Abstract
Background
The impact of acute stent malapposition (ASM) on long‐term clinical outcomes in patients undergoing percutaneous coronary intervention is still controversial. We sought to evaluate predictors and long‐term clinical outcomes of ASM.
Methods and Results
ADAPT‐DES (Assessment of Dual Antiplatelet Therapy With Drug‐Eluting Stents) was a prospective multicenter study of 8663 patients undergoing percutaneous coronary intervention using drug‐eluting stents. In a prespecified intravascular ultrasound–guided substudy, 2072 patients with 2446 culprit lesions had post–percutaneous coronary intervention intravascular ultrasound and were classified according to the presence or absence of ASM. After intravascular ultrasound–guided percutaneous coronary intervention, the overall prevalence of ASM after successful drug‐eluting stents implantation was 14.4% per patient and 12.6% per lesion. Compared to lesions without ASM, lesions with ASM had larger in‐stent lumen areas, larger stent areas, and larger in‐stent vessel areas. A larger mean plaque area along with more attenuated plaque was observed in lesions with ASM versus lesions without ASM. Lesions with ASM had greater proximal and distal reference lumen areas and more distal, but not proximal, reference calcium compared to lesions without ASM. At 2‐year follow‐up, there was no significant difference in the incidence of cardiac death; myocardial infarction; early, late, or very late stent thrombosis; or clinically driven target lesion revascularization in patients with ASM versus those without ASM. Furthermore, ASM was not an independent predictor of 2‐year major adverse cardiac events or target lesion revascularization even when forced into the multivariate model.
Conclusions
In patients treated with intravascular ultrasound–guided drug‐eluting stents implantation, ASM was not associated with adverse clinical events during long‐term follow‐up including, but not limited to, stent thrombosis.
Clinical Trial Registration
URL: https://www.clinicaltrials.gov. Unique identifier: NCT00638794.
Keywords: coronary artery disease, intravascular imaging, malapposition, stent
Subject Categories: Stent, Imaging
Introduction
Acute stent malapposition (ASM) is common at the time of stent implantation1, 2, 3, 4, 5, 6; however, it is still controversial whether ASM impacts long‐term clinical outcomes in patients undergoing percutaneous coronary intervention (PCI). For example, Kimura et al7 reported that although most ASM after sirolimus‐eluting stent implantation did not resolve completely, the incidence of restenosis or thrombosis was not affected. Conversely, Cook et al8 reported that late stent malapposition, potentially representing persistent ASM, was highly prevalent in patients with very late drug‐eluting stent (DES) thrombosis.
ADAPT‐DES (Assessment of Dual Antiplatelet Therapy With Drug‐Eluting Stents) was a large‐scale, prospective, multicenter study designed to assess the relationship between platelet reactivity and other clinical and procedural variables versus subsequent stent thrombosis and adverse clinical events in patients successfully treated (ie, no major periprocedural complications) with DES.9 In a prespecified, prospective intravascular ultrasound (IVUS) substudy, culprit lesions were evaluated and treated using IVUS‐guided PCI.10 The aims of this substudy using the IVUS data from ADAPT‐DES were to (1) determine the prevalence of IVUS‐detected ASM in the current DES era after IVUS‐guided PCI; (2) evaluate the morphological features of ASM; (3) determine the predictors for ASM; and (4) clarify outcomes related to IVUS‐detected ASM, in particular the impact of ASM on stent thrombosis after IVUS‐guided PCI.
Methods
Patient Selection and Imaging
The design, major inclusion and exclusion criteria, end points, and definitions from the ADAPT‐DES study have been previously described in detail.9, 10 In brief, ADAPT‐DES was a prospective, multicenter, observational study of consecutive patients who were treated successfully with 1 or more US Food and Drug Administration‐ or CE mark‐approved DES regardless of patient or lesion complexity. Among 2179 patients enrolled in a prespecified IVUS substudy, 2072 patients with analyzable post‐PCI IVUS were included in the current analysis.10
In the present analysis, major adverse cardiac events (MACE) included cardiac death, myocardial infarction (MI), and stent thrombosis, as defined previously by the Academic Research Consortium and as adjudicated by an independent clinical events committee.9, 10, 11 Clinically driven target lesion revascularization (TLR) was site reported, but not centrally adjudicated.
Angiograms were evaluated visually by operators at the time of the procedure using conventional definitions.12 Angiographic findings included thrombus, calcification, in‐stent restenosis, lesion location and length, reference vessel diameter, pre‐ and post‐PCI lesion diameter stenosis, and Thrombolysis In Myocardial Infarction flow grade.13 Thrombus was defined as a discrete intraluminal filling defect with defined borders, largely separated from the adjacent wall and with or without contrast staining. Calcium was defined as readily visible densities noted within the apparent vascular wall at the site of the stenosis. Angiographic in‐stent restenosis was defined as percentage diameter stenosis >50%.
Clinical follow‐up was done at 2 years. The study was approved by the institutional review board at each participating center; written informed consent was obtained from all patients.
IVUS Image Acquisition and Analysis
Poststenting IVUS was performed using a synthetic aperture array, 20 MHz, 3.2Fr catheter (Eagle Eye; Volcano Corporation, Rancho Cordova, CA) after intracoronary nitroglycerin. The ultrasound catheter was advanced >10 mm beyond the stent into the distal vessel, and the transducer was withdrawn at a pullback speed of 0.5 mm/s to a point >10 mm proximal to the stent. All IVUS studies were archived onto DVD and sent to the IVUS core laboratory (Cardiovascular Research Foundation, New York, NY) for offline quantitative and qualitative analyses by individuals blinded to treatment and clinical outcomes. Quantitative IVUS analyses were performed with validated planimetry software (echoPlaque; INDEC Systems, Inc, Mountain View, CA).
