Abstract
Background
Up to half of patients undergoing percutaneous coronary intervention have multivessel coronary artery disease (MVD) with conflicting data regarding optimal revascularization strategy in such patients. This paper assesses the evidence for complete revascularization (CR) versus incomplete revascularization in patients undergoing percutaneous coronary intervention, and its prognostic impact using meta‐analysis.
Methods and Results
A search of PubMed, EMBASE, MEDLINE, Current Contents Connect, Google Scholar, Cochrane library, Science Direct, and Web of Science was conducted to identify the association of CR in patients with multivessel coronary artery disease undergoing percutaneous coronary intervention with major adverse cardiac events and mortality. Random‐effects meta‐analysis was used to estimate the odds of adverse outcomes. Meta‐regression analysis was conducted to assess the relationship with continuous variables and outcomes. Thirty‐eight publications that included 156 240 patients were identified. Odds of death (OR 0.69, 95% CI 0.61‐0.78), repeat revascularization (OR 0.60, 95% CI 0.45‐0.80), myocardial infarction (OR 0.64, 95% CI 0.50‐0.81), and major adverse cardiac events (OR 0.63, 95% CI 0.50‐0.79) were significantly lower in the patients who underwent CR. These outcomes were unchanged on subgroup analysis regardless of the definition of CR. Similar findings were recorded when CR was studied in the chronic total occlusion (CTO) subgroup (OR 0.65, 95% CI 0.53‐0.80). A meta‐regression analysis revealed a negative relationship between the OR for mortality and the percentage of CR.
Conclusion
CR is associated with reduced risk of mortality and major adverse cardiac events, irrespective of whether an anatomical or a score‐based definition of incomplete revascularization is used, and this magnitude of risk relates to degree of CR. These results have important implications for the interventional management of patients with multivessel coronary artery disease.
Keywords: complete revascularization, incomplete revascularization, major adverse cardiovascular events, mortality, percutaneous coronary intervention
Subject Categories: Percutaneous Coronary Intervention
Introduction
Percutaneous coronary intervention (PCI) is the most common form of coronary revascularization in patients with stable coronary artery disease and acute coronary syndromes (ACS).1 Multivessel coronary artery disease is common and affects more than half of patients who have an ACS.2, 3 In these patients there is a lack of evidence on whether revascularization that is restricted to the culprit artery is sufficient or whether multivessel PCI would lead to an improved prognosis. Angiographically incomplete revascularization (IR) has been considered to be a poor prognostic feature in multiple observational studies and post hoc analyses of randomized controlled trials.4, 5, 6, 7, 8 The only prospective randomized controlled trial (RCT) outside the context of ST‐elevation myocardial infarction (STEMI) comparing the safety, efficacy, and costs of complete versus “culprit” vessel revascularization in multivessel coronary artery disease treated with PCI showed no difference in major adverse cardiovascular event (MACE) rates between the 2 strategies, with a lower cost associated with the culprit‐only strategy in the shorter term, although costs equalized in the longer term.9
Recent data from randomized trials including the PRAMI,10 CvLPRIT,11 and DANAMI‐3‐PRIMULTI trials,12 which recruited patients presenting with STEMI undergoing primary PCI, have shown that multivessel “complete” revascularization is associated with better outcomes than culprit‐only revascularization. However, despite these data, important uncertainties still exist about the optimal strategy for such patients. Furthermore, in patients with stable coronary artery disease, international PCI guidelines do not provide guidance about the performance of complete revascularization (CR) versus IR, although functional assessment of lesions using noninvasive tests or fractional flow reserve (FFR) is recommended to avoid unnecessary treatment of nonsignificant stenosis13, 14, 15 because this is associated with adverse outcomes.
In a previous meta‐analysis by Garcia et al16 including ~90 000 individuals with multivessel disease, incomplete revascularization in 25 938 CABG patients (29% from 16 studies) and 63 945 PCI patients (71% from 24 publications) was associated with increased risk of mortality, myocardial infarction, and repeat revascularization irrespective of the revascularization strategy employed. Since then many studies have been published including large registry data,13, 17 post hoc analyses of randomized trials,6, 18, 19 and observational studies7, 20, 21, 22, 23, 24 to assess effectiveness of complete coronary revascularization.
Our objectives were to assess and update the current evidence for complete revascularization and its prognostic impact in PCI by performing a meta‐analysis of 38 studies including over 150 000 patients (excluding the STEMI and surgical revascularization cohorts).
Methods
Eligibility Criteria
The Preferred Reporting Items for Systematic reviews and Meta‐Analyses (PRISMA) guidelines were followed.25 Studies were selected of patients who underwent PCI, reporting mortality or cardiovascular events among patients with and without complete revascularization with no restriction based on study design or the indication for PCI. Publications that did not report either mortality or MACE were excluded.
Search Strategy
A search was done of PubMed, EMBASE, MEDLINE, Current Contents Connect, Google Scholar, Cochrane library, Science Direct, and Web of Science to October 2016. We used the following search terms: “Complete revascularization” OR “Incomplete revascularization” AND “Percutaneous coronary intervention” OR “PCI.” These keywords were searched as text words as well as exploded medical subject headings when feasible. The search strategy example for MEDLINE is as follows: 1 Complete revascularization.mp. (913), 2 Incomplete revascularization.mp. (358), 3 1 or 2 (1125), 4 exp *Percutaneous Coronary Intervention/(31 192), 5 3 and 4 (292). We excluded the STEMI and surgical revascularization cohorts. The definitions of “complete revascularization” are given in Table 1. Studies in all languages were included. The bibliographies of the included studies and relevant review articles were checked for additional relevant articles.
Table 1.
Definitions of Complete Revascularization
| Anatomical or traditional | All diseased arterial systems with vessel size 1.5 (2.0‐2.25 mm for PCI) with at least 1 significant stenosis >50% receive a stent |
| Functional | All ischemic myocardial territories are grafted (or stented); areas of old infarction with no viable myocardium are not required to be reperfused |
| Numerical | Number of distal anastomoses number of diseased coronary segments/systems |
| Score‐based | Scoring of stenosis in different vessels. Different weight given to different vessels according to number of myocardial segments supplied. A residual score of 0 is usually considered equivalent to CR |
| Physiology‐based | All coronary lesions with FFR less than or equal to 0.75 to 0.80 receive a stent |
CR indicates complete revascularization; FFR, fractional flow reserve; PCI percutaneous coronary intervention.
Study Selection and Data Extraction
Two reviewers (V.N. and M.M.) independently checked all titles and abstracts for studies potentially meeting the inclusion criteria. The full reports of these studies were retrieved, and data were independently extracted on study design, participant characteristics, complete revascularization definition, outcome events, and follow‐up.
Quality Assessment
The Newcastle‐Ottawa Scale (NOS)26 was used as an assessment tool for selection, comparability, and outcome assessment. Study quality was rated on a scale from 1 (very poor) to 9 (high). Publication bias was assessed using an Egger regression model27 and the fail‐safe number method.28
Data Analysis
The program Comprehensive Meta‐analysis (version 2.0) was used to conduct DerSimonian and Laird random‐effects meta‐analysis.29 Risk ratio (RR) and 95% confidence intervals (CI) were calculated. Adjusted or propensity‐matched risk estimates were used when available. Meta‐regression analysis was conducted to assess the relationship with continuous variables and outcomes. The Cochrane Q‐statistic (I2) was used to assess the consistency among studies, with I2<25% considered low, I2>50% moderate, and I2>75% high heterogeneity.30
Results
Study Population
A total of 425 publications were screened; then, 38 relevant studies1 including 156 240 patients met our selection criteria (Figure 1). We excluded previous meta‐analysis16, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66 and trials comparing target lesion revascularization versus upfront revascularization STEMI.10, 11, 12, 67, 68, 69, 70 The studies included were mostly observational and included large registries13, 17, 50, 53 or post hoc analyses of randomized trials such as the SYNTAX trial,6 FAME trial,18 ARTS trial,31 ARTS‐II Study,44 MASS II trial,36 BARI trial, 33 CABRI trial,34 and the ACUITY trial.8 Only 1 randomized single‐center trial9 has been published so far that compares the outcomes of complete and incomplete percutaneous revascularization.
Figure 1.
Flow diagram of included studies. STEMI indicates ST‐elevation myocardial infarction.
The publication dates ranged from 1988 to 2016, and the follow‐up period for patients ranged between 1 and 11 years. The numbers of patients in each study were variable and ranged between 192 and 23 342 individuals. Most of these participants were male, and the percentage of females ranged from 7% to 37%; the mean age reported in the studies varied from 52 to 68 years. The percentage of ACS ranged from 0% to 100%.
Most of the studies used an anatomic definition for complete revascularization. Only 1 study used a functional definition (coronary lesions with fractional flow reserve ≤0.75 to 0.80 received a stent),43 and 7 others utilized a score‐based assessment (SYNTAX score; a residual score of 0 is considered to be complete revascularization) for complete revascularization.5, 6, 17, 19, 21, 22, 23 The percentage of complete revascularization ranged from 17% to 70% with a mean of 42.7%. The study characteristics have been tabulated in Table 2. The results of the studies that evaluated incomplete revascularization and adverse outcomes are shown in Table 3.
Table 2.
