Abstract
Background
In patients with stable coronary artery disease undergoing elective percutaneous coronary intervention, the prognostic value of high‐sensitivity cardiac troponin T (hs‐cTnT) and the influence of sex remain poorly defined.
Methods and Results
Consecutive patients with stable coronary artery disease who underwent elective percutaneous coronary intervention were included. Primary endpoint was all‐cause mortality. Unadjusted hazard ratio (HR) in overall and sex‐specific population and multivariable adjusted HR were calculated by using Cox proportional hazard models. In a total of 5626 patients, elevated hs‐cTnT levels, more than the sex‐specific 99th percentile upper reference limit of normal (URL), were observed in 2221 patients (39%) at baseline. During follow‐up (median, 14.5 months; 25th–75th percentiles, 6.4–27.2 months), 265 patients died. Mortality was higher in patients with the sex‐specific 99th percentile URL compared to those with normal hs‐cTnT (17.3% vs 3.4%; HR=6.10; 95% CI, 4.58–8.14; P<0.001). hs‐cTnT was an independent predictor of mortality in multivariable adjusted models. The C‐statistic was significantly increased by adding hs‐cTnT to the basic prediction model for mortality (0.793–0.815; P<0.001). There was a significant interaction between hs‐cTnT and sex on mortality. Differences in all‐cause mortality between patients with more than the sex‐specific 99th percentile URL and those with normal hs‐cTnT were numerically larger in male than female patients (male, HR=6.45; 95% CI, 4.68–8.87, P<0.001; female, HR=4.29, 95% CI, 2.36–9.03; P<0.001).
Conclusions
In patients with stable coronary artery disease undergoing elective percutaneous coronary intervention, preprocedural hs‐cTnT was a strong predictor of mortality in both men and women.
Keywords: percutaneous coronary intervention, sex, stable coronary artery disease, troponin T
Subject Categories: Biomarkers, Chronic Ischemic Heart Disease, Percutaneous Coronary Intervention
Introduction
Cardiac troponins are broadly used in the diagnosis of acute coronary syndrome (ACS) in patients with acute chest pain, and newly developed high‐sensitivity assays for measurement of circulating cardiac troponins have substantially improved early diagnosis of acute myocardial infarction (MI).1 Cardiac troponins may also be useful as a biomarker for chronic myocardial injury. Elevations of cardiac troponin levels, as measured by high‐sensitivity assays above the normal range, have been observed in the asymptomatic general population and are associated with increased risk of all‐cause and cardiac mortality.2
In patients with known stable coronary artery disease (SCAD), elevation of high‐sensitivity cardiac troponin T (hs‐cTnT) level was reported to be highly correlated with plaque burden, inducible cardiac ischemia, and multiple cardiac structural and functional abnormalities.3, 4, 5 In these patients, hs‐cTnT,6, 7, 8, 9, 10 as well as high‐sensitivity cardiac troponin I (hs‐cTnI),11, 12, 13 is an independent prognostic biomarker for cardiovascular events and all‐cause mortality. However, data are scarce with regard to the prognostic value of high‐sensitivity cardiac troponins in patients with SCAD who undergo percutaneous coronary intervention (PCI). Moreover, sex differences in the association between high‐sensitivity cardiac troponin and mortality have not been investigated in the setting of SCAD patients, despite the fact that significant interaction was observed in other settings.14, 15, 16
Given this background, we thought to assess the prognostic value of hs‐cTnT and the influence of sex in a cohort of patients with SCAD undergoing elective PCI.
Methods
Study Design and Patient Selection
This was a retrospective study of consecutive patients with SCAD undergoing elective PCI between October 2009 and December 2014 at 2 centers in Munich, Germany: Deutsches Herzzentrum München and 1. medizinische Klinik, Klinikum Rechts der Isar. During this period, hs‐cTnT was routinely measured in all patients undergoing PCI. Patients presenting with ACS or with previous MI within 1 month were excluded from this study. This study was conducted in accord with the current revision of the Helsinki Declaration. The study protocol was approved by the institutional ethics committee of the Technische Universität München. Informed written consent was waived by the ethical committee.
PCI and Medication
Commercially available bare‐metal stents, drug‐eluting stents, and bioabsorbable vascular scaffolds were used for PCI. Interventions were performed according to current guidelines. An oral loading dose of 600 mg of clopidogrel was administered to all patients at least 2 hours before the intervention, regardless of whether the patient was taking clopidogrel before being admitted. During the procedure, intravenous aspirin, heparin, or bivalirudin was administered; the use of glycoprotein IIb/IIIa inhibitors was at the discretion of the operator. After the intervention, all patients were prescribed 100 mg/day of aspirin indefinitely, clopidogrel 75 mg/day for at least 6 months, and other cardiac medications at the discretion of the patient's physician.
Quantitative Coronary Angiographic Measurements
Quantitative coronary angiographic analysis at pre‐ and postindex interventions was carried out with a validated automated edge‐detection system (QAngioXA version 7.1; Medis Medical Imaging Systems, Leiden, The Netherlands). Reference vessel diameter, minimal lumen diameter, and percent diameter stenosis were measured.
