Abstract
Background
Extracorporeal cardiopulmonary resuscitation (ECPR) is widely proposed for the treatment of refractory cardiac arrest. It should be associated with coronary angiography if coronary artery disease is suspected. However, the prioritization of care remains unclear in this situation. Our goal was to determine whether coronary reperfusion should be instituted as soon as possible in such situations in a pig model.
Methods and Results
Anesthetized pigs were instrumented and submitted to coronary artery occlusion and ventricular fibrillation. After 5 minutes of untreated cardiac arrest, conventional cardiopulmonary resuscitation (CPR) was started. Fifteen minutes later, ECPR was initiated for a total duration of 240 minutes. Animals randomly underwent either early or late coronary reperfusion at 20 or 120 minutes of ECPR, respectively. This timing was adapted to the kinetic of infarct extension in pigs. Return of spontaneous circulation was determined as organized electrocardiogram rhythm with systolic arterial pressure above 80 mm Hg. During conventional CPR, hemodynamic parameters were not different between groups. Carotid blood flow then increased by 70% after the onset of ECPR in both groups. No animal (0 of 7) elicited return of spontaneous circulation after late reperfusion versus 4 of 7 after early reperfusion (P=0.025). The hemodynamic parameters, such as carotid blood flow, were also improved in early versus late reperfusion groups (113±20 vs 43±17 mL/min after 240 minutes of ECPR, respectively; P=0.030), along with infarct size decrease (71±4% vs 84±2% of the risk zone, respectively; P=0.013).
Conclusions
Early reperfusion improved hemodynamic status and facilitated return of spontaneous circulation in a porcine model of ischemic cardiac arrest treated by ECPR.
Keywords: cardiac arrest, cardiopulmonary resuscitation, ECMO, myocardial infarction, reperfusion
Subject Categories: Basic Science Research, Cardiopulmonary Resuscitation and Emergency Cardiac Care
Introduction
Out‐of‐hospital cardiac arrest is a major public health issue with very poor prognosis and neurological outcome. Chances of survival are extremely low when return of spontaneous circulation (ROSC) is not obtained within 15 to 20 minutes of collapse, despite pursuit of cardiopulmonary resuscitation (CPR).1 In order to improve this prognosis, extracorporeal CPR (ECPR) has recently emerged as a promising therapy for the management of “refractory cardiac arrest.”2, 3 In selected patients, ECPR was shown to increase the chances of ROSC through hemodynamic support and body oxygenation.4 As an example, Maekawa et al reports 3‐month survival rates as high as 29% with ECPR versus 8% with conventional CPR after out‐of‐hospital cardiac arrest of cardiac origin.5 Use of ECPR is now suggested for management of refractory cardiac arrest in the most recent American Heart Association guidelines for CPR and emergency cardiovascular care.6
Beyond the necessity to obtain ROSC, it is also crucial to properly treat the underlying cause of the cardiac arrest. Acute coronary syndrome is the most common etiology, especially in patients presenting out‐of‐hospital cardiac arrest with initial shockable rhythms. In this situation, immediate percutaneous coronary intervention was associated with better survival.7, 8, 9, 10 However, these studies were performed in patients successfully resuscitated with conventional CPR. In the case of refractory cardiac arrest and ECPR, the most recent guidelines do not dictate the prioritization of care, between hemodynamic stabilization in the intensive care unit or rapid transfer to the catheterization laboratory, if a coronary occlusion is suspected. Although immediate coronary reperfusion is recommended if underlying acute myocardial infarction is suspected, little evidence is available during ECPR.11, 12
In the present study, we attempted to determine whether coronary reperfusion should be done as early as possible during ECPR to improve resuscitation efficacy and cardiovascular outcomes in a porcine model of ischemic refractory cardiac arrest. We hypothesized that early reperfusion could facilitate ROSC and improve hemodynamic status. Accordingly, we compared the effect of an early versus late coronary reperfusion during ECPR. We evaluated hemodynamic and biochemical parameters, as well as infarct size. Importantly, myocardial infarction is known to expand much faster in pigs13 than humans and nonhuman primates.14 In this experimental study, we therefore used an accelerated time frame of ischemia and reperfusion as compared to the typical clinical situation and timing. “Early” reperfusion was considered after a total duration of ≈40 minutes of coronary artery occlusion, as compared to 140 minutes in the “late” reperfusion group. This was expected to induce an infarct size of ≈50% to 60% of the risk zone versus more than 80% in both conditions, respectively.
Materials and Methods
The protocol was approved by the French national ethical committee no. 16 (agreement 14/04/15‐6). Animals were hosted in a conventional animal room.
