Abstract
Background
This study assessed the role of surgical ablation for atrial fibrillation (AF) in decreasing tricuspid regurgitation (TR) and right‐sided heart remodeling in patients after mitral valve procedure.
Methods and Results
Between 1994 and 2014, 1568 consecutive patients with AF undergoing mitral valve procedure were identified. In 26.0% (n=408), surgical ablation of AF was used. Propensity‐score matching (PSM) was performed on the basis of 41 known perioperative risk variables. Survival, reoperation, stroke, and moderate‐to‐severe TR, as well as echocardiography indices in long‐term follow‐up, were compared in 406 matched patient pairs (ablated and nonablated groups). The nonablated group showed significantly higher risks of death (hazard ratio [HR], 1.644; 95% CI, 1.081–2.501; P=0.020), reoperation (HR, 2.644; 95% CI, 1.299–5.466; P=0.008), and moderate‐to‐severe TR (HR, 1.436; 95% CI, 1.059–1.948; P=0.020), associated with a significantly deteriorated cardiac function, progression of TR, and right‐sided heart remodeling after 5‐year follow‐up. In a subgroup comparison of ablated patients with sinus rhythm versus AF recurrence, a PSM analysis was performed at the 5‐year follow‐up. The recurrent group showed significantly higher risks of moderate‐to‐severe TR (HR, 2.427; 95% CI, 1.261–4.671; P=0.008). AF recurrence was associated with progressive TR and significant deterioration in right‐sided heart remodeling.
Conclusions
In a retrospective PSM analysis, mitral valve disease with AF was associated with TR progression as well as right‐sided heart remodeling, which are alleviated by surgical ablation.
Keywords: ablation, atrial fibrillation, mitral valve, remodeling, tricuspid regurgitation
Subject Categories: Atrial Fibrillation, Valvular Heart Disease, Cardiovascular Surgery
Introduction
Tricuspid regurgitation (TR) often progresses late after mitral valve (MV) surgery without left‐sided valvular dysfunction.1, 2, 3 TR progression adversely affects long‐term mortality and morbidity. Therefore, prevention of TR is crucial.3, 4, 5, 6
Atrial fibrillation (AF), a common arrhythmia in patients with MV diseases, is often associated with left and right atrial enlargement, resulting in annular dilatation, and TR.7, 8 AF predisposes patients undergoing MV surgery to TR progression, which is prevented by MAZE.9, 10
To reduce the additional time required to perform the standard MAZE III procedure, surgeons have developed alternate energy sources to create atrial ablation lines and minimize the duration of ischemic time and cardiopulmonary bypass in open surgical ablation of AF.11, 12 Long‐term outcomes can be improved by successful ablation in patients with pre‐existing persistent or long‐standing persistent AF treated with MV surgery.13, 14 However, the role of surgical ablation in preventing right‐sided heart remodeling that contributes to late TR long after MV surgery is unclear. Evidence related to TR progression in patients without surgical ablation during long‐term follow‐up is scarce.
In the absence of a randomized trial, we used propensity‐score matching (PSM) to review the long‐term outcomes of TR progression and right‐sided heart remodeling in ablated patients with MV disease versus nonablated patients.
Methods
Study Population
Following the approval of an institutional review board, we conducted a review of the medical records from 1994 to 2014 to identify patients with persistent or long‐standing persistent AF and concomitant MV disease requiring surgical intervention. According to the 2014 American Heart Association/American College of Cardiology/Heart Rhythm Society (AHA‐ACC‐HRS) guidelines, persistent AF was defined as continuous AF for more than 7 days. Long‐standing persistent AF was defined as continuous AF for more than 12 months.15 All patients in this study consented to use of their follow‐up data.
We evaluated 1568 patients (408 in the ablated group and 1160 in the nonablated group) who were discharged alive with inpatient diagnoses of MV disease and persistent or long‐standing persistent AF. The patients in the nonablated and ablated groups were selected from those discharged alive from 1994 to 2003 and 2004 to 2014, respectively. Notably, the differences in duration of patient enrolment in the 2 groups until surgical ablation were widely adopted in our institution.
Surgical Procedures
The procedures were performed using standard cardiopulmonary bypass. In the total cohort, MV repair and replacement were performed successfully in 152 (13.1%) and 1008 (86.9%) patients, respectively. All the 408 patients in the ablated group were treated with a surgical AF biatrial MAZE ablation as we described before.16
The procedures were performed using standard cardiopulmonary bypass. The aorta was then cross‐clamped, the heart was arrested, and the left atrium accessed through a left atriotomy. Pulmonary vein ablation was carried out with the bipolar Cardioablate (Medtronic, Minneapolis, MN) or Atricure clamp (Atricure, West Chester, OH).
All procedures were performed using routine cardiopulmonary bypass with bicaval and aortic cannulation under moderate hypothermia. The procedure was carried out with the bipolar Cardioablate (Medtronic) or Atricure clamp (Atricure). After cardioplegic arrest, a left atrial incision was performed through the interatrial groove. The left atrial appendage was either amputated and sutured afterward, or a circumferential radiofrequency lesion was created around its base and the orifice oversewn from inside the atrium. In addition to the incision in the interatrial groove, isolation of the right pulmonary veins was completed by a circular ablation line. The left pulmonary veins were encircled and a connecting line was performed between both islands of pulmonary veins on the roof, as near to the left atrial roof as possible to avoid injury to the esophagus. An ablation line from the left pulmonary veins to the posterior mitral annulus was then performed with caution so as not to injure the circumflex coronary artery. Cavotricuspid isthmus ablation was then performed to achieve a bidirectional conduction block. Division of the ligament of Marshall was performed in all patients. In the right atrial lesions, the following lesions, other than cavotricuspid isthmus ablation, were added in the right atrium: excision of right atrial appendage; superior vena cava to inferior vena cava; lateral free‐wall lesion complete to anterior‐medial tricuspid valve annulus; and medial free‐wall lesion complete to anterior‐medial tricuspid valve annulus.
Postoperative Antiarrhythmic Drug Use
In the ablated group, an intravenous amiodarone bolus (150 mg), followed by continuous intravenous infusion at 1 mg/kg per hour for 12 hours and then 0.5 mg/kg per hour until patients tolerated oral intake, was routinely administered intraoperatively. Patients then received oral amiodarone (200 mg twice‐daily for 1 week and subsequently 200 mg daily) until 3 months postoperatively.
Rate control in AF is an important strategy. It impacts quality of life, reduces morbidity, and decreases the potential for developing tachycardia‐induced cardiomyopathy. Multiple agents, including beta‐blockers, nondihydropyridine calcium‐channel blockers (CCBs), digoxin, and certain antiarrhythmic drugs, including amiodarone and sotalol, have been used with regard to efficacy in attaining rate control in patients with AF persistence in our institution. When considering which agent(s) to use, the cardiologist would consider the patient's degree of symptoms, hemodynamic status, presence or absence of heart failure, and potential precipitants of AF.
Protocol for Anticoagulation
Oral anticoagulation was given to maintain the international normalized ratio between 2.0 and 2.5 for the first 6 months in all patients, and lifelong in patients who received mechanical valves or who had AF persistence, or both.
Echocardiography
Comprehensive 2‐dimensional (2D) echocardiography was performed with a commercially available system (IE33; Philips Medical Systems, Andover, MA). Standard 2D and Doppler echocardiographic images were acquired in the left lateral decubitus position using a phased‐array transducer in the parasternal and apical views by experienced cardiac sonographers. Three consecutive cardiac cycles were recorded and stored for subsequent offline analysis. Left ventricle end‐diastolic LVEDD and end‐systolic dimensions (LVESD) were measured from parasternal acquisitions. Left ventricle volumes and left ventricle ejection fraction (LVEF) were calculated using Simpson's biplane method according to the guidelines of the American Society of Echocardiography.17 Left and right atrial areas were measured by planimetry at end‐systole from the apical 4‐chamber views. Left atrial volumes were measured by Simpson's biplane method. Color flow was applied in the apical 4‐chamber view to assess severity of TR, which was graded semiquantitatively on a scale from 0 to 4 as follows: 0, none or trace; 1+, jet area/atrial area <10% (mild TR); 2+, jet area/atrial area 10% to 20% (moderate TR); 3+, jet area/atrial area 20% to 33% (moderate‐to‐severe TR); and 4+, jet area/atrial area >33% (severe TR).18, 19 From the apical 4‐chamber view, the right ventricle (RV) end‐systolic and end‐diastolic areas were measured by planimetry with the transducer positioned to maximize the RV area and to include the RV apex. RV fractional area change (RVFAC) was used to determine RV systolic function and was calculated by the following formula: FAC=([diastolic area−systolic area]/diastolic area)×100%.20 RV long‐axis length and RV short‐axis width at the midventricular level were measured as described by Matsunaga and Duran21 and used to calculate the end‐diastolic RV sphericity index (RVSI) as previously described (RVSI=RV long‐axis length/RV short‐axis width).22 Systolic pulmonary artery pressure was measured by echocardiography using the modified Bernoulli equation on the transtricuspid continuous‐wave Doppler signal, while adding right atrial pressure.