Biochemical Measurements
Blood samples were collected into tubes containing lithium‐heparin as anticoagulant on admission to the hospital usually on the same day as the PCI was performed. Within 30 minutes, the blood was centrifuged at room temperature and the plasma supernatant was separated. The plasma concentration of hs‐cTnT was measured with the high‐sensitivity assay (Roche Diagnostics, Indianapolis, IN) on a Cobas e411 immunoanalyzer based on electrochemiliuminescence technology (Roche Diagnostics) according to the instructions of the manufacturer (detection limit of 5 ng/L, 99th percentile in the general population of 14 ng/L and 10% coefficient of variation level of 13 ng/L).
Definitions
Angiographic diagnosis of coronary artery disease was based on the presence of coronary stenosis with ≥75% lumen obstruction in at least 1 of the major coronary arteries or bypass grafts. Glomerular filtration rate (GFR) was estimated using the Modification of Diet in Renal Disease formula. Arterial hypertension was diagnosed in the presence of active treatment with antihypertensive agents or otherwise as a systolic blood pressure of ≥140 mm Hg and/or diastolic blood pressure of ≥90 mm Hg on at least 2 separate occasions. Dyslipidemia was diagnosed in the presence of active treatment with lipid‐lowering agents or total cholesterol value ≥240 mg/dL. Current smokers were defined as those currently smoking any tobacco. Diabetes mellitus was diagnosed in the presence of active treatment with antidiabetic agents or based on current guidelines. We classified patients into 2 groups along with the survival status during follow‐up: a nonsurvival group and a survival group.
Endpoint and Follow‐up
The primary endpoint of interest in this study was all‐cause mortality. The secondary endpoint was cardiac mortality. Subsequent analysis of primary and secondary endpoints was done according to sex. Information on deaths was obtained from hospital records, death certificates, or telephone contact with relatives of the patient or with the attending physician. Clinical follow‐up was performed either by telephone or office visit out to 3 years after the index PCI.
Statistical Methods
Continuous variables are presented as median and interquartile range [25th–75th percentiles] and compared using a Kruskal–Wallis rank‐sum test (attributed to non‐normally distributed data as assessed by the 1‐sample Kolmogorov–Smirnov test). Categorical or binary variables are presented as numbers (percentage) and compared using chi‐squared test or Fisher's exact test. For investigating the prognostic value of hs‐cTnT, overall and sex‐specific survival analyses were performed using the Kaplan–Meier method; the prognostic value of hs‐cTnT was evaluated as dichotomized variable divided by the sex‐specific 99th percentile upper reference limit of normal (URL) as cutoffs (15 ng/L for male and 10 ng/L for female).15 The multivariate Cox proportional hazard model was used to identify the independent correlates of all‐cause mortality using generalized estimating equations to take into account potential cluster effects of multiple lesions in a single patient. The model included the following variables: hs‐cTnT, age, sex, body mass index (BMI), diabetes mellitus, hypertension, dyslipidemia, previous MI, previous bypass surgery, left ventricular ejection fraction (LVEF), GFR, multivessel disease, target vessel, restenotic lesion, reference diameter, and stent type. hs‐cTnT was entered into the model as a continuous variable after logarithmic transformation because of its skewed distribution. BMI, LVEF, GFR, restenotic lesion, and reference diameter were included in the model after imputing missing data using multiple imputation by the chained equations method.17 A potential interaction of hs‐cTnT with sex was evaluated using multiplicative interaction terms between hs‐cTnT and sex and by testing for statistical significance in multivariable Cox proportional hazards model. Potential interactions of hs‐cTnT with age and GFR were also evaluated. The discriminatory power of the multivariable models was assessed by calculating C‐statistics. The multivariable Cox proportional hazard model for all‐cause mortality, except hs‐cTnT, as a variable was used to calculate the C‐statistics of the model with baseline variables. The model with baseline variables and hs‐cTnT and the model with baseline variables, hs‐cTnT, and the interaction terms between hs‐cTnT and sex were also used to calculate each C‐statistics. Bootstrapping (400 samples) was used to calculate the confidence interval (CI) of the C‐statistics and enable the comparison of C‐statistics of the models. The integrated discrimination improvements (IDI) was also calculated to assess the improvement of predictive value after inclusion of hs‐cTnT and the interaction terms between hs‐cTnT and sex into a model with baseline variables. The statistical analysis was performed using the R 2.15.1 Statistical Package (The R foundation for Statistical Computing, Vienna, Austria).
Results
In a total of 5626 patients included in this study, 265 died during follow‐up (median, 14.5 [6.4–27.2] months). Baseline patient characteristics are shown in Table 1. In each group, median concentrations of baseline hs‐cTnT were 30 (17–54) and 10 (10–20) ng/L, respectively. Age, proportions of male, diabetes mellitus, past history of both MI and coronary bypass surgery, and multicoronary vessel disease were significantly higher in the nonsurvival group compared to the survival group. On the other hand, BMI, LVEF, GFR, and prevalence of patients with hypertension and dyslipidemia were significantly higher in the survival group compared to the nonsurvival group.
