Abstract
Background
Atrial fibrillation (AF) is a common cause for hospitalization, but there are limited data regarding acute kidney injury requiring dialysis (AKI‐D) in AF hospitalizations. We aimed to assess temporal trends and outcomes in AF hospitalizations complicated by AKI‐D utilizing a nationally representative database.
Methods and Results
Utilizing the Nationwide Inpatient Sample, AF hospitalizations and AKI‐D were identified using diagnostic and procedure codes. Trends were analyzed overall and within subgroups and utilized multivariable logistic regression to generate adjusted odds ratios (aOR) for predictors and outcomes including mortality and adverse discharge. Between 2003 and 2012, 3751 (0.11%) of 3 497 677 AF hospitalizations were complicated by AKI‐D. The trend increased from 0.3/1000 hospitalizations in 2003 to 1.5/1000 hospitalizations in 2012, with higher increases in males and black patients. Temporal changes in demographics and comorbidities explained a substantial proportion but not the entire trend. Significant comorbidities associated with AKI‐D included mechanical ventilation (aOR 13.12; 95% CI 9.88‐17.43); sepsis (aOR 8.20; 95% CI 6.00‐11.20); and liver failure (aOR 3.72; 95% CI 2.92‐4.75). AKI‐D was associated with higher risk of in‐hospital mortality (aOR 3.54; 95% CI 2.81‐4.47) and adverse discharge (aOR 4.01; 95% CI 3.12‐5.17). Although percentage mortality within AKI‐D decreased over the decade, attributable risk percentage mortality remained stable.
Conclusions
AF hospitalizations complicated by AKI‐D have quintupled over the last decade with differential increase by demographic groups. AKI‐D is associated with significant morbidity and mortality. Without effective AKI‐D therapies, focus should be on early risk stratification and prevention to avoid this devastating complication.
Keywords: acute kidney injury, atrial fibrillation, dialysis, mortality
Subject Categories: Atrial Fibrillation, Nephrology and Kidney, Mortality/Survival
Introduction
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia encountered in clinical practice.1 The prevalence of AF in the general population is up to 1%, and it places a major burden on the healthcare system.2, 3 Affected patients are at increased risk of heart failure and thromboembolic events, leading to increased hospitalizations and ultimately poor outcomes. It is estimated that, in the United States, hospitalizations due to AF have increased by 23% between the years 2000 and 2010.4
It is well known that AF is common in patients with chronic kidney disease (CKD), particularly those with end‐stage renal disease (ESRD) on hemodialysis (HD) and peritoneal dialysis (PD).5 This is partially explained by the fact that both conditions share a number of risk factors such as hypertension, cardiovascular disease, and congestive heart failure. AF in patients with kidney disease not only is highly prevalent but is associated with increased mortality; in ESRD patients with AF the 1‐year mortality rate is nearly double that of ESRD patients without AF.5
Acute kidney injury (AKI) and CKD/ESRD can be considered as inextricably interlinked syndromes.6 The incidence of AKI requiring dialysis (AKI‐D), the most severe form of AKI, has been increasing in the general population as well in specific disease conditions, and thus, the CKD epidemic can in part be attributed to this.7, 8, 9, 10 AF can cause AKI by several mechanisms including acute tubular necrosis from hemodynamic instability and renal ischemia from an embolic event. Additionally, the presence of AF may necessitate additional procedures such as contrast studies and cardioversion, which can also cause AKI. The development of AKI has been linked to worse outcomes in several patient populations. An analysis of the Nationwide Inpatient Sample demonstrated that admissions for AF complicated by AKI were associated with nearly double the odds of mortality.11 However, few studies have examined the association between AF and AKI‐D.12 Most of these studies evaluate the concurrent occurrence of AF and AKI after cardiac surgery and not in patients admitted with a primary diagnosis of AF.13 In order to address this knowledge gap, we utilized a large, nationally representative database to estimate the secular temporal trends of the incidence of AKI‐D among adults hospitalized with AF. We also aimed to describe trends in important demographic subgroups, explore potential reasons for these trends, identify acute and chronic comorbidities associated with AKI‐D, and elucidate its effects on mortality and morbidity.
Methods
Data Sources
We extracted our study cohort from the National Inpatient Sample (NIS) of the Healthcare Cost and Utilization Project (HCUP), Agency for Healthcare Research and Quality.14 We selected the time period from 2003 to 2012 based on availability of complete data, time over a decade, and adequate sample size for modeling. Because of the deidentified, publically available nature of the data, institutional board review approval was not needed.
