Abstract
Background
A lifestyle cardiovascular risk score (LCRS) and a genetic risk score (GRS) have been independently associated with myocardial infarction (MI) in Hispanics/Latinos. Interaction or joint association between these scores has not been examined. Thus, our aim was to assess interactive and joint associations between LCRS and GRS, and each individual lifestyle risk factor, on likelihood of MI.
Methods and Results
Data included 1534 Costa Rican adults with nonfatal acute MI and 1534 matched controls. The LCRS used estimated coefficients as weights for each factor: unhealthy diet, physical inactivity, smoking, elevated waist:hip ratio, low/high alcohol intake, low socioeconomic status. The GRS included 14 MI‐associated risk alleles. Conditional logistic regressions were used to calculate adjusted odds ratios. The odds ratios for MI were 2.72 (2.33, 3.17) per LCRS unit and 1.13 (95% CI 1.06, 1.21) per GRS unit. A significant joint association for highest GRS tertile and highest LCRS tertile and odds of MI was detected (odds ratio=5.43 [3.71, 7.94]; P<1.00×10−7), compared to both lowest tertiles. The odds ratios were 1.74 (1.22, 2.49) under optimal lifestyle and unfavorable genetic profile, and 5.02 (3.46, 7.29) under unhealthy lifestyle but advantageous genetic profile. Significant joint associations were observed for the highest GRS tertile and the highest of each lifestyle component risk category. The interaction term was nonsignificant (P=0.33).
Conclusions
Lifestyle risk factors and genetics are jointly associated with higher odds of MI among Hispanics/Latinos. Individual and combined lifestyle risk factors showed stronger associations. Efforts to improve lifestyle behaviors could help prevent MI regardless of genetic susceptibility.
Keywords: genetics, Hispanics/Latinos, lifestyle, myocardial infarction
Subject Categories: Lifestyle, Genetics, Myocardial Infarction
Introduction
Coronary heart disease (CHD) is the leading cause of death in the Costa Rican population,1, 2 and ischemic heart disease is among the top 3 causes of disability‐adjusted life years.3 These high statistics likely reflect unfavorable changes in lifestyles1, 4 in the last several decades, including unhealthy dietary behaviors as one of the main risk factors.3 In addition to diet, the World Health Organization reported 4 main lifestyle risk factors for such population and risk of noncommunicable diseases: tobacco smoking, alcohol consumption, elevated blood pressure, and obesity.1 Indeed, a lifestyle cardiovascular risk score (LCRS) that included those risk factors—namely, diet, physical activity, smoking, alcohol intake, waist:hip ratio, and socioeconomic status—has been derived in a group of healthy Hispanic/Latino adults living in Costa Rica participating in a myocardial infarction (MI) case–control study, and subsequently validated in the same study among participants with a history of chronic disease, where it was shown to be associated with nearly 3 times the odds of having an MI.5
In addition to lifestyle, compelling evidence indicates that genetic susceptibility alone is part of the etiology of CHD and heart‐related events.6, 7, 8, 9, 10, 11 Genome‐wide association studies have identified chromosomal regions and genetic variants associated with CHD.6, 10, 12 One of the most robust genetic associations for CHD is the chromosome 9p21 region. Several single nucleotide polymorphisms (SNPs) in this region have been associated with increased risk of CHD,6, 10, 12 such as a common sequence variant adjacent to genes CDKN2A and CDKN2B found to be associated with 1.64 times higher risk of MI.10 Recently, we showed that genetic markers in these loci were also associated with this outcome in the same MI case–control study of Costa Rican adults, when analyzed as part of a genetic risk score (GRS).13 Notably, the genetic predisposition conferred by MI risk alleles in the 9p21 region is seen both in persons with a positive family history of MI and in persons with a negative family history, suggesting that these variants add to the effect of a family history, and that different underlying mechanisms may be involved. The specific genes and variants responsible for the autosomal dominant inheritance of MI in families have not been identified.14
It has been documented that the effect of genetic factors may be partly modulated by varying levels of environmental exposures such as diet, physical activity, and smoking.15 Previous gene–environment studies evaluating the risk of MI or related CHD outcomes determined by variations in the 9p21 chromosome showed that this risk could be modified by a prudent diet,16 smoking,17 and vegetable intake.18 Despite the recent progress in this area, the study of complex interactions between multiple genetic and environmental factors has been very scarce, with inconsistent results,19, 20 mostly limited to single environmental components and SNPs, and mostly conducted in European populations. Importantly, genetic background and lifestyle factors tend to be population‐specific; this Hispanic/Latino population experiences unique backgrounds and lifestyle patterns,5, 11, 21, 22 warranting further scrutiny of the contribution of these 2 factors on MI and CHD‐related events among adults in Costa Rica.