Publications Incorporated in the Systematic Review and Meta‐Analysis
| Name | Country | Year | Study Type | CR Definition | IR Definition | Number of Patients | ACS% | Follow Up Years | CR Prevalence | % Female | Mean Age, y | NOS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Appleby et al32 | Canada | 2010 | Observational study | Anatomic | Greater than 70% stenosis in epicardial vessel, assessed angiographically at the end of the procedure | 12 662 | 53 | 3.7 | 35 | 28 | 63 | 7 |
| Bourassa et al33 | USA and Canada | 1999 | Post hoc analysis of the BARI trial | Anatomic: Angiographically significant lesions were defined as ≥50% stenosis in a vessel ≥1.5 mm as measured by electronic calipers | NA | 896 | 63 | 5 | 64 | 23 | 62 | 6 |
| Breeman et al34 | Netherlands | 2001 | Post hoc analysis of the CABRI trial | Anatomic: If all lesions were successfully dilated—ie, if there were no remaining lesions with diameter stenosis <50% and incomplete otherwise | NA | 267 | 25 | 1 | 38 | 19 | 61 | 6 |
| Capodanno et al21 | Italy | 2013 | Observational study | Score‐based: The baseline SYNTAX score and residual SYNTAX score were derived from the summation of the individual scorings for each lesion (defined as ≥50% stenosis in vessel ≥1.5 mm) on angiograms | Residual SYNTAX score >1 | 400 | 62 | 2 | 48.75 | 23 | 67 | 6 |
| Chung et al35 | Korea | 2012 | Observational study | Anatomic: Absence of diameter stenosis ≥50% in major epicardial coronary arteries or their side branches with a diameter ≥2.5 mm after successful PCI during index admission irrespective of the function or viability of relevant myocardium | NA | 845 | 28 | 3.9 | 66.3 | 36.8 | 64 | 6 |
| D'Oliveira Vieira et al36 | Brazil | 2012 | Post hoc analysis of the MASS II trial | Anatomic | NA | 192 | 0 | 10 | 36 | 33 | 59 | 8 |
| Deligonul et al37 | USA | 1988 | Observational study | Anatomic: Successful dilation of all major coronary or branch vessels and absence of residual stenosis ≥50% in a major coronary vessel | NA | 397 | 49 | 2 | 59 | 24 | NA | 6 |
| Gao et al7 | China | 2013 | Observational study | Anatomic: Angiographic CR, which entailed successful angioplasty of all diseased lesions in the major epicardial coronary vessels and their first‐degree side branches (diameter ≥2.5 mm) | Patients not meeting the definition of CR were defined as having IR divided into 4 subgroups: (1) 1 IR vessel with no total occlusion; (2) 1 IR vessel with total occlusion; (3) ≥2 IR vessels with no total occlusion; and (4) 2 IR vessels with total occlusion | 7065 | 61.2 | 1.3 | 16.8 | 20.94 | 58 | 7 |
| Généreux et al6 | Multicenter | 2015 | Post hoc analysis of the SYNTAX trial | Score‐based: The baseline SYNTAX score and residual SYNTAX score were derived from the summation of the individual scorings for each lesion (defined as ≥50% stenosis in vessel ≥1.5 mm) on angiograms |
The SYNTAX Revascularization Index was calculated with the following formula: (Δ SS/baseline SYNTAX score×100). Classified into SRI=100%, SRI 50% to <100%, and SRI <50% |
903 | 28.5 | 5 | 43.5 | 23.7 | 65 | 8 |
| Hambraeus et al13 | Sweden | 2016 | Observational study | Anatomic | Defined as any nontreated significant (at least 60%) stenosis in a coronary artery supplying >10% of the myocardium | 23 342 | 80 | 1 | 35 | 27.2 | 68.1 | 7 |
| Hannan et al4 | USA | 2006 | Observational study | Anatomic: Defined as attempting all lesions with ≥50% stenosis in major epicardial coronary vessels (proximal, mid, and distal right coronary artery, left anterior descending, and left circumflex) either during the index hospitalization or any time within 30 days after discharge from the index hospitalization but before suffering a new myocardial infarction | Patients not meeting the definition of CR were defined to have IR | 21 945 | NA | 3 | 31 | 31 | NA | 6 |
| Hannan et al38 | USA | 2009 | Observational study | Anatomic: Defined as successfully attempting all diseased (≥70% stenosis) lesions in major epicardial coronary vessels (proximal, mid, and distal segments; major left anterior descending diagonals; and circumflex marginal branches) with PCI either during the index hospitalization or at any time within 30 days after discharge from the index hospitalization for PCI but before suffering a new MI. Success was defined as a reduction in stenosis of at least 20% and a residual stenosis of less than 50% | Patients not meeting the definition of CR were defined to have IR | 11 294 | 37 | 1.5 | 31 | 33 | NA | 6 |
| Ijsselmuiden et al9 | Netherlands | 2004 | RCT | Anatomic: Randomly assigned to undergo PCI of either the coronary artery thought to be responsible for ischemia (culprit vessel) or of all ≥50% stenosis (CR) | 219 | 37 | 5 | 50 | 26 | 62 | 9 | |
| Kobayashi et al18 | Multicenter | 2016 | Post hoc analysis of FAME trial | Score‐based: The baseline SYNTAX score and residual SYNTAX score were derived from the summation of the individual scorings for each lesion (defined as ≥50% stenosis in vessel ≥1.5 mm) on angiograms | Residual SYNTAX score of 0, >0 to 4, >4 to 8, and >8, and with SYNTAX revascularization index of 100%, 50% to <100%, and 0% to <50% | 427 | 31.9 | 2 | 14.5 | 25.5 | 64.7 | 8 |
| Kim et al39 | Korea | 2011 | Observational study | Anatomic: Angiographic CR‐1, according to the SYNTAX classification, was defined as angioplasty or grafting in all diseased coronary segments (≥1.5 mm), consisting of the right coronary artery (segments 1, 2, and 3) and its main branches, including the posterior descending artery (segment 4 or 15) and the posterolateral branch (segment 16); the left anterior descending artery (segments 5, 6, 7, and 8) and its major diagonal branches (segment 9 or 10); and the left circumflex artery (segments 11 and 13) and its major obtuse marginal branches (segment 12 or 14). Angiographic CR‐2 was defined as revascularization in all diseased segments ≥2.5 mm in diameter | Patients not meeting these criteria were considered IR patients | 1400 | 42 | 5 | 41 | 29 | 61 | 6 |
| Kip et al40 | USA | 1999 | Post hoc analysis of the BARI trial | Anatomic: Angiographically significant lesions were defined as >50% stenoses in a vessel >1.5 mm, as measured by electronic calipers. A reduction in stenosis of ≥20% with residual stenosis of <50% and TIMI grade 3 flow defined successful lesion dilation | 2047 | NA | 5 | 59 | NA | 61 | 6 | |
| Kloeter et al41 | Switzerland | 2001 | Observational study | Anatomic: No remaining main coronary artery stenosis of >50% | 250 | NA | 2.5 | 60 | 18 | 59 | 6 | |
| Malkin et al22 | United Kingdom | 2013 | Observational study | Score‐based: SYNTAX score and residual SYNTAX score were derived from the summation of the individual scorings for each lesion (defined as ≥50% stenosis in vessel ≥1.5 mm) on angiograms | Residual SYNTAX score of >0 | 353 | 53 | 3.4 | 48.7 | NA | 68 | 7 |
| Malkin et al23 | United Kingdom | 2013 | Observational study | Score‐based: SYNTAX score and residual SYNTAX score were derived from the summation of the individual scorings for each lesion (defined as ≥50% stenosis in vessel ≥1.5 mm) on angiograms | Residual SYNTAX score of >0 | 240 | 38 | 2.6 | 41 | 26 | 66.9 | 7 |
| Mariani et al71 | Italy | 2001 | Observational study | Anatomic: Defined as successful management of all significant stenoses in major epicardial vessels, whereas IR was defined as the residual presence of >50% stenosis in a major segment after the procedure | 208 | 100 | 1 | 24 | 17 | 63 | 6 | |
| Nikolsky et al42 | Israel | 2004 | Observational study | Anatomic | NA | 658 | 22 | 3 | 27 | 27 | 61 | 6 |
| Norwa‐Otto et al43 | Poland | 2010 | Observational study | Functional: CR was defined as successful PCI of all coronary artery lesions with significant narrowing not fulfilling the above criteria | Functionally driven IR was defined as dilation of all segments with >70% stenosis, with the exception of arteries supplying an area of previous transmural MI or a small amount of myocardium | 908 | 33 | 11 | 31 | 18 | 52 | 6 |
| Park et al17 | Korea | 2014 | Observational study | Score‐based: The baseline SYNTAX score and residual SYNTAX score were derived from the summation of the individual scorings for each lesion (defined as ≥50% stenosis in vessel ≥1.5 mm) on angiograms | Residual SYNTAX score of 0, >0 to <7, and >7 | 5088 | 64.5 | 1 | 42.7 | 32 | 62 | 7 |
| Rosner et al8 | USA | 2012 | Post‐hoc analysis of ACUITY trial | Anatomic | Was variably defined as any lesion with a final DS ranging from ≥30% to ≥70% (in 10% increments) with a reference vessel diameter (RVD) ≥2.0 mm by QCA was left untreated after PCI in any epicardial coronary artery | 2954 | 100 | 1 | 63 | 31 | 60 | 8 |
| Sarno et al44 | The Netherlands | 2010 | Post‐hoc analysis of the ARTS‐II Study) | Anatomic: Patients were considered to have CR if all lesions with >50% diameter stenosis had been successfully treated | Those patients in whom attempt was made to treat 1 significant lesion or whose treatment resulted in a final diameter stenosis >50% were considered to have IR | 567 | 45 | 5 | 61.2 | 23 | 62.5 | 6 |
| Sohn et al20 | Korea | 2014 | Observational study | Anatomic: CR was defined as the absence of ≥70% diameter stenosis in major epicardial coronary arteries or their branches with a diameter ≥2.0 mm after successful PCI | 263 | 29 | 3.3 | 57 | 25.8 | 67 | 6 | |
| Song et al45 | Korea | 2012 | Observational study | Anatomic: CR strategy was defined as attempting all lesions with >50% stenosis in major epicardial coronary vessels and their major branches during the index hospitalization | 873 | 48 | 1.5 | 48.9 | 30 | 64 | 6 | |
| Srinivas et al46 | USA | 2007 | Observational study | Anatomic: CR required that at least 1 lesion had to be treated in each of the major territories with diameter stenosis >50% | 1406 | 36.5 | 1 | 22 | 33 | 62 | 6 | |
| Tamburino et al47 | Italy | 2008 | Observational study | Anatomic: Revascularization was defined as complete, when all lesions with >50% diameter stenosis located in segment of at least 2.25 mm diameter, by quantitative coronary analysis, were successfully treated either during the index hospitalization or staged electively within 3 months after the initial procedure | 508 | 50 | 3 | 42 | 21 | 62 | 7 | |
| Valenti et al48 | Italy | 2008 | Observational study | Anatomic: CR was defined as a restoration of TIMI grade 3 flow with residual stenosis <30% on visual assessment in the 3 coronary arteries and their major branches (branch diameter ≥2 mm) | 486 | 37.5 | 2 | 62 | 17 | 68 | 6 | |
| Van den Brand et al31 | Multicenter | 2002 | Post‐hoc analysis of the ARTS Trial | Anatomic: if all lesions of ≥50% diameter stenosis had been successfully treated | If no attempt was made to treat 1 or more significant lesions, or if treatment resulted in a final diameter stenosis ≥50%, these patients were considered to be incompletely revascularized | 576 | 38 | 1 | 70 | 21 | 61.5 | 8 |
| Wu et al5 | USA | 2011 | Observational study | Anatomic: Revascularization was defined as reduction of stenosis to <50% in all diseased (≥70% stenosis) lesions in major epicardial coronary vessels (left anterior descending artery and major diagonals; left circumflex artery and large marginal branches; and right coronary artery and right posterior descending artery) in the index hospitalization or within 30 days after discharge from the index hospitalization before having a new MI. However, if the patient had an MI before the CR was completed, this was not regarded as CR because of the occurrence of an adverse event before CR was attained | When a CR was not achieved during a stenting procedure, it was defined as a procedure with IR. | 13 016 | NA | 8 | 30 | 31 | NA | 6 |
| Wu et al24 | USA | 2013 | Observational study | Anatomic: CR was defined when the postprocedural stenosis in each of the lesions was reduced to <50% in the index hospitalization or within 30 days in staged PCI procedures following discharge from the index hospitalization before the occurrence of a new MI | When CR was not achieved after the stenting procedure in the index admission or within 30 days of discharge, the revascularization was defined as IR | 21 767 | NA | 5 | 31.4 | 33.5 | NA | 7 |
| Yang et al49 | China | 2010 | Observational study | Anatomic: Clinical lesions were defined as >50% stenosis of a main coronary artery or >70% stenosis of its primary branches. The definition of CR was the treatment of all lesions in the main coronary artery and primary branches | Incomplete coronary revascularization (ICR) was defined as treatment of main culprit lesions but not other clinical lesions | 324 | 92 | 1.5 | 22 | 22 | 61 | 6 |
| George et al50 | UK | 2014 | Observational study | Successful PCI to the target CTO and postprocedural obstruction of <50% in all major epicardial coronary arteries | Successful PCI to the target CTO but with residual obstruction of >50% in ≥1 other vessels | 13 443 | NA | 2.65 | NA | 21 | 63.5 | 6 |
| Hannan et al51 | USA | 2016 | Observational study | Defined as a residual stenosis of <50% for all lesions with preprocedural stenoses of at least 70%. The reference category for the variable was successful CTO PCI and CR of all other lesions with preprocedural stenosis of at least 70%. Also, if a CTO or non‐CTO PCI was successful in a staged admission, that patient was regarded as having undergone a successful PCI | NA | 4030 | NA | 1.8 | 61 | 22.4 | 63.2 | 6 |
| Danzi et al52 | Italy | 2013 | Observational study | Defined as a TIMI flow grade 3 with residual stenosis of <30% on visual assessment in the 3 coronary arteries and their major branches (branch diameter of >2 mm) | NA | 120 | 33.3 | 2 | 63.3 | 7.5 | 68 | 6 |
| Chang et al53 | South Korea | 2016 | Prospective cohort study | Absence of diameter stenosis ≥50% in major epicardial coronary arteries or their side branches with a diameter ≥2.5 mm after successful stent implantation during index hospitalization irrespective of the function or viability of relevant myocardium | Not meeting the CR criteria | 3901 | 54.1 | 4.9 | 50 | 30 | 63 | 8 |
ACS indicates acute coronary syndrome; CR, complete revascularization; CTO, chronic total occlusion; IR, incomplete revascularization; NOS, Newcastle‐Ottawa Scale; PCI, percutaneous coronary intervention; SRI, SYNTAX revascularisation index; SYNTAX, Synergy between Percutaneous Coronary Intervention with Taxus and Cardiac Surgery; TIMI, thrombolysis in myocardial infarction.