Study Population and Design
We queried the NIS database using the International Classification of Diseases, 9th Revision, Clinical Modification (ICD‐9‐CM) diagnosis code 427.31 for AF as a primary diagnosis for hospitalizations.15 We included only hospitalizations where patients were ≥18 years of age at time of admission. We defined AKI by ICD‐9‐CM code 584.xx and dialysis procedure by presence of ICD‐9‐CM procedure code of 39.95 or diagnosis code of v56.0 or v56.1.16 We excluded hospitalizations with diagnosis codes for ESRD and those with procedure codes for arteriovenous access creation or revision to avoid misclassification of hospitalizations for hemodialysis initiation. Similarly, we excluded hospitalizations with dialysis codes but no AKI code, assuming that patients were on maintenance hemodialysis. This approach has been used previously and has good sensitivity and specificity.16
Definition of Variables
We included patient level characteristics (age, sex, race, quartile classification of median household income according to ZIP code, primary payer (Medicare/Medicaid, private insurance, self‐pay, or no charge), and hospital level characteristics (hospital location [urban/rural], hospital bed size [small, medium, and large], region [Northeast, Midwest or North Central, South, and West], and teaching status). We defined severity of comorbid conditions using the Deyo modification of the Charlson comorbidity index (CCI).17
Definition of Outcomes
Outcomes of interest included mortality, adverse discharge disposition, length of stay (LoS), and cost of hospitalization (inflation‐adjusted to 2015 price in US dollars).18 Discharge disposition was grouped: (1) home or short‐term facility versus (2) adverse discharge (skilled nursing facility, intermediate care, hospice home, hospice medical facility, long‐term care hospital, certified nursing facility).19
Statistical Analysis
We compared the baseline characteristics of AF hospitalized adults in 2 groups: with and without AKI‐D. We utilized the chi‐squared test for categorical variables, Student t test for normally distributed continuous variables, and Wilcoxon rank‐sum test for non–normally distributed continuous variables. We assessed changes in trends overall and in a priori defined demographic groups using the Cochran Armitage and Cuzick nonparametric trend tests. We also performed a survey regression analysis to explore potential reasons for temporal changes in AKI‐D by fitting a series of sequential models. In the unadjusted model we included only calendar year as the predictor and AKI‐D as the outcome. Additional patient level demographic covariates (age, sex, and race) per year were added in the second model to determine the degree to which they explained temporal trends. Finally, the covariates from models 1 and 2 along with concurrent acute/chronic comorbidities (HIV status, diabetes mellitus type II [DM2], hypertension [HTN], CKD, sepsis, heart failure [HF], chronic liver diseases, liver cancer) and procedures (cardiac catheterizations, catheter ablation of arrhythmias, and mechanical ventilation) per year, which were known risk factors for AKI‐D, were included in a third and final model. We also utilized survey logistic regression to evaluate predictors of AKI‐D and then to estimate the impact of AKI‐D on mortality and adverse discharge.8 Finally, we calculated the trends of mortality over time and the population‐attributable risk of death indicating the proportion of deaths that could potentially be avoided if AKI‐D were eliminated. We constructed final models adjusting for confounders, testing for potential interactions, and ensuring no multicollinearity among covariates.
We performed all association and trend analysis using designated weight values by HCUP to produce nationally representative estimates and considered a 2‐tailed P≤0.01 as statistically significant. We utilized SAS 9.3 (SAS Institute Inc, Cary, NC) for all analyses.
Results
Proportion and Trends of AF Hospitalizations Complicated by AKI‐D
Between the years 2002 to 2012, there were 3 497 677 admissions due to AF. Of these, 3751 (0.11%) were complicated by AKI‐D. By the Cochran‐Armitage test, there was an increasing trend in the hospitalizations complicated by AKI‐D, with a 5‐fold increase from 0.3/1000 hospitalizations in 2003 to 1.5/1000 hospitalizations in 2012 (Figure 1).
Figure 1.
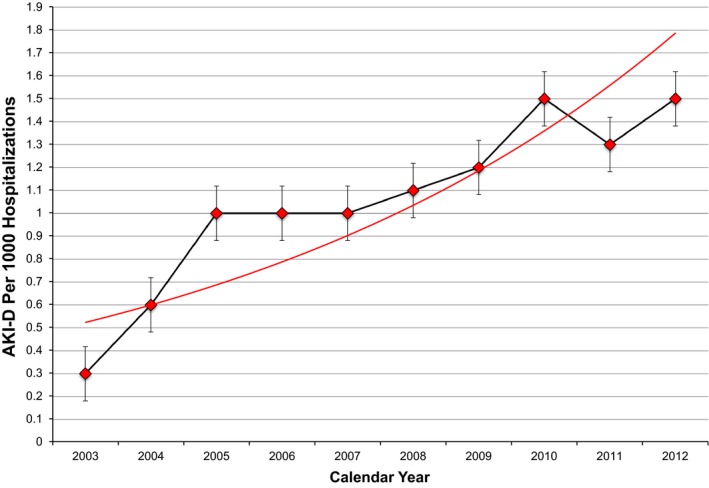
Incidence of acute kidney injury requiring hemodialysis (AKI‐D) by year in atrial fibrillation hospitalizations in the United States from 2003 to 2012. Red dots are incidence for each year, and associated 95% confidence intervals are shown. Red line is best fit line. AKI‐D has increased 5‐fold from 2003 to 2012. Analysis was done using the Cochran‐Armitage trend test.
Explanation of Increase in AKI‐D
In unadjusted analyses, for every 1‐year increase, there was an associated 11% increase in the risk of developing AKI‐D (aOR 1.11, 95% CI 1.09‐1.14) (Table 1). When adjusted for changes in age, sex, race, comorbidities (HIV status, DM2, HTN, CKD, sepsis, HF, chronic liver diseases, liver cancer, CCI), and procedures (cardiac catheterizations, catheter ablation of arrhythmias, and mechanical ventilation) per year, the aOR per year increase decreased to 1.03 (95% CI 1.00‐1.06). However, there remained an unexplained increase of 3% per year.
Table 1.
