Abstract
Background
Biomarker activation in atrial fibrillation (AF) has been widely studied, but the immediate effect of AF initiation remains unclear. We studied the effect of AF initiation on 2 cardiac biomarkers: the N‐terminal fragment of the proB‐type natriuretic peptide (NT‐proBNP), the midregional fragment of the N‐terminal of pro‐atrial natriuretic peptide (MR‐proANP), and 2 extracardiac biomarkers—the copeptin and the midregional portion of proadrenomedullin (MR‐proADM).
Methods and Results
This was a randomized controlled study, including 45 patients with AF who had been referred for radiofrequency ablation to the University Hospital, Linköping, Sweden, between February 2012 and April 2014. Freedom from AF during the 4 days prior to radiofrequency ablation was confirmed by transtelephonic ECGs. Biomarkers were collected from the femoral vein (fv), coronary sinus (CS), and left atrium (LA) prior to AF initiation (baseline) and 30 minutes later. The MR‐proANP and NT‐proBNP concentrations increased in the intervention group compared with the control group 30 minutes after the initiation of AF (MR‐proANP: P fv<0.001, P CS<0.001, P LA<0.001; NT‐proBNP: P LA<0.001). Copeptin levels in patients without ischemic heart disease were decreased after the initiation of AF (P fv=0.003, P CS=0.015, P LA=0.011).
Conclusions
AF is a strong stimulus that results in immediate activation of different biomarkers.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT01553045.
Keywords: atrial fibrillation, biomarkers, radiofrequency ablation
Subject Categories: Arrhythmias
Introduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia, with an estimated prevalence of 2.9% in the Swedish population.1 Both cardiac and extracardiac biomarkers have been associated with clinical events in patients with AF.2
The 2 cardiac biomarkers—the atrial and the N‐terminal proB‐type natriuretic peptide (ANP and NT‐proBNP, respectively)—are synthesized and secreted from atrial and ventricular myocytes3 in response to increased wall tension.4 Although the most common cause of increased NT‐proBNP production is heart failure (HF), elevated concentrations of NT‐proBNP have been reported in patients with AF even without overt HF.5 Moreover, in a community‐based population, NT‐proBNP indicated a substantial risk of developing AF.6 ANP and its precursor protein proANP have not been pursued in clinical studies because they are prone to fragmentation, which means plasma levels can be underestimated.7 Therefore, a stable method for quantifying the midregional fragment of proANP (MR‐proANP) was introduced,7 and the biomarker seems to correlate with the duration of AF episodes.8
Furthermore, there are some extracardiac biomarkers of potential interest in AF. For example, arginine vasopressin (AVP) is an important hormone for the fluid regulation of the body. The plasma concentration of vasopressin increases in patients with HF due to inadequate cardiac output or low blood pressure and increased vascular resistance.9, 10 The C‐terminal fragment of provasopressin (copeptin) is released in equimolar amounts to AVP and is easier to determine.11 Thus, copeptin can serve as a surrogate marker for AVP secretion. Copeptin has proved to be a prognostic biomarker in patients with HF9, 12 and an additional biomarker to conventional troponin to rule out myocardial infarction (MI) early when a highly sensitive troponin assay is not available.13
Adrenomedullin (ADM), an extracardiac peptide with vasoactive and natriuretic properties, has also emerged as a biomarker of potential interest in the prediction of cardiovascular disease risks.14 It is secreted mainly from the adrenal medulla and endothelial cells in response to several hormonal agents and physical stimulants, such as shear stress and stretch.15, 16, 17, 18 Due to the short half‐life of ADM and its immediate binding to receptors, its evaluation in clinical routines is impractical. The MR‐proADM, a product of the parental molecule of ADM, is stable and therefore better suited for clinical use.19 Recent research has shown that MR‐proADM levels predict the recurrence of AF after radiofrequency ablation (RFA).20
Even though these biomarkers have been widely studied in the context of cardiovascular disease and to some extent in AF, to the best of our knowledge, no study has previously evaluated the immediate effect of AF initiation.
The aim of this study is to investigate the response of the biomarkers MR‐proANP, NT‐proBNP, copeptin, and MR‐proADM immediately after the initiation of AF in patients eligible for RFA and in sinus rhythm (SR) prior to the procedure.
Methods
Study Design
This is a randomized, controlled, parallel, single‐center study with an allocation ratio of 2:1 in favor of the interventional group. It is the interventional part of the Symptom burden, Metabolic profile, Ultrasound findings, Rhythm, neurohormonal activation, hemodynamics and health‐related quality of life in patients with atrial Fibrillation (SMURF) study. The design of the SMURF study has been previously presented.21
Participants
The study was conducted between February 2012 and April 2014. All patients with AF referred for RFA to the University Hospital in Linköping, Sweden, were eligible for participation. The inclusion criteria were: (1) patients older than 17 years with paroxysmal or persistent AF, (2) patients referred to the hospital for first‐time RFA treatment, and (3) with sufficient knowledge of the Swedish language to fill out the study questionnaires independently.
Exclusion criteria were patients (1) who previously had undergone catheter or surgical AF ablation, (2) with previous or expected heart surgery, (3) with severe HF with left ventricular (LV) ejection fraction (EF) <35%, (4) with acute coronary syndrome during the past 3 months, and (5) with 1 or more arrhythmia episodes in the 4‐day period prior to RFA.
The Regional Ethical Review Board in Linköping, Sweden, approved the protocol (Registration number 2011/40‐31). All patients gave their written informed consent, and the study participation was documented in the patient's medical record. The study complies with the Declaration of Helsinki.22
Transtelephonic ECG
The transtelephonic ECG registrations were performed twice a day and if symptoms occurred. All ECGs were sent by telephone to a centralized, secure socket layer encrypted digital ECG database on the Internet that can only be accessed by authorized personnel. Rhythm analysis was performed prior to the randomization of eligible patients. The rhythm was classified into the following categories: SR, AF, atrial tachycardia, or “not specified rhythm.”
The system used for this study was developed by Zenicor AB (Stockholm, Sweden) and consisted of a device that can register a short ECG sequence (30 seconds) by placing the thumbs on 2 measuring plates. The method was validated23 and used in order to improve the screening of silent AF in patients with ischemic stroke24 and as an event recorder.
Ablation Procedure
The procedures were performed under conscious sedation as described previously.21 The endpoint of the procedure was an electrical disconnection of all pulmonary veins by antral ablation verified by both entry and exit blocks. In patients with persistent AF, additional ablation was performed in order to create left atrial (LA) lines at the discretion of the operator and verified by pacing maneuvers.
Biomarkers
Blood samples were collected in plastic vials containing ethylenediaminetetraacetic acid (EDTA). The vials were centrifuged at 3100g for 20 minutes at 4°C and then frozen at −70°C. No sample was thawed more than twice.