IVUS measurements were performed millimeter by millimeter beginning 5 mm distal to the distal stent edge and continuing through the stent to a point 5 mm proximal to the proximal stent edge and included the external elastic membrane, lumen, plaque+media (external elastic membrane minus lumen), stent area, and plaque burden (plaque+media area divided by external elastic membrane area). Volumes were calculated using Simpson's rule and reported as normalized volumes (volume divided by analysis length).
A culprit lesion was defined as the lesion that was stented. The slices with the minimum lumen area and minimum stent area within the stented lesion segment were identified and assessed. Proximal and distal 5‐mm‐long segments from each stent edge but before a significant (>1.5 mm in diameter) side branch were defined as the reference segments. Attenuated plaque behind the stent was defined as plaque with >30° ultrasonic attenuation (attenuation of deeper arterial structures) despite the absence of bright calcium behind stent struts.14, 15 Calcified plaque was defined as hyperechoic plaque with acoustic shadowing. ASM was defined as at least 1 stent strut that was clearly (stent struts and adjacent intima seen as distinct entities) separated from the vessel wall with evidence of blood speckle behind the strut but not overlapping a side branch. The location, length, maximum angle, and maximum number of struts per slice in the malapposed segment were recorded. Tissue protrusion was defined as plaque and/or thrombus intrusion through the stent struts into the lumen. Stent edge dissection was defined as a visible tear of the intima at the stent edge.
Statistical Analysis
Baseline clinical characteristics were analyzed on a patient level; angiographic and IVUS characteristics were analyzed on a lesion level. Categorical variables were summarized using percentages and counts and were compared using χ2 statistics or Fisher exact test where appropriate. Patient‐level continuous variables were compared using the t test and shown as mean (SD). All lesion‐level analyses were modeled with a generalized estimating equation approach to correct for multiple lesions from the same patient and shown as least‐squares means with 95% CI. Time‐to‐event data were summarized as Kaplan–Meier estimates. Time‐to‐event outcomes were summarized as Kaplan–Meier failure estimates at 2 years and displayed using cumulative incidence curves. Comparisons for patient‐level outcomes were performed using the log‐rank test. Intra‐observer and interobserver variability for the diagnosis of ASM and maximum ASM angle were assessed using Cohen's kappa statistics and intraclass correlation coefficient, respectively. A P‐value of <0.05 was considered statistically significant. Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
Results
Among 2072 patients with 2446 lesions, the overall prevalence of ASM after successful DES implantation was 14.4% per patient (299 of 2072) and 12.6% per lesion (308 of 2446). In lesions with malapposition, the location of the ASM was at the proximal stent edge in 58.4% (180 of 308), stent body in 23.1% (71 of 308), and distal stent edge in 18.5% (57 of 308). The measurements of ASM (shown as least‐square means, 95% CI) were 2.1 (2.0–2.4) mm in longitudinal length, 2.8 (2.6–3.0) mm2 in maximum ASM area, 0.76 (0.74–0.80) mm in distance between the surface of plaque to stent strut, 126 (123–134) in maximum ASM angle, and 3.5 (3.4–3.7) in maximum number of malapposed struts. Intra‐observer and interobserver variability were good for the diagnosis of ASM (Cohen's kappa; 0.80, 0.80) and maximum ASM angle (intraclass correlation coefficient; 0.90, 0.88).
Baseline Clinical Characteristics
Comparisons of baseline clinical characteristics between patients with versus without ASM have been summarized in Table 1. Patients with ASM were more often men and more frequently had a history of previous MI. Other baseline characteristics such as age, current smoking status, diabetes mellitus, hyperlipidemia, history of renal insufficiency, previous PCI, previous coronary artery bypass graft, presenting clinical syndrome, P2Y12 reaction units, and use of dual antiplatelet therapy including aspirin and a thienopyridine were comparable between the 2 groups.
Table 1.
Clinical Characteristics
| Patients With Acute Malapposition (N=299 Patients) | Patients Without Acute Malapposition (N=1773 Patients) | P Value | |
|---|---|---|---|
| Age, y | 62.8±10.4 | 63.1±10.7 | 0.66 |
| Men | 80.3 (240) | 74.6 (1322) | 0.03 |
| Current smoker | 33.1 (99) | 29.5 (523) | 0.21 |
| Diabetes mellitus | 29.8 (89) | 28.7 (509) | 0.71 |
| Hypertension | 81.6 (244) | 76.5 (1357) | 0.05 |
| Hyperlipidemia | 63.9 (191) | 62.4 (1107) | 0.63 |
| History of renal insufficiencya | 17.8 (53) | 15.7 (276) | 0.34 |
| Body mass index, kg/m2 | 29.0±5.4 | 28.5±5.3 | 0.12 |
| Previous MI | 29.8 (89) | 24.4 (433) | 0.049 |
| Previous PCI | 39.5 (118) | 38.9 (689) | 0.84 |
| Previous CABG | 12.4 (37) | 11.2 (198) | 0.54 |
| Clinical presentation | |||
| ST‐segment elevation MI | 16.1 (48) | 18.2 (322) | 0.38 |
| Non ST‐segment elevation MI | 17.1 (51) | 18.2 (322) | 0.65 |
| Unstable angina | 22.1 (66) | 22.8 (404) | 0.79 |
| Stable coronary artery disease | 44.8 (134) | 40.9 (725) | 0.20 |
| P2Y12 reaction units | 202 (128–264) | 193 (116–257) | 0.14 |
| Use of dual antiplatelet therapyb | |||
| At discharge | 97.7 (292) | 98.1 (1740) | 0.58 |
| At 30 days | 91.0 (272) | 92.2 (1634) | 0.48 |
| At 1 year | 71.9 (215) | 73.1 (1296) | 0.67 |
| At 2 years | 29.1 (87) | 29.4 (522) | 0.90 |
| Statin | |||
| Preadmission (<7 days) | 56.2 (168) | 53.9 (955) | 0.46 |
| At discharge | 94.6 (283) | 93.3 (1654) | 0.38 |
| At 30 days | 89.6 (267) | 89.9 (1588) | 0.89 |
| At 1 year | 88.4 (258) | 86.7 (1515) | 0.43 |
| At 2 years | 85.1 (240) | 83.7 (1413) | 0.54 |
Values are % (n) or mean±SD. CABG indicates coronary artery bypass graft; MI, myocardial infarction; PCI, percutaneous coronary intervention.