Table 1.
Patient Characteristics
| Nonsurvival (n=265) | Survival (n=5361) | P Value | |
|---|---|---|---|
| Age, y | 76.3 (70.8–81.5) | 68.5 (61.0–74.0) | <0.001 |
| BMI, kg/m2 a | 26.9 (24.4–29.3) | 27.3 (24.7–30.1) | 0.03 |
| Male | 223 (84.2) | 4150 (77.4) | 0.01 |
| Hypertension | 168 (63.4) | 4028 (75.1) | <0.001 |
| Current smoker | 38 (14.3) | 771 (14.4) | 0.99 |
| Dyslipidemia | 191 (72.1) | 4251 (79.3) | 0.005 |
| Diabetes mellitus | 103 (38.9) | 1677 (31.3) | 0.01 |
| Insulin dependent | 42 (15.8) | 534 (10.0) | 0.002 |
| Previous MI | 101 (38.1) | 1721 (32.1) | 0.041 |
| Previous bypass surgery | 56 (21.1) | 679 (12.7) | <0.001 |
| LVEF, %b | 50 (38–58) | 58 (50–61) | <0.001 |
| GFR, Glomerular filtration rate, mL/min per 1.73 m2 c | 53.7 (38.8–72.7) | 76.0 (57.3–97.5) | <0.001 |
| Baseline hs‐cTnT, ng/L | 30 (17–54) | 10 (10–20) | <0.001 |
| Multivessel disease | 246 (92.8) | 4601 (85.8) | 0.001 |
Data are shown as median (25th–75th percentiles) or number of patients (%). BMI indicates body mass index; GFR, glomerular filtration rate; hs‐cTnT, high‐sensitivity cardiac troponin T; LVEF, left ventricular ejection fraction; MI, myocardial infarction.
Available for 5605 patients (99.6%).
Available for 3799 patients (67.5%).
Available for 5586 patients (99.3%).
Lesions and procedural characteristics are shown in Table 2. Differences were observed between the 2 groups in the distribution of lesion site and stent type. The proportion of restenotic lesion, reference diameter, and balloon diameter were larger in the nonsurvival group compared to the survival group.
Table 2.
Lesion and Procedural Characteristics
| Nonsurvival (n=422) | Survival (n=8732) | P Value | |
|---|---|---|---|
| Site of lesion | <0.001 | ||
| Left main coronary artery | 29 (6.9) | 344 (3.9) | |
| Left anterior descending | 162 (38.4) | 3677 (42.1) | |
| Left circumflex | 106 (25.1) | 2029 (23.2) | |
| Right coronary artery | 109 (25.8) | 2519 (28.9) | |
| Bypass graft vessel | 16 (3.8) | 163 (1.9) | |
| Restenotic lesion | 66 (15.6) | 842 (9.6) | <0.001 |
| ACC/AHA class B2/C | 313 (74.2) | 6357 (72.8) | 0.54 |
| Chronic total occlusion | 17 (4.0) | 478 (5.5) | 0.20 |
| Bifurcationa | 138 (32.9) | 2940 (33.8) | 0.73 |
| Reference diameter, mmb | 2.97 (2.57–3.40) | 2.86 (2.49–3.33) | 0.008 |
| Preprocedural diameter stenosis, %b | 65.5 (56.1–75.2) | 64.9 (56.0–75.4) | 0.58 |
| Lesion length, mmc | 13.8 (9.1–21.8) | 14.3 (9.5–21.7) | 0.87 |
| Stent type | <0.001 | ||
| Bare‐metal stent | 14 (3.3) | 85 (1.0) | |
| Drug‐eluting stent | 385 (91.2) | 8276 (94.8) | |
| Bioabsorbable vascular scaffold | 23 (5.5) | 371 (4.2) | |
| Balloon diameter, mmd | 3.5 (3.0–4.0) | 3.0 (3.0–3.5) | 0.005 |
| Stent length, mme | 23 (18–33) | 23 (18–33) | 0.51 |
| Postprocedural diameter stenosis, %f | 11.6 (7.9–16.1) | 11.8 (8.1–16.5) | 0.95 |
Data are shown as median (25th–75th percentiles) or number of lesions (%). AHA indicates American Heart Association; ACC, American College of Cardiology.
Available for 9128 lesions (99.7%).
Available for 7163 lesions (78.2%).
Available for 7149 lesions (78.1%).
Available for 9149 lesions (99.9%).
Available for 9139 lesions (99.8%).
Available for 7140 lesions (78.0%).
In terms of medication at discharge from hospital, the prescription rates of statin (93% vs 82%), angiotensin‐converting enzyme inhibitor (68% vs 59%), and beta‐blocker (87% vs 82%) were significantly higher in the nonsurvival group compared to the survival group (Table 3).
Table 3.