Although genetic and environmental factors have been each, independently, associated with MI in a Costa Rican population, the potential interaction or the joint association (combination of independent effects) of an overall GRS and LCRS instead of single genes or lifestyle components has not been explored. Investigating gene–environment interactions is necessary to better understand the biology of the disease and the phenotypic responses that are not mainly explained by genotypic variants. Therefore, our aim was to assess the interactive and joint associations between a LCRS and a GRS in Hispanic/Latino adults living in Costa Rica. As a secondary aim, we evaluated the association of each individual lifestyle risk factor on the likelihood of MI.
Methods
Study Population and Data Collection
The Costa Rica Myocardial Infarction Study is a case–control study where participants were recruited between 1994 and 2004 from 34 counties in the Central Valley of Costa Rica, covering a full range of socioeconomic levels, as well as urban, peri‐urban, and rural lifestyles. Eligible case subjects were adult residents who were diagnosed as survivors of a first MI by 2 independent cardiologists at any of the 6 recruiting hospitals. All cases met the World Health Organization criteria for MI, which required typical symptoms plus either elevations in cardiac enzyme levels or diagnostic changes in the ECG.23 Each case was matched with 1 control for age (±5 years), sex, and area of residence (county level). Controls were randomly selected using the information available at the National Census and Statistics Bureau of Costa Rica and they came from the same source population as the cases. All participants gave informed consent on documents approved by the Human Subjects Committee of the Harvard T. H. Chan School of Public Health and the University of Costa Rica. Detailed description of the study methods was published previously.7, 24, 25
All study participants received home visits during which trained staff collected data on sociodemographic characteristics, lifestyle behaviors (including smoking, physical activity, and alcohol intake), and medical history through questionnaires.26, 27, 28 Dietary exposures were ascertained using a food frequency questionnaire validated against the method of triads with 24‐hour recalls and biomarkers of fatty acids and carotenoids.29 Anthropometric measurements were measured in duplicate while participants wore light clothing, and an average was recorded. Physical activity information was collected using a questionnaire that inquired about the average frequency and time spent on several occupational and leisure time activities during the last year. The questionnaire was previously validated by its ability to predict fitness level among Costa Ricans.26, 28 Energy expenditure for each activity was calculated as the product of frequency, time, and intensity measured in metabolic equivalents of task.27 Adipose tissue α‐linolenic and total trans fatty acids were quantified by gas–liquid chromatography as described previously.30
DNA was extracted from the buffy coat fraction by QIAmp Blood Kit (Qiagen, Chatsworth, CA). Genotyping was performed using the TaqMan Allelic Discrimination system from Applied Biosystems, Inc (Foster City, CA), using custom genotyping assays from ABI's “assays by design” service. Replicate quality‐control samples yielded >99% concordance, and the overall call rate was >95%.