Table 3.
Results of Studies That Evaluated Incomplete Revascularization and Adverse Outcomes
| Study | Results |
|---|---|
| Appleby et al32 | Better survival with CR (87±1% vs 78±1%, P<0.001). Residual disease significant independent predictor of the need for repeat procedures |
| Bourassa et al33 |
CR (n 579) (%) IR (n 317) (%) P Value Death 87.5 84.0 0.13 MI 83.8 84.1 0.91 Repeat revascularization 46.3 42.8 0.48 Angina 79.8 75.6 0.22 |
| Breeman et al34 |
At 1 month PTCA Remaining lesions 0 1 2 ≥3 Death (%) 2.1 0.7 0.8 2.5 MI (%) 4.9 2.8 3.3 5.0 (Repeat revascularization) CABG (%) 2.8 1.4 7.4 20.2 (Repeat revascularization) PTCA (%) 4.2 3.5 5.7 5.9 At 1 year PTCA Remaining lesions 0 1 2 ≥3 Death (%) 5.4 2.0 3.3 5.0 MI (%) 5.4 3.4 4.9 6.7 (Repeat revascularization) CABG (%) 7.4 9.5 18.0 37.0 (Repeat revascularization) PTCA (%) 25.0 22.5 23.8 19.3 |
| Capodanno et al21 | Cardiac mortality at 2 years: 3.3%, 4.5%, and 19.8% in the CR |
| Chung et al35 |
Propensity score‐matched (n=550) Adjusted HR [95% CI] Death 0.66 [0.34‐1.28] Death and MI 0.51 [0.28‐0.95] Death, MI, and repeat revascularization 0.84 [0.60‐1.19] Cardiac death 0.50 [0.18‐1.40] Cardiac death and MI 0.39 [0.16‐0.96] Any adverse cardiac events 0.93 [0.64‐1.35] |
| D'Oliveira Vieira et al36 | A statistically significant difference was observed for the PCI group (CR, 6 individuals died, IR 20 individuals died) |
| Deligonul et al37 |
Outcomes Events/CR Total Events/IR Total Repeat revascularization CABG/PTCA 24/118, 73/255 MI 3/118, 9/255 Death 6/118, 14/255 |
| Gao et al7 | At 36 months, cardiac death was significantly greater in the IR cohort (2.55% vs 1.13%, log‐rank test: P=0.016), but there was no difference in the 3‐year rates of MI, TVR, and MACE between the 2 cohorts. Angiographic IR had a greater risk of cardiac death (adjusted hazard ratio [HR] 2.56, 95% confidence interval [CI] 1.03‐6.41) |
| Généreux et al6 | At 60 months, rates of MACE were linked with IR |
| Hambraeus et al13 | Unadjusted HR (IR compared with CR): repeat revascularization 2.05 (95% CI 1.80‐2.32; P<0.0001); combined endpoint of death/MI, HR was 1.92 (95% CI 1.77‐2.09; P<0.0001) for IR compared with CR |
| Hannan et al4 | Adjusted HR for IR patients compared to CR patients for death was 1.15 (95% CI 1.01‐1.30). Repeat revascularization: 10.09% for CR patients and 11.46% for IR patients (P=0.16) |
| Hannan et al38 | (IR vs CR) 18‐month mortality (adjusted HR 1.23, 95% CI 1.04‐1.45) and 18‐month MI/mortality (adjusted HR 1.27, 95% CI 1.09‐1.47). The adjusted survival rates for CR and IR were 94.9% and 93.8% (P=0.01), and the freedom‐from‐MI rates were 93.3% and 91.7% (P=0.002) |
| Ijsselmuiden et al9 | (IR vs CR) MACE rates at 1 month (14.4% vs 9.3%), 1 year (32.4% vs 26.9%), and 4.6±1.2 years (40.4% vs 34.6%) were similar in both cohorts |
| Kobayashi et al18 | Patients with MACE had comparable RSS and SRI after PCI: RSS 6.0 [IQR 3.0‐10.0] vs 5.0 [IQR 2.0‐9.5], P=0.51; SRI 60.0% [IQR 40.9%‐78.9%] vs 58.8% [IQR 26.7%‐81.8%], P=0.24, respectively. Kaplan‐Meier analysis showed comparable 12‐month rate of MACE with different RSS/SRI (log‐rank P=0.55 and 0.54, respectively) |
| Kim et al39 | (CR vs IR) MACE HR 0.82 (95% CI 0.58‐1.15), MACCE HR 0.90 (95% CI 0.75‐1.09) |
| Kip et al40 |
Outcomes Events/CR Total Events/IR Total Repeat revascularization 328/59, 237/399 Death 55/595, 47/399 |
| Kloeter et al41 |
Outcomes Events/CR Total Events/IR Total Repeat revascularization 10/101 23/149 MI 1/101 1/149 Death 0/101 3/149 Complete revascularization had considerably higher clinical restenosis (35% vs 22%, P=0.02) |
| Malkin et al22 | Complete revascularization was significantly linked with survival (Adjusted OR 3.1, 95% CI 1.7‐5.6) |
| Malkin et al23 |
Outcomes Events/CR Total Events/IR Total Death 6/98 29/142 P<0.001 |
| Mariani et al71 |
Outcomes Events/CR Total Events/IR Total Repeat revascularization 1/49 7/159 MI 4/49 5/159 Death 0/49 2/159 In‐hospital MACE occurred in 10% and 7.5% of patients with CR and IR, respectively (P=NS). At 12 months, the reported MACE was 11.3% and 11.5% of patients with CR and IR, respectively |
| Nikolsky et al42 | Survival in CR was 94.5%, vs 83.0% for those with IR (P<0.001). MI‐free survival was considerably greater in patients with CR than in those with IR (92.9% vs 79.9%, respectively). IR was a prognosticator of mortality (95% CI 1.54‐7.69; P=0.003) |
| Norwa‐Otto et al43 | There was no difference in mortality, cardiovascular deaths, or MI between CR and IR cohorts. The IR had a higher rate of repeat revascularization |
| Park et al17 |
Outcomes Events/CR Total Events/IR Total MACCE 114/2173 297/2915 Death 28/2173 65/2915 Myocardial infarction 4/2173 19/2915 Unplanned revascularization 86/2173 225/2915 Definite/probable stent thrombosis 11/2173 21/2915 |
| Rosner et al8 | (IR vs CR) Unadjusted HR (95% CI) for IR vs CR: death 1.43 (0.90‐2.27); repeat revascularization 1.58 (1.28‐1.96); MI 1.50 (1.18‐1.89); MACE 1.47 (1.24 ‐1.74) |
| Sarno et al44 | MACCEs in the 87% of the CR cohort at 24 months and 75% at 60 months. Definite stent thrombosis occurred in 2.6% of the IR cohort and 3.9% of the CR cohort (P=0.45); definite or probable stent thrombosis occurred in 6.5% in the IR cohort vs 8.6% in the CR cohort (P=0.41) |
| Sohn et al20 | (CR vs IR) MACCE 34.7% vs 45.1%; adjusted hazard ratio [HR] 0.65, 95% CI 0.44‐0.95, P=0.03; all‐cause death adjusted HR 0.48, 95% CI 0.29‐0.80, P<0.01 |
| Song et al45 | (CR vs IR) MACE HR 0.64, 95% CI 0.46‐0.88, P=0.01; revascularization HR 0.61, 95% CI 0.42‐0.90, P=0.01; death HR 0.87, 95% CI 0.48‐1.57, P=0.64; MI HR 0.62, 95% CI 0.23‐1.67, P=0.35. The rate of periprocedural MI and stent thrombosis was comparable in the 2 cohorts (4.7% in the CR group vs 3.6% in the IR group, P=0.42; 1.6% vs 1.3%, P=0.72, respectively) |
| Srinivas et al46 | (CR vs IR) mortality HR 1.10 (95% CI 0.58‐2.10) and repeat revascularization HR 0.92 (96% CI 0.66‐1.29) |
| Tamburino et al47 | (CR vs IR) primary composite endpoint HR 0.43 (0.29‐0.63, P<0.0001), cardiac death HR 0.37 (0.15‐0.92, P=0.03), combination of cardiac death or MI HR 0.34 (0.16‐0.75, P=0.008), and repeat revascularization HR 0.45 (0.29‐0.69, P=0.0003) |
| Valenti et al48 | The survival rates were 91.6% and 87.4% in the CR and IR cohorts, respectively (P=0.025). CR was inversely proportional to mortality (HR 0.44, 95% CI 0.22‐0.87, P=0.021) |
| Van den Brand et al31 |
Outcomes Events/CR Total Events/IR Total Unplanned revascularization 34/406 61/170 Myocardial infarction 20/406 10/170 Death 7/406 6/170 |
| Wu et al5 | Death (HR 1.12, 95% CI 1.01‐1.26, P=0.04). Eight‐year survival was 78.5% and 80.8% for IR and CR (P=0.04). Mortality IR vs CR (adjusted HR 1.16, 95% CI 1.06‐1.26, P=0.001) |
| Wu et al24 | Among 6511 propensity‐matched individuals (IR compared to CR) (79.3% vs 81.4%, P=0.004), and death (HR 1.16, 95% CI 1.06‐1.27). Five‐year survival rate (IR 79.3% vs CR 81.4%, P=0.004) |
| Yang et al49 |
No differences in outcomes between the 2 cohorts at follow‐up. Outcomes Events/CR Total Events/IR Total Repeat revascularization 4/99 17/255 MI 1/99 4/255 Death 3/99 7/255 |
| George et al50 | (CR vs IR) Mortality (adjusted HR 0.70, 95% CI 0.56‐0.87, P=0.002) |
| Hannan et al51 | 2.5‐year Mortality CR vs CR for CTO, incomplete for ≥1 other lesions adjusted HR 1.11 (0.74, 1.68). 2.5‐year mortality CR vs IR for CTO adjusted HR 1.63 (1.28, 2.08), P<0.0001 |
| Danzi et al52 | Two‐year cardiac death‐free survival was better in the CR cohort compared to IR (96 vs 78 P=0.002) |
| Chang et al53 | IR with drug‐eluting stents in multivessel disease was associated with increased MI risk (HR 1.86, 95% CI 1.08‐3.19, P=0.024) and similar risk of death (HR 1.03, 95% CI 0.80‐1.32; P=0.83) compared to CR |
CABG indicates coronary artery bypass grafting; CR, complete revascularization; CTO, chronic total occlusion; IR, incomplete revascularization; MACE, major adverse cardiac events; MI, myocardial infarction; PCI, percutaneous coronary intervention; PTCA, percutaneous transluminal coronary angioplasty; RSS, residual SYNTAX score; SRI, SYNTAX revascularisation index.