The concentrations of proBNP1‐76 (NT‐proBNP) were measured on the Elecsys 2010 platform (Roche Diagnostics, Mannheim, Germany). The total coefficient of variation (CV) was 4.6% at 426.5 pg/mL (n=487) and 3.2% at 2308 pg/mL (n=495). Plasma concentrations of MR‐proADM, copeptin, and MR‐proANP were measured on the Kryptor platform (Brahms AG, Hennigsdorf, Germany). The total CV for copeptin was 4% at a concentration of 15 pmol/L (n=18) and 3.5% at a concentration of 100 pmol/L (n=18). According to the manufacturer, the intra‐assay CV for MR‐proADM was ≤10% for concentrations between 0.2 and 0.5 nmol/L, less than 4% for concentrations between 0.5 and 2 nmol/L, less than 2% for concentrations between 2 and 6 nmol/L, and less than 3.5% for concentrations over 6 nmol/L. The intra‐assay CV for MR‐proANP, according to the manufacturer, was ≤5% for concentrations between 10 and 20 pmol/L, less than 3.5% for concentrations between 20 and 1000 pmol/L, and less than 3.5% for concentrations over 1000 pmol/L.
Subject Measurements
The subject measurements of the study have been described previously.21 In brief, after screening, the eligible patients received written information about the study and were provided with transtelephonic ECG devices. They were instructed to record and send ECG recordings twice daily and extra recordings if any symptoms of arrhythmia occurred during the 4‐day period prior to RFA.
Prior to RFA, the patients received further oral information and signed an informed consent form and were thereby included in the SMURF study. A full baseline evaluation, including medical history, physical examination, and a 12‐lead ECG, was recorded. All patients underwent transthoracic and transesophageal echocardiogram and a computed tomographic scan of the heart, according to the clinical routine.
All registered transtelephonic ECGs sent by the patients were screened, and if these were free from arrhythmias, the respective patients were eligible for randomization.
All patients were catheterized according to clinical routine, and blood samples were drawn from the femoral vein, CS, and LA (baseline sampling) for the analysis of NT‐proBNP, MR‐proANP, copeptin, and MR‐proADM. The catheter position in CS was evaluated in the left anterior oblique projection.
Intervention
After baseline blood sampling and intracardiac pressure measurements, the patients randomized to the active group had AF induced with burst pacing from CS (with cycle length, CL, of 170‐300 milliseconds). AF was maintained for 30 minutes and was immediately reinduced when necessary; thereafter, the blood samples were drawn for biomarker analysis.
The patients randomized to the control group were monitored for 30 minutes in SR after the baseline measurements. Then, new blood samples were drawn.
After the interventional part of the study, RFA was performed as described previously.21 At the end of the procedure, blood samples were drawn from the peripheral vein for biomarker analysis.
Primary Endpoint
The primary endpoints were changes in MR‐proANP, NT‐proBNP, MR‐proADM, and copeptin concentrations after the AF initiation compared with the controls in 3 sample sites, ie, the femoral vein, LA, and CS.
Randomization
The participants were randomized with a 2:1 allocation ratio at the time of catheterization, ie, for every 2 patients randomized to AF initiation, 1 patient was randomized to the control group. Due to the nature of the intervention, blinding of participants or staff was not possible.21
Sample Size
This study was preceded by a small pilot study observing the reaction of MR‐proANP 30 minutes after the initiation of AF. The MR‐proANP concentration was 174 pmol/L (95% confidence interval [CI] 45‐218 pmol/L) at baseline and 253 (64‐308 pmol/L) 30 minutes after AF initiation. The results indicated that 29 patients were required in order to find a statistically significant difference in the MR‐proANP reaction with a power of 90% and type‐1 error of 0.05. However, we increased the number of participants to 45 because we chose another study design (control randomized study) with allocation ratio 2:1, 3 additional biomarkers were tested, the Benjamini‐Hochberg procedure25 was chosen in order to avoid false discoveries due to multiple testing, and possible dropouts were taken into account. The sample size calculation was performed by using a commercially available statistical analysis software package (STATISTICA 10; Statsoft, Dell STATISTICA, Tulsa, OK).
Statistical Methods
For baseline characteristics, continuous variables were expressed as means±standard deviation (SD). Variables that did not have a Gaussian distribution were presented as medians with interquartile range (IQR). Categorical data were presented as counts and percentages. Possible differences in the baseline characteristics between the randomized groups were tested using a t test for normally distributed data, a Mann‐Whitney U test for nonparametric data, and chi‐squared for categorical data.
In order to analyze the primary endpoints, a repeated‐measures analysis of variance (ANOVA) was used. Time with 2 levels (baseline and 30 minutes after the AF initiation) was used as a within‐subjects factor, the randomized group was used as a between‐subjects factor, and changes in biomarkers' levels in the randomized groups over time were studied (time×randomization). A subgroup analysis for patients without ischemic heart disease (IHD) was also performed.
Logarithmic transformation (log10) of NT‐proBNP, MR‐proANP, and copeptin levels was used because these biomarkers were not normally distributed. NT‐proBNP, MR‐proANP, and the copeptin levels were presented as geometric means with a 95% CI. MR‐proADM levels were presented as means with a 95% CI because these were normally distributed.
The repeated‐measures ANOVA was corrected concerning NT‐proBNP for the covariates of heart rate at baseline and age, that concerning MR‐proANP for the covariate ejection fraction <50%, and that concerning copeptin for the covariate of age.
All reported P values were 2‐sided, and a P<0.05 was considered statistically significant. In order to avoid any false discoveries due to multiple testing, the Benjamini‐Hochberg procedure25 was used. The analyses were performed using SPSS 22.0 (SPSS, Chicago, IL).
Results
Participant Flow
During the period from February 2012 to April 2014, 338 patients with AF were referred to the Department of Cardiology at the University Hospital in Linköping, Sweden, and were accepted for first‐time RFA. The inclusion period was closed when the planned number of participants was achieved. The inclusion and randomization flowchart is presented in Figure 1.
Figure 1.
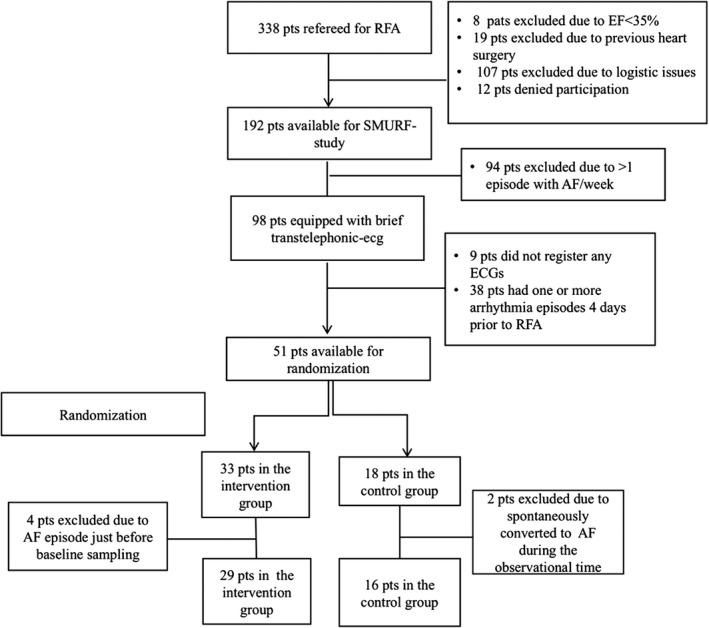
Inclusion and randomization flowchart. AF indicates atrial fibrillation; EF, ejection fraction; pts, patients; RFA, radiofrequency ablation; SMURF, Symptom burden, Metabolic profile, Ultrasound findings, Rhythm, neurohormonal activation, hemodynamics and health‐related quality of life in patients with atrial Fibrillation.