Lifestyle Cardiovascular Risk Score
The LCRS has been defined and validated previously.5 Briefly, the LCRS was calculated by using the estimated coefficients derived from the conditional regression model with MI as outcome and the lifestyle components as predictors, as weights for each of the 6 lifestyle components. The LCRS components (diet, physical activity, smoking, alcohol intake, waist:hip ratio, and socioeconomic status) were selected based on a prior analysis of modifiable MI risk factors in our study population as well as World Health Organization guidelines for healthy lifestyle (nutrition and physical activity).7, 31, 32 The final LCRS was the sum of each component multiplied by the aforementioned regression coefficient. Because the LCRS estimated risk of MI, higher LCRS values reflect a higher risk of MI. The healthy diet component comprised 6 nutrients: saturated fats, dietary cholesterol, polyunsaturated fats, dietary fiber, folate, and total trans fats. In addition, the healthy diet score included the adipose tissue biomarker for α‐linolenic acid as a more reliable measure of long‐term α‐linolenic fatty acid intake, which is a strong cardioprotective marker in this population.33 Quintiles of each dietary component were created and assigned values from 0 (highest risk quintile) to 4 (lowest risk quintile), then summed to create a dietary score ranging from 0 (lowest adherence to the dietary recommendations) to 28 (highest adherence). Physical activity in metabolic equivalents of task was included in the LCRS as a continuous variable, defined as total metabolic equivalents of task expended over a 24‐hour period. Smoking was defined as a dichotomous variable (nonsmokers versus current smokers), and alcohol intake was expressed as g/day. Waist:hip ratio was categorized using the World Health Organization cutoffs for women (<0.85) and men (<0.90).34 Socioeconomic status was calculated as a comprehensive measure of education, occupation, household income, and household possessions,35 and used as a continuous variable in the score.
Genetic Risk Score
The development of the GRS has been previously reported for this population.13 Fourteen SNPs (rs4977574, rs10757274, rs2383206, rs1333049 [CDKN2A/2B]; rs646776, rs599839 [CELSR2‐PSRC1‐SORT1]; rs501120, rs1746048 [CXCL12]; rs2259816 [HNF1A, C12orf43]; rs9818870 [MRAS]; rs2048327 [SLC22A3]; rs3127599 [LPAL2]; rs7767084, and rs10755578 [LPA]) with evidence of association with coronary artery disease and/or MI were assessed to calculate the GRS. The equal contribution to odds of MI in an additive genetic model13 suggested that each SNP was independently associated with MI. The GRS reflected the sum of the number of risk alleles at each locus, and ranged from 0 to 28. Weighed GRS did not change the association with MI.13 In sensitivity analysis, the 3 SNPs showing the strongest association with MI in the Costa Rica study (rs4977574 at CDKN2A/2B; rs646776 at CELSR2‐PSRC1‐SORT1; and rs501120 at CXCL12) were selected to calculate a simplified 3‐SNP GRS, although this may be biased towards a stronger association.
Statistical Analysis
In total, 1534 case–control pairs with complete information on the lifestyle factors included in the LCRS, genotype information, and potential confounders were included in the present study. Means and risk frequencies of health factors by case–control status were compared using paired t test and McNemar's test. Conditional logistic regression analysis matched on sex, age, and area of residence was used to calculate odds ratios and 95% CI of MI in all analyses. When individual lifestyle factors were tested separately, we further adjusted each model for the other lifestyle components. Linear P‐trend was tested across tertiles of LCRS and tertiles GRS. Multiplicative interactions between the GRS (in tertiles) and the LCRS (in tertiles) and each individual component (diet, in tertiles; physical activity, in tertiles; smoking, nonsmokers versus current; alcohol, 0 [not drinkers], 0.1–5.0, 5.1–10, >10 g/day; waist:hip ratio, <0.85 in women, and <0.90 in men; socioeconomic status, in tertiles). The maximum likelihood test was used to compare the models with and without the interactive term. Joint association of the LCRS and the GRS on MI was also tested using conditional logistic regression models. The joint variable was calculated as a combination of the LCRS (in tertiles) and the GRS (in tertiles) resulting in a new variable with 9 categories: corresponding to each combination of low, medium, and high‐risk LCRS plus each of the low, medium, and high‐risk GRS. Additionally, a separate joint analysis was run for each individual lifestyle and genetic risk score (tertile) resulting in 6 separate regressions models. P‐joint of the overall model was calculated. In sensitivity analysis, we used unconditional logistic regression for unmatched case–control (n=1628 control and 1563 cases). Statistical analyses were conducted using SAS version 9.4 (SAS Institute). A significance level of P<0.05 was used.