Medication at Discharge
| Nonsurvival (n=265) | Survival (n=5361) | P Value | |
|---|---|---|---|
| Angiotensin‐converting enzyme inhibitora | 154 (58.6) | 3631 (67.9) | 0.002 |
| Angiotensin II type 1 receptor blockerb | 56 (21.2) | 1320 (24.8) | 0.19 |
| Beta‐blockerc | 216 (81.8) | 4676 (87.5) | 0.008 |
| Statind | 217 (82.2) | 4972 (92.9) | <0.001 |
Data are shown as number (%).
Available for 5608 patients (99.7%).
Available for 5596 patients (99.4%).
Available for 5611 patients (99.7%).
Available for 5614 patients (99.8%).
In the overall population, elevated hs‐cTnT levels more than the sex‐specific 99th URL were observed in 2221 patients (39%) at baseline. The unadjusted incidence of all‐cause mortality at 3 years was significantly higher in patients with elevated hs‐cTnT levels more than the sex‐specific 99th URL compared to those with normal hs‐cTnT levels (17.3% vs 3.4%, respectively; hazard ratio [HR]=6.10; 95% CI, 4.58–8.14; P<0.001; Figure 1). There was also a significant difference between the 2 groups in unadjusted 3‐year cardiac mortality rate (8.7% vs 1.2%, respectively; HR=8.12; 95% CI, 5.06–13.0; P<0.001; Figure 2).
Figure 1.
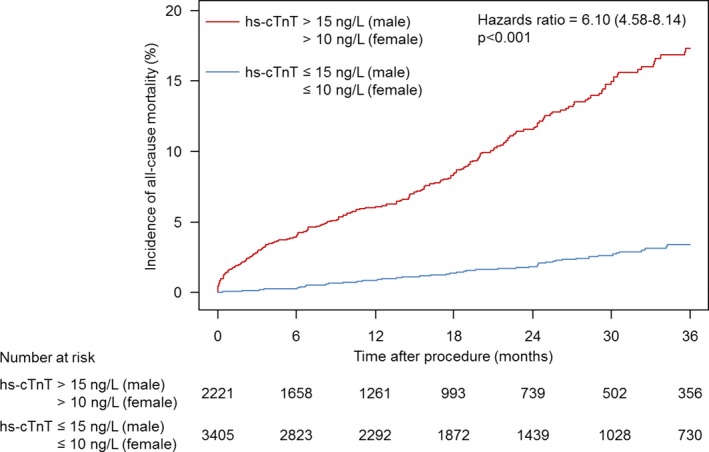
Time‐to‐event curve for incidence of all‐cause mortality. Hazard ratio and P value are derived from Cox proportional hazard models. hs‐cTnT indicates high‐sensitivity cardiac troponin T.
Figure 2.
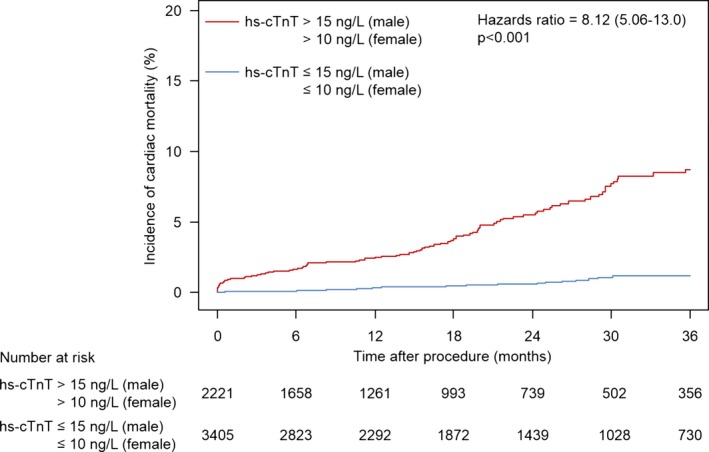
Time‐to‐event curve for incidence of cardiac mortality. Hazard ratio and P value are derived from Cox proportional hazard models. hs‐cTnT indicates high‐sensitivity cardiac troponin T.
After adjustment for other variables in the multivariable Cox proportional hazards model, hs‐cTnT was still an independent predictor of all‐cause mortality at 3 years (HR=1.46 for each unit increase in the natural logarithm; 95% CI, 1.34–1.60; P<0.001; Table 4). Whereas there were no interactions either between age and hs‐cTnT (P interaction=0.49) or GFR and hs‐cTnT (P interaction=0.18) on all‐cause mortality, there was statistically significant interaction between sex and hs‐cTnT concentrations on all‐cause mortality in the multivariable Cox proportional hazards model (P interaction=0.003).
Table 4.