Data Collection and Follow‐up
Patient characteristics, medications, laboratory values, and in‐hospital outcomes of the index surgery were recorded at the time of presentation and extracted from the hospital medical records. All discharged patients in our institution had visits scheduled for 1, 3, and 12 months postoperatively and every 1 year thereafter. At each visit, history, physical examination, chest X‐ray, electrocardiogram, and echocardiogram were obtained. In patients who complained of recurrent arrhythmia, a 24‐hour Holter was used to assess the rhythm status. Patients unable to return to our institution were followed up with a mailed questionnaire or telephone interview. The copies of electrocardiograms and echocardiograms were returned by mail to the referring physicians. Data related to long‐term outcomes were collected by questionnaires, records requested from referring physicians, and extracts from the medical records at follow‐up visits. Patient‐reported responses were cross‐referenced and corroborated with the most current medical records on file. Patients who were not reachable by questionnaires were followed up based on the last recorded visit on file in the medical record. One‐hundred six patients (9.1%) and 16 patients (3.9%) were lost to follow‐up in the nonablated and ablated groups, respectively.
Statistical Analysis
Categorical variables are presented as numbers and percentages and were compared using the chi‐square test and Fisher's exact test. Continuous variables are expressed as mean±SD and were compared using the Student t test or the Mann–Whitney U test. Event‐free survival curves were constructed with Kaplan–Meier estimates and compared using the log‐rank test. Cox proportional hazards analysis was performed to compare hazard rates of outcomes between groups. Linear regression analyses were performed between the time after recurrence and the left atrial diameter (LAD), right atria (RA) area, pulmonary artery systolic pressure (sPAP), tricuspid annulus diameter (TAD), RVSI, and RVFAC to determine coefficients of regression. Accuracy of linear regression curves is expressed as the coefficient of determination (r 2).
To reduce the effect of potential confounding factors in this observational study, PSM was used. The PSM was performed by matching patients in the 2 groups at a 1:1 ratio, without replacement, by the nearest neighbor technique. Toward this end, we used a PSM custom dialog for SPSS.23 (created by F. Thoemmes, Cornell University. Propensity score matching in SPSS. Available from: http://arxiv.org/ftp/arxiv/papers/1201/1201.6385.pdf).
The criterion for matching pairs used a caliper width equal to 0.2 of the pooled SD of the logit of propensity score. All analyses were performed with IBM SPSS‐20 (IBM, Armonk, NY). PSM was performed with IBM SPSS‐20 and R software (version R2.10.1; R Foundation for Statistical Computing, Vienna, Austria). All tests were 2‐tailed, and P<0.05 was considered significant.
Results
Baseline Characteristics
Baseline clinical and echocardiographic characteristics of the ablated and nonablated groups were compared, as shown in Table 1. The ablated group was more likely to show hypertension, a significantly higher CHADS2 score, and a significantly higher TR grade (P<0.05). PSM of the overall cohort yielded 406 matched pairs of patients. In the matched cohort, no significant differences were observed between the 2 groups for any covariates, using statistical methods appropriate for matched data (Table 1; Figure 1).
Table 1.
Baseline Characteristics of Patients Treated With and Without Surgical Ablation
| Overall Cohort | PSM Cohort | |||||
|---|---|---|---|---|---|---|
| Nonablated Group (n=1160) | Ablated Group (n=408) | P Value | Nonablated Group (n=406) | Ablated Group (n=406) | P Value | |
| Age, y | 46.9±14.7 | 46.3±14.5 | 0.321 | 45.9±15.2 | 46.3±14.5 | 0.654 |
| Male | 457 (39.4) | 163 (40.0) | 0.860 | 174 (42.9) | 163 (40.1) | 0.476 |
| BSA, m2 | 1.71±0.23 | 1.70±0.25 | 0.217 | 1.70±0.25 | 1.70±0.25 | 0.764 |
| BMI, kg/m2 | 22.3±3.7 | 22.2±3.8 | 0.599 | 22.3±3.8 | 22.3±3.8 | 0.944 |
| NYHA class | 2.9±0.7 | 2.9±0.7 | 0.942 | 2.9±0.7 | 2.9±0.7 | 0.728 |
| Smoking | 301 (25.9) | 117 (28.7) | 0.298 | 120 (29.6) | 116 (28.6) | 0.817 |
| Diabetes mellitus | 129 (11.1) | 50 (12.3) | 0.528 | 49 (12.1) | 50 (12.3) | 1.000 |
| Hypertension | 242 (20.9) | 110 (27.0) | 0.013 | 101 (24.9) | 108 (26.6) | 0.630 |
| Stroke | 64 (5.5) | 18 (4.4) | 0.439 | 18 (4.4) | 18 (4.4) | 1.000 |
| CAD | 154 (13.3) | 65 (15.9) | 0.185 | 62 (15.3) | 64 (15.8) | 0.923 |
| COPD | 65 (5.6) | 21 (5.1) | 0.801 | 21 (5.2) | 21 (5.2) | 1.000 |
| CRI | 17 (1.5) | 6 (1.5) | 1.000 | 5 (1.2) | 6 (1.5) | 1.000 |
| CHADS2 score | 0.71±1.28 | 0.78±1.43 | 0.048 | 0.75±1.30 | 0.78±1.43 | 0.758 |
| EuroSCORE | 0.78±1.18 | 0.75±1.07 | 0.140 | 0.72±1.04 | 0.75±1.07 | 0.690 |
| AF duration, months | 2.8±0.8 | 2.8±0.8 | 0.114 | 2.8±0.8 | 2.8±0.7 | 0.814 |
| Etiolgy | ||||||
| Rheumatic | 910 (78.4) | 311 (76.2) | 0.368 | 305 (75.1) | 309 (76.1) | 0.806 |
| Degenerative | 157 (13.5) | 59 (14.5) | 0.676 | 64 (15.8) | 59 (14.5) | 0.696 |
| Endocarditis | 54 (4.7) | 24 (5.9) | 0.354 | 23 (5.7) | 24 (5.9) | 1.000 |
| Functional | 39 (3.4) | 14 (3.4) | 1.000 | 14 (3.4) | 14 (3.4) | 1.000 |
| Medication | ||||||
| ACEI | 215 (18.9) | 84 (20.6) | 0.379 | 80 (19.7) | 83 (20.4) | 0.861 |
| ARB | 73 (6.3) | 24 (5.9) | 0.812 | 24 (5.9) | 24 (5.9) | 1.000 |
| CCB | 20 (1.7) | 10 (2.5) | 0.400 | 10 (2.5) | 9 (2.2) | 1.000 |
| Beta‐blocker | 142 (12.2) | 56 (13.7) | 0.436 | 47 (11.6) | 56 (13.8) | 0.399 |
| Digitalis | 266 (22.9) | 90 (22.1) | 0.732 | 95 (23.4) | 90 (22.2) | 0.738 |
| Diuretics | 267 (23.0) | 82 (20.1) | 0.240 | 85 (20.9) | 82 (20.2) | 0.862 |
| Amiodarone | 57 (4.9) | 30 (7.4) | 0.078 | 25 (6.2) | 29 (7.1) | 0.673 |
| LVESD, mm | 34.9±10.7 | 35.3±13.6 | 0.802 | 34.6±8.6 | 35.4±13.6 | 0.360 |