Results
Table 1 shows the general characteristics of the study population by MI status. Compared with controls, cases had a higher LCRS (indicative of higher risk), but lower diet score (indicative of unhealthier diet) and socioeconomic status. Additionally, cases had higher prevalence of smoking, waist:hip ratio, and MI genetic predisposition. No significant differences were found between cases and controls for physical activity or alcohol intake. The GRS was significantly higher among cases.
Table 1.
General Characteristics of Hispanic/Latino Adults Living in Costa Rica for Cases of MI and Population‐Based Controls
| Control, n=1534 | Cases, n=1534 | P Value | |
|---|---|---|---|
| Age, ya | 57.9 (11.1) | 58.1 (10.9) | N/A |
| Female, %a | 24.6 | 24.6 | N/A |
| Area of residence,% rurala | 25.8 | 25.8 | N/A |
| Lifestyle cardiovascular risk scoreb | −0.46 (0.53) | −0.18 (0.57) | 1.0×10−7 |
| Diet scorec | 14.5 (4.8) | 13.5 (5.0) | 1.0×10−7 |
| Physical activity, METSd | 35.3 (14.8) | 34.4 (15.6) | 0.08 |
| Current smokers, % | 35.3 | 64.7 | 1.0×10−7 |
| Alcohol intake, g/day | 6.3 (14.8) | 7.4 (19.1) | 0.06 |
| Elevated waist:hip ratio, %e | 48.1 | 51.9 | 1.0×10−7 |
| Socioeconomic statusf | 9.2 (3.5) | 8.5 (3.5) | 1.4×10−6 |
| Genetic risk scoreg | 13.3 (3.7) | 13.7 (3.6) | 3.9×10−3 |
MET indicates metabolic equivalent of task; MI, myocardial infarction; N/A, not applicable.
Matching variable.
The lifestyle cardiovascular risk score (LCRS) was calculated by multiplying the regression coefficients of each of 6 lifestyle components obtained from the conditional regression with MI as outcome, by the score of each component, then summing them. LCRS estimated risk of MI, therefore higher LCRS values reflect a higher risk of MI.
A composite measure of total dietary intake of saturated fats, cholesterol, polyunsaturated fats, fiber, folate, and adipose tissue α‐linolenic acid (ALA) and total trans fats. The total diet score ranged from 0 (lowest adherence to the dietary recommendations) to 28 (highest adherence).
Physical activity was defined as total METS expended over a 24‐hour period.
Elevated waist:hip ratio was >0.85 for women and >0.90 for men.
Socioeconomic status is a continuous variable that accounts for education, occupation, income, and household possessions. A higher score indicates a higher socioeconomic status.
The genetic risk score (GRS) included the sum of 14 single nucleotide polymorphisms (SNPs) risk alleles: rs4977574, rs10757274, rs2383206, rs1333049 (CDKN2A/2B); rs646776, rs599839 (CELSR2‐PSRC1‐SORT1); rs501120, rs1746048 (CXCL12); rs2259816 (HNF1A, C12orf43); rs9818870 (MRAS); rs2048327 (SLC22A3); rs3127599 (LPAL2); rs7767084 and rs10755578 (LPA).
Both the LCRS and the GRS were associated with higher odds ratio for MI when tested in independent models; however, the point estimates were more than twice as large for the LCRS than the GRS (multivariable odds ratio [95% CI] for third versus first tertile LCRS: 3.71 [3.02, 4.55]; GRS: 1.30 [1.07, 1.59]; Table 2). Similar results were observed when the scores were modeled as continuous (per unit).
Table 2.