Creatinine clearance <60 mL/min calculated with the Cockcroft‐Gault formula.
Aspirin and a thienopyridine.
Angiographic and Procedural Findings
Angiographic and procedural findings are shown in Table 2. ASM was less prevalent in the lesions with thrombus or in‐stent restenosis. Lesions with ASM were longer and had a smaller stent/lumen ratio compared to lesions without ASM.
Table 2.
Angiographic and Procedural Findings
| Lesions With Acute Malapposition (N=307 Lesions) | Lesions Without Acute Malapposition (N=2123 Lesions) | P Value | |
|---|---|---|---|
| Thrombus | 19.2 (59) | 24.1 (511) | 0.045 |
| Calcification | 31.9 (98) | 33.9 (719) | 0.40 |
| Ostial lesion | 9.8 (30) | 12.4 (264) | 0.16 |
| In‐stent restenosis lesion | 4.2 (13) | 8.8 (187) | 0.008 |
| Lesion length, mm | 25.4 (23.5–27.4) | 23.3 (22.6–23.9) | 0.04 |
| Reference vessel diameter, mm | 3.3 (3.2–3.4) | 3.2 (3.2–3.3) | 0.13 |
| Prediameter stenosis (%) | 86.5 (85.2–87.8) | 86.3 (85.8–86.8) | 0.82 |
| Postdiameter stenosis (%) | 0.6 (0.3–1.0) | 0.7 (0.5–0.8) | 0.90 |
| Pre TIMI flow 0 or 1 | 12.1 (37) | 14.3 (303) | 0.26 |
| Final TIMI flow 3 | 99.3 (305) | 99.8 (2119) | 0.15 |
| Total stent length, mm | 30.0 (27.9–32.2) | 28.0 (27.2–28.7) | 0.07 |
| Maximum device diameter, mma | 3.47 (3.40–3.53) | 3.38 (3.35–3.40) | 0.02 |
| Maximum balloon pressure, atm | 16.4 (16.0–16.8) | 16.2 (16.0–16.3) | 0.32 |
| Stent/lumen ratiob | 0.93 (0.91–0.94) | 1.02 (1.01–1.03) | <0.0001 |
Values are % (n) or least‐squares means (95% CI). TIMI indicates thrombolysis in myocardial infarction.
Stent or postdilating balloon.
Maximum stent diameter/mean of proximal and distal reference lumen diameter by intravascular ultrasound.
IVUS Findings
Postprocedure IVUS findings are shown in Table 3. Lesions with ASM were more prevalent in the right coronary artery, had larger in‐stent lumen areas, larger stent areas, and larger in‐stent vessel areas compared with lesions without ASM. A larger mean plaque area along with more attenuated plaque was observed in lesions with ASM versus lesions without ASM; however, the prevalence of tissue protrusion was comparable between the 2 groups. Compared to lesions without ASM, lesions with ASM had greater proximal and distal reference lumen areas and more distal, but not proximal reference calcium. The prevalence of stent edge dissection was similar between the 2 groups.
Table 3.
Poststent Intravascular Ultrasound Findings
| Lesions With Acute Malapposition (N=308 Lesions) | Lesions Without Acute Malapposition (N=2138 Lesions) | P Value | |
|---|---|---|---|
| Lesion location | |||
| Left anterior descending artery | 39.9 (123) | 41.6 (890) | 0.56 |
| Right coronary artery | 38.3 (118) | 30.8 (658) | 0.008 |
| Left circumflex artery | 17.5 (54) | 21.7 (465) | 0.09 |
| Left main coronary artery | 1.9 (6) | 2.5 (54) | 0.53 |
| Saphenous vein graft | 2.3 (7) | 3.3 (71) | 0.48 |
| Stent segment | |||
| Minimum lumen area, mm2 | 6.5 (6.3–6.8) | 6.2 (6.1–6.3) | 0.01 |
| Minimum stent area, mm2 | 6.5 (6.2–6.8) | 6.2 (6.1–6.3) | 0.04 |
| Mean lumen area, mm3/mm | 8.3 (8.0–8.6) | 7.6 (7.5–7.7) | 0.0001 |
| Mean stent area, mm3/mm | 8.1 (7.8–8.4) | 7.7 (7.5–7.8) | 0.01 |
| Mean EEM area, mm3/mm | 18.0 (17.4–18.7) | 15.9 (15.6–16.1) | <0.0001 |
| Mean plaque area, mm3/mm | 10.0 (9.6–10.4) | 8.2 (8.1–8.4) | <0.0001 |
| Attenuated plaque behind stent | 73.7 (227) | 61.0 (1304) | <0.0001 |
| Tissue protrusion | 35.4 (109) | 34.2 (731) | 0.70 |
| Reference segment | |||
| Proximal lumen area, mm2 | 12.6 (12.0–13.2) | 9.8 (9.6–10.0) | <0.0001 |
| Distal lumen area, mm2 | 8.8 (9.3–9.3) | 7.6 (7.4–7.7) | <0.0001 |
| Calcification at proximal reference | 54.1 (150) | 46.7 (899) | 0.14 |
| Calcification at distal reference | 36.8 (109) | 28.0 (583) | 0.002 |
| Edge dissection | 4.5 (14) | 6.9 (147) | 0.13 |
Values are % (n) or least‐squares means (95% CI). EEM indicates external elastic membrane.