Outcomes: Overall and Subgroup Analysis Based on CR Definition and Chronic Total Occlusion Revascularization
There was a significantly lower risk of death with complete revascularization (Figure 2, OR 0.69, 95% CI 0.61‐0.78) among 36 studies2 that reported this outcome. This lower risk of mortality was maintained after performing subgroup analysis based on the anatomic definition of complete revascularization3 (OR 0.69, 95% CI 0.61‐0.79), although this did not reach statistical significance for score‐based definitions6, 8, 18, 19, 24, 39, 44, 46 (OR 0.73, 95% CI 0.50‐1.07). Similar findings were recorded in the complete chronic total occlusion (CTO)‐revascularization cohort (5 studies,4, 38, 48, 50, 52 OR 0.65, 95% CI 0.53‐0.80) and in the non‐CTO cohort (OR 0.71, 95% CI 0.61‐0.82).4
Figure 2.
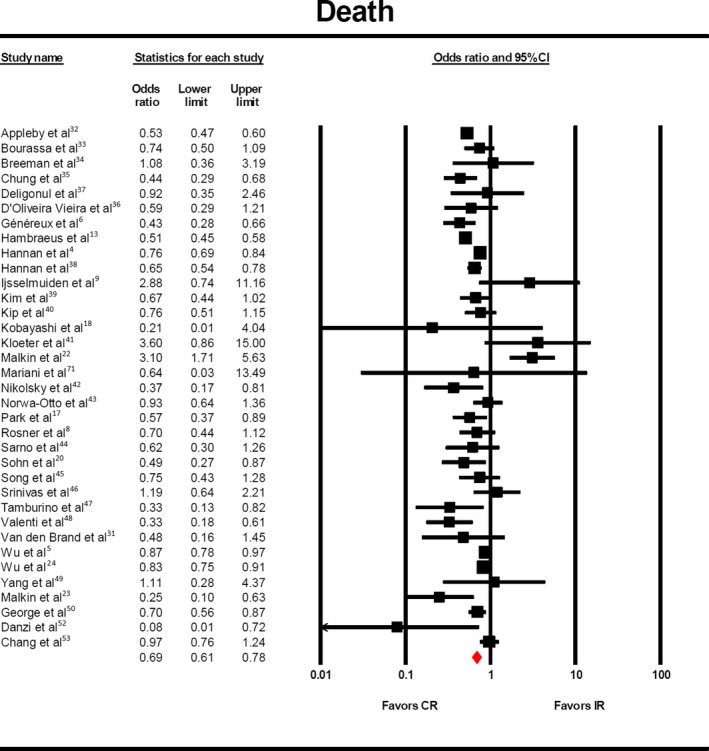
Risk of death with complete vs incomplete revascularization. The odds of death (OR:0.69, 95% CI: 0.61–0.78) was significantly lower in the patients who underwent complete revascularization. CR indicates complete revascularization; IR, incomplete revascularization.
The outcome of repeat revascularization was reported in 17 studies,5 and there was statistically significantly lower rate in complete revascularization populations (Figure 3, OR 0.60, 95% CI 0.45‐0.80). After subgroup analysis with respect to definition of complete revascularization, this benefit was maintained in studies that used anatomic6 (OR 0.58, 95% CI 0.41‐0.82) and score‐based definitions6, 8, 18, 19 (OR 0.64, 95% CI 0.54‐0.76).
Figure 3.
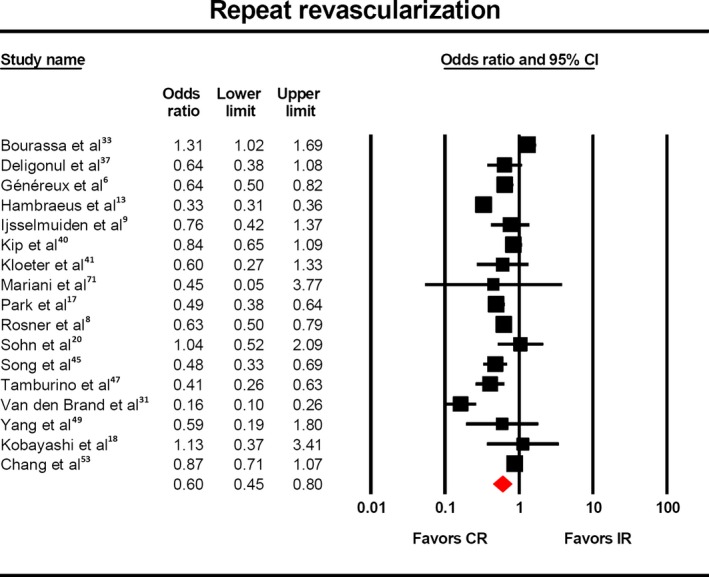
Risk of repeat revascularization with complete vs incomplete revascularization. The odds of repeat revascularization (OR: 0.60, 95%CI: 0.45–0.80) was significantly lower in the patients who underwent complete revascularization. CR indicates complete revascularization; IR, incomplete revascularization.
Myocardial infarction was reported in 17 studies,7 and a statistically significantly lower rate was observed (Figure 4, OR 0.63, 95% CI 0.50‐0.79). This finding was maintained with respect to anatomic8 (OR 0.60, 95% CI 0.45‐0.81) and score‐based definitions6, 8, 18, 19 (OR 0.64, 95% CI 0.51‐0.79) of complete revascularization.
Figure 4.
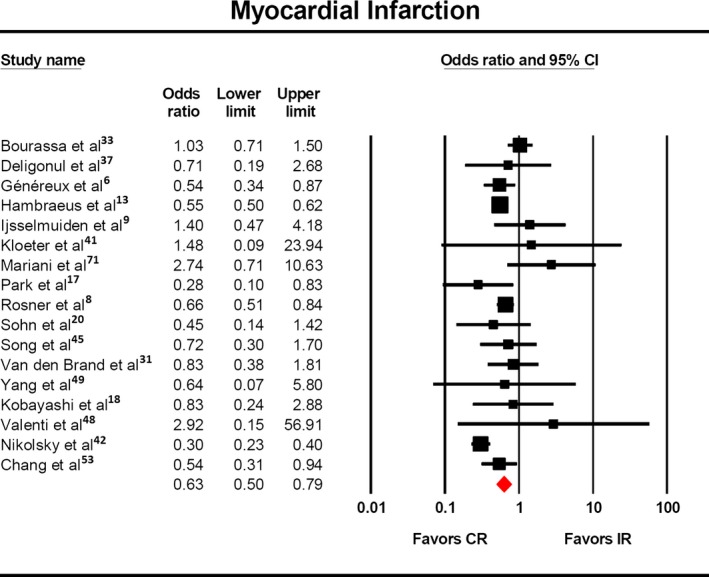
Risk of myocardial infarction with complete vs incomplete revascularization. The odds of myocardial infarction (OR: 0.64, 95% CI: 0.50–0.81) was significantly lower in the patients who underwent complete revascularization. CR indicates complete revascularization; IR, incomplete revascularization.
MACE was reported in 14 studies,9 and a significantly lower rate was observed (Figure 5, OR 0.66, 95% CI 0.51‐0.85). This finding was maintained with respect to anatomic7, 9, 13, 17, 20, 35, 47, 56 (OR 0.64, 95% CI 0.46‐0.89) and score‐based definitions6, 8, 18, 19, 39 (OR 0.68, 95% CI 0.50‐0.93) of complete revascularization.
Figure 5.
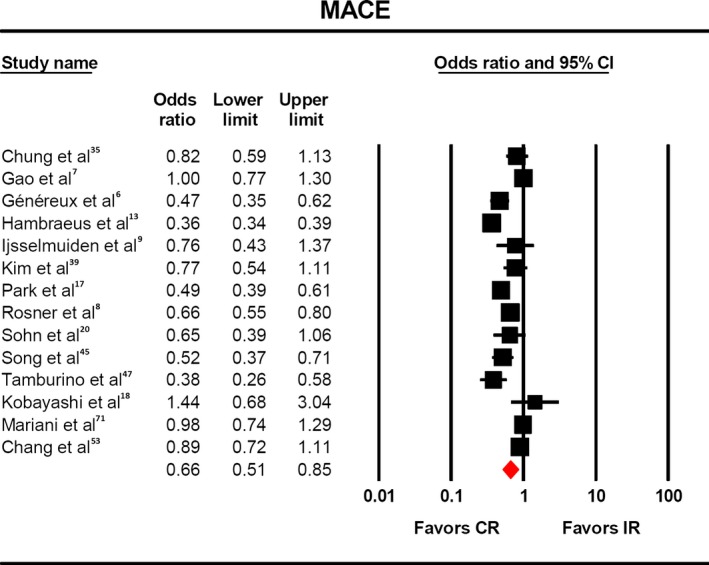
Risk of MACE with complete vs incomplete revascularization. The odds of MACE (OR:0.66, 95% CI: 0.51–0.85) were significantly lower in the patients who underwent complete revascularization. CR indicates complete revascularization; IR, incomplete revascularization, MACE, major adverse cardiac events.
Stent thrombosis was reported in only 3 studies,6, 17, 19, 44 and there was no impact of complete revascularization in its incidence (Figure 6, OR 0.81, 95% CI 0.49‐1.33).
Figure 6.
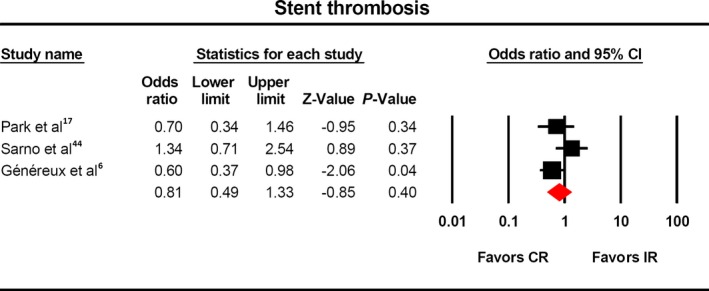
Risk of stent thrombosis with complete vs incomplete revascularization. There was no difference in the rate of stent thrombosis among the two cohorts. CR indicates complete revascularization; IR, incomplete revascularization.