Animal Preparation
Eighteen female pigs crossed between Large White and Landrace were anesthetized with a mixture of ketamine (20 mg/kg, intramuscularly), acepromazine (0.25 mg/kg, intramuscularly), pentobarbital (10 mg/kg, intravenously) and buprenorphine was administered for analgesia (100 μg/kg, intravenously). They were intubated and submitted to conventional mechanical ventilation (FiO2=30%; tidal volume=10 mL/kg; respiratory rate=15–18 cycles/min). They were then instrumented with a carotid output flow probe (PS‐Series Probes, Transonic, NY), 2 fluid‐filled catheters into the left carotid artery and right atrium, and a hand‐made pneumatic occluder around the proximal left descending coronary artery. Two cannulae were also inserted into the right femoral vessels, 1 arterial (15F) and 1 venous (19F; HLS; Maquet, Rastatt, Germany) for further implementation of ECPR. Rectal temperature was maintained at 38.0±0.5°C. Throughout the protocol, carotid blood flow (Flowmeter‐TS420; PS‐Series Probes), arterial blood pressure, and electrocardiogram (ECG) were continuously recorded. Arterial blood samples were collected at baseline and 60 and 240 minutes after ECPR initiation.
Experimental Protocol
The experimental protocol is illustrated in Figure 1. After surgical preparation and stabilization, the pericoronary occluder was inflated to induce coronary artery occlusion. Two minutes later, ventricular fibrillation was induced by passing a transthoracic alternating current (30 V). Ventricular fibrillation was left untreated for 5 minutes, after which conventional CPR was initiated by continuous external chest compressions using an automated device (100 compressions/min; Thumper; Michigan Instruments, Grand Rapids, MI) and asynchronous ventilation (FiO2=100%; respiratory rate=9 cycles/min). After 15 minutes of conventional CPR, animals were shifted to ECPR using a transportable device (Cardiohelp; Maquet). Accordingly, circuit and membrane oxygenator (HLS; Maquet) were primed and heparinized (5000 IU). Extracorporeal blood flow was progressively increased to 50 to 60 mL/kg/min to optimize blood flow and reach the highest possible mean arterial pressure. After 10 minutes of ECPR, animals were divided into 2 experimental groups randomly submitted to early or late coronary reperfusion by deflation of the pericoronary occluder after 20 or 120 minutes of ECPR, respectively. ECPR was maintained for a total duration of 240 minutes. In both groups, fluids were administered using 13 mL/kg of blood and 500 mL/h of saline 0.9%. Norepinephrine was also administered at a fixed rate of 10 μg/kg/min. External defibrillation was attempted every 5 minutes during the first 15 minutes of ECPR and then every 15 minutes until successful defibrillation. As previously described, return of spontaneous heart beat (ROSB) was defined as successful defibrillation with organized ventricular contraction.15 ROSC was defined as an organized ECG rhythm with a systolic blood pressure above 80 mm Hg12, 15, 16 until the end of the protocol. ECPR blood flow was maintained as high as possible to avoid hypotension, whereas it was progressively decreased if ROSC occurred. During ECPR, animals were ventilated with low tidal volumes (7 mL/kg; FiO2=21%) and respiratory rate (10 cycles/min) until ROSB. After ROSB, respiratory parameters were modified depending on blood gases results. At the end of the protocol, animals were sacrificed and the heart was removed for pathological analyses. Myocardial risk zone and infarct size were determined using Evans blue perfusion and triphenyltetrazolium chloride staining, respectively.
Figure 1.
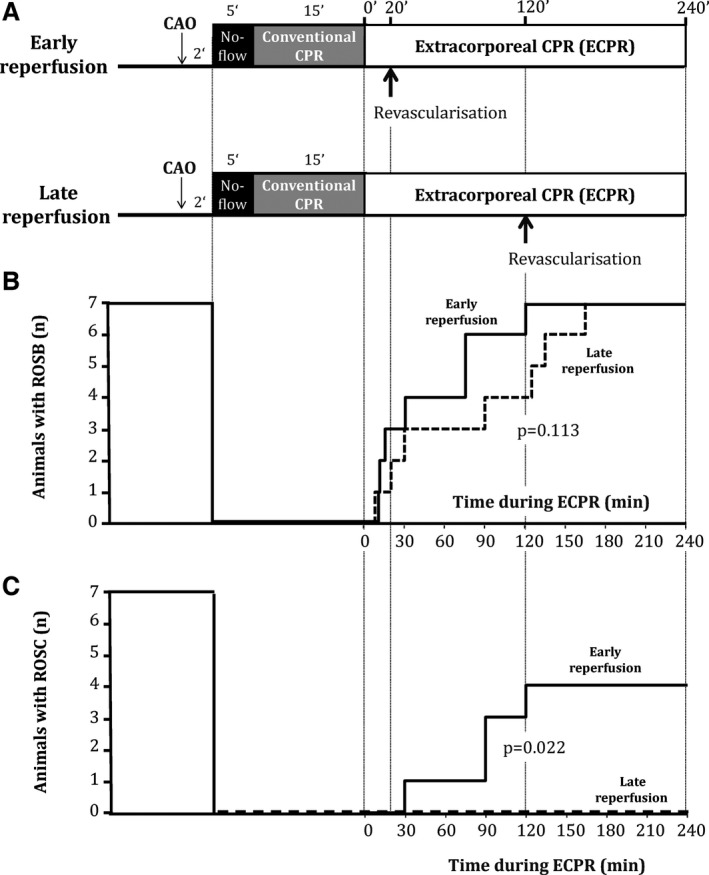
Experimental protocol (A), number of animals eliciting return of spontaneous heart beat (ROSB; B) and number of animals eliciting return of spontaneous circulation (ROSC; C) during extracorporeal cardiopulmonary resuscitation (ECPR). ROSB was defined as successful defibrillation with organized ventricular contraction. ROSC was defined as an organized rhythm with a systolic blood pressure above 80 mm Hg. In (B and C), solid and dashed lines represent the number of animals that achieved ROSC or ROSB in the early versus late reperfusion group, respectively. CAO indicates coronary artery occlusion; CPR, cardiopulmonary resuscitation.