Baseline Characteristics
The study population consisted of 33 men and 12 women with a median age of 59 (IQR 14) years. The mean procedural time was 3 hours and 15 minutes, and the mean fluoroscopy time was 21 minutes. There were no significant differences in the baseline characteristics or baseline biomarker levels between the groups (Tables 1 and 2).
Table 1.
Baseline Characteristics of the 2 Randomized Groups (Control and Intervention Groups)
| Variables | Control Group (N=16) | Induction Group (N=29) | P Value |
|---|---|---|---|
| Age, y | 58 (IQR, 15) | 62 (IQR, 12.8) | 0.104 |
| Female sex | 5 (31%) | 7 (24%) | 0.429 |
| BMI, kg/m2 | 25.8 (IQR, 5.2) | 26.4 (IQR, 7.2) | 0.749 |
| Paroxysmal AF | 7 (44%) | 14 (49%) | 0.509 |
| Months in AF | 36 (IQR, 96) | 54 (IQR, 93) | 0.778 |
| Number of DC conversions/patient with persistent AF | 4.2±2.2 | 4.9±3.9 | 0.627 |
| Hereditya | 9 (56%) | 10 (35%) | 0.109 |
| Hypertension | 9 (56%) | 9 (31%) | 0.091 |
| Diabetes mellitus | 1 (6%) | 2 (7%) | 0.715 |
| Heart failureb | 0 | 2 (7%) | 0.283 |
| CKD (GFR <60 mL/min per 1.73 m2) | 4 (25%) | 3 (10%) | 0.194 |
| SVT | 1 (6%) | 3 (10%) | 0.644 |
| TIA | 2 (13%) | 1 (3%) | 0.244 |
| CHA2DS2VASc | 2 (IQR, 3) | 1 (IQR, 2) | 0.203 |
| β‐Blocker | 10 (66%) | 17 (59%) | 0.799 |
| AAD | 4 (25%) | 14 (48%) | 0.127 |
| Amiodarone | 3 (19%) | 6 (21%) | 0.876 |
| Flecainide | 3 (19%) | 7 (24%) | 0.677 |
| Dronedarone | 0 | 1 (3%) | 0.453 |
| ACEi or ARB | 7 (44%) | 7 (24%) | 0.174 |
| Aldosterone receptor antagonist | 0 | 2 (6.9%) | 0.283 |
| Other diuretics | 3 (19%) | 4 (14%) | 0.661 |
| Statins | 3 (19%) | 10 (35%) | 0.265 |
| HR baseline, bpm | 59±13 | 60±12 | 0.871 |
| Procedural time, minute | 187±35 | 199±43 | 0.338 |
| Fluoroscopy time, minute | 20.8±9.6 | 21.1±6.4 | 0.893 |
| Fluoroscopy dose, cGycm2 | 2260 (IQR, 3061) | 1521 (IQR, 1567) | 0.245 |
| Total delivered RF energy, J | 65 427±19 669 | 65 209±22 493 | 0.974 |
| Complications | 0 | 1 (3%) | 0.453 |
| Primary successful procedure | 16 (100%) | 27 (93%) | 0.283 |
| EF (biplane) | 63±4 | 61±5 | 0.348 |
Normally distributed variables are presented as mean values±SD, nonparametric variables are presented as median values with IQR, and categorical data are presented as counts and percentages. Results from t tests for normally distributed variables, chi‐squared for categorical variables, and Mann‐Whitney U for nonparametric variables are presented, and P<0.05 is considered statistically significant. AAD indicates antiarrhythmic drugs; ACEi, angiotensin‐converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin‐2 receptor blocker; BMI, body mass index; CHA2DS2VASc, congestive heart failure, hypertension, age >75 years, diabetes mellitus, stroke, vascular disease, female sex; CKD, chronic kidney disease; EF, ejection fraction; GFR, glomerular filtration rate; HR, heart rate; IQR, interquartile range; N, number of patients; SD, standard deviation; SVT, supraventricular tachycardia; TIA, transient ischemic attack.
First‐degree relatives with AF.
EF <50%.
Table 2.
Baseline Biomarker Concentrations in Both Randomized Groups
| Control Group | 95% CI | Intervention Group | 95% CI | P Value | |
|---|---|---|---|---|---|
| NT‐proBNPfv (pg/mL) | 97.5 | 61.1 to 155.7 | 63.9 | 45.1 to 90.5 | 0.138 |
| NT‐proBNPCS (pg/mL) | 144.4 | 87 to 239.8 | 82 | 58.1 to 115.7 | 0.06 |
| NT‐proBNPLA (pg/mL) | 94.3 | 59 to 150.8 | 61.4 | 43 to 87.5 | 0.134 |
| MR‐proANPfv (pmol/L) | 114.6 | 77.9 to 151.3 | 98.4 | 82.8 to 113.9 | 0.342 |
| MR‐proANPCS (pmol/L) | 151.5 | 116.1 to 197.8 | 132.5 | 105.9 to 165.9 | 0.451 |
| MR‐proANPLA (pmol/L) | 100.9 | 79.5 to 128.1 | 95.7 | 79.2 to 115.5 | 0.721 |
| Copeptinfv (pmol/L) | 6.68 | 4.95 to 9.01 | 8.21 | 5.5 to 12.27 | 0.471 |
| CopeptinCS (pmol/L) | 7.19 | 5.38 to 9.62 | 9.05 | 5.86 to 14 | 0.464 |
| CopeptinLA (pmol/L) | 6.71 | 4.85 to 9.28 | 8.03 | 5.33 to 12.1 | 0.721 |
| MR‐proADMfv (nmol/L) | 0.654 | 0.565 to 0.734 | 0.583 | 0.53 to 0.636 | 0.134 |
| MR‐proADMCS (nmol/L) | 0.636 | 0.547 to 0.724 | 0.563 | 0.513 to 0.613 | 0.115 |
| MR‐proADMLA (nmol/L) | 0.577 | 0.508 to 0.645 | 0.544 | 0.495 to 0.593 | 0.414 |
NT‐proBNP, MR‐proANP, and copeptin levels are presented as geometric means with 95% CI. MR‐proADM levels are presented as means with 95% CI. Results from t tests are presented, and P<0.05 is considered statistically significant. CI indicates confidence interval; CS, coronary sinus; fv, femoral vein; LA, left atrium; LAm, left atrium mean pressure; MR‐proADM, midregional portion of proadrenomedullin; MR‐proANP, midregional fragment of the N‐terminal precursor of atrial natriuretic peptide; NT‐proBNP, N‐terminal fragment of prodromal B‐type natriuretic peptide.
We were unable to catheterize CS in 6 patients. However, no statistically significant differences were observed between those 6 patients and the 39 patients from whom blood samples were collected from CS (Table S1).
The patients in whom AF was induced were found to have increased HR by 43 beats/min, whereas HR in the control group did not change significantly.
One patient in the intervention group suffered from a cardiac tamponade requiring pericardiocentesis. This complication took place during the RFA, ie, after both baseline blood sampling and the blood sample collection 30 minutes after the intervention. This was the only complication observed.
