528. [PubMed] [Google Scholar]
- 13. Kehrel B, Balleisen L, Kokott R, Mesters R, Stenzinger W, Clemetson KJ, van de Loo J. Deficiency of intact thrombospondin and membrane glycoprotein Ia in platelets with defective collagen‐induced aggregation and spontaneous loss of disorder. Blood. 1988;71:1074–1078. [PubMed] [Google Scholar]
- 14. Santoro SA, Zutter MM. The alpha 2 beta 1 integrin: a collagen receptor on platelets and other cells. Thromb Haemost. 1995;74:813–821. [PubMed] [Google Scholar]
- 15. Nieuwenhuis HK, Akkerman JW, Houdijk WP, Sixma JJ. Human blood platelets showing no response to collagen fail to express surface glycoprotein Ia. Nature. 1985;318:470–472. [DOI] [PubMed] [Google Scholar]
- 16. Kunicki TJ, Orchekowski R, Annis D, Honda Y. Variability of integrin alpha 2 beta 1 activity on human platelets. Blood. 1993;82:2693–2703. [PubMed] [Google Scholar]
- 17. Estavillo D, Ritchie A, Diacovo TG, Cruz MA. Functional analysis of a recombinant glycoprotein Ia/IIa (integrin alpha(2)beta(1)) I domain that inhibits platelet adhesion to collagen and endothelial matrix under flow conditions. J Biol Chem. 1999;274:35921–35926. [DOI] [PubMed] [Google Scholar]
- 18. Kunicki TJ, Kritzik M, Annis DS, Nugent DJ. Hereditary variation in platelet integrin alpha 2 beta 1 density is associated with two silent polymorphisms in the alpha 2 gene coding sequence. Blood. 1997;89:1939–1943. [PubMed] [Google Scholar]
- 19. Antoniades C, Tousoulis D, Vasiliadou C, Stefanadi E, Marinou K, Stefanadis C. Genetic polymorphisms of platelet glycoprotein Ia and the risk for premature myocardial infarction: effects on the release of sCD40L during the acute phase of premature myocardial infarction. J Am Coll Cardiol. 2006;47:1959–1966. [DOI] [PubMed] [Google Scholar]
- 20. Benze G, Heinrich J, Schulte H, Rust S, Nowak‐Gottl U, Tataru MC, Kohler E, Assmann G, Junker R. Association of the GPIa C807T and GPIIIa PLA1/A2 polymorphisms with premature myocardial infarction in men. Eur Heart J. 2002;23:325–330. [DOI] [PubMed] [Google Scholar]
- 21. Santoso S, Kunicki TJ, Kroll H, Haberbosch W, Gardemann A. Association of the platelet glycoprotein Ia C807T gene polymorphism with nonfatal myocardial infarction in younger patients. Blood. 1999;93:2449–2453. [PubMed] [Google Scholar]
- 22. Leone AM, De Stefano V, Burzotta F, Chiusolo P, Casorelli I, Paciaroni K, Rossi E, Sciahbasi A, Testa L, Leone G, Crea F, Andreotti F. Glycoprotein Ia C807T gene polymorphism and increased risk of recurrent acute coronary syndromes: a five year follow up. Heart. 2004;90:567–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Group HTC . HPS2‐THRIVE randomized placebo‐controlled trial in 25 673 high‐risk patients of ER niacin/laropiprant: trial design, pre‐specified muscle and liver outcomes, and reasons for stopping study treatment. Eur Heart J. 2013;34:1279–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Machin D, Campbell M, Fayers P, Pinol A. Sample Size Tables for Clinical Studies. 2nd ed Malden, MA: Blackwell Science; 1997. [Google Scholar]
- 25. Zar JH. Biostatistical Analysis. 2nd ed Englewood Cliffs, NJ: Prentice‐Hall; 1984. [Google Scholar]
- 26. Serruys PW, Morice MC, Kappetein AP, Colombo A, Holmes DR, Mack MJ, Stahle E, Feldman TE, van den Brand M, Bass EJ, Van Dyck N, Leadley K, Dawkins KD, Mohr FW; Investigators S . Percutaneous coronary intervention versus coronary‐artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009;360:961–972. [DOI] [PubMed] [Google Scholar]
- 27. Cannon CP, Brindis RG, Chaitman BR, Cohen DJ, Cross JT Jr, Drozda JP Jr, Fesmire FM, Fintel DJ, Fonarow GC, Fox KA, Gray DT, Harrington RA, Hicks KA, Hollander JE, Krumholz H, Labarthe DR, Long JB, Mascette AM, Meyer C, Peterson ED, Radford MJ, Roe MT, Richmann JB, Selker HP, Shahian DM, Shaw RE, Sprenger S, Swor R, Underberg JA, Van de Werf F, Weiner BH, Weintraub WS. 2013 ACCF/AHA key data elements and definitions for measuring the clinical management and outcomes of patients with acute coronary syndromes and coronary artery disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on clinical data standards (writing committee to develop acute coronary syndromes and coronary artery disease clinical data standards). J Am Coll Cardiol. 2013;61:992–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schaeffeler E, Zanger UM, Eichelbaum M, Asante‐Poku S, Shin JG, Schwab M. Highly multiplexed genotyping of thiopurine S‐methyltransferase variants using MALD‐TOF mass spectrometry: reliable genotyping in different ethnic groups. Clin Chem. 2008;54:1637–1647. [DOI] [PubMed] [Google Scholar]