In a subset with pre‐intervention IVUS (n=812 lesions), the maximum superficial calcium angle was larger in the setting of acute malapposition (138 [121, 154] versus 108 [102, 114], P=0.001) as was the presence of a calcified nodule (12.0% versus 4.8%, P=0.003).
Two‐Year Clinical Outcomes
Two‐year MACE after stent implantation has been summarized in Figure 1 and Table 4. The incidence of cardiac death; MI; early, late, or very late stent thrombosis; and clinically driven TLR was similar between patients with ASM versus those without ASM. Furthermore, ASM was not an independent predictor of 2‐year MACE or TLR, even when ASM was forced into the multivariate model.
Figure 1.
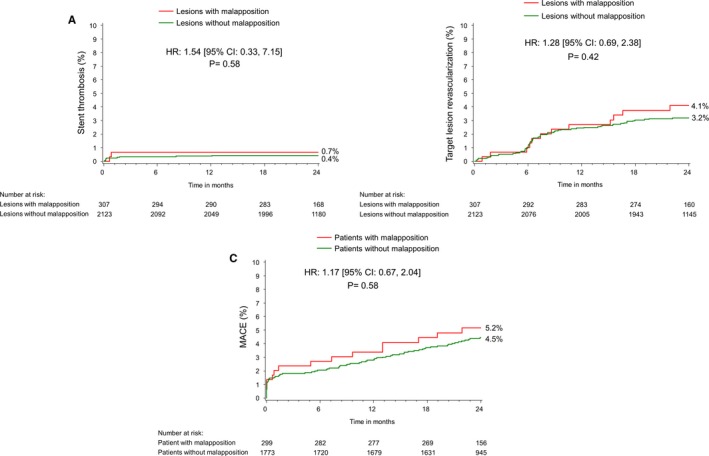
Two‐year Kaplan–Meier cumulative incidence curves for adverse cardiac events: stent thrombosis (ST), TLR, and MACE at 2 years. A, Lesions with or without definite/probable stent thrombosis, (B) shows the lesions with or without target lesion revascularization, and (C) shows patients with or without MACE at 2 years. HR indicates hazard ratio; MACE, major adverse cardiac events; TLR, target lesion revascularization.
Table 4.
Two‐Year Kaplan–Meier Adverse Cardiac Event Rates
| Acute Malapposition | No Acute Malapposition | P Value | |
|---|---|---|---|
| Patient level | N=299 | N=1773 | |
| MACE | 5.2 (15) | 4.5 (77) | 0.58 |
| Cardiac death | 1.7 (5) | 1.4 (24) | 0.64 |
| MI | 4.1 (12) | 3.1 (53) | 0.34 |
| Periprocedural | 1.3 (4) | 1.2 (22) | 0.88 |
| Lesion level | N=307 | N=2123 | |
| TLR | 4.1 (12) | 3.2 (66) | 0.42 |
| Stent thrombosis | 0.65 (2) | 0.43 (9) | 0.58 |
| Within 30 days | 0.65 (2) | 0.24 (5) | 0.22 |
| 31 days to 1 year | 0.0 (0) | 0.14 (3) | 0.51 |
| 1 to 2 years | 0.0 (0) | 0.05 (1) | 0.71 |
MACE indicates major adverse cardiac events; MI, myocardial infarction; TLR, target lesion revascularization.
Values are % (n).
Figure 2 indicates the relationship between minimum stent area and maximum malapposition area stratified by MACE, stent thrombosis, and TLR. No significant difference was observed for the distribution of maximum malapposition area comparing lesions with versus without events. In fact, the largest areas of ASM were seen in lesions without events.
Figure 2.
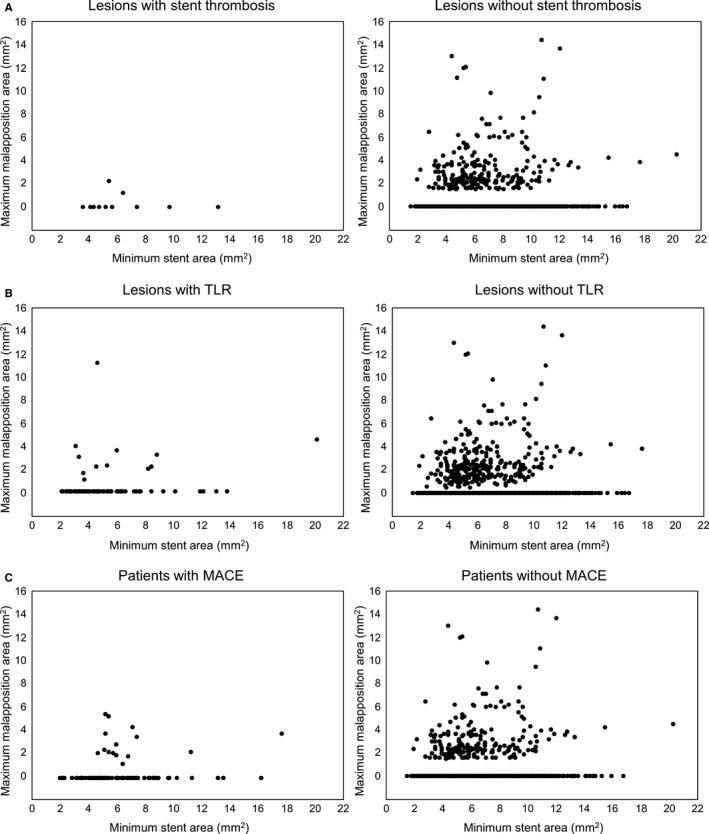
The relationship between minimum stent area and maximum malapposition area stratified by lesions with vs without stent thrombosis or TLR and patients with vs without MACE. A, Lesions with or without definite/probable stent thrombosis, (B) shows the lesions with or without target lesion revascularization, and (C) shows patients with or without MACE at 2 years. In the comparison of TLR or MACE, the larger minimum stent area was associated with the larger maximum malapposition area. Although there were cases with relatively small minimum stent area with large malapposition areas in patients with events, these cases existed in patients without events as well, and no patient with ASM ≥4 mm2 had stent thrombosis. There was no significant difference in distribution of malapposition areas comparing stents with vs without events. ASM indicates acute stent malapposition; MACE, major adverse cardiac events; TLR, target lesion revascularization.
