Abstract
Background
Although several studies have reported an association between atrial fibrillation (AF) and alcohol, the impact of alcohol consumption on the outcome after catheter ablation (CA) for AF has not been discussed. We aimed to elucidate the effect of alcohol consumption on the outcome of CA for paroxysmal AF.
Methods and Results
We examined 1361 consecutive patients with paroxysmal AF (mean age, 61±11 years, 334 women) who underwent CA, including 623 (45.8%) patients who consumed alcohol. The clinical characteristics and outcomes of CA were compared between patients who did and did not consume alcohol. No significant differences were seen in the left atrial size, duration of AF history, and incidence of nonpulmonary vein foci between 2 groups (P=NS). Although the AF recurrence‐free rate after the initial CA was higher in patients who did not consume alcohol (261/623 [41.9%] versus 252/738 [34.1%]; mean follow‐up, 44.4±30.7 months; P=0.003), the outcome after the final CA was similar between 2 groups (patients who consumed alcohol: 111/628 [17.7%] versus patients who did not consume alcohol: 138/738 [18.7%]; mean follow‐up, 53.1±25.8 months; P=0.67). The frequency (hazard ratio 1.07 per 1 day/week increase, CI 1.00–1.15, P=0.04) of alcohol consumption was significantly associated with AF recurrence after CA.
Conclusions
The frequency of alcohol consumption may be associated with AF recurrence after the initial CA for paroxysmal AF, but it may not affect the outcome after the final CA.
Keywords: alcohol, atrial fibrillation, catheter ablation, nonpulmonary vein foci, outcome, recurrent event
Subject Categories: Atrial Fibrillation, Electrophysiology, Lifestyle, Risk Factors, Catheter Ablation and Implantable Cardioverter-Defibrillator
Introduction
Atrial fibrillation (AF) is the most common type of arrhythmia in developed countries. The population with AF will significantly increase in the future with the increasingly aging society.1 In addition to the well‐known risk factors, including hypertension, diabetes mellitus, cardiovascular disease, obesity, metabolic syndrome, and chronic kidney disease, lifestyle characteristics such as alcohol consumption are significantly linked to the incidence and/or prevalence of AF.2, 3, 4, 5 Although catheter ablation (CA) is standard therapy for patients with AF,6, 7, 8 the impact of alcohol consumption on the outcomes of CA has not been discussed. In the present study, we aimed to clarify this impact in patients with paroxysmal AF (PAF).
Methods
Study Population
We enrolled 1361 consecutive patients (mean age, 61±11 years; 334 women) who were referred to our institution between 2005 and 2010 to undergo their first CA session for the treatment of symptomatic PAF refractory to antiarrhythmic drugs. AF was considered PAF when it terminated spontaneously and persisted for <7 days.9 Detailed information of the patients' drinking habit, including the frequency of alcohol consumption, volume of alcohol consumption, type of alcohol consumed, and duration of their drinking history, was collected during hospitalization from patients and their family members. In this study, patients who drank alcohol at least once per week regularly were defined as alcohol consumers. The duration of the drinking history was calculated over the course of the patient's medical history. Our institutional review board approved the study protocol, and all patients provided written informed consent before undergoing the procedures.
Electrophysiological Study
All antiarrhythmic drugs were discontinued for >7 days (amiodarone was discontinued for >1 month) before CA. All patients received anticoagulation medication for >1 month. A 7‐French 20‐ or 14‐pole, 2‐site mapping catheter (Irvine Biomedical Inc, Irvine, CA) was inserted through the right jugular vein and positioned in the coronary sinus for pacing, recording, and internal cardioversion. These procedures were performed with patients under sedation with intravenous propofol or dexmedetomidine in the fasting state.
Catheter Ablation
Our ablation strategy has been described in detail in previous reports.10, 11 Briefly, after performing a transseptal puncture using the 1‐puncture technique, 2 long sheaths were inserted into the left atrium (LA). Pulmonary venography and contrast esophagography examinations were performed to determine the anatomical relationships among the pulmonary vein (PV) ostia, LA, and esophagus. An activated clotting time of 250 to 350 s was maintained with a continuous infusion of heparin during the procedure. Two circular mapping catheters were placed in the superior and inferior PVs, and ipsilateral PVs were circumferentially and extensively ablated under fluoroscopic and electrophysiological guidance. All radiofrequency applications were delivered using an 8‐mm‐tip ablation catheter (Japan Lifeline Inc, Tokyo, Japan) in the temperature control mode at a target temperature of 55°C and maximum power of 35 W on the posterior wall of the LA and 40 W at the anterior aspect of the PVs. The radiofrequency energy was delivered for 30 to 40 s at each site. The esophageal temperature is routinely measured during radiofrequency application to avoid esophagus‐related complications.11, 12, 13 However, radiofrequency application was discontinued in cases in which the esophageal temperature reached 42°C. When the temperature decreased to <38°C, radiofrequency application was initiated again.11 Both an entrance block (ie, the elimination of all PV potentials) and exit block (ie, noncapture of the LA during placement of a circular catheter) were confirmed as end points following a 20‐ to 40‐mg bolus injection of adenosine triphosphate, which unmasked any dormant PV conduction.14 Subsequently, a cavotricuspid isthmus line was created, with a bidirectional conduction block as the end point.15 Isoproterenol (5–20 μg/min) was injected intravenously before the procedure was completed. If sustained or nonsustained AF was reproducibly induced from non‐PV foci, they were focally ablated.16 When non‐PV foci were located in the superior vena cava, the superior vena cava was electrically isolated.17 If spontaneous AF did not occur, rapid atrial pacing was performed to induce AF. After an episode of pacing‐induced AF was sustained, internal cardioversion was attempted to convert the AF to sinus rhythm and confirm whether spontaneous re‐initiation of AF occurred. Linear ablations (left atrial roof and/or bottom and/or mitral isthmus lines) were performed only when AFs from undetermined origins or macroreentrant atrial tachycardia spontaneously occurred, with an end point of a bidirectional conduction block.18, 19
Follow‐Up
Anticoagulation was discontinued after 3 to 6 months in patients who were free of AF recurrence and had no risk factors for thromboembolism. No antiarrhythmic drugs were prescribed. All patients were followed up at 2, 6, 10, 14, 24, 36, and 48 weeks after the ablation procedure; 12‐lead ECG findings were recorded at every visit, and 24‐hour monitoring was performed every 3 months. Thereafter, the patients were followed up every 1 to 3 months at our institution or with their general physician. Patients with heart‐related symptoms were encouraged to undergo a 1‐month event recorder.