Investigated Parameters
The primary endpoint of the study was the rate of ROSC. The secondary endpoints were the rate of ROSB, ECPR flow, infarct size, and carotid blood flow, as a predictor of subsequent neurological sequelae.17, 18 We also investigated arterial blood gases and blood levels of lactate, creatinine, troponine I, protein S100 (PS100), and alanine aminotransferase (ALAT).
Statistical Analyses
Data are expressed as mean±SEM. ROSC and ROSB occurrence were compared between groups using a log‐rank analysis throughout follow‐up. Body weight and infarct size were compared using an unpaired Student t test. Hemodynamic parameters were compared using a 2‐way ANOVA for repeated measures with a Fisher's least significant difference post‐hoc analysis. ANOVA took into account the group effect, time effect, and their interaction. Two distinct analyses were performed (ie, before and after randomization, respectively). The first analysis was performed with values acquired with the following time points: baseline, CPR, and early ECPR. Corresponding post‐hoc analyses were only performed between groups, and between CPR and ECPR, in order to avoid multiple comparisons. The second analysis was performed after randomization and successful defibrillation of all animals with the following time points: 180 and 240 minutes of ECPR. Post‐hoc analyses were then only performed between groups. Finally, biochemical parameters were compared with a Mann–Whitney test at baseline and 60 and 240 minutes after ECPR institution. Analyses were performed using Sigma Stat 3.5 (Systat Software Inc., Chicago, IL). Significant differences were determined at P≤0.05.
Results
Baseline Characteristics
Eighteen swine were enrolled in the present study. Among them, 4 were excluded for technical issues during instrumentation. Fourteen animals were ultimately submitted to ECPR in the 2 experimental groups (ie, 7 animals in both early and late reperfusion groups). Body weights were similar between groups (31.8±1.7 vs 31.7±0.7 kg, respectively), as well as baseline values of hemodynamic and biochemical parameters (Tables 1 and 2). Mean arterial pressure and carotid blood flow were also not different between groups during conventional CPR.
Table 1.
Hemodynamic Parameters in Both Groups at Baseline, During Conventional CPR and 10 Minutes After Initiation of ECPR, That Is, Before Reperfusion and ROSB
| Parameters and Groups | Baseline | Conventional CPR (t=10 minutes) | ECPR (t=10 minutes) | P Value* |
|---|---|---|---|---|
| No. of animals | ||||
| Early reperfusion | 7 | 7 | 7 | — |
| Late reperfusion | 7 | 7 | 7 | |
| Mean arterial blood pressure, mm Hg | ||||
| Early reperfusion | 77±4 | 32±3 | 48±5 | <0.001 |
| Late reperfusion | 81±5 | 33±4 | 46±2 | |
| Maximal arterial blood pressure, mm Hg | ||||
| Early reperfusion | 91±4 | 62±8 | 55±8 | 0.148 (NS) |
| Late reperfusion | 97±6 | 66±16 | 49±3 | |
| Minimal arterial blood pressure, mm Hg | ||||
| Early reperfusion | 65±3 | 9±5 | 45±4* | <0.001 |
| Late reperfusion | 69±4 | 8±4 | 44±2* | |
| Carotid blood flow, mL/min | ||||
| Early reperfusion | 342±33 | 87±10 | 150±21* | 0.012 |
| Late reperfusion | 332±22 | 89±19 | 140±25* | |
| ECPR blood flow, L/min | ||||
| Early reperfusion | — | — | 1.9±0.1 | — |
| Late reperfusion | — | — | 1.7±0.2 | |
| Heart rate, bpm | ||||
| Early reperfusion | 138±18 | — | — | — |
| Late reperfusion | 122±11 | — | — | |
P value represents time effect between ECPR and conventional CPR. No group effect was observed these time points (ie, before group allocation after 10 minutes of ECPR). No comparison was performed versus baseline. Data are expressed as mean±SEM. CPR indicates cardiopulmonary resuscitation; ECPR, extracorporeal cardiopulmonary resuscitation; NS, not significant; ROSB, return of spontaneous beats.
*P<0.05 between conventional CPR and ECPR.
Table 2.