Results of Multivariate Cox Proportional Hazard Models Applied to Assess Predictors of All‐Cause Mortality
| Characteristics | HR [95% CI] | P Value |
|---|---|---|
| hs‐cTnT (for 1‐unit increase in logarithmic scale) | 1.46 [1.34–1.60] | <0.001 |
| Age (for 10‐year increase) | 1.84 [1.44–2.35] | <0.001 |
| BMI (for 5 kg/m2 increase) | 1.09 [0.91–1.30] | 0.33 |
| Female sex | 0.52 [0.35–0.77] | 0.001 |
| Hypertension | 0.51 [0.39–0.68] | <0.001 |
| Dyslipidemia | 0.77 [0.56–1.06] | 0.11 |
| Diabetes mellitus | 1.26 [0.94–1.68] | 0.12 |
| Previous MI, myocardial infarction | 1.49 [1.11–2.00] | 0.008 |
| Previous bypass surgery | 0.94 [0.64–1.40] | 0.77 |
| LVEF (for 10% decrease) | 1.23 [1.10–1.38] | <0.001 |
| GFR (for 30 mL/min per 1.73 m2 decrease) | 1.61 [1.27–2.05] | <0.001 |
| Multivessel disease | 1.11 [0.66–1.87] | 0.70 |
| Target vessel: LMCA (reference=bypass graft vessel) | 1.29 [0.64–2.60] | 0.48 |
| Target vessel: LAD (reference=bypass graft vessel) | 0.81 [0.41–1.63] | 0.56 |
| Target vessel: LCx (reference=bypass graft vessel) | 1.02 [0.52–2.03] | 0.95 |
| Target vessel: RCA (reference=bypass graft vessel) | 0.95 [0.48–1.86] | 0.87 |
| Restenotic lesion | 1.37 [1.01–1.86] | 0.046 |
| Reference diameter (for 0.5‐mm decrease) | 0.97 [0.87–1.07] | 0.50 |
| Stent type: BVS (reference=bare‐metal stent) | 0.42 [0.17–1.02] | 0.06 |
| Stent type: DES (reference=bare‐metal stent) | 0.23 [0.12–0.48] | <0.001 |
Hazard ratios and P values are derived from Cox proportional hazards models. BMI indicates body mass index; BVS, bioresorbable vascular scaffold; DES, drug‐eluting stent; GFR, glomerular filtration rate; HR, hazard ratio; hs‐cTnT, high‐sensitivity cardiac troponin T; LAD, left anterior descending; LCx, left circumflex; LMCA, left main coronary artery; LVEF, left ventricular ejection fraction; MI, myocardial infarction; RCA, right coronary artery.
Unadjusted survival analyses according to sex are shown in Figure 3. All‐cause and cardiac mortality were significantly and widely different between patients with more than the sex‐specific 99th percentile URL and those with normal hs‐cTnT in both males and females.
Figure 3.
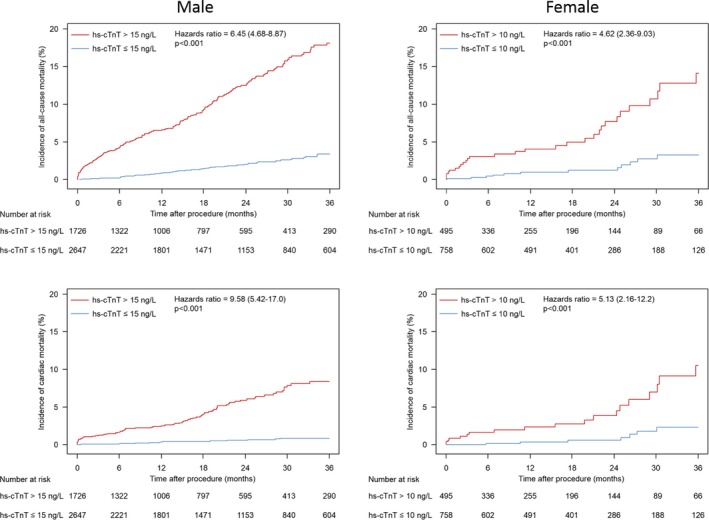
Sex‐specific time‐to‐event curves for incidence of all‐cause mortality and cardiac mortality. Hazard ratios and P values are derived from Cox proportional hazard models. hs‐cTnT indicates high‐sensitivity cardiac troponin T.
C‐statistics of the prediction models for all‐cause mortality are shown in Table 5. Adding hs‐cTnT into the baseline variables improved C‐statistics (0.789–0.813; P<0.001). The IDI was also statistically significant. When the interaction terms between hs‐cTnT and sex was included in the model with baseline variables and hs‐cTnT, C‐statistics was slightly improved (0.813–0.815; P=0.13) with statistically significant IDI (P=0.01).
Table 5.
C‐Statistics of the Prediction Models for All‐Cause Mortality
| Model | C‐Statistics [95% CI] | P Value | IDI | ||
|---|---|---|---|---|---|
| Absolute | Relative (%) | P Value | |||
| Baseline variablesa | 0.789 [0.757–0.819] | ||||
| Baseline variables+hs‐cTnT | 0.813 [0.781–0.838] | <0.001b | 0.033b | 27.7b | <0.001b |
| Baseline variables+hs‐cTnT+interaction terms between hs‐cTnT and sex | 0.815 [0.786–0.841] | 0.13c | 0.004c | 2.5c | 0.01c |
hs‐cTnT indicates high‐sensitivity cardiac troponin T; IDI, integrated discrimination index.