Successful ablation was defined as the absence of any atrial tachyarrhythmias lasting at least 30 s without any antiarrhythmic drugs after a blanking period of 1 month. Repeat ablation was recommended for patients who experienced atrial tachyarrhythmias after this time.
Statistical Analysis
Data were expressed as means±SD for continuous variables, and as frequencies and percentages for categorical variables. To compare the 2 groups, χ2 analysis or Fisher's exact test was used to analyze categorical variables, and an unpaired t test or Wilcoxon's analysis was used to analyze continuous variables. A multivariate Cox proportional analysis was performed to determine significant risk factors and calculate the hazard ratios and 95% CI. The follow‐up period was calculated from the date of the procedure to the date of AF recurrence or censoring. AF recurrence‐free rates were calculated using Kaplan–Meier analysis, and log‐rank statistics were used to make comparisons between the 2 groups. A P<0.05 was considered statistically significant.
Results
Baseline Characteristics
Table 1 shows the baseline characteristics of the study population. Among the 1361 patients (mean age, 61±11 years; 334 women), 623 (45.8%) consumed alcohol. Compared to patients who did not consume alcohol, those who consumed alcohol were significantly younger (P<0.0001), more predominantly male (P<0.0001), had a lower prevalence of structural heart disease (P=0.003), and a history of heart failure (P=0.02) (Table 2). Among patients who consumed alcohol, the mean frequency and volume of alcohol consumption was 5.6 days/week and 192 g/week. The mean duration of patients' drinking history was 37.0±10.0 years. The prevalence of non‐PV foci was similar between the 2 groups at the initial CA.
Table 1.
Baseline Characteristics of the Study Population
| N=1361 | |
|---|---|
| Age, y | 61.2±10.6 |
| Sex (female) (%) | 334 (24.5) |
| BMI, kg/m2 | 23.4±3.0 |
| Duration of AF history, year | 5.03±5.45 |
| Structural heart disease (%) | 231 (17.0) |
| Chronic heart failure (%) | 89 (6.5) |
| Hypertension (%) | 622 (45.7) |
| Age ≥75 (%) | 99 (7.3) |
| Diabetes mellitus (%) | 145 (10.7) |
| Stroke (%) | 106 (7.8) |
| COPD (%) | 17 (1.2) |
| CHADS2 score | 0.9±0.99 |
| Alcohol consumer (%) | 623 (45.8) |
| Duration of drinking history, years | 16.9±19.8 |
| Frequency (day)/week | 2.6±3.1 |
| Volume (g)/week | 88±137 |
| LAD, mm | 37.8±5.0 |
| LVEF, % | 66.2±6.8 |
AF indicates atrial fibrillation; BMI, body mass index; COPD, chronic obstructive pulmonary disease; LAD, left atrial dimension at end‐systole; LVEF, left ventricular ejection fraction.
Table 2.
Comparison Between Patients With and Without Alcohol Consumption
| Alcohol (−) | Alcohol (+) | P Values | |
|---|---|---|---|
| (N=738) | (N=623) | ||
| Age, y | 63.0±10.2 | 59.0±10.6 | <0.0001 |
| Sex, female (%) | 272 (36.9) | 62 (10.0) | <0.0001 |
| BMI, kg/m2 | 23.3±3.2 | 23.6±2.7 | 0.11 |
| Duration of AF history, year | 5.02±5.5 | 5.06±5.39 | 0.89 |
| Structural heart disease (%) | 146 (19.8) | 85 (13.6) | 0.003 |
| Congestive heart failure (%) | 59 (8.0) | 30 (4.8) | 0.02 |
| Hypertension (%) | 349 (47.3) | 273 (43.8) | 0.21 |
| Age ≥75 (%) | 76 (10.3) | 23 (3.7) | <0.0001 |
| Diabetes mellitus (%) | 88 (11.9) | 57 (9.2) | 0.11 |
| Stroke (%) | 59 (8.0) | 47 (7.5) | 0.84 |
| COPD (%) | 13 (1.8) | 4 (0.6) | 0.09 |
| CHADS2 score | 0.9±1.0 | 0.8±0.9 | 0.002 |
| Duration of drinking history, year | 37.0±10.0 | ||
| Alcohol consumption (frequency (day)/week) | 5.6±2.0 | ||
| Alcohol consumption (volume (g)/week) | 192±146 | ||
| LAD, mm | 37.6±5.2 | 37.9±4.9 | 0.21 |
| LVEF, % | 66.5±6.8 | 65.7±6.7 | 0.05 |
| Non‐PV foci ablation (%) | 173 (23.4) | 127 (20.4) | 0.19 |
| Linear ablation (%) | 8 (1.1) | 5 (0.8) | 0.78 |
AF indicates atrial fibrillation; BMI, body mass index; COPD, chronic obstructive pulmonary disease; LAD, left atrial dimension at end‐systole; LVEF, left ventricular ejection fraction; PV, pulmonary vein isolation.