Odds of Myocardial Infarction for the LCRS and the GRS Among Hispanic/Latino Adults Living in Costa Rica
| Lifestyle Cardiovascular Risk Scorea | Genetic Risk Scorea | ||
|---|---|---|---|
| Tertiles (Range) | OR (95% CI) | Tertiles (Range) | OR (95% CI) |
| Low risk (−2.02, −0.61) | Ref. (1.00) | Low risk (8–11) | Ref. (1.00) |
| Medium risk (−0.61, −0.13) | 1.71 (1.41, 2.07) | Medium risk (13–14) | 1.31 (1.09, 1.57) |
| High risk (−0.13, 1.28) | 3.71 (3.02, 4.55) | High risk (16–19) | 1.30 (1.07, 1.59) |
| P‐trend | <1.00×10−7 | 1.24×10−3 | |
| Continuous (per unit) | 2.72 (2.33, 3.17) | 1.13 (1.06, 1.21) | |
Lifestyle cardiovascular risk score (LCRS) tertiles: Low Risk, n=1022; Medium Risk, n=1023, High Risk, n=1023. Genetic risk score (GRS) tertile (14 SNPs): Low Risk, n=889; Medium Risk, n=1283; High Risk, n=896. The LCRS used estimated coefficients as weights for each factor: unhealthy diet, physical inactivity, smoking, elevated waist:hip ratio, low/high alcohol intake, low socioeconomic status. The GRS included the sum of 14 single nucleotide polymorphisms (SNPs) risk alleles: rs4977574, rs10757274, rs2383206, rs1333049 (CDKN2A/2B); rs646776, rs599839 (CELSR2‐PSRC1‐SORT1); rs501120, rs1746048 (CXCL12); rs2259816 (HNF1A, C12orf43); rs9818870 (MRAS); rs2048327 (SLC22A3); rs3127599 (LPAL2); rs7767084 and rs10755578 (LPA). OR, odds ratio.
Matched on age, sex, and area of residence.
An interaction between the GRS and the LCRS for odds of MI was not detected (P=0.33) (Table S1). In the joint analysis, we found that those with the highest GRS and in the highest tertile of the LCRS had 5.43 (3.71, 7.94) higher odds of MI than those in the lowest GRS and the lowest tertile of LCRS (Figure). The odds ratio was 1.74 (1.22, 2.49) under optimal lifestyle and worst genetic risk, while under unhealthy lifestyle and advantageous genetic profile, the odds of MI were 5.02 (3.46, 7.29). Trends for higher odds across higher GRS and LCRS were observed, with the higher odds detected for those in the high LCRS tertile, in general. The joint association was significant for the overall model at P<1.00×10−7 (Table S1).
Figure 1.
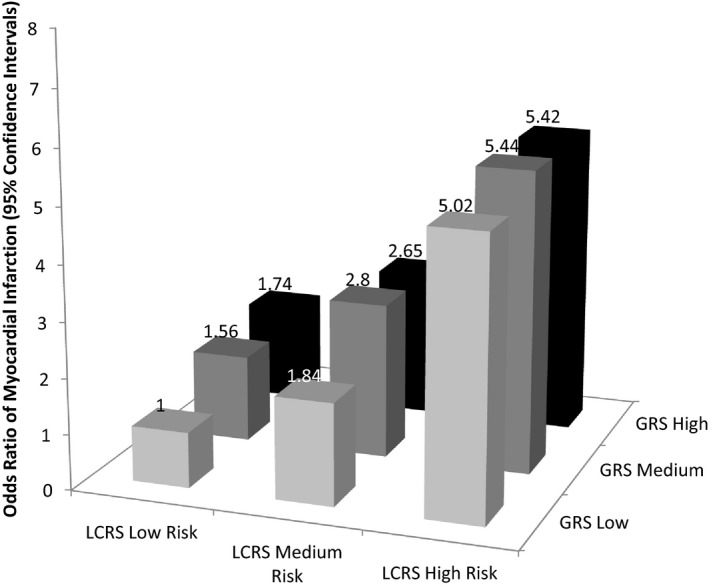
Odds ratio of myocardial infarction risk according to joint classification of lifestyle cardiovascular risk score (LCRS) (in tertiles) and genetic risk score (GRS) (in tertiles) among Hispanic/Latino adults living in Costa Rica. Odds ratios and 95% CI were calculated by using conditional logistic regression model. The analyses were matched on age, sex, and area of residence. P‐joint for overall model 1.00×10−7. The GRS included the sum of 14 single nucleotide polymorphisms risk alleles: rs4977574, rs10757274, rs2383206, rs1333049 (CDKN2A/2B); rs646776, rs599839 (CELSR2‐PSRC1‐SORT1); rs501120, rs1746048 (CXCL12); rs2259816 (HNF1A, C12orf43); rs9818870 (MRAS); rs2048327 (SLC22A3); rs3127599 (LPAL2); rs7767084 and rs10755578 (LPA). The LCRS used estimated coefficients as weights for each factor: unhealthy diet, physical inactivity, smoking, elevated waist:hip ratio, low/high alcohol intake, low socioeconomic status. LCRS+GRS: Low Risk+Low, n=290; Medium Risk+Low, n=266; High Risk+Low, n=305; Low Risk+Medium, n=387; Medium Risk+Medium, n=373; High Risk+Medium, n=356; Low Risk+High, n=345; Medium Risk+High, n=384; High Risk+High, n=362.