Biochemical Parameters in Both Groups Throughout Protocol
| Parameters and Groups | Baseline | ECPR (t=60 minutes) | ECPR (t=240 minutes) | P Value |
|---|---|---|---|---|
| No. of animals | ||||
| Early reperfusion | 7 | 7 | 7 | |
| Late reperfusion | 7 | 7 | 7 | |
| Arterial pH | ||||
| Early reperfusion | 7.36±0.02 | 7.13±0.04 | 7.05±0.01 | 0.620 (NS) |
| Late reperfusion | 7.36±0.02 | 7.14±0.03 | 7.05±0.02 | |
| Arterial pCO2, mm Hg | ||||
| Early reperfusion | 45±2 | 44±4 | 34±4 | 0.128 (NS) |
| Late reperfusion | 45±2 | 45±4 | 26±4 | |
| Arterial pO2, mm Hg | ||||
| Early reperfusion | 147±2 | 354±80 | 295±83 | 0.097 (NS) |
| Late reperfusion | 137±7 | 289±75 | 460±73 | |
| Blood lactates levels, mmol/L | ||||
| Early reperfusion | 3.0±1.6 | 10.0±2.0 | 11.9±2.7* | 0.038 |
| Late reperfusion | 1.9±0.1 | 11.4±0.6 | 16.7±1.0 | |
| Blood creatinine levels, mmol/L | ||||
| Early reperfusion | 146±30 | 210±29 | 171±3* | 0.038 |
| Late reperfusion | 130±6 | 207±12 | 238±15 | |
| Blood ALAT levels, UI/L | ||||
| Early reperfusion | 36.6±5.0 | 45.7±6.5 | 44.0±7.6 | 0.445 (NS) |
| Late reperfusion | 41.7±3.9 | 51.7±6.5 | 54.8±10.4 | |
| Blood PS100 levels, μg/L | ||||
| Early reperfusion | 0.71±0.08 | — | 2.78±0.48 | 0.535 (NS) |
| Late reperfusion | 0.64±0.03 | — | 3.74±0.85 | |
P value represents group effect between Early and Late reperfusion groups at 240 minutes of ECPR. No significant group effect was observed at 60 minutes of ECPR. No comparison was performed among the different time points. Data are expressed as mean±SEM. ALAT indicates alanine aminotransferase; ECPR, extracorporeal cardiopulmonary resuscitation; LDH, lactate deshydrogenase; NS, not significant; PS100, protein S100.
*P<0.05 vs “Late reperfusion” group.
ECPR Improves Hemodynamic Parameters as Compared to Conventional CPR
From the onset of ECPR, mean arterial pressure and carotid blood flow were increased as compared to conventional CPR (Table 1). Carotid blood flow achieved 150±21 mL/min with ECPR as compared to 87±10 mL/min with conventional CPR at t=10 minutes of ECPR in the early reperfusion group. This increase was a direct effect of ECPR because animals were not yet reperfused or defibrillated at this time point. As shown in Table 1, this initial improvement was similar in both groups, as expected, with similar ECPR flows at the beginning of the procedure (1.9±0.1 and 1.7±0.2 L/min in early and late reperfusion groups, respectively). Arterial pH and partial pressures of O2 and CO2 levels were not different among groups throughout the protocol (Table 2).
Early Reperfusion Allows ROSC During ECPR and Limits Shock Status
During ECPR prolongation, all animals progressively achieved ROSB after defibrillation attempts. Mean time to obtain ROSB was slightly shorter in the early versus late reperfusion group (48±17 vs 82±25 minutes, P=0.113), but the difference did not reach statistical significance. ROSB did not always lead to a better hemodynamic status given that ROSC was observed in 4 animals in the early reperfusion group versus 0 in the late reperfusion group (P=0.022 between groups). Arterial pressure was consistently improved in the early versus late reperfusion group (Figure 2). Importantly, this allowed a very rapid decrease of the ECPR flow after ROSB in the early reperfusion group (Figure 3). Two animals could be weaned from ECPR in this group.
Figure 2.
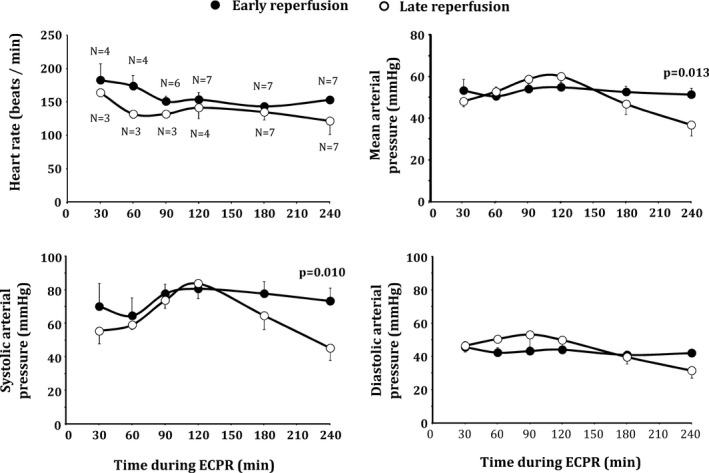
Heart rate and blood pressure after return of spontaneous heart beat (ROSB) during extracorporeal cardiopulmonary resuscitation (ECPR) in both groups. The number of animals with ROSB is shown on the first panel at each time point. Statistical analyses were performed at 180 minutes and 240 of ECPR, after which all animals achieved ROSB. P value corresponds to significant group effect at corresponding time point. No comparison was made among the different time points. Data are expressed as mean±SEM.