Sequential Adjusted Models to Explain Increased Trends of AKI‐D
| Unadjusted Odds Ratio/Year (95% Confidence Interval) | P Value | Adjusted Odds Ratio/Year (95% Confidence Interval)a | P Value | Adjusted Odds Ratio/Year (95% Confidence Interval)b | P Value | |
|---|---|---|---|---|---|---|
| Risk of AKI‐D | 1.11 (1.09‐1.14) | <0.01 | 1.11 (1.08‐1.14) | <0.01 | 1.03 (1.00‐1.06) | 0.02 |
AKI‐D indicates acute kidney injury requiring hemodialysis.
Adjusted for age, sex, and race.
Adjusted for age, sex, race, comorbidities (HIV status, diabetes mellitus, hypertension, CKD, sepsis, heart failure, chronic liver diseases, liver cancer), Charlson Comorbidity Index, and procedures (cardiac ablation, cardiac catheterizations, and mechanical ventilation).
Characteristics of AKI Versus AKI‐D
With respect to patient demographics, AF patients with AKI‐D were older (70.6% >65 years vs 68.9% >65 years, P<0.001), more likely to be male (51.4% vs 47.3%, P<0.001), black (11.6% vs 5.3%, P<0.001), had a higher comorbidity burden as measured by the Charlson Comorbidity Index (78.8% with score of 2 vs 27.4% with score of 2, P<0.001), and had higher All Patient Refined Diagnosis Related Groups (APRDRG) mortality scores (68.1% with score of 4 vs 2.5% with score of 4, P<0.001). They were also more likely to be in the bottom quartile for income (28.2% vs 23.2%, P<0.001), and more likely to have Medicare/Medicaid insurance (78.3% vs 69.3%). Hospital characteristics of AF patients with AKI‐D were notable for being more likely to be large (69.9% vs 61.9%, P<0.001), urban teaching hospitals (51.6% vs 40.3%, P<0.001) located in the Midwest or North Central United States (28.3% vs 26.5%, P=0.01) (Table 2).
Table 2.
Patient and Hospital Level Characteristics of Hospitalizations With and Without AKI‐Da
| AF Patients Without AKI‐D (n=3 493 926) | AF Patients With AKI‐D (n=3751) | P Value | |
|---|---|---|---|
| Patient level characteristics | |||
| Age, y, % | <0.001 | ||
| 18 to 34 | 1.67 | 0.49 | |
| 35 to 49 | 6.87 | 4.57 | |
| 50 to 64 | 22.56 | 24.3 | |
| ≥65 | 68.91 | 70.63 | |
| Sex, % | <0.001 | ||
| Male | 47.35 | 51.41 | |
| Female | 52.65 | 48.59 | |
| Race or ethnicity, % | <0.001 | ||
| White | 67.22 | 63.5 | |
| Black | 5.35 | 11.66 | |
| Hispanic | 3.47 | 4 | |
| Others | 2.7 | 2.81 | |
| Missing | 21.25 | 18.03 | |
| Charlson comorbidity index, % | <0.001 | ||
| 0 | 42.57 | 5.83 | |
| 1 | 29.99 | 15.31 | |
| 2 | 27.43 | 78.86 | |
| APRDRG mortality scale, % | <0.001 | ||
| 1 & 2 | 82.56 | 4.39 | |
| 3 | 13.72 | 27.43 | |
| 4 | 2.53 | 68.06 | |
| Concurrent diagnosis, % | |||
| Diabetes mellitus | 22.75 | 34.92 | <0.001 |
| Hypertension | 65.11 | 71.12 | <0.001 |
| CKDa | 6.48 | 25.29 | <0.001 |
| Acute or chronic liver disease | 2.51 | 19.7 | <0.001 |
| Liver or intrahepatic biliary cancer | 0.06 | 0.24 | <0.001 |
| Sepsis | 0.61 | 23.57 | <0.001 |
| Acute myocardial infarction | 1.45 | 6.02 | <0.001 |
| Primary acute heart failure | 0 | 0 | |
| Cardiac catheterizations | 5.35 | 8.88 | <0.001 |
| Mechanical ventilation | 0.68 | 28.47 | <0.001 |
| Catheter ablation procedure | 4.88 | 4.15 | 0.03 |
| ZIP code incomeb, % | <0.001 | ||
| 0 to 25 percentile | 23.17 | 28.21 | |
| 26 to 50 percentile | 24.4 | 25.96 | |
| 51 to 75 percentile | 22.2 | 21.2 | |
| 76 to 100 percentile | 20.33 | 18.79 | |
| Primary payer, % | <0.001 | ||
| Medicare/Medicaid | 69.36 | 78.3 | |
| Private | 25.46 | 18.16 | |
| Uninsured/self pay | 5.02 | 3.55 | |
| Hospital level characteristics | |||
| Hospital bed size, % | <0.001 | ||
| Small | 13.22 | 8.25 | |
| Medium | 24.36 | 22.18 | |
| Large | 61.93 | 69.09 | |
| Hospital location, % | <0.001 | ||
| Rural | 16.4 | 6.22 | |
| Urban nonteaching | 42.82 | 41.7 | |
| Urban teaching | 40.28 | 51.61 | |
| Hospital region, % | 0.02 | ||
| Northeast | 23.06 | 21.34 | |
| Midwest or North Central | 26.49 | 28.31 | |
| South | 41.13 | 40.71 | |
| West | 8.67 | 8.89 | |
AF indicates atrial fibrillation; AKI‐D, acute kidney injury requiring hemodialysis; APRDRG, all patient refined diagnosis‐related groups; CKD, chronic kidney disease.