AF Initiation and Its Effect on Cardiac Biomarkers
MR‐proANP
Compared with the baseline, the MR‐proANP concentrations showed an increase of 73% in the femoral vein, 70% in CS, and 79% in LA in the intervention group, 30 minutes after the initiation of AF. On the other hand, MR‐proANP concentrations in the control group showed a marginal increase of about 6% in all blood‐sampling locations. The MR‐proANP reactions of the intervention group were statistically significant when compared with the respective control group (femoral vein: P<0.001; CS, P<0.001; LA, P<0.001; Table 3 and Figure 2).
Table 3.
NT‐proBNP, MR‐proANP, Copeptin, and MR‐proADM Reactions in Both Randomized Groups
| Baseline | 95% CI | 30 Minutes After Induction | 95% CI | P time×randomization | |
|---|---|---|---|---|---|
| NT‐proBNPint fv (pg/mL) n=29 | 63.9 | 45.1 to 90.5 | 66 | 46.5 to 93.8 | 0.073 |
| NT‐proBNPcon fv (pg/mL) n=16 | 97.5 | 61.1 to 155.7 | 97.7 | 61.5 to 155.2 | |
| NT‐proBNPint CS (pg/mL) n=26 | 82 | 58.1 to 115.7 | 92.7 | 69.4 to 123.9 | 0.882 |
| NT‐proBNPcon CS (pg/mL) n=13 | 144.4 | 87 to 239.8 | 158.2 | 93.5 to 268 | |
| NT‐proBNPint LA (pg/mL) n=29 | 61.4 | 43 to 87.5 | 65.3 | 46.2 to 92.3 | <0.001a |
| NT‐proBNPcon LA (pg/mL) n=16 | 94.3 | 59 to 150.8 | 92.9 | 58.8 to 146.9 | |
| MR‐proANPint fv (pmol/L) n=29 | 98.4 | 82.8 to 113.9 | 152 | 125.7 to 183.8 | <0.001a |
| MR‐proANPcon fv (pmol/L) n=16 | 102.7 | 77.9 to 151.3 | 108.3 | 85.2 to 137.8 | |
| MR‐proANPint CS (pmol/L) n=26 | 132.5 | 105.9 to 165.9 | 234.2 | 197.7 to 277.4 | <0.001a |
| MR‐proANPcon CS (pmol/L) n=13 | 151.5 | 116.1 to 197.8 | 161.7 | 123.2 to 213.6 | |
| MR‐proANPint LA (pmol/L) n=29 | 95.7 | 79.2 to 115.5 | 165.5 | 136.6 to 200.5 | <0.001a |
| MR‐proANPcon LA (pmol/L) n=16 | 100.9 | 79.5 to 128.1 | 106.2 | 82.7 to 136.4 | |
| Copeptinint fv (pmol/L) n=29 | 8.21 | 5.5 to 12.27 | 6.53 | 4.36 to 9.78 | 0.245 |
| Copeptincon fv (pmol/L) n=16 | 6.68 | 4.95 to 9.01 | 6.92 | 4.86 to 9.84 | |
| Copeptinint CS (pmol/L) n=26 | 9.05 | 5.86 to 14 | 6.77 | 4.39 to 10.46 | 0.225 |
| Copeptincon CS (pmol/L) n=13 | 7.19 | 5.38 to 9.62 | 7.13 | 4.78 to 10.63 | |
| Copeptinint LA (pmol/L) n=29 | 8.03 | 5.33 to 12.1 | 6.33 | 4.23 to 9.48 | 0.202 |
| Copeptincon LA (pmol/L) n=16 | 6.71 | 4.85 to 9.28 | 6.44 | 4.23 to 9.46 | |
| MR‐proADMint fv (nmol/L) n=29 | 0.583 | 0.53 to 0.636 | 0.568 | 0.522 to 0.614 | 0.363 |
| MR‐proADMcon fv (nmol/L) n=16 | 0.654 | 0.565 to 0.734 | 0.655 | 0.562 to 0.747 | |
| MR‐proADMint CS (nmol/L) n=26 | 0.563 | 0.513 to 0.613 | 0.558 | 0.507 to 0.61 | 0.61 |
| MR‐proADMcon CS (nmol/L) n=13 | 0.636 | 0.547 to 0.724 | 0.628 | 0.528 to 0.728 | |
| MR‐proADMint LA (nmol/L) n=29 | 0.544 | 0.495 to 0.593 | 0.537 | 0.492 to 0.582 | 0.037 |
| MR‐proADMcon LA (nmol/L) n=16 | 0.577 | 0.508 to 0.645 | 0.605 | 0.538 to 0.673 |
NT‐proBNP, MR‐proANP, and copeptin levels are presented as geometric means with 95% CI; MR‐proADM levels are presented as means with 95% CI. Results from repeated‐measure ANOVA within‐subjects contrast tests are presented after correction for false discovery rates due to multiple testing. ANOVA indicates analysis of variance; CI, confidence interval; con, control group; CS, coronary sinus; fv, femoral vein; int, intervention group; LA, left atrium; MR‐proADM, midregional portion of proadrenomedullin; MR‐proANP, midregional fragment of the N‐terminal precursor of atrial natriuretic peptide; n, number of patients available for analysis; NT‐proBNP, N‐terminal pro B‐type natriuretic peptide.
Statistical significant P values after correction for false discovery rate.
Figure 2.
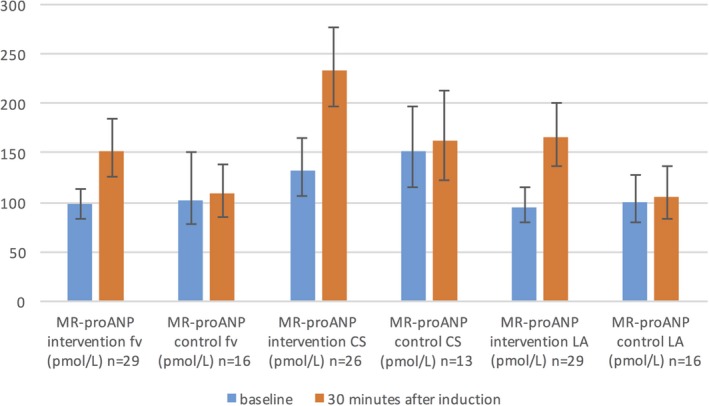
MR‐proANP reaction in randomized groups in fv, CS, and LA. MR‐proANP levels are presented as geometric means with 95% CI. Results from repeated‐measure ANOVA within‐subjects contrast tests between randomization groups based on interaction with time: P time×randomization fv<0.001; P time×randomization CS<0.001; P time×randomization LA<0.001. AF indicates atrial fibrillation; ANOVA, analysis of variance; CI, confidence interval; CS, coronary sinus; fv, femoral vein; LA, left atrium; MR‐proANP, mid‐regional fragment of the N‐terminal precursor of atrial natriuretic peptide; n, number of patients available for analyses.
NT‐proBNP
The AF initiation caused a significant increase in the NT‐proBNP concentration in LA after 30 minutes of AF compared with the baseline, but no significant change was observed in the NT‐proBNP concentrations in the control group in the same sampling locations. The NT‐proBNP reaction in the intervention group was statistically significant compared with the reaction in the control group in the same sampling site (P<0.001) (Figure 3 and Table 3).
Figure 3.