Figure 3.
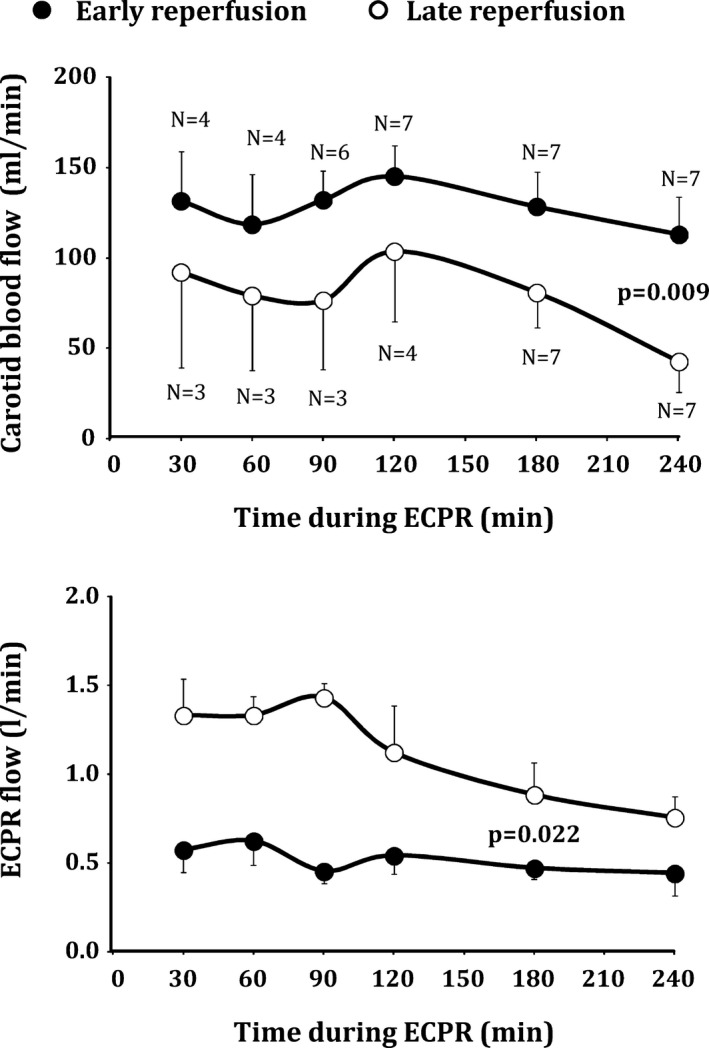
Carotid and extracorporeal cardiopulmonary resuscitation (ECPR) blood flow after resumption of spontaneous heart beat (ROSB) in both groups. Statistical analyses were only performed at 180 minutes and 240 of ECPR, after which all animals achieved ROSB. P value corresponds to significant group effect at corresponding time point. No comparison was made among the different time points. Data are expressed as mean±SEM.
In the late reperfusion group, ECPR flow remained high and showed greater dependency on circulatory support. However, it was impossible to maintain high ECPR flow at the end of the follow‐up (240 minutes of ECPR), because a flow limitation occurred progressively attributed to the severe shock status and venous return limitation. No animals were weaned from ECPR in this group.
Early Reperfusion Improves Cerebral Perfusion After ECPR and Limits Myocardial Infarct Size
In line with improved hemodynamic status, carotid blood flow was significantly higher in the early versus late reperfusion group after ROSB (eg, 113±20 vs 43±17 mL/min in both groups at the end of the procedure, respectively; P=0.009). The decreased shock status in the early reperfusion group was also supported by the attenuation of lactate and creatinine blood levels at the end of the follow‐up as compared to late reperfusion. ALAT blood levels were not different among groups, but they elicited only a very mild increase after cardiac arrest. PS100 blood levels were also not significantly different between groups.
Importantly, infarct size was significantly reduced in the early versus late reperfusion group (71±4% vs 84±2%, respectively; P=0.013) despite a similar risk zone (31±3% vs 30±4%, respectively).
Discussion
The present study shows that early coronary reperfusion can improve hemodynamics and limit shock status during ECPR in a pig model of refractory cardiac arrest. This was shown by an improved rate of ROSC, enhanced cerebral perfusion, and reduced infarct size. The novelty of the study is to evaluate ECPR during experimental cardiac arrest with concomitant coronary artery occlusion and reperfusion. To our knowledge, previous experimental studies were performed in “non ischemic” hearts13, 19, 20 or without reperfusion.12 Our results suggest that revascularization should be prioritized during ECPR when an ischemic origin is suspected.