Discussion
In a previous report from ADAPT‐DES, IVUS guidance was associated with a reduction in stent thrombosis, MI, and MACE within 1 year after DES implantation.10 The current study used the IVUS data from ADAPT‐DES to assess the impact of residual ASM on late events. The major findings of the present study were the following. (1) The overall prevalence of residual IVUS‐detected ASM was 14.4% per patient and 12.6% per lesion. (2) Patients with ASM were more often men and more frequently had a history of previous MI. Lesions with ASM had larger in‐stent lumen areas, larger stent areas, and larger vessel areas. Lesions with ASM had greater proximal and distal reference lumen areas and more distal but not proximal reference calcium. (3) At 2‐year follow‐up, the rate of early, late, or very late stent thrombosis was very low (0.65%) and was similar to that of patients without ASM (0.43%); and the largest areas of ASM were in patients without stent thrombosis.
Prevalence of ASM
In patients with stable angina, the prevalence of IVUS‐detected ASM was reported to be 11.5% after paclitaxel‐eluting stent implantation,16 17.9% to 25% after sirolimus‐eluting stent implantation,7, 17 and 12.5% after zotarolimus‐eluting stent implantation18; however, these data were accumulated primarily in stable patients. Guo et al1 reported that ASM occurred in 34.3% of paclitaxel‐eluting stent‐treated lesions and 40.3% of bare metal stent (BMS)–treated lesions in patients presenting with ST‐segment elevation MI. Similarly, van der Hoeven et al2 reported that in patients with ST‐segment elevation MI, ASM was found in 38.5% of sirolimus‐eluting stent‐treated patients and 33.8% of BMS‐treated patients. In the current study the prevalence of IVUS‐detected ASM was 14.4% (299 of 2072 patients), lower than reported by Hoffmann et al,17 but similar to the report by Kandzari et al.18 Of note, in the current study <20% of patients presented with an ST‐segment elevation MI.
IVUS may underreport the prevalence of ASM. Studies using optical coherence tomography (OCT) have reported a frequency of ASM that has ranged from 39.1% to 83.7%,19, 20, 21, 22 suggesting that it is ubiquitous after implantation if more sensitive methods are used to assess its frequency. Any finding seen in the majority of stents is of limited value in predicting adverse events.
In addition and as previously reported, IVUS‐detected ASM was typically corrected with additional balloon dilations10; and ADAPT‐DES did not contain a blinded IVUS arm in which the findings were hidden from the interventionalists. Therefore, the current prevalence of ASM may be less than in patients treated with angiographic guidance alone.
Predictors for ASM
Previous IVUS studies demonstrated that factors such as thrombus,1, 2 calcification,7 and larger reference diameters23 may be predictors of ASM. Severe diameter stenosis and subsequent differences between the luminal diameter of the most stenotic segment and that of the reference vessel also increase the frequency of ASM.19 Heavily calcified lesions are prone to ASM, despite high‐pressure balloon dilation or rotational atherectomy.22, 23 In our study, there was a higher prevalence of ASM in the right coronary artery. This may be because of the large size of the right coronary artery, the presence of more calcium, and (in the setting of previous MI) potential residual thrombi. Superficial calcium and the presence of a calcified nodule were also more common in ASM in the subset with pre‐intervention IVUS.
Outcomes Related to ASM
Some retrospective studies have related ASM to stent thrombosis, but most of these studies enrolled only patients with stent thrombosis and in small numbers.24, 25, 26, 27 Conversely, Guo et al1 reported that 40% of ASM resolved at 13‐month follow‐up because of negative remodeling; this was similar in paclitaxel‐eluting stents and BMS with no deaths or stent thromboses related to the presence of stent malapposition at 1‐year follow‐up in either group. Im et al19 reported that 31% of lesions with ASM remained malapposed at the time of 6‐month follow‐up OCT examination; the clinical outcomes of OCT‐detected ASM were favorable. Steinberg et al23 reported that at 9‐month follow‐up the MACE rates were similar for BMS with versus without ASM (3.8% versus 15.2%, log‐rank P=0.13) as well as for paclitaxel‐eluting stents with versus without ASM (11.6% versus 8.8%, log‐rank P=0.45). At 2‐year follow‐up combining BMS and paclitaxel‐eluting stents, there were no significant differences in MACE rates for patients with ASM (17.1%) versus no ASM. In the recent OCT studies of Prati et al21 (49.3% of ASM with stent to adjacent vessel distance >200 μm in 984 lesions of 832 patients) and Soeda et al22 (39.1% of ASM in 900 lesions of 786 patients), OCT‐detected ASM was not a predictor of 1‐year MACE.