Both populations were compared utilizing a chi‐squared test, Wilcoxon rank‐sum test, or survey regression depending on the distributions of individual variables.
Quartile classification of the estimated median household income of residents in the patient's ZIP code. These values are derived from ZIP code‐demographic data obtained from Claritas.
Trends in Demographic Subgroups
In the age group greater than 50 years, the percentage of AF admissions complicated by AKI‐D increased by 5‐fold from 0.3/1000 hospitalizations in 2003 to 1.6/1000 hospitalizations in 2012. However, more strikingly, there was a marked rise of AKI‐D in the younger age group (18‐49) from 0 in 2003 to 0.8/1000 hospitalizations in 2012 (Figure 2A). Males and females had near similar AKI‐D rates in 2002. However, males had greater increase (0.3‐1.7/1000 hospitalizations) compared to females (0.3‐1.3/1000 hospitalizations) (Figure 2B). Blacks had greater overall rates as well as a more rapid increase in trends compared to whites, Hispanics, and other groups (Figure 2C).
Figure 2.
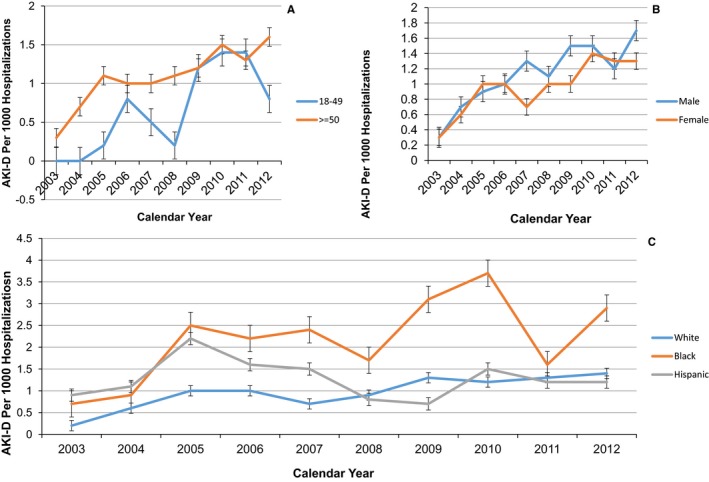
Yearly incidence of acute kidney injury requiring hemodialysis (AKI‐D) by age (A), sex (B), and race or ethnicity (C) in atrial fibrillation hospitalizations in the United States from 2003 to 2012. Dots are incidence for each year, and brackets are associated standard errors. A, The younger age group in 2003 had 0% AKI‐D and then had a rapid rise to until 2011 and then a drop off in 2012. Both groups increased at similar rates. B, Although both males and females started at similar rates of AKI‐D in 2003, males had a more rapid rise in AKI‐D incidence. C, Black patients had both higher absolute rates and higher rise in rates from 2003 to 2012. Analysis was done using the Cochran‐Armitage trend test.
Significant Associations of AKI‐D
Table 3 shows associations of comorbidities and procedures with AKI‐D. The strongest predictors were requiring mechanical ventilation (aOR 13.12; 95% CI 9.88‐17.43), sepsis (aOR 8.20; 95% CI 6.00‐11.20), and liver dysfunction (aOR 3.72; 95% CI 2.92‐4.75). Interestingly, catheter ablations for arrhythmias (aOR 0.92; 95% CI 0.60‐1.40) and other cardiac procedures such as cardiac catheterizations (aOR 1.24; 95% CI 0.91‐1.68) were not associated with AKI‐D.
Table 3.
Adjusted Associations of Comorbidities With AKI‐D in AF Hospitalizations
| Adjusted Odds Ratio (95% Confidence Interval)a | P Value | |
|---|---|---|
| Comorbidities | ||
| Hypertension | 1.52 (1.25‐1.85) | <0.001 |
| Diabetes mellitus | 0.96 (0.80‐1.14) | 0.61 |
| CKD | 1.40 (1.12‐2.97) | <0.001 |
| Sepsis | 8.20 (6.00‐11.20) | <0.001 |
| Heart failure | 1.48 (1.19‐1.72) | <0.001 |
| Liver disease | 3.72 (2.92‐4.75) | <0.01 |
| Procedures | ||
| Cardiac catheterization | 1.24 (0.91‐1.68) | 0.18 |
| Catheter ablation | 0.92 (0.60‐1.40) | 0.68 |
| Mechanical ventilation | 13.12 (9.88‐17.43) | <0.001 |
AF indicates atrial fibrillation; AKI‐D, acute kidney injury requiring hemodialysis; CKD, chronic kidney disease.
Associations adjusted for patient age, sex, race, year of admission, comorbidities of hypertension, HIV status, diabetes mellitus, chronic kidney disease, heart failure, liver disease, Charlson Comorbidity Index, sepsis on admission, and procedures of cardiac catheterization, catheter ablation, and mechanical ventilation.