| LVEDD, mm | 51.3±7.0 | 51.3±9.4 | 0.098 | 51.2±10.1 | 51.4±9.5 | 0.859 |
| sPAP, mm Hg | 41.5±8.8 | 40.5±8.7 | 0.192 | 40.6±8.7 | 40.5±8.7 | 0.877 |
| LAD, mm | 55.9±12.4 | 55.5±12.6 | 0.953 | 55.4±11.8 | 55.6±12.6 | 0.843 |
| LVEF, % | 59.0±7.6 | 58.6±7.9 | 0.424 | 58.0±7.9 | 58.7±7.9 | 0.275 |
| RAA, mm2 | 19.6±4.3 | 19.5±4.3 | 0.528 | 19.9±4.2 | 19.5±4.3 | 0.167 |
| RVSI | 2.0±0.6 | 1.9±0.4 | 0.967 | 1.9±0.6 | 1.9±0.4 | 0.874 |
| RVFAC, % | 42.5±6.0 | 42.6±6.3 | 0.141 | 42.5±6.1 | 42.6±6.3 | 0.857 |
| TAD, cm | 37.1±3.5 | 36.9±3.6 | 0.271 | 37.1±3.6 | 36.9±3.6 | 0.442 |
| TR grade | 1.1±0.6 | 1.1±0.7 | 0.039 | 1.1±0.6 | 1.2±0.7 | 0.661 |
| CABG | 56 (4.8) | 19 (4.7) | 1.000 | 19 (4.7) | 19 (4.7) | 1.000 |
| MV repair | 152 (13.1) | 40 (9.8) | 0.095 | 36 (8.9) | 40 (9.9) | 0.718 |
| MV replacement | 1008 (86.9) | 368 (90.2) | 0.095 | 370 (91.1) | 366 (90.1) | 0.718 |
| Anuloplasty ring | 152 (13.1) | 40 (9.8) | 0.187 | 36 (8.9) | 40 (9.9) | 0.880 |
| Mechanic valve | 708 (61.0) | 264 (64.7) | 0.187 | 265 (65.3) | 264 (65.0) | 0.880 |
| Bioprosthetic valve | 300 (25.9) | 104 (25.5) | 0.187 | 105 (25.9) | 102 (25.1) | 0.880 |
| CPB, minute | 105.2±38.1 | 109.5±37.6 | 0.873 | 110.1±38.2 | 109.3±37.6 | 0.778 |
| ACT, minute | 68.9±28.1 | 72.0±28.6 | 0.403 | 71.3±28.9 | 71.8±28.6 | 0.839 |
ACEI indicates angiotensin‐converting enzyme inhibitor; ACT, aortic clamp time; AF, atrial fibrillation; ARB, angiotensin receptor blocker; BMI, body mass index; BSA, body surface area; CABG, coronary artery bypass graft; CAD, coronary artery disease; CCB, calcium‐channel blocker; COPD, chronic obstructive pulmonary disease; CPB, cardiopulmonary bypass; CRI, chronic renal insufficiency; LAD, left atrial diameter; LVEDD, left ventricle end‐diastolic dimension; LVEF, left ventricle ejection fraction; LVESD, left ventricle end‐systolic dimension; MV, mitral valve; NYHA, New York Heart Association; PSM, propensity‐score matching; RAA, right atrial area; RVFAC, right ventricle fractional area change; RVSI, right ventricle sphericity index; sPAP, pulmonary artery systolic pressure; TAD, tricuspid annulus diameter; TR, tricuspid regurgitation.
Figure 1.
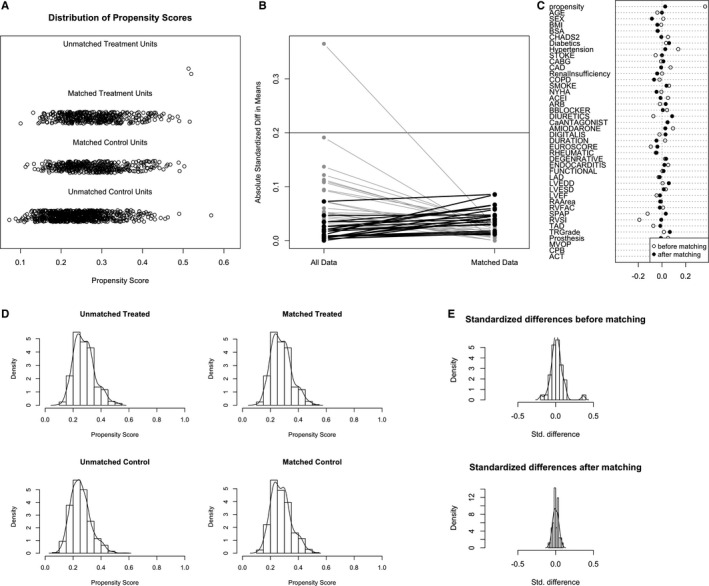
Propensity‐score matching for the total cohort (n=1568, 408 in ablated group, and 1160 in the nonablated group). A, Dot plot of patients in either matched or unmatched groups. B, Line plot of standardized differences before and after matching. C, Dot plot of standardized mean differences (Cohen's d) for all covariates before and after matching. D, Distribution of propensity scores of ablated (“treated”) and nonablated patients (“control”) before and after matching with overlaid kernel density estimate. E, Histograms with overlaid kernel density estimates of standardized differences before and after matching. ACEI indicates angiotensin‐converting enzyme inhibitor; ACT, aortic clamp time; ARB, angiotensin receptor blocker; BMI, body mass index; BSA, body surface area; CABG, coronary artery bypass graft; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; CPB, cardiopulmonary bypass; LAD, left atrial diameter; LVEDD, left ventricle end‐diastolic dimension; LVEF, left ventricle ejection fraction; LVESD, left ventricle end‐systolic dimension; MVOP, mitral valve operation; NYHA, New York Heart Association; RA, right atrial; RVFAC, right ventricle fractional area change; RVSI, right ventricle sphericity index; sPAP, pulmonary artery systolic pressure; Standardized Diff, standardized difference; Std. difference, standardized difference; TAD, tricuspid annulus diameter; TR, tricuspid regurgitation.
Comparison of Outcomes in the Overall Cohort
Median follow‐up was 10.7 years (interquartile range [IQR], 10.7–14.0) in the nonablated group and 7.5 years (IQR, 6.3–8.9) in the ablated group (P<0.001). During the follow‐up, there were 32 deaths in the ablated group and 253 in the nonablated group. Causes of death were sudden cardiac death in 9 patients, congestive heart failure (CHF) in 12, operative mortality after redo surgery in 2, acute myocardial infarction in 2, stroke in 3, traffic accident in 1, and unknown cause in 3 in the ablated group, whereas sudden cardiac death occurred in 95, CHF in 59, operative mortality after redo surgery in 33, acute myocardial infarction in 12, stroke in 32, malignancy in 2, and unknown cause in 20 in the nonablated group. Risk of mortality (hazard ratio [HR], 1.504; 95% CI: 1.066–2.008; P=0.0232) and stroke (HR, 2.607; 95% CI: 1.072–4.069; P=0.033) was significantly higher in the nonablated group. Estimated actuarial 10‐year survival rates were 88.9% in the ablated group and 82.0% in the nonablated group (P=0.022; Figure 2A and 2B).
Figure 2.
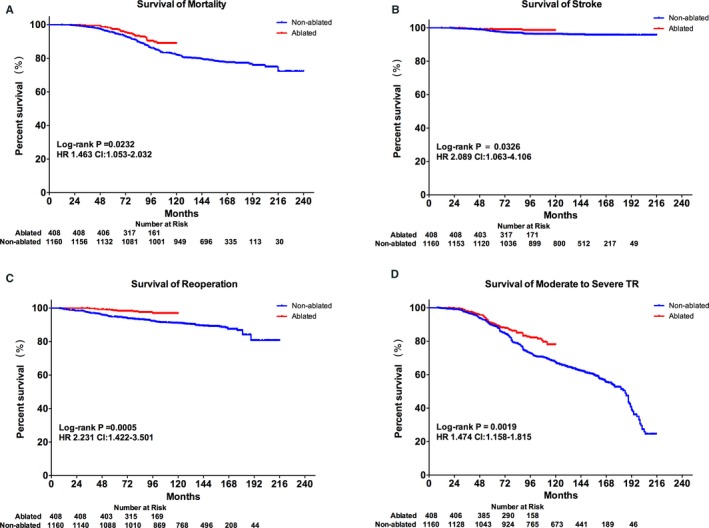
Comparison of outcomes in the overall cohort. Kaplan–Meier curves showing patient survival: overall mortality (A), stroke (B), reoperation (C), and moderate‐to‐severe tricuspid regurgitation (D) in the cohort. HR indicates hazard ratio; TR, tricuspid regurgitation.
During follow‐up, there were 119 patients in the ablated group and 10 in the nonablated group required reoperation. Causes of reoperation include aortic valve replacement AVR in 13 patients, coronary artery bypass graft (CABG) in 6, recurrent mitral regurgitation (MR) in 15, infective endocarditis (IE) in 9, paravalvular leak (PVL) in 22, and severe TR in 52 in the nonablated group and AVR in 2 patients, CABG in 1, recurrent MR in 1, IE in 3, PVL in 2, and of severe TR in 1 in the ablated group. Survival of reoperation at 10 years was 96.9% in the ablated group compared with 91.0% in the nonablated group (HR, 2.886; 95% CI, 1.443–3.448; P=0.0004; Figure 2C). Furthermore, survival of moderate‐to‐severe TR at 10 years was significantly higher in 78.0% in the ablated group compared to 61.2% in the nonablated group (HR, 1.474; 95% CI, 1.158–1.815; P=0.0019; Figure 2D).