Including age, sex, body mass index, diabetes mellitus, hypertension, dyslipidemia, previous myocardial infarction, previous bypass surgery, left ventricular ejection fraction, glomerular filtration rate, multivessel disease, target vessel, restenotic lesion, reference diameter, and stent type.
Compared with the model with baseline variables.
Compared with the model with baseline variables+hs‐cTnT.
Discussion
This large cohort study showed 3 main findings. First, in patients with SCAD, who were treated with elective PCI, incidence of all‐cause and cardiac mortality were significantly higher in patients with elevated hs‐cTnT levels more than the sex‐specific 99th URL compared to those with normal hs‐cTnT levels. Second, continuous hs‐cTnT was an independent predictor for all‐cause mortality in the multivariate Cox proportional hazard model, and C‐statistics for predicting all‐cause mortality was improved by including hs‐cTnT as a variable in the standard model. Third, there was a significant interaction between sex and hs‐cTnT on all‐cause mortality in the multivariable adjusted model; differences between high and normal hs‐cTnT appeared to be more marked in male than in female patients, though they remained significant in both sex.
Circulating cardiac troponin concentrations can be elevated by various causes, including nonpathological mechanisms,18 and spontaneous elevation of high‐sensitivity cardiac troponins without obvious myocardial necrosis is well recognized.19 In addition, post‐PCI cardiac troponin is of little value as a prognostic factor compared to pre‐PCI level.12, 20 On the other hand, chronic elevation of high‐sensitivity cardiac troponin level was associated with chronic myocardial injury in the asymptomatic population21 and was an independent predictor of composite major cardiac adverse events in diabetic patients with SCAD.9 Furthermore, although a strong association between hs‐cTnT and coronary artery plaque burden, or inducible cardiac ischemia, in patients with SCAD has been identified,3, 4, 5 prompt revascularization did not affect the concentration of hs‐cTnT at follow‐up or clinical outcomes.9 These reports suggest that pre‐PCI hs‐cTnT could be an important biomarker for patients with SCAD undergoing elective PCI, because elevation above the normal range may be reflective of the underlying overall plaque burden and/or chronic cardiac injury, irrespective of which lesions will be treated with coronary angioplasty.
Our study demonstrated a strong prognostic value of hs‐cTnT for the risk to die of any cause and cardiac mortality in patients with SCAD who underwent elective PCI. When the sex‐specific 99th percentile URL was used as a cutoff, all‐cause and cardiac mortality after index PCI were higher in patients with elevated hs‐cTnT levels than in those with normal hs‐cTnT levels. Our results are in line with a recent analysis by Zanchin et al.22 In a cohort of 2029 patients undergoing PCI, they found that mortality within 1 year occurred more frequently in patients with elevated hs‐cTnT (7.7% vs 1.4%; HR, 5.73; 95% CI, 3.34–9.83; P<0.001). Similar observations were also observed in the setting of diabetic patients with SCAD who underwent revascularization.9 In another report, hs‐cTnI showed a similar prognostic value in patients undergoing elective PCI for SCAD.11 Our findings further validate the utility of cardiac troponins as a discriminative prognostic marker associated with all‐cause and cardiac mortality in patients undergoing elective PCI for SCAD.
In the current study, a statistically significant interaction between sex and hs‐cTnT on mortality was observed; there were more‐pronounced differences between high and normal hs‐cTnT groups in male than female patients. A similar trend has also been observed in the setting of general populations with elevated high‐sensitivity cardiac troponins in some reports.23, 24 On the other hand, contradictory findings were reported in other studies; a stronger prognostic value of high sensitivity cardiac troponins was observed in females than males in a general population setting,14 older adults,15 and patients with non‐ST‐elevation ACS.16 Because of the variability in the findings reported by previous studies investigating the relationship between sex differences in patients with elevated cardiac troponin, further validation studies are needed to determine the effect of sex on the prognostic value of high‐sensitivity cardiac troponins for all‐cause and cardiac mortality.
Our study has a number of strengths. First, to the best of our knowledge, this is the largest study investigating the prognostic value of hs‐cTnT in the setting of SCAD patients. Second, the distribution of the concentration of pre‐PCI hs‐cTnT in this study is likely to reflect a real‐world population attributed to systematic inclusion of consecutive patients. Third, our study provides precise data on lesion and procedural characteristics including quantitative coronary angiography analysis.
Our study also has several important limitations. First, because of the unbalanced proportion of male and female patients, our findings related to the effect of sex on the prognostic value of hs‐cTnT should be interpreted with caution. Second, our study did not include some variables related to cardiovascular outcomes (eg, the New York Heart Association functional classification, brain natriuretic peptide, or high‐sensitivity C‐reactive protein) into multivariable analysis. Third, although we show survival analysis out to 3 years, it should be noted that the median follow‐up of patients in our study was 14.5 months and the proportion of patients with complete follow‐up at 3 years was relatively low.