Association of AKI‐D on Mortality and Discharge
Table 4 shows the association of AKI‐D with mortality and adverse discharge. The mortality proportion in hospitalizations with AKI‐D was 21.9% compared to less than 1% without AKI‐D. After adjustment for confounders, the adjusted mortality was still nearly 4‐fold higher in those with AKI‐D than in those without (aOR 3.54; 95% CI 2.81‐4.47). Similarly, hospitalizations with AKI‐D had twice the proportion of adverse discharge (55.6% vs 21.7%). Adjusted odds for adverse discharge were 4‐fold with AKI‐D compared to without (aOR 4.01; 95% CI 3.12‐5.17).
Table 4.
Adjusted Associations of Mortality and Adverse Discharge in AF Hospitalizations With and Without AKI‐Da
| Proportion Without AKI‐D | Proportion With AKI‐D | Unadjusted Odds Ratio (95% CI) | Adjusted Odds Ratio (95% CI) | |
|---|---|---|---|---|
| Mortality | 0.96 | 23.2 | 31.19 (25.72‐37.83) | 3.54 (2.81‐4.47) |
| Adverse discharge | 21.7 | 55.6 | 7.91 (6.60‐9.48) | 4.01 (3.12‐ 5.17) |
AF indicates atrial fibrillation; AKI‐D, acute kidney injury requiring hemodialysis.
Adjusted for age, sex, race, comorbidities of hypertension, HIV status, diabetes mellitus, chronic kidney disease, heart failure, liver disease, Charlson Comorbidity Index, sepsis on admission, and procedures of cardiac catheterization, catheter ablation, and mechanical ventilation.
AF hospitalizations with AKI‐D had a median LoS of 16 days (range 13.6‐22.7) versus 3.53 (range 3.33‐3.65) days for those without. The median cost in AF hospitalizations for AKI‐D, $44 558 ($36 337‐$58 387), was also significantly higher compared to those without AKI‐D, $8405 ($7644‐$8659). Although the trends for LoS and cost stayed reasonably stable from 2003 to 2012, they were much higher throughout for AF hospitalizations with AKI‐D compared to those without (Figure 3).
Figure 3.
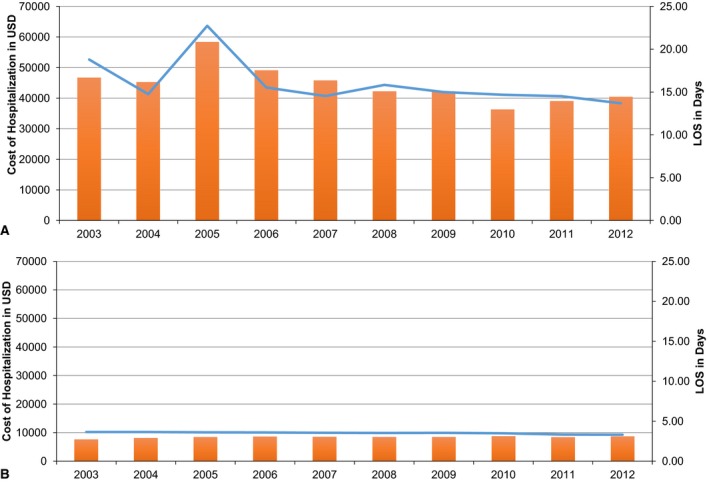
Yearly trends of cost and length of stay (LoS) in atrial fibrillation hospitalizations with (A) and without (B) acute kidney injury requiring hemodialysis (AKI‐D). Blue line represents LoS (right axis), and orange bars represent cost in US dollars. There is no significant change in LoS or cost in either group from 2003 to 2012. However, both LoS and cost were substantially higher in the AKI‐D group.
Trends of Mortality and Attributable Mortality Due to AKI‐D
The percentage mortality in AF hospitalizations with and without AKI‐D slightly decreased from 2003 to 2012 (Table 5). However, this decrease in percentage mortality was offset by an increase in AKI‐D per year, resulting in a slight increase in attributable risk percent (95.49 in 2003 to 96.31 in 2012) (Table 5).
Table 5.
Yearly Trends of Percentage Mortality and Attributable Risk of Mortality With AKI‐D in AF Hospitalizations
| Year | Percentage Mortality Without AKI‐D | Percentage Mortality With AKI‐D | Attributable Risk Percentage of Mortality From AKI‐D |
|---|---|---|---|
| 2003 | 1.12 | 20 | 95.49 |
| 2004 | 1.14 | 27 | 96.9 |
| 2005 | 1.06 | 26.7 | 99.93 |
| 2006 | 0.99 | 26.3 | 97.19 |
| 2007 | 0.96 | 31.4 | 97.89 |
| 2008 | 0.90 | 26.3 | 97.47 |
| 2009 | 0.84 | 28.1 | 97.84 |
| 2010 | 0.85 | 13.3 | 94.37 |
| 2011 | 0.89 | 13.7 | 94.37 |
| 2012 | 0.80 | 18 | 96.31 |
AF indicates atrial fibrillation; AKI‐D, acute kidney injury requiring hemodialysis.