We examined interaction and joint associations for each component of the LCRS separately and controlling for each other (ie, adjusted for the other lifestyles in the LCRS). Significant joint associations (all P<0.01) were found for the overall model when comparing the highest joint risk versus lowest risk (reference group) categories of all components: 1.87 (1.30, 2.68) for diet, 2.11 (1.47, 3.04) for physical activity, 3.16 (2.30, 4.35) for smoking, 2.77 (1.77, 4.34) for waist:hip ratio, and 1.58 (1.13, 2.21) for socioeconomic status (Table 3). While the overall model was significant for alcohol intake, the odds of MI among those in the highest category of alcohol consumption and highest genetic risk were not significant 1.25 (0.87, 1.81). None of the individual lifestyle components were found to interact significantly with the GRS. Similar results were also observed for the joint association of the lifestyle score and GRS using the 3‐SNP score (Tables S2 through S4). Additionally, all results were similar when we conducted the analysis using unconditional logistic regression (data not shown).
Table 3.
Joint and Interaction Associations of Individual Lifestyle Cardiovascular Risk Factors With the Genetic Risk Score (GRS) (in Tertiles) on Myocardial Infarction Among Hispanic/Latino Adults Living in Costa Rica
| GRS Tertiles | |||||
|---|---|---|---|---|---|
| T1=GRS 14 SNPs Low Risk | T2=GRS 14 SNPs Medium Risk | T3=GRS 14 SNPs High Risk | P‐Overall Joint Model | P for Interaction | |
| Diet scorea | |||||
| High adherence | 1 | 1.32 (0.95, 1.84) | 1.38 (0.98, 1.94) | 5.8×10−4 | 0.36 |
| Medium adherence | 1.04 (0.73, 1.47) | 1.67 (1.21, 2.30) | 1.67 (1.18, 2.35) | ||
| Low adherence | 1.62 (1.12, 2.34) | 1.86 (1.32, 2.61) | 1.87 (1.30, 2.68) | ||
| Physical activityb | |||||
| High | 1 | 1.56 (1.12, 2.17) | 1.52 (1.06, 2.18) | 6.85×10−4 | 0.53 |
| Medium | 1.13 (0.79, 1.62) | 1.68 (1.20, 2.35) | 1.63 (1.14, 2.32) | ||
| Low | 1.73 (1.22, 2.47) | 1.88 (1.35, 2.64) | 2.11 (1.47, 3.04) | ||
| Smoking | |||||
| Never | 1 | 1.45 (1.16, 1.83) | 1.51 (1.19, 1.92) | <1.00×10−7 | 0.36 |
| Current | 2.85 (2.08, 3.90) | 3.30 (2.50, 4.42) | 3.16 (2.30, 4.35) | ||
| Alcohol consumptionc | |||||
| Never | 1 | 1.35 (1.06, 1.72) | 1.33 (1.02, 1.72) | 1.92×10−4 | 0.87 |
| Low | 0.78 (0.50, 1.22) | 1.06 (0.73, 1.54) | 1.15 (0.73, 1.80) | ||
| Moderate | 0.60 (0.34, 1.04) | 0.68 (0.42, 1.09) | 0.63 (0.38, 1.05) | ||
| High | 0.68 (0.47, 0.99) | 1.02 (0.72, 1.44) | 1.25 (0.87, 1.81) | ||
| Waist:hip ratiod | |||||
| Normal | 1 | 1.37 (0.80, 2.35) | 1.32 (0.75, 2.34) | <1.00×10−7 | 0.98 |
| Elevated | 2.01 (1.28, 3.14) | 2.72 (1.74, 4.3) | 2.77 (1.77, 4.34) | ||
| Socioeconomic statuse | |||||
| High | 1 | 1.26 (0.91, 1.74) | <1.00×10−7 | 0.29 | |
| Medium | 1.04 (0.74, 1.45) | 1.63 (1.19, 2.25) | 1.25 (0.88, 1.77) | ||
| Low | 1.46 (0.99, 2.15) | 1.73 (1.22, 2.47) | 1.58 (1.13, 2.21) | ||