In a subgroup analysis of 2 studies8, 71 that reported on outcomes in patients who exclusively had acute coronary syndromes (ACS), no significant benefit was observed (OR 0.71, 95% CI 0.44‐1.11) in mortality or MACE (OR 0.79, 95% CI 0.54‐1.17).
Regression Analysis Based on Proportion of CR
A regression analysis was conducted, and a negative relationship was observed between the mortality and the percentage of CR. From the regression model, there was very strong evidence that the OR of mortality was inversely related to CR with a P<0.001 (df=34). Log OR of mortality decreased by 1.25 (95% CI −1.64 to −0.88) for every 1% increase in CR. There was no relationship between the odds ratio of mortality and year of publication.
Heterogeneity and Publication Bias
There was significant heterogeneity noted among the different studies that could be explained by diverse population groups. The degree of heterogeneity reduced to a minimal amount once subgroup analysis was performed based on a score‐based definition of complete revascularization, suggesting similar study designs and population cohorts. The results have been summarized in Table 4. There was no publication bias identified using the Egger regression model.
Table 4.
Pooled OR and 95% CI for the Studies Included in the Meta‐Analysis
| Outcome | Subgroup Analysis | OR | 95% CI | I2 | P Value |
|---|---|---|---|---|---|
| Death | All | 0.69 | 0.61 to 0.78 | 77.03 | <0.001 |
| Anatomic | 0.69 | 0.61 to 0.79 | 80.60 | <0.001 | |
| Scored based | 0.73 | 0.50 to 1.07 | 60.81 | 0.03 | |
| CTO | 0.65 | 0.53 to 0.80 | 68.13 | <0.001 | |
| Non‐CTO | 0.71 | 0.61 to 0.82 | 78.6 | <0.001 | |
| ACS | 0.71 | 0.44 to 1.11 | 0 | 0.95 | |
| Repeat revascularization | All | 0.60 | 0.45 to 0.80 | 92.87 | <0.001 |
| Anatomic | 0.58 | 0.41 to 0.82 | 94.23 | <0.001 | |
| Scored based | 0.64 | 0.54 to 0.76 | 0 | 0.59 | |
| MI | All | 0.63 | 0.50 to 0.79 | 62.4 | <0.001 |
| Anatomic | 0.60 | 0.45 to 0.81 | 65.86 | 0.07 | |
| Scored based | 0.64 | 0.51 to 0.79 | 0.00 | 0.72 | |
| MACE | All | 0.66 | 0.51 to 0.85 | 93.29 | <0.001 |
| Anatomic | 0.64 | 0.46 to 0.89 | 94.5 | <0.001 | |
| Scored based | 0.68 | 0.50 to 0.93 | 70.87 | 0.02 | |
| ACS | 0.79 | 0.54 to 1.17 | 81.86 | 0.02 | |
| Stent thrombosis | All | 0.81 | 0.49 to 1.33 | 49.2 | 0.14 |
ACS indicates acute coronary syndrome; CI, confidence interval; CTO, chronic total occlusion; I2, Cochrane Q‐statistic; MACE, major adverse cardiovascular event; MI, myocardial infarction; OR, odds ratio.
Discussion
In our meta‐analysis of 38 studies including over 156 240 patients undergoing PCI, we observed that fewer than half of all patients with multivessel coronary artery disease have CR. We observed that CR is associated with a lower rate of mortality, myocardial infarction, and MACE, irrespective of whether an anatomical or a score‐based definition of IR was used, and that the magnitude of risk relates to degree of CR on meta‐regression. Our analysis builds on the work done by Garcia et al16 by placing a focus on PCI and including new studies.
There are several reasons why IR might not be achieved in PCI including patient clinical characteristics, lesion characteristics, failed PCI, and operator choice. Independent predictors of IR include advanced age, race, impaired LV function, previous MI, and comorbidities such as peripheral arterial disease, heart failure, diabetes, and renal failure.38 The most common lesion/anatomical characteristics for not achieving CR with PCI in SYNTAX were the presence of CTO (OR 2.46, 95% CI 1.81‐3.39; P<0.01), bifurcation disease (RR 1.44, 95% CI 1.09‐1.89; P<0.01), and diffuse disease or small vessels (<2 mm) (RR 1.53, 95% CI 1.12‐2.10, P<0.008).72
Previous studies have shown that patients with IR have a greater prevalence of adverse clinical characteristics, are older, and have more complex lesions than patients with CR.8, 35, 38, 39, 73 These adverse procedural characteristics might contribute to the associations reported. Most of the studies included in this analysis are derived from registry data; hence, the decision not to undertake CR by the operator may reflect uncaptured comorbid conditions/general frailty of the patient and so act as a surrogate of poor health status of the patients, which will contribute to the poorer outcomes reported. Although nearly all of the studies have adjusted for differences in baseline characteristics, the possibility of unmeasured confounding, particularly in studies derived from registry data, is significant. Furthermore, the increased risk associated with IR may relate to the complexity/extensiveness of coronary artery disease at baseline. For example, a post hoc analysis of the ARTS trial revealed that IR was associated with worse outcomes only in patients in the highest SYNTAX score tertile, whereas in the low and middle tertiles IR was not an independent predictor of adverse outcomes.44
Our analysis does not allow comparison of outcomes of patients undergoing IR in different settings such as elective versus the ACS because the majority of studies do not report outcomes by clinical presentation. The subgroup analysis of ACS of 2 studies8, 71 did not show any difference in mortality or MACE between the 2 cohorts. The studies were heterogeneous, and 1 of them71 was not large enough to detect the difference among the cohorts. Nevertheless, it has been demonstrated that complete revascularization in STEMI confers survival benefit.10, 11, 12 More recently, for example, the DANAMI‐3‐PRIMULTI trial reported a 44% reduction in the primary endpoint of all‐cause mortality, nonfatal myocardial infarction, and repeat revascularization (HR 0.56, 95% CI 0.38‐0.83; P=0.004). Following these trials, multiple meta‐analyses56, 63, 65, 74, 75, 76 have suggested a significant survival advantage in complete revascularization in patients with STEMI. Similarly, in the post hoc analysis of the ACUITY Trial,8 which included 2954 ACS patients, IR is associated with an increased risk of MACE. Unstable angina accounted for approximately one third of the patients in the SYNTAX6 and FAME trials.18, 77, 78 In the post hoc analysis18 of the FAME trial, CR was compared to IR in patients who underwent FFR‐guided PCI. There was no significant difference in survival between stable and unstable individuals at 24 months, indicating a consistent treatment effect with the FFR intervention. Further, a post hoc analysis of the SYNTAX trial6 performed subgroup analysis based on SYNTAX Revascularization Index <70% versus >70%. The OR for patients with unstable angina was 3.25 (95% CI 3.37‐11.25), clearly indicating a survival advantage for patients with CR.
Our meta‐regression analysis suggests that outcomes relate to the degree of CR, in agreement with several previous studies. Indeed, post hoc analysis of the SYNTAX trial suggests higher degrees of IR, as measured by the SYNTAX revascularization index, were associated with increased 5‐year cardiac death, AMI, and major adverse cardiac or cerebrovascular events (MACCE).6 Similarly, Park et al17 showed in the EXCELLENT registry that patient‐orientated composite endpoint rates (POCE) increased with increasing residual syntax score tertiles. Finally, CTO revascularization has been a matter of debate in recent years.50, 79, 80, 81 Contemporary evidence from a large UK registry of 13 443 individuals with CTO50 suggests that complete revascularization had a survival advantage over partial revascularization with a hazard ratio of 0.70 (95% CI 0.56‐0.87). Our study confirms survival benefit regarding complete revascularization of CTO with an OR of 0.69 (95% CI 0.61‐0.78). Similarly, a meta‐analysis of 7288 patients81 suggested that successful CTO recanalization had a survival advantage and reduced surgical revascularization.
Limitations
There are several limitations associated with our analysis. First, although we report an association between IR and adverse clinical outcomes, we cannot infer a causal relationship. We have shown an association between IR and adverse outcomes, but it cannot be assumed that treating such patients with IR with additional PCI to attain CR would improve their prognosis. Second, for anatomical based definitions of IR, there are no universally accepted definitions of lesion “significance” with studies defining significant lesions as those with diameter stenosis (DS) varying between ≥50% and ≥70% in vessels of diameter ≥1.5 mm in some studies to ≥2.5 mm in other studies. Many of the studies included in this analysis used visual assessment to define lesion severity, which is known to have greater interobserver variability and to overestimate percentage DS compared with quantitative coronary angiograph (QCA).82 Interestingly, a post hoc analysis of the ACUITY trial using QCA illustrated that even when DS≥30% was used to define a significant lesion, IR was independently associated with an increased risk of MACE (HR 1.36, 95% CI 1.11‐1.68), although the risk increased with increasing DS thresholds (for DS threshold of ≥70%, HR 1.59, 95% CI 1.30‐1.93). Score‐based definitions of IR, such as the residual SYNTAX score, overcome some of the limitations around differences in anatomical definitions of lesion significance used across studies, allowing comparisons to be made more easily. In the current analysis we report a similar prognostic impact of IR irrespective of whether this is defined by anatomical or score‐based definitions.
Third, contemporary studies have shown that the functional significance of lesions on the basis of fractional flow reserve is a more important determinant of future cardiac events than anatomical/angiographic appearances.18, 83, 84, 85 Operators may choose not to revascularize lesions due to their functional nonsignificance or location within vessels supplying infarcted and nonviable myocardium. A recent post hoc analysis of the FAME study18 demonstrated that IR (as defined by residual SYNTAX score and SYNTAX revascularization index) was not associated with adverse outcome in the setting of complete functional revascularization, supporting the hypothesis that functional CR is more important than anatomical CR. The remaining studies that report outcomes following IR included in this analysis (with the exception of the aforementioned study18) do not differentiate between the anatomical and the functional significance of incompletely revascularized lesions. The differences in the prognostic impact of incomplete revascularization across the different studies analyzed in this meta‐analysis may relate to the above limitations, mainly variability in the definition of what is considered to be a significant coronary lesion, the site of the lesion, whether the lesions that were not revascularized were in infarcted nonviable territories or were functionally significant, the sample size of the cohort studied, and whether this would be adequately powered to detect a statistically significant difference and the nature of the cohort studied. Finally, most of the studies included in this analysis are derived from registry data; hence, the decision not to undertake CR by the operator may reflect uncaptured comorbidity or general frailty of the patient and so act as a surrogate of poor health status of the patients that will contribute to the poorer outcomes reported. Although nearly all of the studies have adjusted for differences in baseline characteristics, there remains a possibility of unmeasured confounding.