Discussion
We utilized a large nationally representative sample of hospitalizations to assess trends, predictors, and outcomes for AKI‐D in AF hospitalizations. We have found that the proportion of AKI‐D in patients hospitalized with AF has increased 5‐fold over the last decade, and that AKI‐D is associated with 4‐fold higher mortality and 5‐fold higher adverse discharge rate. Although, percentage mortality decreased slightly per year, this was offset by rising incidence, leading to stable mortality attributable to AKI‐D. While older adults (>50) continue to have rising rates of AKI‐D, the younger population (18‐49), which toward the beginning of our study period had no occurrence of AKI‐D, now contributes a significant proportion to the burden of this disease. Although LoS and hospitalization cost have not changed significantly during the period of 2003‐2012, AKI‐D is associated with a much longer LoS and higher hospitalization cost. Additionally, we identified chronic and acute conditions that were predictors of AKI‐D such as HTN, DM, liver dysfunction, mechanical ventilation, and sepsis.
The data on AKI‐D in AF hospitalizations are limited because studies looking at AF and AKI‐D have been only small single‐center observational studies.12, 13 Our finding of increasing incidence of AKI‐D in the AF population coincides with the results of a recent study in the general population that found that the incidence of AKI‐D is increasing with an unadjusted average increase of 10% per year; however, patient demographic and comorbidities seemed to account for the temporal trends in the general population.20, 21 In our analysis even after adjustment for multiple similar covariates, there remained an unexplained 3% increase in AKI‐D per year. One possible explanation for this finding is that we have not identified factors driving this increase, including medication/nephrotoxin use, which is not captured by this database.
We also discovered multiple significant differences between non‐AKI‐D and AKI‐D groups including older age and higher comorbidity burden. It is well observed that patients with DM, HTN, HF, sepsis, and liver disease are much more likely to develop AKI in various settings as compared to patients without, which is concordant with our findings.7, 22, 23, 24, 25 Also, conditions such as sepsis predispose to the development of AKI. In fact, studies report that AKI represented the most frequent organ failure in patients with sepsis, and furthermore, the incidence of AKI‐D complicating sepsis has been rising, possibly because of overall increasing complexity of patients with severe sepsis and progressively earlier initiation of dialysis for AKI in these patients. Furthermore, DM, HTN, and liver disease are risk factors for CKD.23, 24, 25 CKD was one of the strongest predictors for AKI‐D, and as the prevalence of CKD increases with age, and CKD patients have more comorbidities than the general population, this finding may be reflective of the higher prevalence of underlying CKD in the AKI‐D patients. In addition, not only is CKD strongly associated with AKI, but in patients with CKD who develop AKI, there is a high chance of requiring dialysis.6, 26
We, along with others, have found that male sex has higher risk of AKI and AKI‐D.27, 28 Although the exact mechanism of this sex disparity in AKI incidence is unclear, animal models suggest an association with the modulation of sex hormone production.29 Studies in the general population have also found that blacks were more likely to develop AKI and AKI‐D despite being younger and having higher baseline estimated glomerular filtration rates (eGFR). This racial disparity seemed to be driven by socioeconomic factors such as income and insurance type.20, 30 We have found similar findings in the blacks who are hospitalized with AF, which supports the need for increased screening for AKI in this high‐risk population. Although we did not specifically examine socioeconomic factors in blacks, we found that in our cohort, patients in the lower income group and those with public insurance were more likely to develop AKI‐D. Multiple studies in the CKD population have demonstrated that lower‐income groups have worse quality of life, faster progression of kidney disease, and are referred to nephrology care with lower eGFR.31, 32, 33 However, studies linking socioeconomic factors and insurance type to AKI and AKI‐D incidence and outcomes are lacking and need to be conducted in the future.
We did not find an association between cardiac catheterizations and AKI‐D, which is surprising given that intravenous contrast exposure is a well‐established risk factor for AKI.34 Because cardiac catheterization done in the AF setting is generally not emergent, this allows time for recognition and institution of appropriate preventative measures for contrast‐induced nephropathy. This may partially explain why we did not observe these procedures to be predictors of AKI‐D. Through extensive literature review, we could not identify any study evaluating the association between catheter ablation for AF and AKI. Further studies are needed to explore this finding and to elucidate whether cardiac catheterization is a risk factor for AKI in AF.
In the United States it is estimated that acute dialysis is associated with an average increase of $39 087 per hospitalization, and thus the total costs associated with AKI‐D in patients with AF was $25 million for the year 2012 alone.35 To prevent this devastating complication, it is imperative that we identify patients at risk for AKI‐D early in the hospital course and institute preventative measures including avoiding nephrotoxins, use of minimal contrast, and hydration prior to the insult.
Because prior studies in AF and AKI‐D were small single‐center studies, we used a nationally representative sample to allow for power and true national generalizability. However, we do recognize several limitations such as the use of administrative data instead of laboratory data to identify AKI‐D. Therefore, we chose to focus only on AKI‐D as opposed to all AKI, for which the validity of codes might be less questionable.36 We are unable to exclude the more liberal use of acute dialysis over time; however, the incidence of laboratory‐defined AKI has been increasing over time.21, 27, 37 Also, we could not capture information on nephrotoxin, drug toxicity, or medication use. Because the data were deidentified, we could not identify patients with multiple admissions or those who had recurrent episodes of AKI‐D; however, we suspect that this number is likely small and would not qualitatively change our results.
Conclusions
We have observed a significant increase in the incidence of AKI‐D among individuals hospitalized for AF between 2003 and 2012, with differential increases among demographic subgroups. AKI‐D was associated with significant mortality and morbidity, and although mortality decreased slightly over time, the attributable mortality remained stable. These data highlight the public health burden of AKI‐D in AF and the need for better strategies for early AKI detection, appropriate risk stratification, and timely institution of preventive therapies to prevent progression of AKI and its consequences in patients hospitalized with AF.