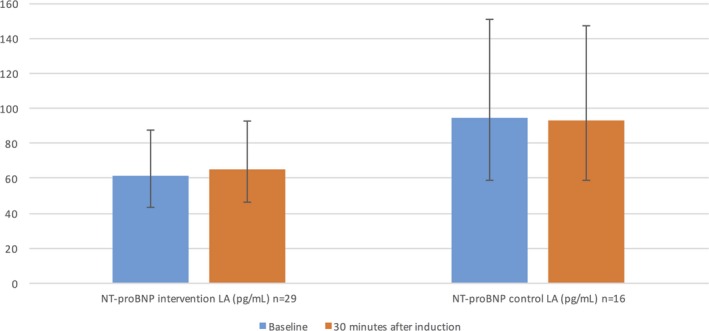
NT‐proBNP reaction in randomized groups in LA. NT‐proBNP levels are presented as geometric means with 95% CI. P time×randomization<0.001 (from repeated‐measure ANOVA within‐subjects contrast test between randomized groups based on interaction with time). AF indicates atrial fibrillation; CI, confidence interval; LA, left atrium; n, number of patients available for analysis; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide.
The reactions in NT‐proBNP concentrations in the femoral vein were not statistically different between the randomized groups (P=0.073; Table 3). However, a marked increase in the NT‐proBNP concentration was observed in the intervention group directly at the end of RFA compared with the baseline in the femoral vein. On the other hand, the NT‐proBNP concentrations in the control group did not change significantly directly after the end of the RFA. The latter reaction in the intervention group was statistically significant when compared with the control group (P<0.001) (Table 4, Figure 4). The complication of pericardial effusion was entered as a covariate in this analysis without any significant impact on the result.
Table 4.
NT‐proBNP Reaction in Both Randomized Groups Directly After RFA Compared to Baseline
| Baseline | 95% CI | Directly After RFA | 95% CI | P time×randomization | |
|---|---|---|---|---|---|
| NT‐proBNPint fv (pg/mL) n=29 | 63.9 | 45.1 to 90.5 | 86.9 | 63 to 116.7 | <0.001a |
| NT‐proBNPcon fv (pg/mL) n=16 | 97.5 | 61.1 to 155.7 | 102.1 | 65.6 to 158.9 |
NT‐proBNP levels are presented as geometric means with 95% CI. Results from repeated‐measure ANOVA within‐subjects contrast tests are presented and considered statistically significant after Benjamini‐Hochberg correction. ANOVA indicates analysis of variance; CI, confidence interval; con, control group; fv, femoral vein; int, intervention group; n, number of patients available for analysis; NT‐proBNP, N‐terminal pro B‐type natriuretic peptide.
Statistical significant P values after correction for false discovery rate.
Figure 4.
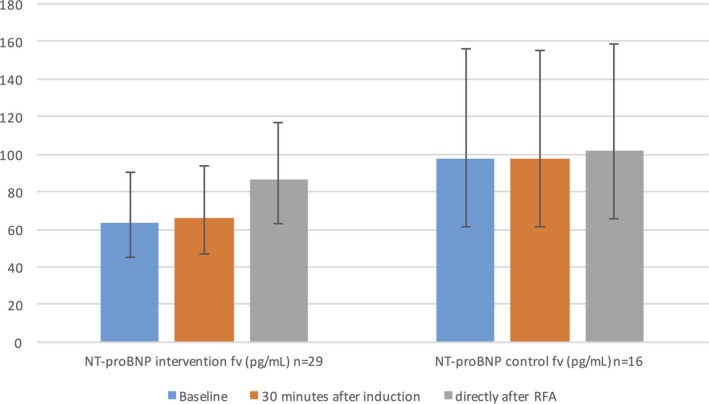
NT‐proBNP reaction in randomization groups in femoral vein. NT‐proBNP levels are presented as geometric means with 95% CI. P time×randomization<0.001 (from repeated‐measure ANOVA within‐subjects contrast test between randomized groups based on interaction with time). AF indicates atrial fibrillation; CI, confidence interval; fv, femoral vein; group; n, number of patients available for analysis; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide.
No differences in the NT‐proBNP reactions were observed between the randomized groups in the CS (P=0.882; Table 3).
AF Initiation and Its Effect on Extracardiac Biomarkers
Copeptin
Copeptin concentrations did not change significantly 30 minutes after AF initiation compared with the control group (femoral vein P=0.245; CS P=0.225; LA P=0.202; Table 3).
However, it was notable that the copeptin concentration after AF initiation in 2 patients with IHD included in the intervention group increased by 34.2 and 8.2 pmol/L, while that in the patient with IHD in the control group slightly decreased by 2 pmol/L in the femoral vein. Excluding these 3 patients from the analyses (nintervention=27; ncontrols=15), we observed that patients without IHD had decreased copeptin concentrations 30 minutes after AF initiation compared with the baseline, although the concentrations in the control group did not change. The copeptin reactions of the intervention group were statistically significant compared with the controls in the respective sampling sites (femoral vein P=0.003; CS P=0.015; LA P=0.011) (Table 5 and Figure 5).
Table 5.
Copeptin Level Reactions in Patients Without IHD in fv, CS, and LA in Both Randomized Groups
| Baseline | 95% CI | 30 Minutes After the Induction | 95% CI | P time×randomization | |
|---|---|---|---|---|---|
| Copeptinint fv (pmol/L) n=27 | 8.23 | 5.34 to 12.7 | 5.9 | 3.94 to 8.85 | 0.003a |
| Copeptincon fv (pmol/L) n=15 | 6.41 | 4.7 to 8.5 | 6.63 | 4.59 to 9.56 | |
| Copeptinint CS (pmol/L) n=24 | 9.04 | 5.64 to 14.5 | 6 | 3.94 to 9.1 | 0.015a |
| Copeptincon CS (pmol/L) n=12 | 6.79 | 5.1 to 9.04 | 6.78 | 4.45 to 10.35 | |
| Copeptinint LA (pmol/L) n=27 | 7.93 | 5.1 to 12.35 | 5.67 | 3.84 to 8.37 | 0.011a |
| Copeptincon LA (pmol/L) n=15 | 6.37 | 4.59 to 8.83 | 6.18 | 4.31 to 8.86 |
Copeptin levels are presented as geometric means with 95% CI. The results from repeated‐measure ANOVA within‐subjectsȧ contrast tests after correction for false discovery rates due to multiple testing. ANOVA indicates analysis of variance; CI, confidence intervals; con, control group; CS, coronary sinus; fv, femoral vein; IHD, ischemic heart disease; int, intervention group; LA, left atrium; n, number of patients available for analysis.
Statistical significant P values after correction for false discovery rate.
Figure 5.
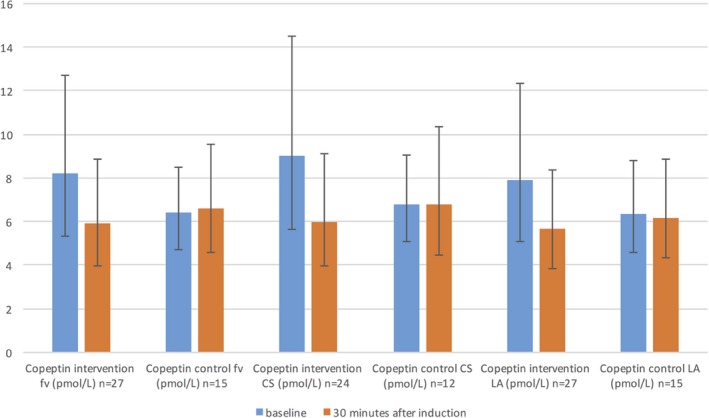
Copeptin reaction in randomized groups in patients without IHD in femoral vein, CS, and LA. Copeptin levels are presented as geometric means with 95% CI. Results from repeated‐measure ANOVA within‐subjects contrast tests between randomized groups based on interaction with time: P time×randomization fv<0.003; P time×randomization CS<0.015; P time×randomization LA<0.011. AF indicates atrial fibrillation; ANOVA, analysis of variance; CI, confidence interval; CS, coronary sinus; fv, femoral vein; IHD, ischemic heart disease; LA, left atrium; n, number of patients available for analysis.