Outcomes After the CA Session
Ablation was successfully performed in all patients during the initial CA procedure. Mean durations of the CA procedure and fluoroscopy procedure were 184±44 minutes and 80±26 minutes, respectively, and no significant difference was seen between patients who did and did not consume alcohol. Although the prevalence of non‐PV foci at the initial CA was higher in women, this did not differ between patients who did and did not consume alcohol. During the initial CA, 300 (22.0%) patients required additional focal ablation for non‐PV AF foci (patients who did not consume alcohol: 173/738 [23.4%] versus patients who consumed alcohol: 127/623 [20.4%], P=0.19). Ten (0.7%) patients underwent linear ablation for macroreentrant tachycardias that spontaneously initiated during the CA procedures (7 patients), posterior wall isolation with roof line and bottom line to isolate multiple AF foci (2), and atrial modification for unmapable, multiple foci (1). Forty‐three (3.2%) procedure‐related major complications occurred (patients who consumed alcohol: 25/738 [3.4%] versus patients who did not consume alcohol: 18/623 [2.9%], P=0.64); 7 patients had ischemic stroke (including a transient ischemic attack), 21 had cardiac effusion (including tamponade), 2 had venous thrombosis, 1 had myocardial infarction, 9 had phrenic nerve injury, and 3 had vagal nerve injury.
During a mean follow‐up of 44.4±30.7 months (median 52.8 months, range 1–111.3 months) after the first CA session, 513 (37.7%) of 1361 patients (patients who consumed alcohol: 261/623 [41.9%], patients who did not consume alcohol: 252/738 [34.1%]; P=0.003) had AF recurrence. The sinus rhythm maintenance rates at 3, 5, and 7 years after the first CA session were 64.9%, 58.5%, and 56.1% in the patients who consumed alcohol, and 69.6%, 66.1%, and 61.7% in patients who did not consume alcohol consumption, respectively. AF recurred more frequently in patients who consumed alcohol after the initial CA (P=0.0009), as shown in Figure 1A.
Figure 1.
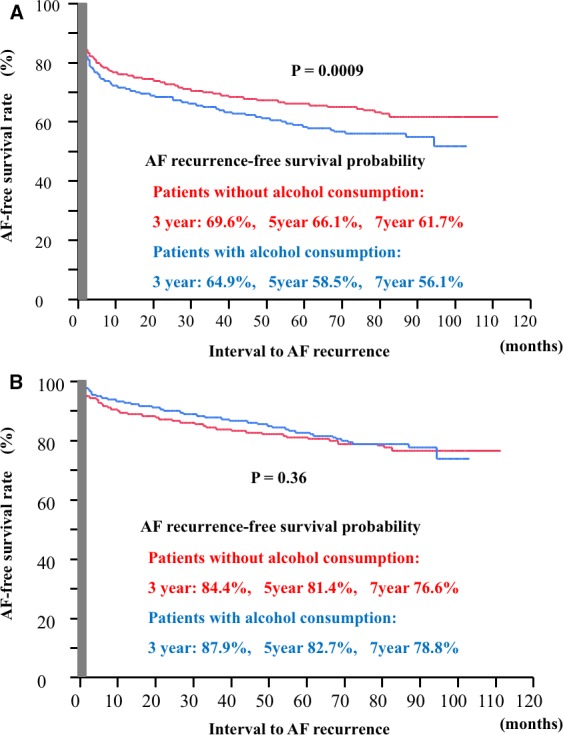
AF‐free survival curve after the initial CA (A) and the final CA (B). AF indicates atrial fibrillation; CA, catheter ablation. AF indicates atrial fibrillation.
Among 360 patients who underwent the second CA, 277 (76.9%) patients showed reconnection between the PVs and LA (patients who did not consume alcohol: 119/175 [68.0%] versus patients who consumed alcohol: 158/185 [85.4%], P<0.0001). In addition, non‐PV AF foci were identified in 161 (44.7%) patients (patients who did not consume alcohol: 92/175 [52.6%] versus patients who consumed alcohol: 69/185 [37.3%], P<0.004).
During a mean follow‐up of 53.1±25.8 months (median 55.7 months, range 1–111.3 months) after the final CA session, 250 (18.4%) of 1361 patients (patients who did not consume alcohol: 111/628 [17.7%], patients who consumed alcohol: 138/738 [18.7%]; P=0.67) had AF recurrence. The sinus rhythm maintenance rates at 1, 3, and 5 years after the first CA session were 84.4%, 81.4%, and 76.6% in patients who consumed alcohol, and 87.9%, 82.7%, and 78.8% in patients who did not consume alcohol, respectively; thus, the rates were similar between the 2 groups (Figure 1B).