Sources of Funding
Dr Chan is supported in part by the NIH (5T32DK007757 – 18)
Disclosures
None.
(J Am Heart Assoc. 2016;5:e004509 doi: 10.1161/JAHA.116.004509)
References
- 1. Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. JAMA. 2001;285:2370–2375. [DOI] [PubMed] [Google Scholar]
- 2. Feinberg WM, Blackshear JL, Laupacis A, Kronmal R, Hart RG. Prevalence, age distribution, and gender of patients with atrial fibrillation. Analysis and implications. Arch Intern Med. 1995;155:469–473. [PubMed] [Google Scholar]
- 3. Ball J, Carrington MJ, McMurray JJ, Stewart S. Atrial fibrillation: profile and burden of an evolving epidemic in the 21st century. Int J Cardiol. 2013;167:1807–1824. [DOI] [PubMed] [Google Scholar]
- 4. Patel NJ, Deshmukh A, Pant S, Singh V, Patel N, Arora S, Shah N, Chothani A, Savani GT, Mehta K, Parikh V, Rathod A, Badheka AO, Lafferty J, Kowalski M, Mehta JL, Mitrani RD, Viles‐Gonzalez JF, Paydak H. Contemporary trends of hospitalization for atrial fibrillation in the United States, 2000 through 2010: implications for healthcare planning. Circulation. 2014;129:2371–2379. [DOI] [PubMed] [Google Scholar]
- 5. Winkelmayer WC, Patrick AR, Liu J, Brookhart MA, Setoguchi S. The increasing prevalence of atrial fibrillation among hemodialysis patients. J Am Soc Nephrol. 2011;22:349–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chawla LS, Kimmel PL. Acute kidney injury and chronic kidney disease: an integrated clinical syndrome. Kidney Int. 2012;82:516–524. [DOI] [PubMed] [Google Scholar]
- 7. Nadkarni GN, Patel A, Simoes PK, Yacoub R, Annapureddy N, Kamat S, Konstantinidis I, Perumalswami P, Branch A, Coca SG, Wyatt CM. Dialysis‐requiring acute kidney injury among hospitalized adults with documented hepatitis C virus infection: a nationwide inpatient sample analysis. J Viral Hepat. 2016;23:32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nadkarni GN, Patel AA, Konstantinidis I, Mahajan A, Agarwal SK, Kamat S, Annapureddy N, Benjo A, Thakar CV. Dialysis requiring acute kidney injury in acute cerebrovascular accident hospitalizations. Stroke. 2015;46:3226–3231. [DOI] [PubMed] [Google Scholar]
- 9. Nadkarni GN, Patel AA, Yacoub R, Benjo AM, Konstantinidis I, Annapureddy N, Agarwal SK, Simoes PK, Kamat S, Menon MC, Wyatt CM. The burden of dialysis‐requiring acute kidney injury among hospitalized adults with HIV infection: a nationwide inpatient sample analysis. AIDS. 2015;29:1061–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nadkarni GN, Simoes PK, Patel A, Patel S, Yacoub R, Konstantinidis I, Kamat S, Annapureddy N, Parikh CR, Coca SG. National trends of acute kidney injury requiring dialysis in decompensated cirrhosis hospitalizations in the United States. Hepatol Int. 2016;10:525–531. [DOI] [PubMed] [Google Scholar]
- 11. Yedlapati SH, Youna U, Stewart SH. Acute kidney injury is associated with high mortality in patients with atrial fibrillation. Circulation. 2015;132:A17300. [Google Scholar]
- 12. Hellman Y, Cohen MJ, Leibowitz D, Loncar S, Gozal D, Haviv YS, Haber G, Afifi M, Rosenheck S, Lotan C, Pollak A, Gilon D. The incidence and prognosis of renal dysfunction following cardioversion of atrial fibrillation. Cardiology. 2013;124:184–189. [DOI] [PubMed] [Google Scholar]
- 13. Ng RR, Tan GH, Liu W, Ti LK, Chew ST. The association of acute kidney injury and atrial fibrillation after cardiac surgery in an Asian prospective cohort study. Medicine (Baltimore). 2016;95:e3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. HCUP Nationwide Inpatient Sample (NIS). Healthcare Cost and Utilization Project (HCUP). Rockville, MD: Agency for Healthcare Research and Quality; 2011. Available at: www.Hcup-us.Ahrq.Gov/nisoverview.jsp. Accessed April 12, 2016. [Google Scholar]
- 15. Jensen PN, Johnson K, Floyd J, Heckbert SR, Carnahan R, Dublin S. A systematic review of validated methods for identifying atrial fibrillation using administrative data. Pharmacoepidemiol Drug Saf. 2012;21(suppl 1):141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Waikar SS, Wald R, Chertow GM, Curhan GC, Winkelmayer WC, Liangos O, Sosa MA, Jaber BL. Validity of International Classification of Diseases, ninth revision, clinical modification codes for acute renal failure. J Am Soc Nephrol. 2006;17:1688–1694. [DOI] [PubMed] [Google Scholar]
- 17. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 18. Calculator UI. US Inflation Calculator. 2016. Available at http://www.usinflationcalculator.com/. Accessed April 1, 2016.