MR‐proADM
The MR‐proADM reactions did not differ significantly between the randomized groups in different sites after correction for false discovery due to multiple testing (femoral vein P=0.363; CS P=0.610; LA P=0.037; Table 3).
Discussion
In this study we examined the effects of induced AF lasting for at least 30 minutes on 2 biomarkers with cardiac origin (NT‐proBNP and MR‐proANP) and 2 biomarkers with extracardiac origin (copeptin and MR‐proADM). This is the first study to show that 30 minutes of AF resulted in an increase in the cardiac biomarker concentrations. Therefore, these findings allow us to better understand the pathophysiology behind AF.
AF Initiation and Its Effect on Cardiac Biomarkers
MR‐proANP
Interestingly, we observed an immediate increase in the MR‐proANP concentrations in all sampling sites after the initiation of AF. The proANP is stored in secretory granules in the atrial myocytes and splits into ANP and NT‐proANP fragments on secretion in response to increased wall tension.26 A previous study has shown that the MR‐proANP concentration was elevated in patients with AF but without HF, compared with controls. It also showed that the MR‐proANP concentration increased during the initial days to weeks of AF initiation and then gradually decreased when AF persisted.8 The increased atrial wall tension and volume overload were believed to be the key stimuli for the secretion of natriuretic peptides.27, 28, 29 Another recent study proposed the high‐frequency contraction of atrial myocytes resulting in a local inflammation as a possible additional stimulus in patients with persistent AF.30 Furthermore, the immediate increase in MR‐proANP observed in our study can be explained by the fact that ANP releases as rapidly as 2 minutes from the application of the stimulus.31
NT‐proBNP
Our results demonstrated a minor but significant increase in NT‐proBNP concentration in LA 30 minutes after initiation of AF and an increase in NT‐proBNP level in femoral vein directly after the RFA, compared with the baseline. The NT‐proBNP concentration increases in plasma in patients with so‐called “lone AF”5 (AF without cardiac comorbidity), possibly due to the increased atrial wall tension and possible atrial inflammation.3 Consequently the restoration of sinus rhythm decreases the plasma BNP concentration.32 A study by Suzuki et al33 on cultured rat atrial cells showed that BNP is secreted directly after synthesis; however, some synthesized BNP may be stored in a small subset of atrial granules. Goetze et al argued that the BNP precursor is also stored in the atrial granule.3 From these data, it could be inferred that the modest increase in NT‐proBNP concentration 30 minutes after the initiation of AF could be attributed to the limited amount of NT‐proBNP that is stored in granules and could thus be directly released after the initiation of AF.
The marked increase, compared with controls, observed in peripheral blood directly after RFA should be discussed. The major stimulus is probably the increased wall tension due to the hemodynamic changes during AF but may also be due to the increased volume as a result of the volume given during the RFA. The RFA process itself could be a factor behind the increase in NT‐proBNP due to myocardial injury and volume overload,34, 35, 36 but this effect is equal in both study groups, as all patients underwent RFA. Thus, this could not explain the different reactions between the randomized groups.
Furthermore, Inoue et al37 have suggested that BNP is secreted mostly from atrial cells in patients with AF, which can probably explain why we found an isolated and significant increase of NT‐proBNP levels in LA 30 minutes after AF initiation but no significant changes in NT‐proBNP levels in CS and peripheral blood.
AF Initiation and Its Effect on Extracardiac Biomarkers
Interestingly, the copeptin levels in patients without IHD were decreased after the initiation of AF.
Copeptin and AVP are produced in the hypothalamus and released from the neurohypophysis in response to a drop in blood pressure, changes in osmotic pressure, and endogenous stress (eg, acute MI).38 The physiological relevance of copeptin is unknown, but AVP can lead to the release of adrenocorticotropic hormone (ACTH), water retention, and vasoconstriction as part of the volume regulation in which AVP has a major role.38
In our study we found an increase in the diastolic blood pressure (DBP) by 7 mm Hg after 30 minutes of AF in the intervention group. This reaction was significantly different from that of the control group (Figure 6). The increase in DBP as a result of AF was also confirmed in a previous study.39 Thus, it can be assumed that the decrease in copeptin concentration after the initiation of AF can be attributed to the increase in DBP.38, 40 It is also well known that the copeptin concentration increases following an acute MI,9 and the increase in copeptin in the 2 patients with IHD could possibly be attributed to ischemia following elevated heart rate.
Figure 6.
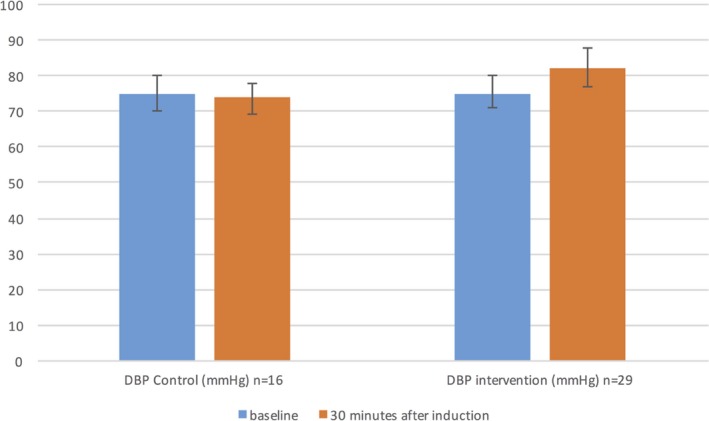
DBP reaction in both randomized groups. AF indicates atrial fibrillation; DBP, diastolic blood pressure; n, number of patients available for analysis.
The reaction on MR‐proADM concentration after AF induction did not differ between the randomized groups after adjustment for false discovery rate. Considering the increased shear stress and inflammation due to AF initiation, an increase of MR‐proADM levels could have been expected, which our results failed to show.
Clinical and Investigational Importance and Future Perspectives
To the best of our knowledge this is the first randomized study with a control arm showing the association of novel biomarkers following 30 minutes of induced atrial fibrillation.
Our study showed that MR‐proANP levels increased in all blood sampling sites 30 minutes after AF initiation. A previous study by Meune et al has shown that the MR‐proANP level correlated with the duration of AF episodes.8 Thus, MR‐proANP can be used as a biomarker of AF. New studies are needed in order to verify if MR‐proANP can serve as an indicator for AF duration <48 hours and thus identify candidates for cardioversion without the otherwise mandatory pretreatment with anticoagulants.
Another interesting finding was the increase in NT‐proBNP in LA 30 minutes after the initiation of AF, and in peripheral blood at the end of the RFA procedure. More studies are needed in order to find out whether the increase of the NT‐proBNP levels is due to a direct release from the LA myocardial granules or the result of an early production. Furthermore, the early increase of MR‐proANP and NT‐proBNP is of potential clinical importance because it shows that both natriuretic peptides can serve as biomarkers of the initiation/start of AF.