ECPR Is Efficient for the Treatment of Refractory Cardiac Arrest
Experimental evidence on refractory cardiac arrest is rather scarce. Most studies were done in non ischemic refractory cardiac arrest, after which it has been well demonstrated that ECPR increases the chances of ROSC.16, 19, 20 Here, we confirm that ECPR improves hemodynamic parameters given that mean arterial pressure and carotid blood flow increased by more than 50% when compared with conventional CPR, respectively. In a previous report, Reynolds et al reported consistent results with a comparison of hemodynamic parameters during ECPR and conventional CPR in pigs submitted to 8 or 15 minutes of ventricular fibrillation and to 30 to 60 minutes of conventional CPR before ECPR.16 They showed that mean arterial pressure, coronary perfusion pressure, and amplitude spectrum area of ventricular fibrillation were significantly improved by ECPR. The rate of ROSC was also higher when animals underwent early ECPR, emphasizing the relevance of a rapid initiation of ECPR after cardiac arrest. Mlček et al also showed that hemodynamic and biological alterations could be rapidly restored when ECPR was started immediately after a prolonged cardiac arrest of 20 minutes in swine.19 This is consistent with recent clinical reports starting ECPR as early as possible after cardiac arrest.21
To our knowledge, only 1 study was performed with ECPR after ischemic cardiac arrest in sheep.12 This study showed that ECPR was more efficient than conventional CPR to achieve ROSC, but the impact of reperfusion was not tested. In the present study, we attempted to mimic the typical clinical situation in which ECPR could be combined to reperfusion.4 Preliminary and unpublished data confirmed the severity of our model, which, in the absence of ECPR, led to unsuccessful CPR, even after prolonged conventional CPR and coronary revascularization. With ECPR, all animals were successfully defibrillated and obtained ROSB, again showing the potency of ECPR versus conventional CPR, independently from the time of reperfusion.
Reperfusion Is Essential After Cardiac Arrest, Even During ECPR
In the present study, the major finding is the strong cardiovascular improvement with early versus late reperfusion during ECPR. In the group with late reperfusion, we chose a total duration of ischemia of more than 140 minutes, which led to high infarct size and minimal salvage at reperfusion in pigs.22 In such conditions, animals were initially defibrillated, but none achieved ROSC. A dramatic shock status was evidenced by a low arterial pressure, decreased cerebral perfusion, and high blood lactate levels, despite fluid and vasopressors administration. In addition, it was progressively impossible to maintain high ECPR flow given that venous return was insufficient. This could be related to a progressive no reflow that seems to occur after prolonged cardiac arrest given that thrombolytic drugs were shown to improve the outcome in pigs during ECPR.15 This shows that cardiovascular recovery was not possible in our study with late reperfusion, despite successful defibrillation after ECPR.
Importantly, we observed a dramatic contrast in the group with early reperfusion. In that group, reperfusion was started after 20 minutes of ECPR and a total ischemic duration of 42 minutes. This short duration was chosen to induce an infarction of ≈50% to 60% of the risk zone because infarct expands faster in pigs than humans.13 Interestingly, we observed higher infarct size than expected (71±4%) as a possible consequence of worsened infarction after cardiac arrest and ECPR. Therefore, the actual decrease in infarct size was limited with early versus late reperfusion (only −15%), but led to major improvements on systemic hemodynamics and cerebral perfusion. This allowed ROSC in 4 of 7 animals and weaning in 2/7 (vs 0 with late reperfusion). Overall, this demonstrates that ECPR potentiated by early reperfusion could lead to rapid cardiovascular improvement, whereas ECPR loses a large part of its benefit if reperfusion is delayed.
Sideris et al found similar results in pigs resuscitated after ischemic cardiac arrest and conventional CPR.23 In this study, early reperfusion increased survival and improved neurological recovery after 24 hours. More important, this is consistent with clinical reports showing that immediate coronary angiography improves survival in comatose victims of cardiac arrest after resuscitation with conventional CPR.7, 8 This was recently confirmed by a cohort study from the large PROCAT registry8 and a meta‐analysis.9
Clinical Perspectives
Today, there are no guidelines regarding the immediate care of cardiac arrest patients undergoing ECPR. There is little clinical evidence of the necessity to obtain early reperfusion during post‐arrest ECPR.11 Indeed, patients can be very unstable and one might speculate that initial intensive care and stabilization should be done before revascularization. The present study suggests that revascularization should be done as early as possible to allow the cardiovascular benefits of ECPR. In other words, the patient should have a coronary angiography as soon as possible, supporting ECPR implementation as close as possible to a cath lab. The use of dedicated valves for cannula could also facilitate coronary angiography without need for repeated arterial access. It could be particularly relevant in patients with very poor pulsatility.