Drinking Habit
The frequency and volume of alcohol consumed was significantly correlated (R 2=0.64, P<0.0001). In addition, among 623 patients who consumed alcohol, 402 (64.5%) patients consumed alcohol every day. In general, men consumed more alcohol than women did. Also, compared to older patients, those younger than 60 years old consumed significantly larger amounts of alcohol more frequently, and this tendency was typical in men (Figure 2). Furthermore, the drinking habit was not changed in 1336 (98.2%) patients at the 1‐year follow‐up; even in patients with AF recurrence, the drinking habit did not change significantly, because the symptoms and frequency of the burden of AF were more or less decreased compared to that before the CA procedure.
Figure 2.
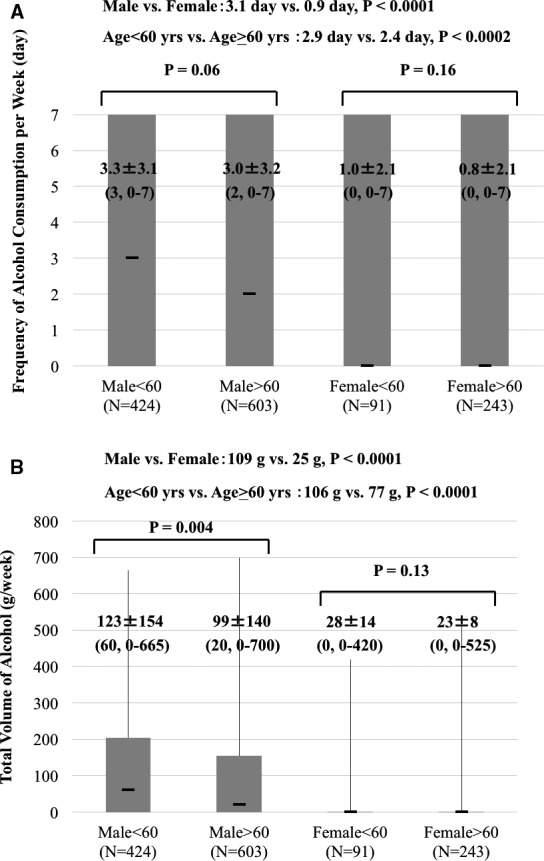
Comparison of alcohol consumption between male and female, age <60 and age ≥60. Frequency of alcohol consumption (A) and total volume of alcohol (B) are described in mean±SD (median, range).
As for the type of alcohol, 288 (46.2%), 272 (43.7%), 16 (2.6%), and 47 (7.5%) patients consumed beer, Japanese sake, whisky, and wine, respectively. However, these difference did not affect the outcome of CA (P=0.36).
Clinical Predictors of AF Recurrence After CA
Results of univariate analysis showed that the duration of the AF history (P<0.0001), presence of structural heart disease (P=0.0004), left atrial diameter (P=0.002), and the duration of drinking history (P=0.002), and frequency (P=0.0002) and volume (P=0.001) of weekly alcohol consumption were significantly associated with AF recurrence after the initial CA session (Table 3). Additionally, in multivariate analysis, the duration of the AF history, presence of structural heart disease, and left atrial diameter remained as significant predictors of AF recurrence after the initial CA (Table 4). Regarding alcohol consumption, the duration of drinking history and the weekly alcohol consumption volume did not remain as significant predictors in multivariate analysis; however, only the weekly frequency of alcohol consumption was associated with AF recurrence after the initial CA (hazard ratio 1.07 per 1 day/week increase, CI 1.00–1.15, P=0.04). Although patients with alcohol consumption generally showed a worse outcome after the initial CA (Figure 1), the risk of AF recurrence remarkably increased in patients who consumed alcohol more frequently than 5 times/week (Figure 3).
Table 3.
Clinical Predictors of AF Recurrence (Univariate)
| P Values | HR | 95% CI | |
|---|---|---|---|
| Age, 1‐year increase | 0.3 | 1 | 0.99 to 1.01 |
| Sex (female) | 0.64 | 1.05 | 0.86 to 1.28 |
| BMI, 1 kg/m2 increase | 0.74 | 1 | 0.97 to 1.02 |
| Duration of AF history, 1‐year increase | <0.0001 | 1.03 | 1.02 to 1.04 |
| Structural heart disease | 0.0004 | 1.48 | 1.19 to 1.81 |
| Congestive heart failure | 0.12 | 1.3 | 0.93 to 1.76 |
| Hypertension | 0.78 | 0.98 | 0.82 to 1.03 |
| Age ≥75 | 0.69 | 1.07 | 0.76 to 1.46 |
| Diabetes mellitus | 0.27 | 1.17 | 0.88 to 1.51 |
| Stroke | 0.63 | 1.08 | 0.78 to 1.46 |
| COPD | 0.32 | 0.66 | 0.23 to 1.42 |
| CHADS(2) score | 0.34 | 1.04 | 0.96 to 1.13 |
| LAD, 10‐mm increase | 0.002 | 1.32 | 1.10 to 1.57 |
| LVEF, 10% increase | 0.8 | 0.98 | 0.87 to 1.79 |
| Duration of drinking history, 5‐year increase | 0.002 | 1.51 | 1.16 to 1.95 |
| Alcohol consumption, 1 day/week increase | 0.0002 | 1.05 | 1.02 to 1.08 |
| Alcohol consumption, 100 g/week increase | 0.001 | 1.1 | 1.04 to 1.16 |
AF indicates atrial fibrillation; BMI, body mass index; COPD, chronic obstructive pulmonary disease; HR, hazard ratio; LAD, left atrial dimension at end‐systole; LVEF, left ventricular ejection fraction.
Table 4.