One interesting observation was the decrease in copeptin in all patients apart from those 2 with IHD. In those 2 patients an increase in copeptin was observed instead. As this was only an observation, more studies are needed in order to confirm it.
Limitations
Our study was a single‐center randomized study with a moderate sample size. Our sample consisted of a relatively healthy group of patients with few comorbidities, and the extrapolation of our results to a general population with AF has to be done with caution. Moreover, the initiation of AF by burst pacing is a simulation model of the real pathophysiological mechanism. Examining the levels of these biomarkers 1 or 2 hours after the AF initiation could have provided us with better knowledge of their reactions, but this was considered unethical and unsafe. Finally, performing sample collection post‐RFA in CS or LA could have provided more information but was not included in the protocol due to the further increase in procedure time and the addition of a new intervention (new catheterization of CS), which would have increased the risk of complications.
Conclusions
AF is a strong stimulus that causes an immediate activation of different biomarkers and has an immediate effect on hemodynamics. The NT‐proBNP and MR‐proANP concentrations increased after the initiation of AF, whereas the copeptin level decreased in patients without IHD; however, no significant changes were detected in the MR‐proADM concentration. This is the first study to examine the results of both cardiac and extracardiac biomarkers immediately after AF initiation. More research regarding the mechanisms affecting the balance of these biomarkers and their effects is needed in order to better evaluate the results.
Sources of Funding
The SMURF study is supported by grants from ALF‐grants (agreement on medical training and research grants between County Council of Östergötland and Linköping University), the Carldavid Jönsson Research Foundation, the Heart Foundation, Linköping University, and by unrestricted grants from Biosense Webster and Johnson & Johnson.
Disclosures
None.
Supporting information
Table S1. Baseline Characteristics of Patients Included in the Analyses of Biomarkers in CS and Those We Were Unable to Catheterize CS
(J Am Heart Assoc. 2016;5:e003957 doi: 10.1161/JAHA.116.003957)
References
- 1. Friberg L, Bergfeldt L. Atrial fibrillation prevalence revisited. J Intern Med. 2013;274:461–468. [DOI] [PubMed] [Google Scholar]
- 2. Hijazi Z, Oldgren J, Siegbahn A, Granger CB, Wallentin L. Biomarkers in atrial fibrillation: a clinical review. Eur Heart J. 2013;34:1475–1480. [DOI] [PubMed] [Google Scholar]
- 3. Goetze JP, Friis‐Hansen L, Rehfeld JF, Nilsson B, Svendsen JH. Atrial secretion of B‐type natriuretic peptide. Eur Heart J. 2006;27:1648–1650. [DOI] [PubMed] [Google Scholar]
- 4. Yasue H, Yoshimura M, Sumida H, Kikuta K, Kugiyama K, Jougasaki M, Ogawa H, Okumura K, Mukoyama M, Nakao K. Localization and mechanism of secretion of B‐type natriuretic peptide in comparison with those of A‐type natriuretic peptide in normal subjects and patients with heart failure. Circulation. 1994;90:195–203. [DOI] [PubMed] [Google Scholar]
- 5. Ellinor PT, Low AF, Patton KK, Shea MA, Macrae CA. Discordant atrial natriuretic peptide and brain natriuretic peptide levels in lone atrial fibrillation. J Am Coll Cardiol. 2005;45:82–86. [DOI] [PubMed] [Google Scholar]
- 6. Patton KK, Ellinor PT, Heckbert SR, Christenson RH, DeFilippi C, Gottdiener JS, Kronmal RA. N‐Terminal pro‐B‐type natriuretic peptide is a major predictor of the development of atrial fibrillation: the Cardiovascular Health Study. Circulation. 2009;120:1768–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Morgenthaler NG, Struck J, Thomas B, Bergmann A. Immunoluminometric assay for the midregion of pro‐atrial natriuretic peptide in human plasma. Clin Chem. 2004;50:234–236. [DOI] [PubMed] [Google Scholar]
- 8. Meune C, Vermillet A, Wahbi K, Guerin S, Aelion H, Weber S, Chenevier‐Gobeaux C. Mid‐regional pro atrial natriuretic peptide allows the accurate identification of patients with atrial fibrillation of short time of onset: a pilot study. Clin Biochem. 2011;44:1315–1319. [DOI] [PubMed] [Google Scholar]
- 9. Voors AA, von Haehling S, Anker SD, Hillege HL, Struck J, Hartmann O, Bergmann A, Squire I, van Veldhuisen DJ, Dickstein K; OPTIMAAL Investigators . C‐terminal provasopressin (copeptin) is a strong prognostic marker in patients with heart failure after an acute myocardial infarction: results from the OPTIMAAL study. Eur Heart J. 2009;30:1187–1194. [DOI] [PubMed] [Google Scholar]
- 10. Chatterjee K. Neurohormonal activation in congestive heart failure and the role of vasopressin. Am J Cardiol. 2005;95:8B–13B. [DOI] [PubMed] [Google Scholar]
- 11. Morgenthaler NG, Struck J, Alonso C, Bergmann A. Assay for the measurement of copeptin, a stable peptide derived from the precursor of vasopressin. Clin Chem. 2006;52:112–119. [DOI] [PubMed] [Google Scholar]
- 12. Alehagen U, Dahlstrom U, Rehfeld JF, Goetze JP. Association of copeptin and N‐terminal proBNP concentrations with risk of cardiovascular death in older patients with symptoms of heart failure. JAMA. 2011;305:2088–2095. [DOI] [PubMed] [Google Scholar]
- 13. Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, Bax JJ, Borger MA, Brotons C, Chew DP, Gencer B, Hasenfuss G, Kjeldsen K, Lancellotti P, Landmesser U, Mehilli J, Mukherjee D, Storey RF, Windecker S, Baumgartner H, Gaemperli O, Achenbach S, Agewall S, Badimon L, Baigent C, Bueno H, Bugiardini R, Carerj S, Casselman F, Cuisset T, Erol C, Fitzsimons D, Halle M, Hamm C, Hildick‐Smith D, Huber K, Iliodromitis E, James S, Lewis BS, Lip GY, Piepoli MF, Richter D, Rosemann T, Sechtem U, Steg PG, Vrints C, Luis Zamorano J; Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST‐Segment Elevation of the European Society of Cardiology . 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST‐Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J. 2016;37:267–315. [DOI] [PubMed] [Google Scholar]
- 14. Sabatine MS, Morrow DA, de Lemos JA, Omland T, Sloan S, Jarolim P, Solomon SD, Pfeffer MA, Braunwald E. Evaluation of multiple biomarkers of cardiovascular stress for risk prediction and guiding medical therapy in patients with stable coronary disease. Circulation. 2012;125:233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jougasaki M, Burnett JC Jr. Adrenomedullin: potential in physiology and pathophysiology. Life Sci. 2000;66:855–872. [DOI] [PubMed] [Google Scholar]