The current study showed similar results. At 2‐year follow‐up, the rates of committee‐adjudicated stent thrombosis (early, late, very late, and overall) and of site‐reported, clinically driven TLR were not significantly increased in patients with residual ASM after IVUS‐guided DES implantation. Furthermore, there was no significant difference regarding lesions with the largest areas of ASM comparing those with versus without cardiovascular events; and the largest areas of ASM were in patients who did not have events, especially patients with stent thrombosis (Figure 2). Finally, cardiac death and MI did not differ between patients with versus without ASM.
Procedural Predictors of Stent Thrombosis
Previous reports showed that stent thrombosis may be associated with stent underexpansion,28, 29 smaller lumens, and more dissections,28, 30 but not with ASM28, 29 or tissue protrusion.28 In the current study, the final minimum stent area measured 6.3 (6.2–6.3) mm2 (least‐squares means, 95% CI) and was numerically larger in lesions with ASM with few stents meeting standard criteria for underexpansion. Thus, ASM was unrelated to events as long as stents were well expanded.
Limitations
First, this was a cross‐sectional analysis; IVUS was not performed at follow‐up; therefore, the current study cannot be used to determine the natural history of ASM other than its relationship to clinical events during a 2‐year follow‐up period. Second, the resolution of the IVUS catheter used was relatively low; the current study may have missed some ASM. Third, TLR was site reported and not adjudicated by an independent clinical events committee. Fourth, as was true of almost all studies of IVUS (or OCT)–detected ASM, operators were not blinded to the images, and large areas of ASM were typically treated using larger postdilation balloons.10 However, no significant difference was found regarding lesions with the largest areas of ASM comparing those with versus without cardiovascular events; in fact, stents with the largest areas of ASM were in patients who did not have events, especially patients with stent thrombosis (Figure 2). Fifth, the current analysis included only patients who were in the IVUS‐guided arm of ADAPT‐DES. It is highly likely that rates of ASM would be have been higher in the angiographically guided PCI arm. Indeed, much of the ASM observed in this study was small. Thus, the lack of an association between ASM and events may have been related to operator attempts to minimize ASM. Sixth, the present study included only patients who underwent PCI without major periprocedural complications (criteria for inclusion in the overall ADAPT‐DES study). Finally, the low event rate makes it difficult to compare patients with versus without ASM (type 2 error).
Conclusions
The present large‐scale IVUS substudy from ADAPT‐DES showed that IVUS‐detected ASM was not associated with adverse clinical events during long‐term follow‐up including, but not limited to, stent thrombosis.
Disclosures
Dr Wang has received research grant support from Boston Scientific. Dr Mintz has received research grant support from Volcano, Boston Scientific, and InfraReDx; honoraria from Boston Scientific and ACIST; and he is a consultant for Boston Scientific and ACIST. Dr Witzenbichler is a consultant for Volcano. Dr Metzger has received symposium honoraria from Abbott Vascular and Boston Scientific. Dr Rinaldi is on an advisory board for Abbott Vascular, Boston Scientific, and Edwards Lifesciences. Dr Duffy is a consultant/speaker for Philips Medical/Volcano Corporation. Dr Weisz is a member of an advisory board for AngioSlide, AstraZeneca, Corindus, Filterlex, M.I. Medical Incentive, Medtronic, Medivizor, TriSol, and Vectorious. Dr Stuckey is on an advisory board for Boston Scientific and has received speaker honoraria from Boston Scientific and Eli Lilly/Daiichi‐Sankyo. Dr Kirtane has received institutional research grants to Columbia University from Boston Scientific, Medtronic, Abbott Vascular, Abiomed, St. Jude Medical, Vascular Dynamics, and Eli Lilly. Dr Maehara has received grant support from Boston Scientific; is a consultant for Boston Scientific and ACIST; and has received speaker fees from St. Jude Medical. All other authors have no relationships to disclose.
(J Am Heart Assoc. 2016;5:e004438 doi: 10.1161/JAHA.116.004438)
References
- 1. Guo N, Maehara A, Mintz GS, He Y, Xu K, Wu X, Lansky AJ, Witzenbichler B, Guagliumi G, Brodie B, Kellett MA Jr, Dressler O, Parise H, Mehran R, Stone GW. Incidence, mechanisms, predictors, and clinical impact of acute and late stent malapposition after primary intervention in patients with acute myocardial infarction: an intravascular ultrasound substudy of the Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction (HORIZONS‐AMI) trial. Circulation. 2010;122:1077–1084. [DOI] [PubMed] [Google Scholar]