Clinical Predictors of AF Recurrence (Multivariate)
| P Values | HR | 95% CI | |
|---|---|---|---|
| Duration of AF history, 1‐year increase | <0.0001 | 1.03 | 1.02 to 1.04 |
| Structural heart disease | 0.003 | 1.43 | 1.13 to 1.79 |
| Congestive heart failure | 0.5 | 1.13 | 0.79 to 1.57 |
| LAD, per 10‐mm increase | 0.04 | 1.21 | 1.01 to 1.46 |
| Alcohol consumption, 1 day/week increase | 0.04 | 1.07 | 1.00 to 1.15 |
| Alcohol consumption, 100 g/week increase | 0.92 | 1.01 | 0.91 to 1.11 |
| Duration of drinking history, 5‐year increase | 0.55 | 0.99 | 0.94 to 1.03 |
AF indicates atrial fibrillation; HR, hazard ratio; LAD, left atrial dimension at end‐systole.
Figure 3.
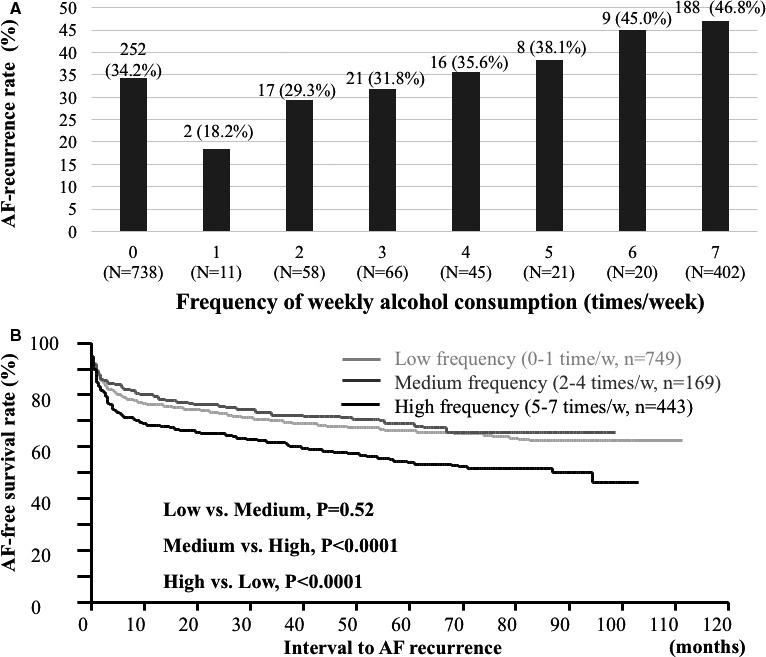
The association between the weekly frequency of alcohol consumption and AF‐recurrence rate (A). AF‐free survival rate remarkably increased in patients who consume alcohol more frequently than 5 times/week (B). AF indicates atrial fibrillation.
Discussion
Major Findings
In the present study, we demonstrated the following: (1) alcohol consumption was strongly associated with the outcome of AF recurrence after the initial CA; however, the impact of alcohol consumption did not affect the outcome after the final CA; (2) most patients did not change their drinking habit after the initial CA procedure; (3) although the frequency and the volume of weekly alcohol consumption were well correlated, the frequency individually affected the outcome of the initial CA; and (4) alcohol consumption was not associated with the incidence of non‐PV AF foci in PAF patients.
The Impact of Alcohol Consumption on the Outcome of CA
Although alcohol consumption was strongly associated with the outcome of AF recurrence after the initial CA, the impact did not remain after the final CA. Several reports4, 5, 20 have described the mechanism of how the consumption of alcohol causes AF. First, there is a link between chronic alcohol abuse and alcoholic cardiomyopathy,4, 21 which possibly leads to cardiac arrhythmias such as AF. Second, transient triggers,5, 22 including a hyperadrenergic state (increased levels of serum catecholamines),20 impaired vagal tone (decreased heart rate variability),23 electrolyte imbalances,20 and various electrophysiological changes in atrial cells (eg, an increase in intra‐atrial conduction time represented by a length of the P‐wave, reduction in the refractory period, and negative inotropic effect through calcium‐channel inhibition in ventricular cells)24, 25, 26 are associated with the occurrence of AF. Regarding the outcome after AF ablation in patients with PAF, the latter factors seem to have more of an effect than the former factor, because neither the duration of the drinking history nor the volume of alcohol consumption, which could have a severe chronic effect, significantly affected the outcome in multivariate analysis even though these factors were remarkably related to the outcome in univariate analysis. Instead, the frequency of weekly alcohol consumption, which could be a transient trigger, remained a significant predictor of AF recurrence after the initial CA even under the multivariate analysis. Although the volume of alcohol consumption or the duration of the drinking history could be correlated with the frequency of alcohol consumption, the frequency may significantly affect the outcome of the initial AF ablation. The similar atrial size and prevalence of non‐PV foci between patients who did and did not consume alcohol may also imply that atrial remodeling is not as severe when AF is paroxysmal, even in patients who consumed alcohol. Indeed, some studies have reported an association between alcohol consumption and mechanical remodeling of the heart21, 27; however, unfortunately in the present study, no tools were used to ascertain changes in the substrate (eg, cardiac magnetic resonance imaging, voltage mapping, or an assessment of areas of fractionation).