In conclusion, preprocedural hs‐cTnT was a strong predictor of mortality in the setting of patients with SCAD undergoing elective PCI. Differences in mortality between high and normal hs‐cTnT were more marked in men. Routine evaluation of hs‐cTnT before elective PCI seems to permit risk stratification for mortality in patients during subsequent follow‐up. Further studies are needed to investigate the effect of sex on the association between hs‐cTnT and mortality.
Disclosures
Colleran reports receiving support from the Irish Board for Training in Cardiovascular Medicine sponsored by MSD. Kastrati reports holding patents in relation to drug‐eluting stent technology. Byrne reports receiving lecture fees from B. Braun Melsungen AG, Biotronik, and Boston Scientific and institutional research grants from Boston Scientific and Heartflow. The other authors have no conflicts of interest to declare.
(J Am Heart Assoc. 2016;5:e004464 doi: 10.1161/JAHA.116.004464)
References
- 1. Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, Bax JJ, Borger MA, Brotons C, Chew DP, Gencer B, Hasenfuss G, Kjeldsen K, Lancellotti P, Landmesser U, Mehilli J, Mukherjee D, Storey RF, Windecker S, Baumgartner H, Gaemperli O, Achenbach S, Agewall S, Badimon L, Baigent C, Bueno H, Bugiardini R, Carerj S, Casselman F, Cuisset T, Erol Ç, Fitzsimons D, Halle M, Hamm C, Hildick‐Smith D, Huber K, Iliodromitis E, James S, Lewis BS, Lip GY, Piepoli MF, Richter D, Rosemann T, Sechtem U, Steg PG, Vrints C, Luis Zamorano J. 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST‐Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J. 2016;37:267–315. [DOI] [PubMed] [Google Scholar]
- 2. Sze J, Mooney J, Barzi F, Hillis GS, Chow CK. Cardiac troponin and its relationship to cardiovascular outcomes in community populations—a systematic review and meta‐analysis. Heart Lung Circ. 2016;25:217–228. [DOI] [PubMed] [Google Scholar]
- 3. Oemrawsingh RM, Cheng JM, García‐García HM, Kardys I, van Schaik RH, Regar E, van Geuns RJ, Serruys PW, Boersma E, Akkerhuis KM. High‐sensitivity troponin T in relation to coronary plaque characteristics in patients with stable coronary artery disease; results of the ATHEROREMO‐IVUS study. Atherosclerosis. 2016;247:135–141. [DOI] [PubMed] [Google Scholar]
- 4. Caselli C, Prontera C, Liga R, De Graaf MA, Gaemperli O, Lorenzoni V, Ragusa R, Marinelli M, Del Ry S, Rovai D, Giannessi D, Aguade‐Bruix S, Clemente A, Bax JJ, Lombardi M, Sicari R, Zamorano J, Scholte AJ, Kaufmann PA, Knuuti J, Underwood SR, Clerico A, Neglia D. Effect of coronary atherosclerosis and myocardial ischemia on plasma levels of high‐sensitivity troponin T and NT‐proBNP in patients with stable angina. Arterioscler Thromb Vasc Biol. 2016;36:757–764. [DOI] [PubMed] [Google Scholar]
- 5. Beatty AL, Ku IA, Christenson RH, DeFilippi CR, Schiller NB, Whooley MA. High‐sensitivity cardiac troponin T levels and secondary events in outpatients with coronary heart disease from the Heart and Soul Study. JAMA Intern Med. 2013;173:763–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Omland T, de Lemos JA, Sabatine MS, Christophi CA, Rice MM, Jablonski KA, Tjora S, Domanski MJ, Gersh BJ, Rouleau JL, Pfeffer MA, Braunwald E. A sensitive cardiac troponin T assay in stable coronary artery disease. N Engl J Med. 2009;361:2538–2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Koenig W, Breitling LP, Hahmann H, Wüsten B, Brenner H, Rothenbacher D. Cardiac troponin T measured by a high‐sensitivity assay predicts recurrent cardiovascular events in stable coronary heart disease patients with 8‐year follow‐up. Clin Chem. 2012;58:1215–1224. [DOI] [PubMed] [Google Scholar]
- 8. Lyngbæk S, Winkel P, Gøtze JP, Kastrup J, Gluud C, Kolmos HJ, Kjøller E, Jensen GB, Hansen JF, Hildebrandt P, Hilden J. Risk stratification in stable coronary artery disease is possible at cardiac troponin levels below conventional detection and is improved by use of N‐terminal pro‐B‐type natriuretic peptide. Eur J Prev Cardiol. 2014;21:1275–1284. [DOI] [PubMed] [Google Scholar]