- 2. van der Hoeven BL, Liem SS, Dijkstra J, Bergheanu SC, Putter H, Antoni ML, Atsma DE, Bootsma M, Zeppenfeld K, Jukema JW, Schalij MJ. Stent malapposition after sirolimus‐eluting and bare‐metal stent implantation in patients with ST‐segment elevation myocardial infarction: acute and 9‐month intravascular ultrasound results of the MISSION! intervention study. JACC Cardiovasc Interv. 2008;1:192–201. [DOI] [PubMed] [Google Scholar]
- 3. Kim BK, Shin DH, Kim JS, Ko YG, Choi D, Jang Y, Hong MK. Randomized comparison of acute stent malapposition between platinum‐chromium versus cobalt‐chromium everolimus‐eluting stents. Int J Cardiovasc Imaging. 2015;31:269–277. [DOI] [PubMed] [Google Scholar]
- 4. Kim JS, Ha J, Kim BK, Shin DH, Ko YG, Choi D, Jang Y, Hong MK. The relationship between post‐stent strut apposition and follow‐up strut coverage assessed by a contour plot optical coherence tomography analysis. JACC Cardiovasc Interv. 2014;7:641–651. [DOI] [PubMed] [Google Scholar]
- 5. Foin N, Gutierrez‐Chico JL, Nakatani S, Torii R, Bourantas CV, Sen S, Nijjer S, Petraco R, Kousera C, Ghione M, Onuma Y, Garcia‐Garcia HM, Francis DP, Wong P, Di Mario C, Davies JE, Serruys PW. Incomplete stent apposition causes high shear flow disturbances and delay in neointimal coverage as a function of strut to wall detachment distance: implications for the management of incomplete stent apposition. Circ Cardiovasc Interv. 2014;7:180–189. [DOI] [PubMed] [Google Scholar]
- 6. Brown AJ, McCormick LM, Braganza DM, Bennett MR, Hoole SP, West NE. Expansion and malapposition characteristics after bioresorbable vascular scaffold implantation. Catheter Cardiovasc Interv. 2014;84:37–45. [DOI] [PubMed] [Google Scholar]
- 7. Kimura M, Mintz GS, Carlier S, Takebayashi H, Fujii K, Sano K, Yasuda T, Costa RA, Costa JR Jr, Quen J, Tanaka K, Lui J, Weisz G, Moussa I, Dangas G, Mehran R, Lansky AJ, Kreps EM, Collins M, Stone GW, Moses JW, Leon MB. Outcome after acute incomplete sirolimus‐eluting stent apposition as assessed by serial intravascular ultrasound. Am J Cardiol. 2006;98:436–442. [DOI] [PubMed] [Google Scholar]
- 8. Cook S, Wenaweser P, Togni M, Billinger M, Morger C, Seiler C, Vogel R, Hess O, Meier B, Windecker S. Incomplete stent apposition and very late stent thrombosis after drug‐eluting stent implantation. Circulation. 2007;115:2426–2434. [DOI] [PubMed] [Google Scholar]
- 9. Stone GW, Witzenbichler B, Weisz G, Rinaldi MJ, Neumann FJ, Metzger DC, Henry TD, Cox DA, Duffy PL, Mazzaferri E, Gurbel PA, Xu K, Parise H, Kirtane AJ, Brodie BR, Mehran R, Stuckey TD. Platelet reactivity and clinical outcomes after coronary artery implantation of drug‐eluting stents (ADAPT‐DES): a prospective multicentre registry study. Lancet. 2013;382:614–623. [DOI] [PubMed] [Google Scholar]
- 10. Witzenbichler B, Maehara A, Weisz G, Neumann FJ, Rinaldi MJ, Metzger DC, Henry TD, Cox DA, Duffy PL, Brodie BR, Stuckey TD, Mazzaferri EL Jr, Xu K, Parise H, Mehran R, Mintz GS, Stone GW. Relationship between intravascular ultrasound guidance and clinical outcomes after drug‐eluting stents: the assessment of dual antiplatelet therapy with drug‐eluting stents (ADAPT‐DES) study. Circulation. 2014;129:463–470. [DOI] [PubMed] [Google Scholar]
- 11. Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, Steg PG, Morel MA, Mauri L, Vranckx P, McFadden E, Lansky A, Hamon M, Krucoff MW, Serruys PW. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115:2344–2351. [DOI] [PubMed] [Google Scholar]
- 12. Genereux P, Redfors B, Witzenbichler B, Maehara A, Yadav M, Weisz G, Francese DP, Parvataneni R, Brener SJ, Mehran R, Kirtane AJ, Stone GW. Angiographic predictors of 2‐year stent thrombosis in patients receiving drug‐eluting stents: insights from the ADAPT‐DES study. Catheter Cardiovasc Interv. 2016, doi: 10.1002/ccd.26409. Available at: http://onlinelibrary.wiley.com/doi/10.1002/ccd.26409/full. Accessed December 17, 2016. [DOI] [PubMed] [Google Scholar]
- 13. The Thrombolysis in Myocardial Infarction (TIMI) trial. Phase I findings. TIMI Study Group. N Engl J Med. 1985;312:932–936. [DOI] [PubMed] [Google Scholar]
- 14. Wu X, Mintz GS, Xu K, Lansky AJ, Witzenbichler B, Guagliumi G, Brodie B, Kellett MA Jr, Dressler O, Parise H, Mehran R, Stone GW, Maehara A. The relationship between attenuated plaque identified by intravascular ultrasound and no‐reflow after stenting in acute myocardial infarction: the HORIZONS‐AMI (Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction) trial. JACC Cardiovasc Interv. 2011;4:495–502. [DOI] [PubMed] [Google Scholar]
- 15. Xu K, Mintz GS, Kubo T, Wu X, Guo N, Yang J, Witzenbichler B, Guagliumi G, Brodie B, Dressler O, Cristea E, Parise H, Mehran R, Stone GW, Maehara A. Long‐term follow‐up of attenuated plaques in patients with acute myocardial infarction: an intravascular ultrasound substudy of the HORIZONS‐AMI trial. Circ Cardiovasc Interv. 2012;5:185–192. [DOI] [PubMed] [Google Scholar]
- 16. Tanabe K, Serruys PW, Degertekin M, Grube E, Guagliumi G, Urbaszek W, Bonnier J, Lablanche JM, Siminiak T, Nordrehaug J, Figulla H, Drzewiecki J, Banning A, Hauptmann K, Dudek D, Bruining N, Hamers R, Hoye A, Ligthart JM, Disco C, Koglin J, Russell ME, Colombo A. Incomplete stent apposition after implantation of paclitaxel‐eluting stents or bare metal stents: insights from the randomized TAXUS II trial. Circulation. 2005;111:900–905. [DOI] [PubMed] [Google Scholar]