Interestingly, 43.7% of severe TR significantly contributed to reoperation in the nonablated group during the first 10‐year follow‐up (χ2=25.66; P=0.04; Figure 3).
Figure 3.

Combination chart distribution of reoperation. Combination chart showing the extent of reoperation in the nonablated (A) and ablated groups (B) in the overall cohort. AVR indicates aortic valve replacement; CABG, coronary artery bypass graft; HR, hazard ratio; IE, infective endocarditis; MR, mitral regurgitation; PVL, paravalvular leak; TR, tricuspid regurgitation.
Comparison of Outcomes in the PSM Cohort
Among the 406 PSM pairs, the nonablated group showed significantly higher rates of mortality (HR, 1.569; 95% CI, 1.106–2.314; P=0.0188), reoperation (HR, 1.855; 95% CI, 1.072–3.631; P=0.050), and moderate‐to‐severe TR (HR, 1.376; 95% CI, 1.083–1.874; P=0.04; Figure 4). The Cox regression model also revealed significantly higher risks of death (HR, 1.644; 95% CI, 1.081–2.501; P=0.020), reoperation (HR, 2.644; 95% CI, 1.299–5.466; P=0.008), and moderate‐to‐severe TR (HR, 1.436; 95% CI, 1.059–1.948; P=0.020) in the nonablated group compared to the ablated group (Table 2).
Figure 4.
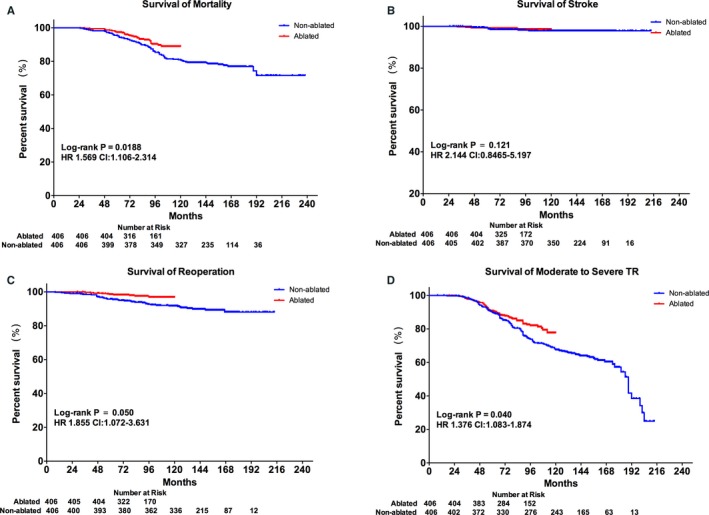
Comparison of outcomes in the PSM cohort. Kaplan–Meier curves of patient survival: overall mortality (A), stroke (B), reoperation (C), and moderate‐to‐severe tricuspid regurgitation (D) in the PSM cohort. HR indicates hazard ratio; PSM, propensity‐score matching; TR, tricuspid regurgitation.
Table 2.
HRs for Clinical Outcomes of Ablated Group Compared to Nonablated Group in the PSM Cohort
| No. of Events | PSM | |||
|---|---|---|---|---|
| Non‐Ablated Group (n=406) | Ablated Group (n=406) | HR (95% CI) | P Value | |
| Death | 91 | 32 | 1.644 (1.081–2.501) | 0.020 |
| Stroke | 8 | 5 | 1.412 (0.458–4.351) | 0.548 |
| Reoperation | 38 | 10 | 2.664 (1.299–5.466) | 0.008 |
| Moderate‐to‐severe TR | 152 | 66 | 1.436 (1.059–1.948) | 0.020 |
HR indicates hazard ratio; PSM, propensity‐score matching; TR, tricuspid regurgitation.
At the 5‐year follow‐up, the ablated group showed a significantly lower percentage of New York Heart Association Functional Classification (NYHA) class than II, LAD, and percentage of diuretic use (P<0.05) compared with its baseline. The nonablated group demonstrated a significantly higher percentage of NYHA class than II, LAD, RA area, and TAD (P<0.05) and a significantly lower RVSI, RVFAC, and percentage of diuretic use (P<0.05) at the 5‐year follow‐up compared with baseline (Figure 5). Further, the nonablated group displayed a significantly higher percentage of NYHA class than II, LAD, RA area, TAD, and percentage of diuretic use (P<0.05) and had a significantly lower RVSI and RVFAC at the 5‐year follow‐up (P<0.05) compared to the ablated group (Figure 5). Notably, the comparison between 2 groups was limited in the first 5‐year follow‐up, which was different in the 2 groups, although only the nonablated group completed the second 5‐year follow‐up.
Figure 5.
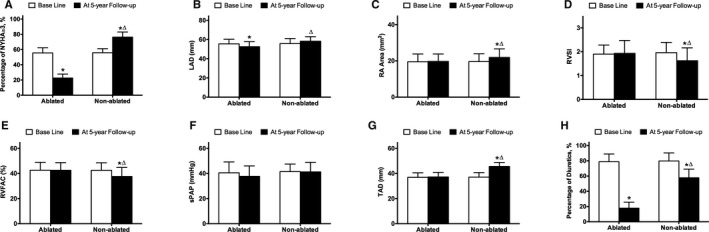
Indices of heart function and echocardiography at baseline and after 5 years of follow‐up in the PSM cohort. A, Percentage of NYHA more than II at baseline and after 5 years of follow‐up in the PSM cohort. B, LAD at baseline and after 5 years of follow‐up in the PSM cohort. C, RA Area at baseline and after 5 years of follow‐up in the PSM cohort. D, RVSI at baseline and after 5 years of follow‐up in the PSM cohort. E, RVFAC at baseline and after 5 years of follow‐up in the PSM cohort. F, sPAP at baseline and after 5 years of follow‐up in the PSM cohort. G, TAD at baseline and after 5 years of follow‐up in the PSM cohort. H, Percentage of Diuretics use at baseline and after 5 years of follow‐up in the PSM cohort. LAD indicates left atrial diameter; NYHA, New York Heart Association; PSM, propensity‐score matching; RA, right atrial; RVFAC, right ventricle fraction area change; RVSI, right ventricle sphericity index; sPAP, pulmonary artery systolic pressure; TAD, tricuspid annulus diameter.
*P<0.05 versus baseline.
▵ P<0.05 versus ablated group.
Subgroup Analysis in the Ablated Group
Recurrence of AF was documented if AF was noted on 24‐hour Holter monitoring or electrocardiography longer than 3 months after the procedure.14 Any episode of recurrent AF after 3 months was classified as permanent failure. In the ablated group, there were 151 patients with AF recurrence at the last follow‐up, the survival in recurrent AF was 91±1%, 66±2%, and 61±3% at 1, 5, and 10 years, respectively (Figure 6).
Figure 6.
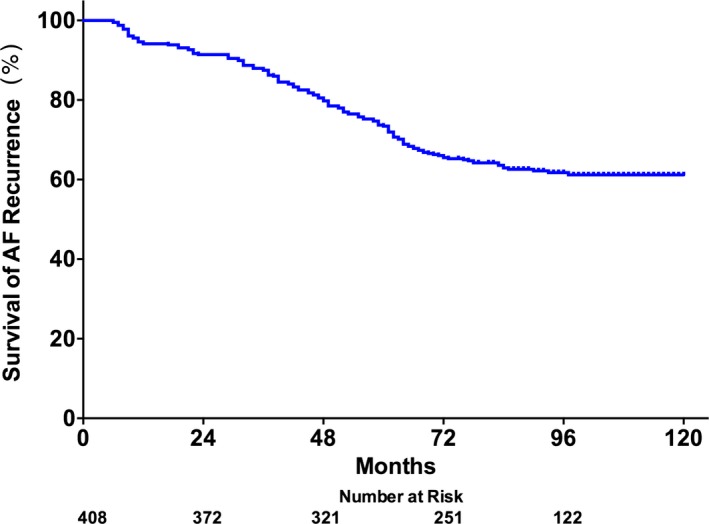
Kaplan–Meier curves for the survival of atrial fibrillation recurrence in the ablated group. AF indicates atrial fibrillation.