- 29. Savage B, Almus‐Jacobs F, Ruggeri ZM. Specific synergy of multiple substrate‐receptor interactions in platelet thrombus formation under flow. Cell. 1998;94:657–666. [DOI] [PubMed] [Google Scholar]
- 30. Rinder CS, Mathew JP, Rinder HM, Greg Howe J, Fontes M, Crouch J, Pfau S, Patel P, Smith BR; Multicenter Study of Perioperative Ischemia Research G . Platelet PLA2 polymorphism and platelet activation are associated with increased troponin I release after cardiopulmonary bypass. Anesthesiology. 2002;97:1118–1122. [DOI] [PubMed] [Google Scholar]
- 31. Zotz RB, Klein M, Dauben HP, Moser C, Gams E, Scharf RE. Prospective analysis after coronary‐artery bypass grafting: platelet GP IIIa polymorphism (HPA‐1B/PIA2) is a risk factor for bypass occlusion, myocardial infarction, and death. Thromb Haemost. 2000;83:404–407. [PubMed] [Google Scholar]
- 32. Kiefer TL, Becker RC. Inhibitors of platelet adhesion. Circulation. 2009;120:2488–2495. [DOI] [PubMed] [Google Scholar]
- 33. Jacquelin B, Tarantino MD, Kritzik M, Rozenshteyn D, Koziol JA, Nurden AT, Kunicki TJ. Allele‐dependent transcriptional regulation of the human integrin alpha2 gene. Blood. 2001;97:1721–1726. [DOI] [PubMed] [Google Scholar]
- 34. Samaha FF, Hibbard C, Sacks J, Chen H, Varello MA, George T, Kahn ML. Measurement of platelet collagen receptor density in human subjects. Arterioscler Thromb Vasc Biol. 2004;24:e181–e182. [DOI] [PubMed] [Google Scholar]
- 35. Kunicki TJ, Nugent DJ. The genetics of normal platelet reactivity. Blood. 2010;116:2627–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kritzik M, Savage B, Nugent DJ, Santoso S, Ruggeri ZM, Kunicki TJ. Nucleotide polymorphisms in the alpha2 gene define multiple alleles that are associated with differences in platelet alpha2 beta1 density. Blood. 1998;92:2382–2388. [PubMed] [Google Scholar]
- 37. Nieuwenhuis HK, Sakariassen KS, Houdijk WP, Nievelstein PF, Sixma JJ. Deficiency of platelet membrane glycoprotein Ia associated with a decreased platelet adhesion to subendothelium: a defect in platelet spreading. Blood. 1986;68:692–695. [PubMed] [Google Scholar]
- 38. Shabalina SA, Spiridonov NA, Kashina A. Sounds of silence: synonymous nucleotides as a key to biological regulation and complexity. Nucleic Acids Res. 2013;41:2073–2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sorensen MA, Kurland CG, Pedersen S. Codon usage determines translation rate in Escherichia coli . J Mol Biol. 1989;207:365–377. [DOI] [PubMed] [Google Scholar]
- 40. Sauna ZE, Kimchi‐Sarfaty C. Understanding the contribution of synonymous mutations to human disease. Nat Rev Genet. 2011;12:683–691. [DOI] [PubMed] [Google Scholar]
- 41. Yu D, Green B, Tolleson WH, Jin Y, Mei N, Guo Y, Deng H, Pogribny I, Ning B. MicroRNA hsa‐miR‐29a‐3p modulates CYP2C19 in human liver cells. Biochem Pharmacol. 2015;98:215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hausser J, Syed AP, Bilen B, Zavolan M. Analysis of CDS‐located miRNA target sites suggests that they can effectively inhibit translation. Genome Res. 2013;23:604–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Revascularization procedures.
Table S1. Information of Primers
Table S2. Genotype Information and Hardy–Weinberg Equilibrium Tests for GPIA rs1126643
Table S3. Candidate Risk Factors
Table S4. Baseline Information of Functional Study Cohort
Figure S1. Kaplan–Meier survival curves of GPIA rs1126643 for discovery cohort and replication cohort grouped by CC, CT, and TT genotypes.
Figure S2. Platelet counts and aggregation rate grouped by CC, CT, and TT genotypes.
Figure S3. GPIA mRNA and protein expression levels grouped by CC, CT, and TT genotypes.