In a recent single‐center study, Stub et al demonstrated that the combination of ECPR, revascularization, and hypothermia4 could lead to a global survival rate with good neurological outcome as high as 54% in a limited number of patients with in‐ or out‐of‐hospital cardiac arrest (14 of 26 patients). It is now important to determine the individual benefit of each particular treatment. Prospective trials are now required to rationalize the treatments of those patients.
Study Limitations
Our study presents several limitations. As an example, we used a schematic design, which does not directly mimic the typical timeline in the clinical arena. As previously discussed, our rationale was mostly directed to obtain a certain level of infarct size, taking into account the rapid infarct expansion in pigs. Moreover, we were not able to follow the animals for a longer duration. Our goal was to investigate the acute hemodynamic parameters and not long‐term survival and neurological recovery. However, it was previously demonstrated that hemodynamic stability and improved carotid blood flow was somehow related to better survival and neurological outcome on the longer term.17, 18 Further studies will be required to confirm these findings after ECPR and refractory cardiac arrest.24
Conclusion
In a porcine model of cardiac arrest, early coronary reperfusion was associated with better hemodynamic status and increased chances of ROSC during ECPR. This supports the use of rapid reperfusion in this situation, rather than delaying revascularization for hemodynamic stabilization. These findings support the need for clinical trials to confirm these results in patients.
Sources of Funding
The study was funded by the “Fondation de l'Avenir” (Paris, France; RMA‐2015‐039), Inserm (Paris, France), Ecole Nationale Vétérinaire d'Alfort (Maisons‐Alfort, France), Region Ile‐de France (CORDDIM, Paris, France), and Université Paris‐Est Créteil (Créteil, France).
Disclosures
None.
(J Am Heart Assoc. 2016;5:e004588 doi: 10.1161/JAHA.116.004588)
References
- 1. Reynolds JC, Frisch A, Rittenberger JC, Callaway CW. Duration of resuscitation efforts and functional outcome after out‐of‐hospital cardiac arrest: when should we change to novel therapies? Circulation. 2013;128:2488–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Morimura N, Sakamoto T, Nagao K, Asai Y, Yokota H, Tahara Y, Atsumi T, Nara S, Hase M. Extracorporeal cardiopulmonary resuscitation for out‐of‐hospital cardiac arrest: a review of the Japanese literature. Resuscitation. 2011;82:10–14. [DOI] [PubMed] [Google Scholar]
- 3. Patroniti N, Sangalli F, Avalli L. Post‐cardiac arrest extracorporeal life support. Best Pract Res Clin Anaesthesiol. 2015;29:497–508. [DOI] [PubMed] [Google Scholar]
- 4. Stub D, Bernard S, Pellegrino V, Smith K, Walker T, Sheldrake J, Hockings L, Shaw J, Duffy SJ, Burrell A, Cameron P, Smit DV, Kaye DM. Refractory cardiac arrest treated with mechanical CPR, hypothermia, ECMO and early reperfusion (the CHEER trial). Resuscitation. 2015;86:88–94. [DOI] [PubMed] [Google Scholar]
- 5. Maekawa K, Tanno K, Hase M, Mori K, Asai Y. Extracorporeal cardiopulmonary resuscitation for patients with out‐of‐hospital cardiac arrest of cardiac origin: a propensity‐matched study and predictor analysis. Crit Care Med. 2013;41:1186–1196. [DOI] [PubMed] [Google Scholar]
- 6. Neumar RW, Shuster M, Callaway CW, Gent LM, Atkins DL, Bhanji F, Brooks SC, de Caen AR, Donnino MW, Ferrer JME. Part 1: executive summary: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015;132:S315–S367. [DOI] [PubMed] [Google Scholar]
- 7. Spaulding CM, Joly LM, Rosenberg A, Monchi M, Weber SN, Dhainaut JF, Carli P. Immediate coronary angiography in survivors of out‐of‐hospital cardiac arrest. N Engl J Med. 1997;336:1629–1633. [DOI] [PubMed] [Google Scholar]
- 8. Dumas F, Cariou A, Manzo‐Silberman S, Grimaldi D, Vivien B, Rosencher J, Empana JP, Carli P, Mira JP, Jouven X, Spaulding C. Immediate percutaneous coronary intervention is associated with better survival after out‐of‐hospital cardiac arrest: insights from the PROCAT (Parisian Region Out of hospital Cardiac ArresT) registry. Circ Cardiovasc Interv. 2010;3:200–207. [DOI] [PubMed] [Google Scholar]
- 9. Camuglia AC, Randhawa VK, Lavi S, Walters DL. Cardiac catheterization is associated with superior outcomes for survivors of out of hospital cardiac arrest: review and meta‐analysis. Resuscitation. 2014;85:1533–1540. [DOI] [PubMed] [Google Scholar]