Accordingly, the populations in the ablated group were divided into 2 subgroups: patients with sinus rhythm (SR; SR group) and those who had AF recurrence (recurrent group). In an effort to assess whether recurrence of AF was a cause of TR and right‐sided heart remodeling, 116 patients with AF recurrence were matched with 116 patients in SR group after 5 years (Table 3 and Figure 7). In these 116 PSM pairs, the SR group showed significantly high survival in moderate‐to‐severe TR (HR, 2.838; 95% CI, 1.433–4.877; P=0.0007; Figure 8). The Cox regression model also revealed that the recurrent group had significantly higher risks of moderate‐to‐severe TR (HR, 2.427; 95% CI, 1.261–4.671; P=0.008) than the SR group after PSM.
Table 3.
Baseline Characteristics of Successful Versus Unsuccessful AF Ablated Patients in Ablated Group
| Overall Cohort | PSM Cohort | |||||
|---|---|---|---|---|---|---|
| SR Group (n=257) | Recurrent Group (n=151) | P Value | SR Group (n=116) | Recurrent Group (n=116) | P Value | |
| Age, y | 45.8±14.4 | 47.2±14.6 | 0.363 | 44.4±14.8 | 46.7±14.4 | 0.469 |
| Male | 101 (39.3) | 62 (41.1) | 0.754 | 47 (40.5) | 47 (40.5) | 1.000 |
| BSA, m2 | 1.70±0.24 | 1.70±0.26 | 0.914 | 1.70±0.23 | 1.70±0.27 | 0.959 |
| BMI, kg/m2 | 22.2±3.8 | 22.4±3.8 | 0.739 | 21.9±3.6 | 22.4±3.8 | 0.343 |
| NYHA class | 2.9±0.7 | 2.9±0.7 | 0.924 | 2.9±0.7 | 2.9±0.7 | 0.923 |
| Smoking | 67 (26.1) | 50 (33.1) | 0.298 | 37 (31.9) | 31 (26.7) | 0.471 |
| Diabetes mellitus | 35 (13.6) | 15 (9.9) | 0.348 | 13 (11.2) | 12 (10.3) | 1.000 |
| Hypertension | 64 (24.9) | 46 (30.5) | 0.248 | 32 (27.6) | 34 (29.3) | 0.884 |
| Stroke | 8 (3.1) | 10 (6.6) | 0.132 | 6 (5.2) | 6 (5.2) | 1.000 |
| CAD | 40 (15.6) | 25 (16.6) | 0.781 | 17 (14.7) | 19 (16.4) | 0.856 |
| COPD | 13 (5.1) | 8 (5.3) | 0.801 | 9 (7.8) | 6 (5.2) | 0.595 |
| CRI | 3 (1.2) | 3 (2.0) | 0.674 | 3 (2.6) | 3 (2.6) | 1.000 |
| CHADS2 score | 0.74±1.38 | 0.86±1.50 | 0.391 | 0.88±1.53 | 0.88±1.42 | 0.722 |
| EuroSCORE | 0.78±1.09 | 0.69±1.03 | 0.416 | 0.74±1.17 | 0.71±1.00 | 0.809 |
| AF duration, months | 2.8±0.8 | 2.8±0.7 | 0.343 | 2.8±0.7 | 2.8±0.7 | 0.647 |
| Etiology | ||||||
| Rheumatic | 195 (75.9) | 116 (76.8) | 0.904 | 87 (75.0) | 87 (75.0) | 1.000 |
| Degenerative | 39 (15.2) | 20 (13.2) | 0.663 | 20 (17.2) | 18 (15.5) | 0.859 |
| Endocarditis | 14 (5.4) | 10 (6.6) | 0.666 | 6 (5.2) | 7 (6.0) | 1.000 |
| Functional | 9 (3.5) | 5 (3.3) | 1.000 | 3 (2.6) | 4 (3.4) | 1.000 |
| Medication | ||||||
| ACEI | 53 (20.6) | 31 (20.5) | 1.000 | 22 (19.0) | 24 (20.7) | 0.869 |
| ARB | 17 (6.6) | 7 (4.6) | 0.516 | 7 (6.0) | 6 (5.2) | 1.000 |
| CCB | 7 (2.7) | 3 (2.0) | 0.751 | 3 (2.6) | 3 (2.6) | 1.000 |
| Beta‐blocker | 36 (14.0) | 20 (13.2) | 0.882 | 15 (12.9) | 16 (13.8) | 1.000 |
| Digitalis | 52 (20.2) | 38 (25.2) | 0.732 | 28 (24.1) | 22 (19.0) | 0.425 |
| Diuretics | 55 (21.4) | 27 (17.9) | 0.443 | 23 (19.8) | 21 (18.1) | 0.867 |
| Amiodarone | 21 (8.2) | 9 (6.0) | 0.440 | 8 (6.9) | 7 (6.0) | 1.000 |
| LVESD, mm | 35.2±8.2 | 35.6±19.7 | 0.791 | 35.9±8.4 | 34.6±8.3 | 0.234 |
| LVEDD, mm | 51.7±9.6 | 50.7±9.1 | 0.330 | 52.0±9.7 | 51.3±9.4 | 0.613 |
| sPAP, mm Hg | 40.8±8.5 | 40.0±9.0 | 0.356 | 40.6±8.7 | 40.9±9.0 | 0.653 |
| LAD, mm | 55.5±13.0 | 55.5±12.1 | 0.956 | 57.0±13.7 | 55.7±12.7 | 0.453 |
| LVEF, % | 58.4±8.0 | 59.0±7.8 | 0.472 | 58.1±8.6 | 58.2±8.1 | 0.881 |
| RAA, mm2 | 19.3±4.2 | 19.9±4.4 | 0.170 | 19.6.5±3.9 | 19.8±4.4 | 0.745 |
| RVSI | 1.8±0.4 | 2.0±0.4 | 0.000 | 1.9±0.4 | 1.9±0.4 | 0.435 |
| RVFAC, % | 41.9±6.1 | 43.8±6.4 | 0.003 | 43.4±6.1 | 43.4±6.1 | 0.949 |
| TAD, cm | 37.0±3.7 | 36.7±3.5 | 0.566 | 36.6±3.6 | 36.6±3.5 | 0.991 |
| TR grade | 1.1±0.7 | 1.1±0.7 | 0.698 | 1.1±0.7 | 1.1±0.6 | 0.920 |
| CABG | 13 (5.1) | 6 (4.0) | 1.000 | 4 (3.4) | 6 (5.2) | 0.748 |
| MV repair | 28 (10.9) | 12 (7.9) | 0.391 | 7 (6.0) | 12 (10.3) | 0.339 |
| MV replacement | 229 (89.1) | 139 (92.1) | 0.391 | 109 (94.0) | 104 (89.7) | 0.339 |
| Anuloplasty ring | 28 (10.9) | 12 (7.9) | 0.421 | 7 (6.0) | 12 (10.3) | 0.487 |
| Mechanic valve | 168 (65.4) | 96 (63.6) | 0.421 | 76 (65.5) | 73 (62.9) | 0.487 |
| Bioprosthetic valve | 61 (23.7) | 43 (28.5) | 0.421 | 33 (28.4) | 31 (26.7) | 0.487 |
| CPB, minute | 113.0±35.6 | 103.4±40.2 | 0.012 | 106.8±35.7 | 107.1±39.1 | 0.943 |
| ACT, minute | 74.2±7.6 | 68.1±29.9 | 0.035 | 69.8±27.2 | 70.3±29.1 | 0.891 |
ACEI indicates angiotensin‐converting enzyme inhibitor; ACT, aortic clamp time; AF, atrial fibrillation; ARB, angiotensin receptor blocker; BMI, body mass index; BSA, body surface area; CABG, coronary artery bypass graft; CAD, coronary artery disease; CCB, calcium‐channel blocker; COPD, chronic obstructive pulmonary disease; CPB, cardiopulmonary bypass; CRI, chronic renal insufficiency; LAD, left atrial diameter; LVEDD, left ventricle end‐diastolic dimension; LVEF, left ventricle ejection fraction; LVESD, left ventricle end‐systolic dimension; MV, mitral valve; NYHA, New York Heart Association; PSM, propensity‐score matching; RAA, right atrial area; RVFAC, right ventricle fractional area change; RVSI, right ventricle sphericity index; sPAP, pulmonary artery systolic pressure; SR, sinus rhythm; TAD, tricuspid annulus diameter; TR, tricuspid regurgitation.
Figure 7.