Relationship Between Alcohol Consumption and AF Foci During Repeat CA
Findings regarding the second CA procedure demonstrated that patients who consumed alcohol had a significantly higher PV reconnection but significantly lower non‐PV AF foci. The higher PV reconnection should not be because of the difficulty in achieving complete PV isolation in patients who consumed alcohol. Jiang et al28 examined 32 patients without AF recurrence after the index CA, and they demonstrated that PV conduction recovered in 90.6% of these patients, which implies that some patients, even those without AF recurrence, may still be potential candidates for PV‐related AF. We speculate that frequent triggers due to alcohol consumption activate these PVs, and they likely play a role in inducing the clinical recurrence of AF. Furthermore, the outcome of ablation was similar after the final CA between patients who did and did not consume alcohol, which suggests that even if there are more triggers from PVs due to frequent alcohol consumption, triggered PV firing should be confined to the veins and thus should not affect the left atrium, if PVs are completely isolated. Although we believe that our speculation is reasonable, further study is required to confirm our theory.
The lower non‐PV AF foci during the second CA in patients who consumed alcohol may be explained by comparing the baseline clinical characteristics. Some groups reported that chronic alcohol consumption negatively affected atrial substrate and cause atrial remodeling to progress21, 27 that might be associated with AF generation or persistence. Although this theory seems reasonable, atrial remodeling may be caused by the effect of alcohol and other multifactorial factors. As our data show in the present study, patients who did not consume alcohol were relatively older, more predominantly women, and they had more structural heart disease and heart failure; these factors have been reported to be associated with non‐PV foci in several studies.29, 30, 31, 32, 33 We believe that these negative effects may exceed the positive effect of avoiding alcohol in these patients.
Clinical Implications
The present study's findings demonstrate a strong association between alcohol consumption and AF recurrence after the initial CA. In addition, the frequency of alcohol consumption rather than the volume of alcohol consumption or the duration of drinking history affects the outcome. Although most patients do not change their drinking habit even after CA, cessation of alcohol intake may improve the outcome after the initial CA. However, this should be confirmed in a further prospective study.
Limitations
First, despite our efforts to perform a 1‐month event recording in any patient who reported symptoms, asymptomatic AF episodes could have been missed in these patients and the recurrence rate of atrial arrhythmias could have been higher. Second, although AF that terminated within the first week spontaneously or even by electrical or chemical means was defined as paroxysmal AF, these interventions were usually performed within 48 hours. In addition, the results should be different in patients with persistent AF, so the findings of the present study may not always apply to patients with persistent AF. Third, during the study period, an 8‐mm tip, nonirrigation catheter was used instead of a 3.5‐mm tip, irrigation catheter with contact force measurement capabilities, because irrigation catheters were not available in Japan and an electroanatomical mapping system was not used during the procedures. Advances in these technologies may improve the outcome of CA. Thus, the results of our study may not be generalized to areas where these technologies are used. Fourth, although substrate modification may not be necessary and even proarrhythmic, we rarely performed linear ablation as a substrate modification in this study. Fifth, we set a 1‐month blanking period in this study, because the patient series was from 2005 when the Heart Rhythm Society guideline did not yet report the appropriate blanking period; this should be considered when comparing our study's results to another recent study's findings. Finally, inducing non‐PV AF foci under an isoproterenol infusion and burst pacing/cardioversion protocol may be associated with the risk of inducing nonclinical AF foci or unmasking clinical AF foci. However, results of the comparison between patients who did and did not consume alcohol would not be altered, because this likelihood would be equal in both groups.
Conclusions
The frequency of alcohol consumption may be associated with AF recurrence after the initial CA for PAF. Although the frequent transient triggers due to alcohol consumption may increase the recurrence rate of AF, the outcome of CA will be better when PVs are completely isolated after the final CA.
Disclosures
None.
(J Am Heart Assoc. 2016;5:e004149 doi: 10.1161/JAHA.116.004149)
References