In conclusion, our analysis of data derived from over 150 000 patients undergoing PCI suggests that fewer than half of all patients with multivessel coronary artery disease have CR following PCI. We observe that CR is associated with decreased incidence of mortality, myocardial infarction, and MACE, irrespective of whether an anatomical or a score‐based definition of IR was used and that the magnitude of risk relates to degree of CR. The findings of our analysis have several practical implications for interventional cardiologists. Our reported associations between IR and adverse clinical outcomes suggest that in patients with MVD, consideration should be given to the degree of CR that can be achieved by PCI when discussing choice of revascularization modality within the heart team, in addition to consideration of lesion complexity, functional significance, patient characteristics, and syntax score in line with current international recommendations.86 At the very least these data speak of the need for further carefully conducted randomized trials to address this question.
Author Contributions
Mamas conceived and planned the study. Nagaraja and Ooi performed the search, screened relevant studies, extracted data from the studies, and performed the analysis. Mamas wrote the first draft of the paper. All authors contributed to the interpretation of the findings and reporting of the work and edited the manuscript for significant intellectual content.
Sources of Funding
This work was funded by the North Staffs Heart Committee.
Disclosures
None.
Acknowledgments
The authors acknowledge the North Staffs Heart Committee for providing support for this study.
(J Am Heart Assoc. 2016;5:e004598 doi: 10.1161/JAHA.116.004598)
Notes
References 4, 5, 6, 7, 8, 9, 13, 17, 18, 20, 21, 22, 23, 24, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 71.
References 4, 5, 6, 7, 8, 9, 13, 17, 18, 20, 21, 22, 23, 24, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 52, 53.
References 4, 7, 9, 13, 17, 20, 21, 22, 23, 24, 31, 32, 33, 34, 35, 36, 37, 38, 40, 41, 42, 43, 45, 47, 48, 49, 50, 52, 53.
References
- 1. Iqbal J, Serruys PW, Taggart DP. Optimal revascularization for complex coronary artery disease. Nat Rev Cardiol. 2013;10:635–647. [DOI] [PubMed] [Google Scholar]
- 2. Fröbert O, Lagerqvist B, Olivecrona GK, Omerovic E, Gudnason T, Maeng M, Aasa M, Angerås O, Calais F, Danielewicz M, Erlinge D, Hellsten L, Jensen U, Johansson AC, Kåregren A, Nilsson J, Robertson L, Sandhall L, Sjögren I, Ostlund O, Harnek J, James SK. Thrombus aspiration during ST‐segment elevation myocardial infarction. N Engl J Med. 2013;369:1587–1597. [DOI] [PubMed] [Google Scholar]
- 3. Wallentin L, Lagerqvist B, Husted S, Kontny F, Stahle E, Swahn E. Outcome at 1 year after an invasive compared with a non‐invasive strategy in unstable coronary‐artery disease: the FRISC II invasive randomised trial. FRISC II Investigators. Fast Revascularisation during Instability in Coronary artery disease. Lancet. 2000;356:9–16. [DOI] [PubMed] [Google Scholar]
- 4. Hannan EL, Racz M, Holmes DR, King SB III, Walford G, Ambrose JA, Sharma S, Katz S, Clark LT, Jones RH. Impact of completeness of percutaneous coronary intervention revascularization on long‐term outcomes in the stent era. Circulation. 2006;113:2406–2412. [DOI] [PubMed] [Google Scholar]
- 5. Wu C, Dyer AM, King SB III, Walford G, Homes DR Jr, Stamato NJ, Venditti FJ, Sharma SK, Fergus I, Jacobs AK, Hannan EL. Impact of incomplete revascularization on long‐term mortality after coronary stenting. Circ Cardiovasc Interv. 2011;4:413–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Généreux P, Campos CM, Farooq V, Bourantas CV, Mohr FW, Colombo A, Morel MA, Feldman TE, Holmes DR Jr, Mack MJ, Morice MC, Kappetein AP, Palmerini T, Stone GW, Serruys PW. Validation of the SYNTAX revascularization index to quantify reasonable level of incomplete revascularization after percutaneous coronary intervention. Am J Cardiol. 2015;116:174–186. [DOI] [PubMed] [Google Scholar]
- 7. Gao Z, Xu B, Yang YJ, Yuan JQ, Chen J, Chen JL, Qiao SB, Wu YJ, Yan HB, Gao RL. Long‐term outcomes of complete versus incomplete revascularization after drug‐eluting stent implantation in patients with multivessel coronary disease. Catheter Cardiovasc Interv. 2013;82:343–349. [DOI] [PubMed] [Google Scholar]
- 8. Rosner GF, Kirtane AJ, Genereux P, Lansky AJ, Cristea E, Gersh BJ, Weisz G, Parise H, Fahy M, Mehran R, Stone GW. Impact of the presence and extent of incomplete angiographic revascularization after percutaneous coronary intervention in acute coronary syndromes: the Acute Catheterization and Urgent Intervention Triage Strategy (ACUITY) trial. Circulation. 2012;125:2613–2620. [DOI] [PubMed] [Google Scholar]
- 9. Ijsselmuiden AJ, Ezechiels J, Westendorp IC, Tijssen JG, Kiemeneij F, Slagboom T, van der Wieken R, Tangelder G, Serruys PW, Laarman G. Complete versus culprit vessel percutaneous coronary intervention in multivessel disease: a randomized comparison. Am Heart J. 2004;148:467–474. [DOI] [PubMed] [Google Scholar]
- 10. Wald DS, Morris JK, Wald NJ, Chase AJ, Edwards RJ, Hughes LO, Berry C, Oldroyd KG. Randomized trial of preventive angioplasty in myocardial infarction. N Engl J Med. 2013;369:1115–1123. [DOI] [PubMed] [Google Scholar]
- 11. Gershlick AH, Khan JN, Kelly DJ, Greenwood JP, Sasikaran T, Curzen N, Blackman DJ, Dalby M, Fairbrother KL, Banya W, Wang D, Flather M, Hetherington SL, Kelion AD, Talwar S, Gunning M, Hall R, Swanton H, McCann GP. Randomized trial of complete versus lesion‐only revascularization in patients undergoing primary percutaneous coronary intervention for STEMI and multivessel disease: the CvLPRIT trial. J Am Coll Cardiol. 2015;65:963–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Engstrom T, Kelbaek H, Helqvist S, Hofsten DE, Klovgarrd L, Holmvang L, Jorgensen E, Pedersen F, Saunamaki K, Clemmensen P, de Backer O, Ravkilde J, Tilsted HH, Villadsen AB, Aaroe J, Jensen SE, Raungaard B, Kober L. Complete revascularisation versus treatment of the culprit lesion only in patients with ST‐segment elevation myocardial infarction and multivessel disease (DANAMI‐3‐PRIMULTI): an open‐label, randomised controlled trial. Lancet. 2015;386:665–671. [DOI] [PubMed] [Google Scholar]
- 13. Hambraeus K, Jensevik K, Lagerqvist B, Lindahl B, Carlsson R, Farzaneh‐Far R, Kellerth T, Omerovic E, Stone G, Varenhorst C, James S. Long‐term outcome of incomplete revascularization after percutaneous coronary intervention in SCAAR (Swedish Coronary Angiography and Angioplasty Registry). JACC Cardiovasc Interv. 2016;9:207–215. [DOI] [PubMed] [Google Scholar]
- 14. Task Force Members , Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Budaj A, Bugiardini R, Crea F, Cuisset T, Di Mario C, Ferreira JR, Gersh BJ, Gitt AK, Hulot JS, Marx N, Opie LH, Pfisterer M, Prescott E, Ruschitzka F, Sabaté M, Senior R, Taggart DP, van der Wall EE, Vrints CJ; ESC Committee for Practice Guidelines , Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S; Document Reviewers , Knuuti J, Valgimigli M, Bueno H, Claeys MJ, Donner‐Banzhoff N, Erol C, Frank H, Funck‐Brentano C, Gaemperli O, Gonzalez‐Juanatey JR, Hamilos M, Hasdai D, Husted S, James SK, Kervinen K, Kolh P, Kristensen SD, Lancellotti P, Maggioni AP, Piepoli MF, Pries AR, Romeo F, Rydén L, Simoons ML, Sirnes PA, Steg PG, Timmis A, Wijns W, Windecker S, Yildirir A, Zamorano JL. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34:2949–3003. [DOI] [PubMed] [Google Scholar]
- 15. Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, Chambers CE, Ellis SG, Guyton RA, Hollenberg SM, Khot UN, Lange RA, Mauri L, Mehran R, Moussa ID, Mukherjee D, Nallamothu BK, Ting HH. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol. 2011;58:e44–e122. [DOI] [PubMed] [Google Scholar]
- 16. Garcia S, Sandoval Y, Roukoz H, Adabaq S, Canoniero M, Yannopoulos D, Brilaskis ES. Outcomes after complete versus incomplete revascularization of patients with multivessel coronary artery disease: a meta‐analysis of 89,883 patients enrolled in randomized clinical trials and observational studies. J Am Coll Cardiol. 2013;62:1421–1431. [DOI] [PubMed] [Google Scholar]
- 17. Park KW, Kang J, Kang SH, Ahn HS, Kang HJ, Koo BK, Chae IH, Youn TJ, Oh BH, Park YB, Kandzari D, Kim HS. The impact of residual coronary lesions on clinical outcomes after percutaneous coronary intervention: residual SYNTAX score after percutaneous coronary intervention in patients from the Efficacy of Xience/Promus versus Cypher in rEducing Late Loss after stENTing (EXCELLENT) registry. Am Heart J. 2014;167:384–392.e5. [DOI] [PubMed] [Google Scholar]
- 18. Kobayashi Y, Nam CW, Tonino PA, Kimura T, De Bruyne B, Lijls NH, Fearon WF. The prognostic value of residual coronary stenoses after functionally complete revascularization. J Am Coll Cardiol. 2016;67:1701–1711. [DOI] [PubMed] [Google Scholar]
- 19. Genereux P, Campos CM, Yadav M, Palmerini T, Caixeta A, Xu K, Francese DP, Dangas GD, Mehran R, Leon MB, Serruys PW, Stone GW. Reasonable incomplete revascularisation after percutaneous coronary intervention: the SYNTAX Revascularisation Index. EuroIntervention. 2015;11:634–642. [DOI] [PubMed] [Google Scholar]
- 20. Sohn GH, Yang JH, Choi SH, Song YB, Hahn JY, Choi JH, Gwon HC, Lee SH. Long‐term outcomes of complete versus incomplete revascularization for patients with multivessel coronary artery disease and left ventricular systolic dysfunction in drug‐eluting stent era. J Korean Med Sci. 2014;29:1501–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Capodanno D, Chisari A, Giacoppo D, Bonura S, Lavanco V, Capranzano P, Caqqeqi A, Ministeri M, Tamburino C. Objectifying the impact of incomplete revascularization by repeat angiographic risk assessment with the residual SYNTAX score after left main coronary artery percutaneous coronary intervention. Catheter Cardiovasc Interv. 2013;82:333–340. [DOI] [PubMed] [Google Scholar]
- 22. Malkin CJ, Ghobrial MS, Raina T, Siotia A, Morton AC, Gunn J. Impact of incomplete revascularization in patients undergoing PCI for unprotected left main stem stenosis. Catheter Cardiovasc Interv. 2013;81:939–946. [DOI] [PubMed] [Google Scholar]
- 23. Malkin CJ, George V, Ghobrial MS, Krishnan A, Siotia A, Raina T, Morton AC, Gunn J. Residual SYNTAX score after PCI for triple vessel coronary artery disease: quantifying the adverse effect of incomplete revascularisation. EuroIntervention. 2013;8:1286–1295. [DOI] [PubMed] [Google Scholar]
- 24. Wu C, Dyer AM, Walford G, Holmes DR Jr, King SB III, Stamato NJ, Sharma S, Jacobs AK, Venditti FJ, Hannan EL. Incomplete revascularization is associated with greater risk of long‐term mortality after stenting in the era of first generation drug‐eluting stents. Am J Cardiol. 2013;112:775–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–1012. [DOI] [PubMed] [Google Scholar]
- 26. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed December 2, 2016.