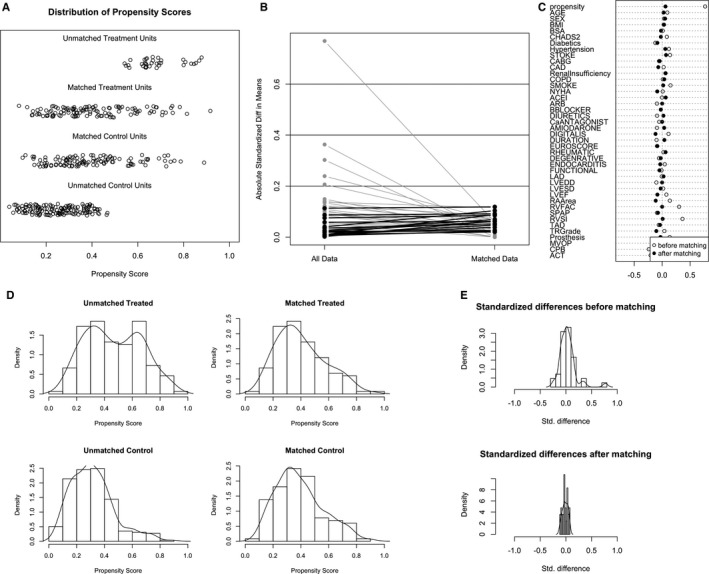
Propensity‐score matching for subgroup analysis in the ablated group (n=408, 257 in SR group, and 151 in recurrent group). A, Dot plot of patients in either matched or unmatched groups. B, Line plot of standardized differences before and after matching. C, Dot plot of standardized mean differences (Cohen's d) for all covariates before and after matching. D, Distribution of propensity scores of recurrent patients (“treated”) and SR patients (“control”) before and after matching with overlaid kernel density estimate. E, Histograms with overlaid kernel density estimates of standardized differences before and after matching. ACEI indicates angiotensin‐converting enzyme inhibitor; ACT, aortic clamp time; ARB, angiotensin receptor blocker; BMI, body mass index; BSA, body surface area; CABG, coronary artery bypass graft; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; CPB, cardiopulmonary bypass; LAD, left atrial diameter; LVEDD, left ventricle end‐diastolic dimension; LVEF, left ventricle ejection fraction; LVESD, left ventricle end‐systolic dimension; MVOP, mitral valve operation; NYHA, New York Heart Association; RA, right atrial; RVFAC, right ventricle fractional area change; RVSI, right ventricle sphericity index; sPAP, pulmonary artery systolic pressure; SR, sinus rhythm; Standardized Diff, standardized difference; Std. difference, standardized difference; TAD, tricuspid annulus diameter; TR, tricuspid regurgitation.
Figure 8.
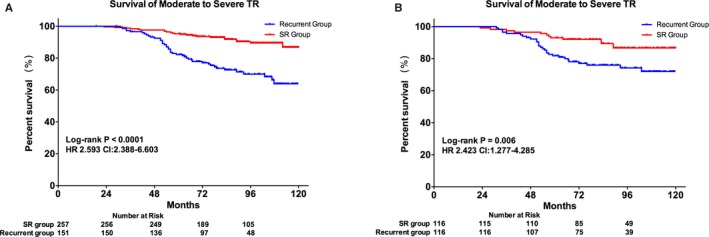
Kaplan–Meier curves for the survival of moderate‐to‐severe tricuspid regurgitation in (A), overall cohort and (B), PSM cohort of ablated group. HR indicates hazard ratio; PSM, propensity‐score matching; SR, sinus rhythm; TR, tricuspid regurgitation.
At the 5‐year follow‐up, the SR group showed a significantly lower percentage of NYHA class more than II, LAD, and percentage of diuretic use (P<0.05) compared with its baseline. The recurrent group had a significantly higher TAD (P<0.05) and a significantly lower percentage of diuretic use (P<0.05) at the 5‐year follow‐up compared with the baseline (Figure 9). Furthermore, the recurrent group had a significantly higher percentage of NYHA class than II, LAD, RA area, TAD, and percentage of diuretic use (P<0.05) compared to the SR group (Figure 9).
Figure 9.
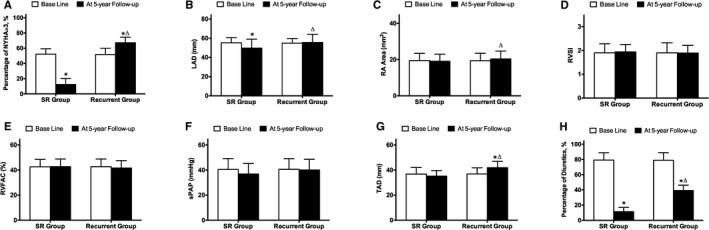
Indices of heart function and echocardiography at baseline and after 5 years of follow‐up in the PSM cohort of the ablated group. A, Percentage of NYHA more than II at baseline and after 5 years of follow‐up. B, LAD at baseline and after 5 years of follow‐up. C, RA Area at baseline and after 5 years of follow‐up. D, RVSI at baseline and after 5 years of follow‐up. E, RVFAC at baseline and after 5 years of follow‐up. F, sPAP at baseline and after 5 years of follow‐up. G, TAD at baseline and after 5 years of follow‐up. H, Percentage of diuretics use at baseline and after 5 years of follow‐up. LAD indicates left atrial diameter; NYHA, New York Heart Association; PSM, propensity‐score matching; RA, right atria; RVFAC, right ventricle fraction area change; RVSI, right ventricle sphericity index; sPAP, pulmonary artery systolic pressure; SR, sinus rhythm; TAD, tricuspid annulus diameter.
*P<0.05 versus baseline.
▵ P<0.05 versus SR group.
To determine the impact of AF recurrence on the LAD and the remodeling of right‐sided heart, we analyzed the relationship between time after recurrence and the LAD, RA area, sPAP, TAD, RVSI, and RVFAC at the last follow‐up. Significant positive correlations existed between time after recurrence and LAD, RA area, and TAD, respectively (Figure 7). Significant negative correlations were noted between time after recurrence and RVSI and RVFAC, respectively (Figure 10).
Figure 10.
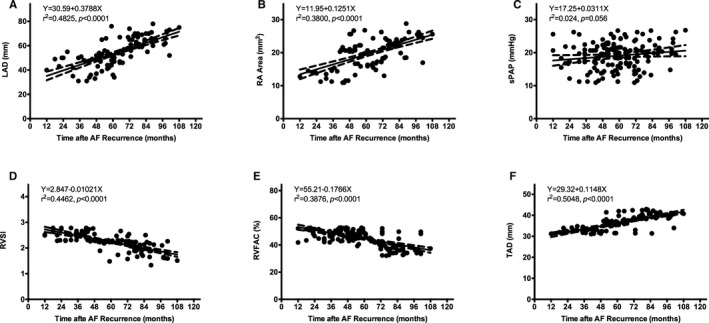
Correlations between the time after recurrence and (A), LAD; (B), RA area; (C), sPAP; (D), RVSI; (D), RVFAC, and (F), TAD. AF indicates atrial fibrillation; LAD, left atrial diameter; RA, right atria; RVFAC, right ventricle fraction area change; RVSI, right ventricle sphericity index; sPAP, pulmonary artery systolic pressure; TAD, tricuspid annulus diameter.
Discussion
Our study, which, to the best of our knowledge, is the first PSM study, demonstrates that ablation of AF during cardiac surgery in patients with MV disease not only resulted in successful restoration of SR, but also prevented TR progression and remodeling of the right‐sided heart. It improved survival compared to nonablated patients or those ablated but remaining in AF.
Development of significant TR late after MV procedure is frequent and varies between 9% and 49%.21, 24, 25 These patients represent a challenging population because they often experience severe symptoms of CHF. Conservative treatment options are limited, and redo surgery carries a high risk.26, 27 Previous studies showed that sPAP increase and permanent AF were the most powerful risk factors for TR progression in patients with low prevalence of rheumatic heart disease and preserved LVEF.2, 28 Our PSM analysis demonstrates that overall survival, reoperation, and moderate‐to‐severe TR in ablated patients were better than those in nonablated patients. TR was a significant and leading cause of reoperation in the nonablated group in the first 10‐year follow‐up, whereas patients in the ablated group had a significant lower risk of reoperation for TR in the follow‐up. Our findings are consistent with those of Kim et al,9 who reported that AF predisposes patients undergoing MV surgery to TR progression, which is prevented by MAZE. In another study, Song et al24 reported that presence of AF at the time of left‐sided heart valve surgery was an independent predictor of delayed, but significant, TR. Therefore, long‐standing persistent AF is a serious condition and contributes to development of severe TR and right‐sided heart remodeling and, possibly, to decreased heart function and long‐term survival.