- 16. Sugo S, Minamino N, Kangawa K, Miyamoto K, Kitamura K, Sakata J, Eto T, Matsuo H. Endothelial cells actively synthesize and secrete adrenomedullin. Biochem Biophys Res Commun. 1994;201:1160–1166. [DOI] [PubMed] [Google Scholar]
- 17. Sugo S, Minamino N, Shoji H, Kangawa K, Kitamura K, Eto T, Matsuo H. Production and secretion of adrenomedullin from vascular smooth muscle cells: augmented production by tumor necrosis factor‐alpha. Biochem Biophys Res Commun. 1994;203:719–726. [DOI] [PubMed] [Google Scholar]
- 18. Chun TH, Itoh H, Ogawa Y, Tamura N, Takaya K, Igaki T, Yamashita J, Doi K, Inoue M, Masatsugu K, Korenaga R, Ando J, Nakao K. Shear stress augments expression of C‐type natriuretic peptide and adrenomedullin. Hypertension. 1997;29:1296–1302. [DOI] [PubMed] [Google Scholar]
- 19. Morgenthaler NG, Struck J, Alonso C, Bergmann A. Measurement of midregional proadrenomedullin in plasma with an immunoluminometric assay. Clin Chem. 2005;51:1823–1829. [DOI] [PubMed] [Google Scholar]
- 20. Parwani AS, von Haehling S, Kolodziejski AI, Huemer M, Wutzler A, Attanasio P, Stojakovic T, Scharnagl H, Haverkamp W, Boldt LH. Mid‐regional proadrenomedullin levels predict recurrence of atrial fibrillation after catheter ablation. Int J Cardiol. 2015;180:129–133. [DOI] [PubMed] [Google Scholar]
- 21. Charitakis E, Walfridsson U, Nystrom F, Nylander E, Stromberg A, Alehagen U, Walfridsson H. Symptom burden, Metabolic profile, Ultrasound findings, Rhythm, neurohormonal activation, haemodynamics and health‐related quality of life in patients with atrial Fibrillation (SMURF): a protocol for an observational study with a randomised interventional component. BMJ Open. 2015;5:e008723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rickham PP. Human experimentation. Code of ethics of the World Medical Association. Declaration of Helsinki. Br Med J. 1964;2:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hendrikx T, Rosenqvist M, Wester P, Sandstrom H, Hornsten R. Intermittent short ECG recording is more effective than 24‐hour Holter ECG in detection of arrhythmias. BMC Cardiovasc Disord. 2014;14:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Doliwa Sobocinski P, Anggardh Rooth E, Frykman Kull V, von Arbin M, Wallen H, Rosenqvist M. Improved screening for silent atrial fibrillation after ischaemic stroke. Europace. 2012;14:1112–1116. [DOI] [PubMed] [Google Scholar]
- 25. Benjamini Y, Hochberg Y. Controlling the false discovery rate—a practical and powerful approach to multiple testing. J Roy Stat Soc Ser B Methodol. 1995;57:289–300. [Google Scholar]
- 26. Mukoyama M, Nakao K, Hosoda K, Suga S, Saito Y, Ogawa Y, Shirakami G, Jougasaki M, Obata K, Yasue H, Kambayashi Y, Inouye K, Imura H. Brain natriuretic peptide as a novel cardiac hormone in humans. Evidence for an exquisite dual natriuretic peptide system, atrial natriuretic peptide and brain natriuretic peptide. J Clin Invest. 1991;87:1402–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Levin ER, Gardner DG, Samson WK. Natriuretic peptides. N Engl J Med. 1998;339:321–328. [DOI] [PubMed] [Google Scholar]
- 28. Edwards BS, Zimmerman RS, Schwab TR, Heublein DM, Burnett JC Jr. Atrial stretch, not pressure, is the principal determinant controlling the acute release of atrial natriuretic factor. Circ Res. 1988;62:191–195. [DOI] [PubMed] [Google Scholar]
- 29. Brookes CI, Kemp MW, Hooper J, Oldershaw PJ, Moat NE. Plasma brain natriuretic peptide concentrations in patients with chronic mitral regurgitation. J Heart Valve Dis. 1997;6:608–612. [PubMed] [Google Scholar]
- 30. Mollmann H, Weber M, Elsasser A, Nef H, Dill T, Rixe J, Schmitt J, Sperzel J, Hamm CW. NT‐ProBNP predicts rhythm stability after cardioversion of lone atrial fibrillation. Circ J. 2008;72:921–925. [DOI] [PubMed] [Google Scholar]
- 31. Lindberg BF, Hambraeus G, Wollmer P, Andersson KE. Release of atrial natriuretic peptide during pulmonary artery clamping in man. Clin Physiol. 1997;17:449–458. [DOI] [PubMed] [Google Scholar]
- 32. Wozakowska‐Kaplon B. Effect of sinus rhythm restoration on plasma brain natriuretic peptide in patients with atrial fibrillation. Am J Cardiol. 2004;93:1555–1558. [DOI] [PubMed] [Google Scholar]
- 33. Suzuki E, Hirata Y, Kohmoto O, Sugimoto T, Hayakawa H, Matsuoka H, Sugimoto T, Kojima M, Kangawa K, Minamino N. Cellular mechanisms for synthesis and secretion of atrial natriuretic peptide and brain natriuretic peptide in cultured rat atrial cells. Circ Res. 1992;71:1039–1048. [DOI] [PubMed] [Google Scholar]
- 34. Chen L, Wei T, Zeng C, Chen Q, Shi Z, Wang L. Effect of radiofrequency catheter ablation on plasma B‐type natriuretic peptide. Pacing Clin Electrophysiol. 2005;28:200–204. [DOI] [PubMed] [Google Scholar]
- 35. Seiler J, Steven D, Roberts‐Thomson KC, Inada K, Tedrow UB, Michaud GF, Stevenson WG. The effect of open‐irrigated radiofrequency catheter ablation of atrial fibrillation on left atrial pressure and B‐type natriuretic peptide. Pacing Clin Electrophysiol. 2014;37:616–623. [DOI] [PubMed] [Google Scholar]
- 36. Charitakis E, Walfridsson H, Alehagen U. Short‐term influence of radiofrequency ablation on NT‐proBNP, MR‐proANP, copeptin, and MR‐proADM in patients with atrial fibrillation: data from the observational SMURF study. J Am Heart Assoc. 2016;5:e003557 doi: 10.1161/JAHA.116.003557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Inoue S, Murakami Y, Sano K, Katoh H, Shimada T. Atrium as a source of brain natriuretic polypeptide in patients with atrial fibrillation. J Card Fail. 2000;6:92–96. [DOI] [PubMed] [Google Scholar]
- 38. Morgenthaler NG. Copeptin: a biomarker of cardiovascular and renal function. Congest Heart Fail. 2010;16(suppl 1):S37–S44. [DOI] [PubMed] [Google Scholar]
- 39. Alboni P, Scarfo S, Fuca G, Paparella N, Yannacopulu P. Hemodynamics of idiopathic paroxysmal atrial fibrillation. Pacing Clin Electrophysiol. 1995;18:980–985. [DOI] [PubMed] [Google Scholar]
- 40. Morgenthaler NG, Muller B, Struck J, Bergmann A, Redl H, Christ‐Crain M. Copeptin, a stable peptide of the arginine vasopressin precursor, is elevated in hemorrhagic and septic shock. Shock. 2007;28:219–226. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline Characteristics of Patients Included in the Analyses of Biomarkers in CS and Those We Were Unable to Catheterize CS