- 27. Egger M, Smith GD, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Orwin R. A fail‐safe N for effect size in meta‐analysis. J Educ Stat. 1983;8:157–159. [Google Scholar]
- 29. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials. 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 30. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ. 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. van den Brand MJ, Rensing BJ, Morel MA, Foley DP, de Valk V, Breeman A, Suryapranata H, Haalebos MM, Wijns W, Wellens F, Balcon R, Magee P, Ribeiro E, Buffolo E, Unger F, Serruys PW. The effect of completeness of revascularization on event‐free survival at one year in the ARTS trial. J Am Coll Cardiol. 2002;39:559–564. [DOI] [PubMed] [Google Scholar]
- 32. Appleby CE, Mackie K, Dzavik V, Ivanov J. Late outcomes following percutaneous coronary interventions: results from a large, observational registry. Can J Cardiol. 2010;26:e218–e224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bourassa MG, Kip KE, Jacobs AK, Jones RH, Sopko G, Rosen AD, Sharaf BL, Schwartz L, Chaitman BR, Alderman EL, Holmes DR, Roubin GS, Detre KM, Frye RL. Is a strategy of intended incomplete percutaneous transluminal coronary angioplasty revascularization acceptable in nondiabetic patients who are candidates for coronary artery bypass graft surgery? The Bypass Angioplasty Revascularization Investigation (BARI). J Am Coll Cardiol. 1999;33:1627–1636. [DOI] [PubMed] [Google Scholar]
- 34. Breeman A, Boersma E, van den Brand MJ, van Herwerden L, Serruys PW. Completeness of revascularisation by percutaneous coronary intervention. Neth Heart J. 2001;9:3–9. [PMC free article] [PubMed] [Google Scholar]
- 35. Chung JW, Park KH, Lee MH, Park KW, Park JS, Kang HJ, Koo BK, Kwon YW, Kim HS. Benefit of complete revascularization in patients with multivessel coronary disease in the drug‐eluting stent era. Circ J. 2012;76:1624–1630. [DOI] [PubMed] [Google Scholar]
- 36. Vieira RD, Hueb W, Gersh BJ, Lima EG, Pereira AC, Rezende PC, Garzillo CL, Hueb AC, Favarato D, Soares PR, Ramires JA, Kalil Filho R. Effect of complete revascularization on 10‐year survival of patients with stable multivessel coronary artery disease: MASS II trial. Circulation. 2012;126:S158–S163. [DOI] [PubMed] [Google Scholar]
- 37. Deligonul U, Vandormael MG, Kern MJ, Zelman R, Galan K, Chaitman BR. Coronary angioplasty: a therapeutic option for symptomatic patients with two and three vessel coronary disease. J Am Coll Cardiol. 1988;11:1173–1179. [DOI] [PubMed] [Google Scholar]
- 38. Hannan EL, Wu C, Walford G, Holmes DR, Jones RH, Sharma S, King SB III. Incomplete revascularization in the era of drug‐eluting stents: impact on adverse outcomes. JACC Cardiovasc Interv. 2009;2:17–25. [DOI] [PubMed] [Google Scholar]
- 39. Kim YH, Park DW, Lee JY, Kim WJ, Yun SC, Ahn JM, Song HG, Oh JH, Park JS, Kang SJ, Lee SW, Lee CW, Park SW, Park SJ. Impact of angiographic complete revascularization after drug‐eluting stent implantation or coronary artery bypass graft surgery for multivessel coronary artery disease. Circulation. 2011;123:2373–2381. [DOI] [PubMed] [Google Scholar]
- 40. Kip KE, Bourassa MG, Jacobs AK, Schwartz L, Feit F, Alderman EL, Weiner BH, Weiss MB, Kellett MA Jr, Sharaf BL, Dimas AP, Jones RH, Sopko G, Detre KM. Influence of pre‐PTCA strategy and initial PTCA result in patients with multivessel disease: the Bypass Angioplasty Revascularization Investigation (BARI). Circulation. 1999;100:910–917. [DOI] [PubMed] [Google Scholar]
- 41. Kloeter UC, Jander NG, Buser PT, Osswald S, Mueller‐Brand J, Pfisterer ME. Long‐term outcome of angioplasty for multivessel coronary disease: importance and price of complete revascularization. Int J Cardiol. 2001;79:197–205. [DOI] [PubMed] [Google Scholar]
- 42. Nikolsky E, Gruberg L, Patil CV, Roguin A, Kepeliovich M, Petcherski S, Boulos M, Grenadier E, Amikam S, Linn S, Markiewicz W, Beyar R. Percutaneous coronary interventions in diabetic patients: is complete revascularization important? J Invasive Cardiol. 2004;16:102–106. [PubMed] [Google Scholar]
- 43. Norwa‐Otto B, Kadziela J, Malek LA, Debski A, Witkowski A, Demkow M, Ruzyllo W. Functionally driven complete vs incomplete revascularisation in multivessel coronary artery disease—long‐term results from a large cohort. Kardiol Pol. 2010;68:1344–1350. [PubMed] [Google Scholar]
- 44. Sarno G, Garg S, Onuma Y, Gutierrez‐Chico JL, van den Brand MJ, Rensing BJ, Morel MA, Serruys PW. Impact of completeness of revascularization on the five‐year outcome in percutaneous coronary intervention and coronary artery bypass graft patients (from the ARTS‐II study). Am J Cardiol. 2010;106:1369–1375. [DOI] [PubMed] [Google Scholar]
- 45. Song YB, Lee SY, Hahn JY, Choi SH, Choi JH, Lee SH, Hong KP, Park JE, Gwon HC. Complete versus incomplete revascularization for treatment of multivessel coronary artery disease in the drug‐eluting stent era. Heart Vessels. 2012;27:433–442. [DOI] [PubMed] [Google Scholar]
- 46. Srinivas VS, Selzer F, Wilensky RL, Holmes DR, Cohen HA, Monrad ES, Jacobs AK, Kelsey SF, Williams DO, Kip KE. Completeness of revascularization for multivessel coronary artery disease and its effect on one‐year outcome: a report from the NHLBI Dynamic Registry. J Interv Cardiol. 2007;20:373–380. [DOI] [PubMed] [Google Scholar]
- 47. Tamburino C, Angiolillo DJ, Capranzano P, Dimopoulos K, La Manna A, Barbaqallo R, Tagliareni F, Magiafico S, Guzman LA, Galassi AR, Bass TA. Complete versus incomplete revascularization in patients with multivessel disease undergoing percutaneous coronary intervention with drug‐eluting stents. Catheter Cardiovasc Interv. 2008;72:448–456. [DOI] [PubMed] [Google Scholar]
- 48. Valenti R, Migliorini A, Signorini U, Vergara R, Parodi G, Carrabba N, Cerisano G, Antoniucci D. Impact of complete revascularization with percutaneous coronary intervention on survival in patients with at least one chronic total occlusion. Eur Heart J. 2008;29:2336–2342. [DOI] [PubMed] [Google Scholar]
- 49. Yang HH, Chen Y, Gao CY. The influence of complete coronary revascularization on long‐term outcomes in patients with multivessel coronary heart disease undergoing successful percutaneous coronary intervention. J Int Med Res. 2010;38:1106–1112. [DOI] [PubMed] [Google Scholar]
- 50. George S, Cockburn J, Clayton TC, Ludman P, Cotton J, Spratt J, Redwood S, de Belder M, de Belder A, Hill J, Hove A, Palmer N, Rathore S, Gershlick A, Di Mario C, Hildick‐Smith D. Long‐term follow‐up of elective chronic total coronary occlusion angioplasty: analysis from the U.K. Central Cardiac Audit Database. J Am Coll Cardiol. 2014;64:235–243. [DOI] [PubMed] [Google Scholar]
- 51. Hannan EL, Zhong Y, Jacobs AK, Stamato NJ, Berger PB, Walford G, Sharma S, Venditti FJ, King SB III. Patients with chronic total occlusions undergoing percutaneous coronary interventions: characteristics, success, and outcomes. Circ Cardiovasc Interv. 2016;9:e003586. [DOI] [PubMed] [Google Scholar]
- 52. Danzi GB, Valenti R, Migliorini A, Parodi G, Vergara R, Antoniucci D. Percutaneous coronary intervention for multiple chronic total occlusions. Am J Cardiol. 2013;112:1849–1853. [DOI] [PubMed] [Google Scholar]
- 53. Chang M, Ahn JM, Kim N, Lee PH, Roh JH, Yoon SH, Kang SJ, Lee SW, Kim YH, Lee CW, Park SW, Park DW, Park SJ. Complete versus incomplete revascularization in patients with multivessel coronary artery disease treated with drug‐eluting stents. Am Heart J. 2016;179:157–165. [DOI] [PubMed] [Google Scholar]
- 54. Villablanca PA, Briceno DF, Massera D, Hlinomaz O, Lombardo M, Bortnick AE, Menegus MA, Pyo RT, Garcia MJ, Mookadam F, Ramakrishna H, Wiley J, Faggioni M, Dangas GD. Culprit‐lesion only versus complete multivessel percutaneous intervention in ST‐elevation myocardial infarction: a systematic review and meta‐analysis of randomized trials. Int J Cardiol. 2016;220:251–259. [DOI] [PubMed] [Google Scholar]
- 55. Spencer FA, Sekercioglu N, Prasad M, Lopes LC, Guyatt GH. Culprit vessel versus immediate complete revascularization in patients with ST‐segment myocardial infarction‐a systematic review. Am Heart J. 2015;170:1133–1139. [DOI] [PubMed] [Google Scholar]
- 56. Song YJ, Shin HC, Yang JI, Lee HY, Jin HY, Seo JS, Yang TH, Kim DK, Kim DS, Jang JS. Preventive versus culprit‐only percutaneous coronary intervention in ST‐elevation myocardial infarction patients with multivessel disease: a meta‐analysis. J Interv Cardiol. 2015;28:1–13. [DOI] [PubMed] [Google Scholar]
- 57. Sethi A, Bahekar A, Bhuriya R, Singh S, Ahmed A, Khosla S. Complete versus culprit only revascularization in acute ST elevation myocardial infarction: a meta‐analysis. Catheter Cardiovasc Interv. 2011;77:163–170. [DOI] [PubMed] [Google Scholar]
- 58. Sekercioglu N, Spencer FA, Lopes LC, Guyatt GH. Culprit vessel only vs immediate complete revascularization in patients with acute ST‐segment elevation myocardial infarction: systematic review and meta‐analysis. Clin Cardiol. 2014;37:765–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sardar P, Chatterjee S, Giri J, Alfonso F, Helmy T, Ledley GS, Magalhaes MA, Mukherjee D, Waksman R. Intervention strategies for multi‐vessel disease in patients with ST‐segment elevation myocardial infarction: a meta‐analysis of randomized trials. Int J Cardiol. 2015;179:225–227. [DOI] [PubMed] [Google Scholar]