Furthermore, in our study, nonablated patients showed significantly increased LAD, RA area, and TAD, compared with baseline, and deteriorated RV function at the 5‐year follow‐up. In the absence of significant increase in sPAP, it is likely that longer duration of AF leads to larger atrium and hence larger TAD, resulting in progressive TR and eventually impaired RV function, as previously observed in patients with idiopathic TR.29 TR results in right‐sided heart volume overload and, presumably, initiates a circle of progressive annular dilation and right‐sided heart remodeling and worsening TR. This vicious circle results in clinical deterioration, which can be predicted by measuring decreased RVSI and RVFAC. The interaction between TR and RV function appears to be bidirectional: Not only does TR initiate RV dysfunction, but RV dysfunction affects the severity of TR. RV dysfunction is secondary to TR and vice versa.
Despite the increase of TR and further RA dilatation, sPAP remained unchanged within the 5‐year follow‐up in the nonablated group, indicating that the TR per se leads to dilatation of both RA and RV, irrespective of pulmonary arterial pressure. Thus, our results confirm the findings of several previous studies demonstrating that atrial remodeling is central to the progression of TR, based on the follow‐up data in our nonablated group of patients.29, 30, 31, 32 In these patients, RA area progressively increased over a period of 10 years in association with tricuspid annulus (TA) dilatation. As the TAD increased, TR severity worsened because of progressive systolic coaptation loss. This observation supports the causal relationship between atrial remodeling and severe TR. In brief, long‐standing AF causes progressive atrial remodeling and leads to atrial dilation. The dilated atrium enlarges the TA and causes TR attributed to systolic coaptation loss. The resulting TR further dilates the atrium, resulting in progressive annular dilation. Therefore, a vicious circle in which “TR begets TR” is established.
Among patients undergoing MV surgery, 30% to 50% manifest AF.7, 33, 34 Current AHA‐ACC‐HRS guidelines state that it is reasonable to perform AF ablation in selected patients undergoing other types of cardiac surgery.15 In our subgroup analysis, we found that survival of AF recurrence was 91±1%, 66±2%, and 61±3% at 1, 5, and 10 years, respectively. Our results are consistent with those of surgical ablation studies, suggesting that ablation is associated with increased survival rate in AF recurrence.8, 11, 35 It is important to note that patients with successful SR restoration showed better survival in moderate‐to‐severe TR compared with those who had recurrence. Although patients with recurrence only had slightly increased values of LAD, RA area, and TAD compared with their baseline, these values were significantly larger than those of patients with SR at the 5‐year follow‐up. Furthermore, the increase in TAD, RA area, and TAD and the decrease of RVSI and RVFAC are related to time after recurrence. It also suggested that atrial remodeling and TA dilation attributed to long‐standing AF may lead to severe TR and deteriorated heart function.
Previous studies have demonstrated that successful SR restoration improved survival of stroke.34, 36 However, interestingly, our PSM analysis shows that in the nonablated group, stroke survival was similar to those in ablated patients. Remarkably, in both groups, nearly 90% of patients underwent mechanical MV replacement, which entailed chronic anticoagulation therapy. Long‐term use of warfarin, which reduced risk of stroke, may cause similar incidence of late stroke.
Several limitations of the study need to be acknowledged. First, this study is limited by its retrospective nature; therefore, it was subject to all the biases inherent to this type of analysis. Patients treated with or without surgical ablation were not randomized, leading to important differences between them. Although we attempted to compensate for these differences using PSM, assumptions were made in the development of the scoring system, and there might have been residual unmeasured confounding. Therefore, the possibility of confounding factors or even the role of chance underlying the association between surgical ablation and outcomes could not be excluded. According to current recommendations, surgical ablation is widely adopted in patients with AF who also had MV disease requiring surgical intervention. Surgical ablation alone is contraindicated if the procedure was too complex or the patients were too ill and the risk outweighed the potential benefit. Generally, these patients were likely to have worse survival and were not eligible for enrollment in this study. Therefore, patients in the nonablated groups were selected from 1994 to 2003, when surgical ablation was not adopted. Thus, in the earliest nonablated patients, there can be as many as 20 years of follow‐up, but for those in the ablated group at the end of the study period, an average 5‐year follow‐up was relatively short to detect important long‐term problems with surgical ablation, including progression of TR and right‐sided heart remodeling. These data are used because they are realistic in terms of the actual survival rates they yield. Factors such as surgical skills and myocardial protection in the 2 groups at different time periods might cause differences in long‐term survival. Other limitations include lack of knowledge of TR progression and right‐sided heart remodeling in non‐AF patients with MV disease, which was matched in this study. Finally, the current study was based on 2D echocardiographic images, and, conceivably, more‐accurate data involving TR grade and right‐sided heart remodeling may be available from three‐dimensional echocardiography and magnetic resonance imaging.37 However, we believe that our observations are clinically relevant. The method is the most practical technique in “real‐life” practice in almost all hospitals.
Conclusion
Our retrospective PSM analysis demonstrated that patients with MV disease who had a history of persistent or long‐standing persistent AF manifested progression of TR as well as right‐sided heart remodeling, which are alleviated by surgical ablation. Further prospective, randomized, clinical trials are warranted to validate our results.
Sources of Funding
This study was supported by the National Natural Science Foundation of China (Grant No. 81370294, 81570291).
Disclosures
None.
(J Am Heart Assoc. 2016;5:e004213 doi: 10.1161/JAHA.116.004213)
Contributor Information
Jiangang Wang, Email: jiangangwang@ccmu.edu.cn.
Xu Meng, Email: mxu@263.net.
References
- 1. Rogers JH, Bolling SF. The tricuspid valve: current perspective and evolving management of tricuspid regurgitation. Circulation. 2009;119:2718–2725. [DOI] [PubMed] [Google Scholar]
- 2. Shiran A, Sagie A. Tricuspid regurgitation in mitral valve disease. J Am Coll Cardiol. 2009;53:401–408. [DOI] [PubMed] [Google Scholar]
- 3. Cheng R, Azarbal A, Currier J, Thomson LEJ, Hamilton MA, Esmailian F, Azarbal B. Tricuspid regurgitation, the forgotten valvular lesion‐a contemporary review of etiology, prevalence, and management options. Rev Cardiovasc Med. 2015;16:171–181. [DOI] [PubMed] [Google Scholar]
- 4. Chikwe J, Itagaki S, Anyanwu A, Adams DH. Impact of concomitant tricuspid annuloplasty on tricuspid regurgitation, right ventricular function, and pulmonary artery hypertension after repair of mitral valve prolapse. J Am Coll Cardiol. 2015;65:1931–1938. [DOI] [PubMed] [Google Scholar]
- 5. Bertrand PB, Koppers G, Verbrugge FH, Mullens W, Vandervoort P, Dion R, Verhaert D. Tricuspid annuloplasty concomitant with mitral valve surgery: effects on right ventricular remodeling. J Thorac Cardiovasc Surg. 2014;147:1256–1264. [DOI] [PubMed] [Google Scholar]
- 6. Teman NR, Huffman LC, Krajacic M, Pagani FD, Haft JW, Bolling SF. “Prophylactic” tricuspid repair for functional tricuspid regurgitation. Ann Thorac Surg. 2014;97:1520–1524. [DOI] [PubMed] [Google Scholar]