- 10. Noc M, Fajadet J, Lassen JF, Kala P, MacCarthy P, Olivecrona GK, Windecker S, Spaulding C. Invasive coronary treatment strategies for out‐of‐hospital cardiac arrest: a consensus statement from the European association for percutaneous cardiovascular interventions (EAPCI)/stent for life (SFL) groups. EuroIntervention. 2014;10:31–37. [DOI] [PubMed] [Google Scholar]
- 11. Kagawa E, Dote K, Kato M, Sasaki S, Nakano Y, Kajikawa M, Higashi A, Itakura A, Sera A, Inoue I, Kawagoe T, Ishihara M, Shimatani Y, Kurisu S. Should we emergently revascularize occluded coronaries for cardiac arrest? Rapid‐response extracorporeal membrane oxygenation and intra‐arrest percutaneous coronary intervention. Circulation. 2012;126:1605–1613. [DOI] [PubMed] [Google Scholar]
- 12. Stub D, Byrne M, Pellegrino V, Kaye DM. Extracorporeal membrane oxygenation to support cardiopulmonary resuscitation in a sheep model of refractory ischaemic cardiac arrest. Heart Lung Circ. 2013;22:421–427. [DOI] [PubMed] [Google Scholar]
- 13. Jablonowski R, Engblom H, Kanski M, Nordlund D, Koul S, van der Pals J, Englund E, Heiberg E, Erlinge D, Carlsson M, Arheden H. Contrast‐enhanced CMR overestimates early myocardial infarct size: mechanistic insights using ECV measurements on day 1 and day 7. JACC Cardiovasc Imaging. 2015;8:1379–1389. [DOI] [PubMed] [Google Scholar]
- 14. Shen YT, Fallon JT, Iwase M, Vatner SF. Innate protection of baboon myocardium: effects of coronary artery occlusion and reperfusion. Am J Physiol. 1996;270:H1812–H1818. [DOI] [PubMed] [Google Scholar]
- 15. Spinelli E, Davis RP, Ren X, Sheth PS, Tooley TR, Iyengar A, Sowell B, Owens GE, Bocks ML, Jacobs TL. Thrombolytic‐enhanced extracorporeal cardiopulmonary resuscitation after prolonged cardiac arrest. Crit Care Med. 2016;44:e58–e69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Reynolds JC, Salcido DD, Sundermann ML, Koller AC, Menegazzi JJ. Extracorporeal life support during cardiac arrest resuscitation in a porcine model of ventricular fibrillation. J Extra Corpor Technol. 2013;45:33–39. [PMC free article] [PubMed] [Google Scholar]
- 17. Yang F, Jia Z, Xing J, Wang Z, Liu Y, Hao X, Jiang CJ, Wang H, Jia M, Hou XT. Effects of intra‐aortic balloon pump on cerebral blood flow during peripheral venoarterial extracorporeal membrane oxygenation support. J Transl Med. 2014;12:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Metzger AK, Herman M, McKnite S, Tang W, Yannopoulos D. Improved cerebral perfusion pressures and 24‐hr neurological survival in a porcine model of cardiac arrest with active compression‐decompression cardiopulmonary resuscitation and augmentation of negative intrathoracic pressure. Crit Care Med. 2012;40:1851–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mlček M, Ošťádal P, Bělohlávek J, Havránek Š, Hrachovina M, Huptych M, Hála P, Hrachovina V, Neužil P, Kittnar O. Hemodynamic and metabolic parameters during prolonged cardiac arrest and reperfusion by extracorporeal circulation. Physiol Res. 2012;61:S57–S65. [DOI] [PubMed] [Google Scholar]
- 20. Bergan HA, Halvorsen PS, Skulstad H, Edvardsen T, Fosse E, Bugge JF. Successful ECMO‐cardiopulmonary resuscitation with the associated post‐arrest cardiac dysfunction as demonstrated by MRI. Intensive Care Med Exp. 2015;3:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kim SJ, Jung JS, Park JH, Park JS, Hong YS, Lee SW. An optimal transition time to extracorporeal cardiopulmonary resuscitation for predicting good neurological outcome in patients with out‐of‐hospital cardiac arrest: a propensity‐matched study. Crit Care. 2014;18:535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Miyazaki S, Fujiwara H, Onodera T, Kihara Y, Matsuda M, Wu DJ, Nakamura Y, Kumada T, Sasayama S, Kawai C. Quantitative analysis of contraction band and coagulation necrosis after ischemia and reperfusion in the porcine heart. Circulation. 1987;75:1074–1082. [DOI] [PubMed] [Google Scholar]
- 23. Sideris G, Magkoutis N, Sharma A, Rees J, McKnite S, Caldwell E, Sarraf M, Henry P, Lurie K, Garcia S, Yannopoulos D. Early coronary revascularization improves 24 h survival and neurological function after ischemic cardiac arrest. A randomized animal study. Resuscitation. 2014;85:292–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bartos JA, Matsuura TR, Sarraf M, Youngquist ST, McKnite SH, Rees JN, Sloper DT, Bates FS, Segal N, Debaty G. Bundled postconditioning therapies improve hemodynamics and neurologic recovery after 17 min of untreated cardiac arrest. Resuscitation. 2015;87:7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]