- 60. Navarese EP, De Servi S, Buffon A, Suryapranata H, De Luca G. Clinical impact of simultaneous complete revascularization vs. culprit only primary angioplasty in patients with ST‐elevation myocardial infarction and multivessel disease: a meta‐analysis. J Thromb Thrombolysis. 2011;31:217–225. [DOI] [PubMed] [Google Scholar]
- 61. Moretti C, D'Ascenzo F, Quadri G, Omede P, Montefusco A, Taha S, Cerrato C, Chen SL, Biondi‐Zoccai G, Gaita F. Management of multivessel coronary disease in STEMI patients: a systematic review and meta‐analysis. Int J Cardiol. 2015;179:552–557. [DOI] [PubMed] [Google Scholar]
- 62. Lu C, Huang H, Li J, Zhao J, Zhang Q, Zeng Z, Chen Y. Complete versus culprit‐only revascularization during primary percutaneous coronary intervention in ST‐elevation myocardial infarction patients with multivessel disease: a meta‐analysis. Kaohsiung J Med Sci. 2013;29:140–149. [DOI] [PubMed] [Google Scholar]
- 63. Kowalewski M, Schulze V, Berti S, Waksman R, Kubica J, Kolodziejcazk M, Buffon A, Suryapranata H, Burbel PA, Kelm M, Pawliszak W, Anisimowicz L, Navarese EP. Complete revascularisation in ST‐elevation myocardial infarction and multivessel disease: meta‐analysis of randomised controlled trials. Heart. 2015;101:1309–1317. [DOI] [PubMed] [Google Scholar]
- 64. Bainey KR, Welsh RC, Toklu B, Bangalore S. Complete vs culprit‐only percutaneous coronary intervention in STEMI with multivessel disease: a meta‐analysis and trial sequential analysis of randomized trials. Can J Cardiol. 2016;32:1542–1551. [DOI] [PubMed] [Google Scholar]
- 65. Bainey KR, Mehta SR, Lai T, Welsh RC. Complete vs culprit‐only revascularization for patients with multivessel disease undergoing primary percutaneous coronary intervention for ST‐segment elevation myocardial infarction: a systematic review and meta‐analysis. Am Heart J. 2014;167:1–14.e2. [DOI] [PubMed] [Google Scholar]
- 66. Anantha Narayanan M, Reddy YN, Sundaram V, Reddy YN, Baskaran J, Agnihotri K, Badheka A, Patel N, Deshmukh A. What is the optimal approach to a non‐ culprit stenosis after ST‐elevation myocardial infarction—conservative therapy or upfront revascularization? An updated meta‐analysis of randomized trials. Int J Cardiol. 2016;216:18–24. [DOI] [PubMed] [Google Scholar]
- 67. Di Mario C, Sansa M, Airoldi F, Shaiban I, Manari A, Patronio A, Piccaluga E, De Servi S, Ramondo A, Colusso S, Formosa A, Cernigliaro C, Colombo A, Monzini N, Bonardi MA. Single vs multivessel treatment during primary angioplasty: results of the multicentre randomised HEpacoat for cuLPrit or multivessel stenting for Acute Myocardial Infarction (HELP AMI) Study. Int J Cardiovasc Intervent. 2004;6:128–133. [DOI] [PubMed] [Google Scholar]
- 68. Ghani A, Dambrink JH, van ‘t Hof AW, Ottervanger JP, Gosselink AT, Hoorntje JC. Treatment of non‐culprit lesions detected during primary PCI: long‐term follow‐up of a randomised clinical trial. Neth Heart J. 2012;20:347–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ochala A, Smolka GA, Wojakowski W, Dudek D, Dziewierz A, Krolikowski Z, Gasior Z, Tendera M. The function of the left ventricle after complete multivessel one‐stage percutaneous coronary intervention in patients with acute myocardial infarction. J Invasive Cardiol. 2004;16:699–702. [PubMed] [Google Scholar]
- 70. Politi L, Sgura F, Rossi R, Monopoli D, Guerri E, Leuzzi C, Bursi F, Sangiorgi GM, Modena MG. A randomised trial of target‐vessel versus multi‐vessel revascularisation in ST‐elevation myocardial infarction: major adverse cardiac events during long‐term follow‐up. Heart. 2010;96:662–667. [DOI] [PubMed] [Google Scholar]
- 71. Mariani G, De Servi S, Dellavalle A, Repetto S, Chierchia S, D'Urbano M, Repetto A, Klersy C. Complete or incomplete percutaneous coronary revascularization in patients with unstable angina in stent era: are early and one‐year results different? Catheter Cardiovasc Interv. 2001;54:448–453. [DOI] [PubMed] [Google Scholar]
- 72. Head SJ, Mack MJ, Holmes DR Jr, Mohr FW, Morice MC, Serruys PW, Kappetein AP. Incidence, predictors and outcomes of incomplete revascularization after percutaneous coronary intervention and coronary artery bypass grafting: a subgroup analysis of 3‐year SYNTAX data. Eur J Cardiothorac Surg. 2012;41:535–541. [DOI] [PubMed] [Google Scholar]
- 73. Farooq V, Serruys PW, Bourantas CV, Zhang Y, Muramatsu T, Feldman T, Holmes DR, Mack M, Morice MC, Ståhle E, Colombo A, de Vries T, Morel MA, Dawkins KD, Kappetein AP, Mohr FW. Quantification of incomplete revascularization and its association with five‐year mortality in the synergy between percutaneous coronary intervention with taxus and cardiac surgery (SYNTAX) trial validation of the residual SYNTAX score. Circulation. 2013;128:141–151. [DOI] [PubMed] [Google Scholar]
- 74. Sarathy K, Nagaraja V, Kapur A, Szirt R, Raval J, Eslick GD, Burgess D, Denniss AR. Target‐vessel versus multivessel revascularisation in ST‐elevation myocardial infarction: a meta‐analysis of randomised trials. Heart Lung Circ. 2015;24:327–334. [DOI] [PubMed] [Google Scholar]
- 75. Dahal K, Rijal J, Panta R, Lee J, Azrin M, Lootens R. Multi‐vessel versus culprit‐vessel and staged percutaneous coronary intervention in STEMI patients with multivessel disease: a meta‐analysis of randomized controlled trials. Cardiovasc Revasc Med. 2014;15:408–413. [DOI] [PubMed] [Google Scholar]
- 76. El‐Hayek GE, Gershlick AH, Hong MK, Casso Deominquez A, Banning A, Afshar AE, Herzoq E, Tamis‐Holland JE. Meta‐analysis of randomized controlled trials comparing multivessel versus culprit‐only revascularization for patients with ST‐segment elevation myocardial infarction and multivessel disease undergoing primary percutaneous coronary intervention. Am J Cardiol. 2015;115:1481–1486. [DOI] [PubMed] [Google Scholar]
- 77. Tonino PAL, De Bruyne B, Pijls NH, Siebert U, Ikeno F, van't Veer M, Klauss V, Manoraran G, Engstrom T, Oldroyd KG, Ver Lee PN, MacCarthy PA, Fearon WF. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360:213–224. [DOI] [PubMed] [Google Scholar]
- 78. Pijls NH, Fearon WF, Tonino PA, Siebert U, Ikeno F, Bornschein B, van't Veer M, Klauss V, Manoharan G, Engstrom T, Oldroyd KG, Ver Lee PN, MacCarthy PA, De Bruyne B. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention in patients with multivessel coronary artery disease: 2‐year follow‐up of the FAME (Fractional Flow Reserve Versus Angiography for Multivessel Evaluation) study. J Am Coll Cardiol. 2010;56:177–184. [DOI] [PubMed] [Google Scholar]
- 79. Mahmud E. Chronic total occlusion revascularization: Achilles’ heel or golden opportunity for PCI? J Am Coll Cardiol. 2014;64:244–246. [DOI] [PubMed] [Google Scholar]
- 80. Khan MF, Wendel CS, Thai HM, Movahed MR. Effects of percutaneous revascularization of chronic total occlusions on clinical outcomes: a meta‐analysis comparing successful versus failed percutaneous intervention for chronic total occlusion. Catheter Cardiovasc Interv. 2013;82:95–107. [DOI] [PubMed] [Google Scholar]
- 81. Joyal D, Afilalo J, Rinfret S. Effectiveness of recanalization of chronic total occlusions: a systematic review and meta‐analysis. Am Heart J. 2010;160:179–187. [DOI] [PubMed] [Google Scholar]
- 82. Desmet W, Willems J, Van Lierde J, Piessens J. Discrepancy between visual estimation and computer‐assisted measurement of lesion severity before and after coronary angioplasty. Cathet Cardiovasc Diagn. 1994;31:192–198. [DOI] [PubMed] [Google Scholar]
- 83. De Bruyne B, Pijls NH, Kalesan B, Barbato E, Tonino PA, Piroth Z, Jagic N, Möbius‐Winkler S, Rioufol G, Witt N, Kala P, MacCarthy P, Engström T, Oldroyd KG, Mavromatis K, Manoharan G, Verlee P, Frobert O, Curzen N, Johnson JB, Jüni P, Fearon WF. Fractional flow reserve‐guided PCI for stable coronary artery disease. N Engl J Med. 2014;371:1208–1217. [DOI] [PubMed] [Google Scholar]
- 84. De Bruyne B, Pijls NH, Kalesan B, Barbato E, Tonino PA, Piroth Z, Jagic N, Möbius‐Winkler S, Rioufol G, Witt N, Kala P, MacCarthy P, Engström T, Oldroyd KG, Mavromatis K, Manoharan G, Verlee P, Frobert O, Curzen N, Johnson JB, Jüni P, Fearon WF. Fractional flow reserve‐guided PCI versus medical therapy in stable coronary disease. N Engl J Med. 2012;367:991–1001. [DOI] [PubMed] [Google Scholar]
- 85. Pijls NH, van Schaardenburgh P, Manoharan G, Boersma E, Bech JW, van't Veer M, Bar F, Hoorntje J, Koolen J, Wijns W, de Bruyne B. Percutaneous coronary intervention of functionally nonsignificant stenosis: 5‐year follow‐up of the DEFER Study. J Am Coll Cardiol. 2007;49:2105–2111. [DOI] [PubMed] [Google Scholar]
- 86. Authors/Task Force members , Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, Filippatos G, Hamm C, Head SJ, Jüni P, Kappetein AP, Kastrati A, Knuuti J, Landmesser U, Laufer G, Neumann FJ, Richter DJ, Schauerte P, Sousa Uva M, Stefanini GG, Taggart DP, Torracca L, Valgimigli M, Wijns W, Witkowski A. 2014 ESC/EACTS guidelines on myocardial revascularization: the Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio‐Thoracic Surgery (EACTS). Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2014;35:2541–2619. [DOI] [PubMed] [Google Scholar]