- 17. Hoffmann R, Guagliumi G, Musumeci G, Reimers B, Petronio AS, Disco C, Amoroso G, Moses JW, Fitzgerald PJ, Schofer J, Leon MB, Breithardt G. Vascular response to sirolimus‐eluting stents delivered with a nonaggressive implantation technique: comparison of intravascular ultrasound results from the multicenter, randomized E‐SIRIUS, and SIRIUS trials. Catheter Cardiovasc Interv. 2005;66:499–506. [DOI] [PubMed] [Google Scholar]
- 18. Kandzari DE, Leon MB, Popma JJ, Fitzgerald PJ, O'Shaughnessy C, Ball MW, Turco M, Applegate RJ, Gurbel PA, Midei MG, Badre SS, Mauri L, Thompson KP, LeNarz LA, Kuntz RE. Comparison of zotarolimus‐eluting and sirolimus‐eluting stents in patients with native coronary artery disease: a randomized controlled trial. J Am Coll Cardiol. 2006;48:2440–2447. [DOI] [PubMed] [Google Scholar]
- 19. Im E, Kim BK, Ko YG, Shin DH, Kim JS, Choi D, Jang Y, Hong MK. Incidences, predictors, and clinical outcomes of acute and late stent malapposition detected by optical coherence tomography after drug‐eluting stent implantation. Circ Cardiovasc Interv. 2014;7:88–96. [DOI] [PubMed] [Google Scholar]
- 20. Gutierrez‐Chico JL, Wykrzykowska J, Nuesch E, van Geuns RJ, Koch KT, Koolen JJ, di Mario C, Windecker S, van Es GA, Gobbens P, Juni P, Regar E, Serruys PW. Vascular tissue reaction to acute malapposition in human coronary arteries: sequential assessment with optical coherence tomography. Circ Cardiovasc Interv. 2012;5:20–29, S21–28. [DOI] [PubMed] [Google Scholar]
- 21. Prati F, Romagnoli E, Burzotta F, Limbruno U, Gatto L, La Manna A, Versaci F, Marco V, Di Vito L, Imola F, Paoletti G, Trani C, Tamburino C, Tavazzi L, Mintz GS. Clinical impact of OCT findings during PCI: the CLI‐OPCI II study. JACC Cardiovasc Imaging. 2015;8:1297–1305. [DOI] [PubMed] [Google Scholar]
- 22. Soeda T, Uemura S, Park SJ, Jang Y, Lee S, Cho JM, Kim SJ, Vergallo R, Minami Y, Ong DS, Gao L, Lee H, Zhang S, Yu B, Saito Y, Jang IK. Incidence and clinical significance of poststent optical coherence tomography findings: one‐year follow‐up study from a multicenter registry. Circulation. 2015;132:1020–1029. [DOI] [PubMed] [Google Scholar]
- 23. Steinberg DH, Mintz GS, Mandinov L, Yu A, Ellis SG, Grube E, Dawkins KD, Ormiston J, Turco MA, Stone GW, Weissman NJ. Long‐term impact of routinely detected early and late incomplete stent apposition: an integrated intravascular ultrasound analysis of the TAXUS IV, V, and VI and TAXUS ATLAS workhorse, long lesion, and direct stent studies. JACC Cardiovasc Interv. 2010;3:486–494. [DOI] [PubMed] [Google Scholar]
- 24. Tanigawa J, Barlis P, Di Mario C. Heavily calcified coronary lesions preclude strut apposition despite high pressure balloon dilatation and rotational atherectomy: in‐vivo demonstration with optical coherence tomography. Circ J. 2008;72:157–160. [DOI] [PubMed] [Google Scholar]
- 25. Alfonso F, Suarez A, Angiolillo DJ, Sabate M, Escaned J, Moreno R, Hernandez R, Banuelos C, Macaya C. Findings of intravascular ultrasound during acute stent thrombosis. Heart. 2004;90:1455–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Uren NG, Schwarzacher SP, Metz JA, Lee DP, Honda Y, Yeung AC, Fitzgerald PJ, Yock PG. Predictors and outcomes of stent thrombosis: an intravascular ultrasound registry. Eur Heart J. 2002;23:124–132. [DOI] [PubMed] [Google Scholar]
- 27. Inoue T, Shinke T, Otake H, Nakagawa M, Hariki H, Osue T, Iwasaki M, Taniguchi Y, Nishio R, Hiranuma N, Konishi A, Kinutani H, Kuroda M, Hirata K. Impact of strut‐vessel distance and underlying plaque type on the resolution of acute strut malapposition: serial optimal coherence tomography analysis after everolimus‐eluting stent implantation. Int J Cardiovasc Imaging. 2014;30:857–865. [DOI] [PubMed] [Google Scholar]
- 28. Fujii K, Carlier SG, Mintz GS, Yang YM, Moussa I, Weisz G, Dangas G, Mehran R, Lansky AJ, Kreps EM, Collins M, Stone GW, Moses JW, Leon MB. Stent underexpansion and residual reference segment stenosis are related to stent thrombosis after sirolimus‐eluting stent implantation: an intravascular ultrasound study. J Am Coll Cardiol. 2005;45:995–998. [DOI] [PubMed] [Google Scholar]
- 29. Prati F, Kodama T, Romagnoli E, Gatto L, Di Vito L, Ramazzotti V, Chisari A, Marco V, Cremonesi A, Parodi G, Albertucci M, Alfonso F. Suboptimal stent deployment is associated with subacute stent thrombosis: optical coherence tomography insights from a multicenter matched study. From the CLI Foundation investigators: the CLI‐THRO study. Am Heart J. 2015;169:249–256. [DOI] [PubMed] [Google Scholar]
- 30. Cheneau E, Satler LF, Escolar E, Suddath WO, Kent KM, Weissman NJ, Waksman R, Pichard AD. Underexpansion of sirolimus‐eluting stents: incidence and relationship to delivery pressure. Catheter Cardiovasc Interv. 2005;65:222–226. [DOI] [PubMed] [Google Scholar]