- 1. Inoue H, Fujiki A, Origasa H, Ogawa S, Okumura K, Kubota I, Aizawa Y, Yamashita T, Atarashi H, Horie M, Ohe T, Doi Y, Shimizu A, Chishaki A, Saikawa T, Yano K, Kitabatake A, Mitamura H, Kodama I, Kamakura S. Prevalence of atrial fibrillation in the general population of Japan: an analysis based on periodic health examination. Int J Cardiol. 2009;137:102–107. [DOI] [PubMed] [Google Scholar]
- 2. Miyasaka Y, Barnes ME, Bailey KR, Cha SS, Gersh BJ, Seward JB, Seward JB, Tsang TS. Mortality trends in patients diagnosed with first atrial fibrillation: a 21‐year community‐based study. J Am Coll Cardiol. 2007;49:986–992. [DOI] [PubMed] [Google Scholar]
- 3. Watanabe H, Tanabe N, Watanabe T, Darbar D, Roden DM, Sasaki S, Aizawa Y. Metabolic syndrome and risk of development of atrial fibrillation: the Niigata preventive medicine study. Circulation. 2008;117:1255–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Larsson SC, Drca N, Wolk A. Alcohol consumption and risk of atrial fibrillation: a prospective study and dose‐response meta‐analysis. J Am Coll Cardiol. 2014;64:281–289. [DOI] [PubMed] [Google Scholar]
- 5. Kodama S, Saito K, Tanaka S, Horikawa C, Saito A, Heianza Y, Anasako Y, Nishigaki Y, Yachi Y, Iida KT, Ohashi Y, Yamada N, Sone H. Alcohol consumption and risk of atrial fibrillation: a meta‐analysis. J Am Coll Cardiol. 2011;57:427–436. [DOI] [PubMed] [Google Scholar]
- 6. Haïssaguerre M, Jaïs P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Métayer P, Clémenty J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–666. [DOI] [PubMed] [Google Scholar]
- 7. Bhargava M, Di Biase L, Mohanty P, Prasad S, Martin DO, Williams‐Andrews M, Wazni OM, Burkhardt JD, Cummings JE, Khaykin Y, Verma A, Hao S, Beheiry S, Hongo R, Rossillo A, Raviele A, Bonso A, Themistoclakis S, Stewart K, Saliba WI, Schweikert RA, Natale A. Impact of type of atrial fibrillation and repeat catheter ablation on long‐term freedom from atrial fibrillation: results from a multicenter study. Heart Rhythm. 2009;6:1403–1412. [DOI] [PubMed] [Google Scholar]
- 8. Ouyang F, Bänsch D, Ernst S, Schaumann A, Hachiya H, Chen M, Chun J, Falk P, Khanedani A, Antz M, Kuck KH. Complete isolation of left atrium surrounding the pulmonary veins: new insights from the double‐Lasso technique in paroxysmal atrial fibrillation. Circulation. 2004;110:2090–2096. [DOI] [PubMed] [Google Scholar]
- 9. European Heart Rhythm Association (EHRA); European Cardiac Arrhythmia Society (ECAS); American College of Cardiology (ACC); American Heart Association (AHA); Society of Thoracic Surgeons (STS) , Calkins H, Brugada J, Packer DL, Cappato R, Chen SA, Crijns HJ, Damiano RJ Jr, Davies DW, Haines DE, Haissaguerre M, Iesaka Y, Jackman W, Jais P, Kottkamp H, Kuck KH, Lindsay BD, Marchlinski FE, McCarthy PM, Mont JL, Morady F, Nademanee K, Natale A, Pappone C, Prystowsky E, Raviele A, Ruskin JN, Shemin RJ. HRS/EHRA/ECAS expert consensus statement on catheter surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow‐up. A report of the Heart Rhythm Society (HRS) Task Force on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2007;4:816–861. [DOI] [PubMed] [Google Scholar]
- 10. Miyazaki S, Kuwahara T, Kobori A, Takahashi Y, Takei A, Sato A, Isobe M, Takahashi A. Preprocedural predictors of atrial fibrillation recurrence following pulmonary vein antrum isolation in patients with paroxysmal atrial fibrillation: long‐term follow‐up results. J Cardiovasc Electrophysiol. 2011;22:621–625. [DOI] [PubMed] [Google Scholar]
- 11. Kuwahara T, Takahashi A, Kobori A, Miyazaki S, Takahashi Y, Takei A, Nozato T, Hikita H, Sato A, Aonuma K. Safe and effective ablation of atrial fibrillation: importance of esophageal temperature monitoring to avoid periesophageal nerve injury as a complication of pulmonary vein isolation. J Cardiovasc Electrophysiol. 2009;20:1–6. [DOI] [PubMed] [Google Scholar]
- 12. Redfearn DP, Trim GM, Skanes AC, Petrellis B, Krahn AD, Yee R, Klein GJ. Esophageal temperature monitoring during radiofrequency ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 2005;16:589–593. [DOI] [PubMed] [Google Scholar]
- 13. Pappone C, Oral H, Santinelli V, Vicedomini G, Lang CC, Manguso F, Torracca L, Benussi S, Alfieri O, Hong R, Lau W, Hirata K, Shikuma N, Hall B, Morady F. Atrio‐esophageal fistula as a complication of percutaneous transcatheter ablation of atrial fibrillation. Circulation. 2004;109:2724–2726. [DOI] [PubMed] [Google Scholar]
- 14. Hachiya H, Hirao K, Takahashi A, Nagata Y, Suzuki K, Maeda S, Sasaki T, Kawabata M, Isobe M, Iesaka Y. Clinical implications of reconnection between the left atrium and isolated pulmonary veins provoked by adenosine triphosphate after extensive encircling pulmonary vein isolation. J Cardiovasc Electrophysiol. 2007;18:1–7. [DOI] [PubMed] [Google Scholar]
- 15. Miyazaki S, Takahashi A, Kuwahara T, Kobori A, Yokoyama Y, Nozato T, Sato A, Aonuma K, Hirao K, Isobe M. Randomized comparison of the continuous vs. point‐by‐point radiofrequency ablation of the cavotricuspid isthmus for atrial flutter. Circ J. 2007;71:1922–1926. [DOI] [PubMed] [Google Scholar]