- 7. Gillinov AM, Gelijns AC, Parides MK, DeRose JJ Jr, Moskowitz AJ, Voisine P, Ailawadi G, Bouchard D, Smith PK, Mack MJ, Acker MA, Mullen JC, Rose EA, Chang HL, Puskas JD, Couderc J‐P, Gardner TJ, Varghese R, Horvath KA, Bolling SF, Michler RE, Geller NL, Ascheim DD, Miller MA, Bagiella E, Moquete EG, Williams P, Taddei‐Peters WC, O'Gara PT, Blackstone EH, Argenziano M. Surgical ablation of atrial fibrillation during mitral‐valve surgery. N Engl J Med. 2015;372:1399–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gelsomino S, La Meir M, Van Breugel HNAM, Renzulli A, Rostagno C, Lorusso R, Parise O, Lozekoot PWJ, Klop IDG, Kumar N, Lucà F, Matteucci F, Serraino F, Santè P, Caciolli S, Vizzardi E, De Jong M, Crijns HJGM, Gensini GF, Maessen JG. Surgical ablation in patients undergoing mitral valve surgery: impact of lesion set and surgical techniques on long‐term success. Europace. 2016;18:1528–1537. [DOI] [PubMed] [Google Scholar]
- 9. Kim H‐K, Kim Y‐J, Kim K‐I, Jo S‐H, Kim K‐B, Ahn H, Sohn D‐W, Oh B‐H, Lee M‐M, Park Y‐B, Choi Y‐S. Impact of the Maze operation combined with left‐sided valve surgery on the change in tricuspid regurgitation over time. Circulation. 2005;112:I14–I19. [DOI] [PubMed] [Google Scholar]
- 10. Stulak JM, Schaff HV, Dearani JA, Orszulak TA, Daly RC, Sundt TM III. Restoration of sinus rhythm by the Maze procedure halts progression of tricuspid regurgitation after mitral surgery. Ann Thorac Surg. 2008;86:40–45. [DOI] [PubMed] [Google Scholar]
- 11. Pinho‐Gomes AC, Amorim MJ, Oliveira SM, Leite‐Moreira AF. Surgical treatment of atrial fibrillation: an updated review. Eur J Cardiothorac Surg. 2014;46:167–178. [DOI] [PubMed] [Google Scholar]
- 12. Dunning J, Nagendran M, Alfieri OR, Elia S, Kappetein AP, Lockowandt U, Sarris GE, Kolh PH; on behalf of the EACTS Clinical Guidelines Committee , Mahiben M, Nicholas S, Christian C, Phillip X, Robert G, Yang C, David M, Sumoyee B. Guideline for the surgical treatment of atrial fibrillation. Eur J Cardiothorac Surg. 2013;44:777–791. [DOI] [PubMed] [Google Scholar]
- 13. Knecht S, Sticherling C, von Felten S, Conen D, Schaer B, Ammann P, Altmann D, Osswald S, Kühne M. Long‐term comparison of cryoballoon and radiofrequency ablation of paroxysmal atrial fibrillation: a propensity score matched analysis. Int J Cardiol. 2014;176:645–650. [DOI] [PubMed] [Google Scholar]
- 14. Kim JB, Yun TJ, Chung CH, Choo SJ, Song H, Lee JW. Long‐term outcome of modified Maze procedure combined with mitral valve surgery: analysis of outcomes according to type of mitral valve surgery. J Thorac Cardiovasc Surg. 2010;139:111–117. [DOI] [PubMed] [Google Scholar]
- 15. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW; ACC/AHA Task Force Members . 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130:e199–e267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang J, Meng X, Li H, Cui Y, Han J, Xu C. Prospective randomized comparison of left atrial and biatrial radiofrequency ablation in the treatment of atrial fibrillation. Eur J Cardiothorac Surg. 2009;35:116–122. [DOI] [PubMed] [Google Scholar]
- 17. Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, Gutgesell H, Reichek N, Sahn D, Schnittger I. Recommendations for quantitation of the left ventricle by two‐dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two‐Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358–367. [DOI] [PubMed] [Google Scholar]
- 18. Chopra HK, Nanda NC, Fan P, Kapur KK, Goyal R, Daruwalla D, Pacifico A. Can two‐dimensional echocardiography and Doppler color flow mapping identify the need for tricuspid valve repair? J Am Coll Cardiol. 1989;14:1266–1274. [DOI] [PubMed] [Google Scholar]
- 19. Miyatake K, Okamoto M, Kinoshita N, Ohta M, Kozuka T, Sakakibara H, Nimura Y. Evaluation of tricuspid regurgitation by pulsed Doppler and two‐dimensional echocardiography. Circulation. 1982;66:777–784. [DOI] [PubMed] [Google Scholar]
- 20. Kaul S, Tei C, Hopkins JM, Shah PM. Assessment of right ventricular function using two‐dimensional echocardiography. Am Heart J. 1984;107:526–531. [DOI] [PubMed] [Google Scholar]
- 21. Matsunaga A, Duran CMG. Progression of tricuspid regurgitation after repaired functional ischemic mitral regurgitation. Circulation. 2005;112:I453–I457. [DOI] [PubMed] [Google Scholar]
- 22. Kim H‐K, Kim Y‐J, Park J‐S, Kim KH, Kim K‐B, Ahn H, Sohn D‐W, Oh B‐H, Park Y‐B, Choi Y‐S. Determinants of the severity of functional tricuspid regurgitation. Am J Cardiol. 2006;98:236–242. [DOI] [PubMed] [Google Scholar]
- 23. Thoemmes FJ, Kim ES. A systematic review of propensity score methods in the social sciences. Multivariate Behav Res. 2011;46:90–118. [DOI] [PubMed] [Google Scholar]
- 24. Song H, Kim M‐J, Chung CH, Choo SJ, Song MG, Song JM, Kang DH, Lee JW, Song JK. Factors associated with development of late significant tricuspid regurgitation after successful left‐sided valve surgery. Heart. 2009;95:931–936. [DOI] [PubMed] [Google Scholar]
- 25. Izumi C, Iga K, Konishi T. Progression of isolated tricuspid regurgitation late after mitral valve surgery for rheumatic mitral valve disease. J Heart Valve Dis. 2002;11:353–356. [PubMed] [Google Scholar]
- 26. King RM, Schaff HV, Danielson GK, Gersh BJ, Orszulak TA, Piehler JM, Puga FJ, Pluth JR. Surgery for tricuspid regurgitation late after mitral valve replacement. Circulation. 1984;70:I193–I197. [PubMed] [Google Scholar]
- 27. Kuwaki K, Morishita K, Tsukamoto M, Abe T. Tricuspid valve surgery for functional tricuspid valve regurgitation associated with left‐sided valvular disease. Eur J Cardiothorac Surg. 2001;20:577–582. [DOI] [PubMed] [Google Scholar]
- 28. Shiran A, Najjar R, Adawi S, Aronson D. Risk factors for progression of functional tricuspid regurgitation. Am J Cardiol. 2014;113:995–1000. [DOI] [PubMed] [Google Scholar]
- 29. Yamasaki N, Kondo F, Kubo T, Okawa M, Matsumura Y, Kitaoka H, Yabe T, Furuno T, Doi Y. Severe tricuspid regurgitation in the aged: atrial remodeling associated with long‐standing atrial fibrillation. J Cardiol. 2006;48:315–323. [PubMed] [Google Scholar]
- 30. Najib MQ, Vinales KL, Vittala SS, Challa S, Lee HR, Chaliki HP. Predictors for the development of severe tricuspid regurgitation with anatomically normal valve in patients with atrial fibrillation. Echocardiography. 2012;29:140–146. [DOI] [PubMed] [Google Scholar]
- 31. Park J‐H, Shin S‐H, Lee M‐J, Lee M‐D, Shim H‐I, Yoon J, Oh S, Kim D‐H, Park S‐D, Kwon S‐W, Woo S‐I, Park K‐S, Kwan J. Clinical and echocardiographic factors affecting tricuspid regurgitation severity in the patients with lone atrial fibrillation. J Cardiovasc Ultrasound. 2015;23:136–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Teixeira R, Monteiro R, Garcia J, Baptista R, Ribeiro M, Cardim N, Gonçalves L. The relationship between tricuspid regurgitation severity and right atrial mechanics: a speckle tracking echocardiography study. Int J Cardiovasc Imaging. 2015;31:1–11. [DOI] [PubMed] [Google Scholar]
- 33. Lee R, McCarthy PM, Wang EC, Vaduganathan M, Kruse J, Malaisrie SC, McGee EC. Midterm survival in patients treated for atrial fibrillation: a propensity‐matched comparison to patients without a history of atrial fibrillation. J Thorac Cardiovasc Surg. 2012;143:1341–1351, discussion 1350–1. [DOI] [PubMed] [Google Scholar]
- 34. Hussein AA, Wazni OM, Harb S, Joseph L, Chamsi‐Pasha M, Bhargava M, Martin DO, Dresing T, Callahan T, Kanj M, Natale A, Lindsay BD, Saliba WI. Radiofrequency ablation of atrial fibrillation in patients with mechanical mitral valve prostheses. J Am Coll Cardiol. 2011;58:596–602. [DOI] [PubMed] [Google Scholar]
- 35. Lawrance CP, Henn MC, Damiano RJ. Surgery for atrial fibrillation. Heart Fail Clin. 2016;12:235–243. [DOI] [PubMed] [Google Scholar]
- 36. Philpott JM, Zemlin CW, Cox JL, Stirling M, Mack M, Hooker RL, Morris A, Heimansohn DA, Longoria J, Gandhi DB, McCarthy PM. The ABLATE Trial: safety and efficacy of Cox Maze‐IV using a bipolar radiofrequency ablation system. Ann Thorac Surg. 2015;100:1541–1546, discussion 1547–8. [DOI] [PubMed] [Google Scholar]
- 37. Huttin O, Voilliot D, Mandry D, Venner C, Juillière Y, Selton‐Suty C. All you need to know about the tricuspid valve: tricuspid valve imaging and tricuspid regurgitation analysis. Arch Cardiovasc Dis. 2016;109:67–80. [DOI] [PubMed] [Google Scholar]