- 19. Hoh BL, Chi YY, Waters MF, Mocco J, Barker FG. Effect of weekend compared with weekday stroke admission on thrombolytic use, in‐hospital mortality, discharge disposition, hospital charges, and length of stay in the Nationwide Inpatient Sample Database, 2002 to 2007. Stroke. 2010;41:2323–2328. [DOI] [PubMed] [Google Scholar]
- 20. Hsu RK, McCulloch CE, Dudley RA, Lo LJ, Hsu CY. Temporal changes in incidence of dialysis‐requiring AKI. J Am Soc Nephrol. 2013;24:37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hsu RK, McCulloch CE, Heung M, Saran R, Shahinian VB, Pavkov ME, Burrows NR, Powe NR, Hsu CY; Centers for Disease Control and Prevention Chronic Kidney Disease Surveillance Team . Exploring potential reasons for the temporal trend in dialysis‐requiring AKI in the United States. Clin J Am Soc Nephrol. 2016;11:14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Girman CJ, Kou TD, Brodovicz K, Alexander CM, O'Neill EA, Engel S, Williams‐Herman DE, Katz L. Risk of acute renal failure in patients with type 2 diabetes mellitus. Diabet Med. 2012;29:614–621. [DOI] [PubMed] [Google Scholar]
- 23. Bagshaw SM, George C, Bellomo R; ANZICS Database Management Committee . Early acute kidney injury and sepsis: a multicentre evaluation. Crit Care. 2008;12:R47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kumar G, Kumar N, Taneja A, Kaleekal T, Tarima S, McGinley E, Jimenez E, Mohan A, Khan RA, Whittle J, Jacobs E, Nanchal R; Milwaukee Initiative in Critical Care Outcomes Research Group of Investigators . Nationwide trends of severe sepsis in the 21st century (2000–2007). Chest. 2011;140:1223–1231. [DOI] [PubMed] [Google Scholar]
- 25. Sakhuja A, Kumar G, Gupta S, Mittal T, Taneja A, Nanchal RS. Acute kidney injury requiring dialysis in severe sepsis. Am J Respir Crit Care Med. 2015;192:951–957. [DOI] [PubMed] [Google Scholar]
- 26. Hsu CY, Chertow GM, McCulloch CE, Fan D, Ordonez JD, Go AS. Nonrecovery of kidney function and death after acute or chronic renal failure. Clin J Am Soc Nephrol. 2009;4:891–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hsu CY, McCulloch CE, Fan D, Ordonez JD, Chertow GM, Go AS. Community‐based incidence of acute renal failure. Kidney Int. 2007;72:208–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hatakeyama Y, Horino T, Kataoka H, Matsumoto T, Ode K, Shimamura Y, Ogata K, Inoue K, Taniguchi Y, Terada Y, Okuhara Y. Incidence of acute kidney injury among patients with chronic kidney disease: a single‐center retrospective database analysis. Clin Exp Nephrol. 2016. doi: 10.1007/s10157‐016‐1243‐2. Available at: http://link.springer.com/article/10.1007/s10157-016-1243-2. Accessed December 7, 2016. [DOI] [PubMed] [Google Scholar]
- 29. Robert R, Ghazali DA, Favreau F, Mauco G, Hauet T, Goujon JM. Gender difference and sex hormone production in rodent renal ischemia reperfusion injury and repair. J Inflamm. 2011;8:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Grams ME, Matsushita K, Sang Y, Estrella MM, Foster MC, Tin A, Kao WH, Coresh J. Explaining the racial difference in AKI incidence. J Am Soc Nephrol. 2014;25:1834–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Plantinga L, Johansen KL, Schillinger D, Powe NR. Lower socioeconomic status and disability among US adults with chronic kidney disease, 1999–2008. Prev Chronic Dis. 2011;9:110052. [PMC free article] [PubMed] [Google Scholar]
- 32. Hidalgo G, Ng DK, Moxey‐Mims M, Minnick ML, Blydt‐Hansen T, Warady BA, Furth SL. Association of income level with kidney disease severity and progression among children and adolescents with CKD: a report from the Chronic Kidney Disease in Children (CKiD) Study. Am J Kidney Dis. 2013;62:1087–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lemos CF, Rodrigues MP, Veiga JR. Family income is associated with quality of life in patients with chronic kidney disease in the pre‐dialysis phase: a cross sectional study. Health Qual Life Outcomes. 2015;13:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Keaney JJ, Hannon CM, Murray PT. Contrast‐induced acute kidney injury: how much contrast is safe? Nephrol Dial Transplant. 2013;28:1376–1383. [DOI] [PubMed] [Google Scholar]
- 35. Dasta JF, Kane‐Gill SL, Durtschi AJ, Pathak DS, Kellum JA. Costs and outcomes of acute kidney injury (AKI) following cardiac surgery. Nephrol Dial Transplant. 2008;23:1970–1974. [DOI] [PubMed] [Google Scholar]
- 36. Grams ME, Waikar SS, MacMahon B, Whelton S, Ballew SH, Coresh J. Performance and limitations of administrative data in the identification of AKI. Clin J Am Soc Nephrol. 2014;9:682–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Siew ED, Davenport A. The growth of acute kidney injury: a rising tide or just closer attention to detail? Kidney Int. 2015;87:46–61. [DOI] [PMC free article] [PubMed] [Google Scholar]