- 9. Everett BM, Brooks MM, Vlachos HE, Chaitman BR, Frye RL, Bhatt DL. Troponin and cardiac events in stable ischemic heart disease and diabetes. N Engl J Med. 2015;373:610–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lepojärvi ES, Piira OP, Kiviniemi AM, Miettinen JA, Kenttä T, Ukkola O, Tulppo MP, Huikuri HV, Junttila MJ. Usefulness of highly sensitive troponin as a predictor of short‐term outcome in patients with diabetes mellitus and stable coronary artery disease (from the ARTEMIS Study). Am J Cardiol. 2016;117:515–521. [DOI] [PubMed] [Google Scholar]
- 11. Omland T, Pfeffer MA, Solomon SD, de Lemos JA, Røsjø H, Šaltyt≐ Benth J, Maggioni A, Domanski MJ, Rouleau JL, Sabatine MS, Braunwald E. Prognostic value of cardiac troponin I measured with a highly sensitive assay in patients with stable coronary artery disease. J Am Coll Cardiol. 2013;61:1240–1249. [DOI] [PubMed] [Google Scholar]
- 12. Lupi A, Rognoni A, Lazzero M, Rolla R, Pergolini P, Bellomo G, Rossi L, Sante Bongo A, Jaffe AS. Below normal pre‐procedural cardiac troponin I levels are associated with an adverse prognosis after percutaneous coronary interventions. EuroIntervention. 2016;11:1380–1388. [DOI] [PubMed] [Google Scholar]
- 13. Carda R, Aceña Á, Pello A, Cristóbal C, Tarín N, Huelmos A, Alonso J, Asensio D, Lorenzo Ó, Martín‐Ventura JL, Blanco‐Colio L, Farré J, López Bescós L, Egido J, Tuñón J. The prognostic value of high‐sensitive troponin I in stable coronary artery disease depends on age and other clinical variables. Cardiology. 2015;132:1–8. [DOI] [PubMed] [Google Scholar]
- 14. Omland T, de Lemos JA, Holmen OL, Dalen H, Benth JŠ, Nygård S, Hveem K, Røsjø H. Impact of sex on the prognostic value of high‐sensitivity cardiac troponin I in the general population: the HUNT study. Clin Chem. 2015;61:646–656. [DOI] [PubMed] [Google Scholar]
- 15. Dallmeier D, Denkinger M, Peter R, Rapp K, Jaffe AS, Koenig W, Rothenbacher D. Sex‐specific associations of established and emerging cardiac biomarkers with all‐cause mortality in older adults: the ActiFE study. Clin Chem. 2015;61:389–399. [DOI] [PubMed] [Google Scholar]
- 16. Eggers KM, Johnston N, James S, Lindahl B, Venge P. Cardiac troponin I levels in patients with non‐ST‐elevation acute coronary syndrome‐the importance of gender. Am Heart J. 2014;168:317–324.e1. [DOI] [PubMed] [Google Scholar]
- 17. van Buuren S, Groothuis‐Oudshoorn K. mice: Multivariate imputation by chained equations in R. J Stat Softw. 2011;45:1–67. [Google Scholar]
- 18. White HD. Pathobiology of troponin elevations: do elevations occur with myocardial ischemia as well as necrosis? J Am Coll Cardiol. 2011;57:2406–2408. [DOI] [PubMed] [Google Scholar]
- 19. Turer AT, Addo TA, Martin JL, Sabatine MS, Lewis GD, Gerszten RE, Keeley EC, Cigarroa JE, Lange RA, Hillis LD, de Lemos JA. Myocardial ischemia induced by rapid atrial pacing causes troponin T release detectable by a highly sensitive assay: insights from a coronary sinus sampling study. J Am Coll Cardiol. 2011;57:2398–2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Miller WL, Garratt KN, Burritt MF, Lennon RJ, Reeder GS, Jaffe AS. Baseline troponin level: key to understanding the importance of post‐PCI troponin elevations. Eur Heart J. 2006;27:1061–1069. [DOI] [PubMed] [Google Scholar]
- 21. Barbier CE, Themudo R, Bjerner T, Johansson L, Lindahl B, Venge P, Lind L, Ahlström H. Cardiac troponin I associated with the development of unrecognized myocardial infarctions detected with MRI. Clin Chem. 2014;60:1327–1335. [DOI] [PubMed] [Google Scholar]
- 22. Zanchin T, Räber L, Koskinas KC, Piccolo R, Jüni P, Pilgrim T, Stortecky S, Khattab AA, Wenaweser P, Bloechlinger S, Moschovitis A, Frenk A, Moro C, Meier B, Fiedler GM, Heg D, Windecker S. Preprocedural high‐sensitivity cardiac troponin T and clinical outcomes in patients with stable coronary artery disease undergoing elective percutaneous coronary intervention. Circ Cardiovasc Interv. 2016;9:e003202. [DOI] [PubMed] [Google Scholar]
- 23. de Lemos JA, Drazner MH, Omland T, Ayers CR, Khera A, Rohatgi A, Hashim I, Berry JD, Das SR, Morrow DA, McGuire DK. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA. 2010;304:2503–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Eggers KM, Johnston N, Lind L, Venge P, Lindahl B. Cardiac troponin I levels in an elderly population from the community—the implications of sex. Clin Biochem. 2015;48:751–756. [DOI] [PubMed] [Google Scholar]