- 16. Lin WS, Tai CT, Hsieh MH, Tsai CF, Lin YK, Tsao HM, Huang JL, Yu WC, Yang SP, Ding YA, Chang MS, Chen SA. Catheter ablation of paroxysmal atrial fibrillation initiated by non‐pulmonary vein ectopy. Circulation. 2003;107:3176–3183. [DOI] [PubMed] [Google Scholar]
- 17. Tsai CF, Tai CT, Hsieh MH, Lin WS, Yu WC, Ueng KC, Ding YA, Chang MS, Chen SA. Initiation of atrial fibrillation by ectopic beats originating from the superior vena cava: electrophysiological characteristics and results of radiofrequency ablation. Circulation. 2000;102:67–74. [DOI] [PubMed] [Google Scholar]
- 18. Jaïs P, Hocini M, Hsu LF, Sanders P, Scavee C, Weerasooriya R, Macle L, Raybaud F, Garrigue S, Shah DC, Le Metayer P, Clémenty J, Haïssaguerre M. Technique and results of linear ablation at the mitral isthmus. Circulation. 2004;110:2996–3002. [DOI] [PubMed] [Google Scholar]
- 19. Hocini M, Jaïs P, Sanders P, Takahashi Y, Rotter M, Rostock T, Hsu LF, Sacher F, Reuter S, Clémenty J, Haïssaguerre M. Techniques, evaluation, and consequences of linear block at the left atrial roof in paroxysmal atrial fibrillation: a prospective randomized study. Circulation. 2005;112:3688–3696. [DOI] [PubMed] [Google Scholar]
- 20. Denison H, Jern S, Jagenburg R, Wendestam C, Wallerstedt S. Influence of increased adrenergic activity and magnesium depletion on cardiac rhythm in alcohol withdrawal. Br Heart J. 1994;72:554–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Piano MR. Alcoholic cardiomyopathy: incidence, clinical characteristics, and pathophysiology. Chest. 2002;121:1638–1650. [DOI] [PubMed] [Google Scholar]
- 22. Suzuki S. Alcohol and atrial fibrillation. Circ J. 2014;78:839–840. [DOI] [PubMed] [Google Scholar]
- 23. Mandyam MC, Vedantham V, Scheinman MM, Tseng ZH, Badhwar N, Lee BK, Lee RJ, Gerstenfeld EP, Olgin JE, Marcus GM. Alcohol and vagal tone as triggers for paroxysmal atrial fibrillation. Am J Cardiol. 2012;110:364–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Marcus GM, Smith LM, Whiteman D, Tseng ZH, Badhwar N, Lee BK, Lee RJ, Scheinman MM, Olgin JE. Alcohol intake is significantly associated with atrial flutter in patients under 60 years of age and a shorter right atrial effective refractory period. Pacing Clin Electrophysiol. 2008;31:266–272. [DOI] [PubMed] [Google Scholar]
- 25. Steinbigler P, Haberl R, Konig B, Steinbeck G. P‐wave signal averaging identifies patients prone to alcohol‐induced paroxysmal atrial fibrillation. Am J Cardiol. 2003;91:491–494. [DOI] [PubMed] [Google Scholar]
- 26. Habuchi Y, Furukawa T, Tanaka H, Lu LL, Morikawa J, Yoshimura M. Ethanol inhibition of Ca2+ and Na+ currents in the guinea‐pig heart. Eur J Pharmacol. 1995;292:143–149. [DOI] [PubMed] [Google Scholar]
- 27. Ettinger PO, Wu CF, De La Cruz C Jr, Weisse AB, Ahmed SS, Regan TJ. Arrhythmias and the “Holiday Heart”: alcohol‐associated cardiac rhythm disorders. Am Heart J. 1978;95:555–562. [DOI] [PubMed] [Google Scholar]
- 28. Jiang RH, Po SS, Tung R, Liu Q, Sheng X, Zhang ZW, Sun YX, Yu L, Zhang P, Fu GS, Jiang CY. Incidence of pulmonary vein conduction recovery in patients without clinical recurrence after ablation of paroxysmal atrial fibrillation: mechanistic implications. Heart Rhythm. 2014;11:969–976. [DOI] [PubMed] [Google Scholar]
- 29. Lee SH, Tai CT, Hsieh MH, Tsao HM, Lin YJ, Chang SL, Huang JL, Lee KT, Chen YJ, Cheng JJ, Chen SA. Predictors of non‐pulmonary vein ectopic beats initiating paroxysmal atrial fibrillation: implication for catheter ablation. J Am Coll Cardiol. 2005;46:1054–1059. [DOI] [PubMed] [Google Scholar]
- 30. Takigawa M, Kuwahara T, Takahashi A, Watari Y, Okubo K, Takahashi Y, Takagi K, Kuroda S, Osaka Y, Kawaguchi N, Yamao K, Nakashima E, Sugiyama T, Akiyama D, Kamiishi T, Kimura S, Hikita H, Hirao K, Isobe M. Differences in catheter ablation of paroxysmal atrial fibrillation between males and females. Int J Cardiol. 2013;168:1984–1991. [DOI] [PubMed] [Google Scholar]
- 31. Miyazaki S, Taniguchi H, Kusa S, Ichihara N, Nakamura H, Hachiya H, Iesaka Y. Factors predicting an arrhythmogenic superior vena cava in atrial fibrillation ablation: insight into the mechanism. Heart Rhythm. 2014;11:1560–1566. [DOI] [PubMed] [Google Scholar]
- 32. Zhao Y, Di Biase L, Trivedi C, Mohanty S, Bai R, Mohanty P, Gianni C, Santangeli P, Horton R, Sanchez J, Gallinghouse GJ, Zagrodzky J, Hongo R, Beheiry S, Lakkireddy D, Reddy M, Hranitzky P, Al‐Ahmad A, Elayi C, Burkhardt JD, Natale A. Importance of non‐pulmonary vein triggers ablation to achieve long‐term freedom from paroxysmal atrial fibrillation in patients with low ejection fraction. Heart Rhythm. 2016;13:141–149. [DOI] [PubMed] [Google Scholar]
- 33. Takigawa M, Takahashi A, Kuwahara T, Okubo K, Takahashi Y, Nakashima E, Watari Y, Yamao K, Nakajima J, Takagi K, Kimura S, Hikita H, Hirao K, Isobe M. Impact of non‐pulmonary vein foci on the outcome of the second session of catheter ablation for paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol. 2015;26:739–746. [DOI] [PubMed] [Google Scholar]