Matched on age, sex, area of residence and adjusted for the other lifestyle components. The GRS included the sum of 14 SNPs (single nucleotide polymorphisms) risk alleles: rs4977574, rs10757274, rs2383206, rs1333049 (CDKN2A/2B); rs646776, rs599839 (CELSR2‐PSRC1‐SORT1); rs501120, rs1746048 (CXCL12); rs2259816 (HNF1A, C12orf43); rs9818870 (MRAS); rs2048327 (SLC22A3); rs3127599 (LPAL2); rs7767084 and rs10755578 (LPA).
A composite measure of total dietary intake of saturated fats, cholesterol, polyunsaturated fats, fiber, folate, and adipose tissue α‐linolenic acid (ALA) and total trans fats. The total diet score range from 0 (lowest adherence to the dietary recommendations) to 28 (highest adherence).
Physical activity was defined as total metabolic equivalents of task (METS) expended over a 24‐hour period.
Alcohol consumption categories were: never=0, low=0.1 to 5.0 g/day, moderate=5.1 to 10 g/day, and high as >10 g/day.
Elevated waist:hip ratios were >0.85 for women and >0.90 for men.
Socioeconomic status is a continuous variable that accounts for education, occupation, income, and household possessions. A higher score indicates a higher socioeconomic status.
Discussion
In this population of Hispanic/Latino adults living in Costa Rica, we found that poor predictive lifestyle behaviors were associated with higher odds of MI, in line with previous studies.7, 9, 11, 36, 37, 38 The association for the GRS and higher odds of MI was consistent with previous literature analyzing SNPs in the chromosome 9p21 and higher risk of CHD and mortality.6, 10, 12 Moreover, we observed that participants with the highest genetic risk in combination with the highest lifestyle risk factors (both all‐together as well as individually) had higher odds of MI. Although the 2 scores contributed independently, the association of the LCRS on MI risk was stronger than the GRS, suggesting that lifestyle factors can contribute to MI more than genetic predisposition. Moreover, the genetic predisposition may be blunted if a healthy lifestyle is attained.
In our study, no statistically significant interactions were found. However, we found a statistically significant joint association between the GRS and the LCRS. Those participants in the highest risk category for the GRS and the LCRS had 5.4 times higher risk of MI, mostly due to the lifestyle. Our results underscore that MI is less likely to occur for individuals with an optimal healthy lifestyle that comprise various behaviors, even when having a high‐risk genetic profile, than for those having the more advantageous GRS but an unhealthy lifestyle. Prior investigation studying the combined association of genes and other independent factors such as body mass index, waist:hip ratio, physical activity, diet or other nutrients or food components, on risk of diabetes20 and other cardiometabolic39 outcomes has also found significant joint associations while failing to find significant interactions.20, 39 Yet, as the majority of studies had small sample sizes, it is difficult to know whether the nonsignificant interaction is attributable to low power or no true interaction.40 For example, a large study of 8114 individuals (3820 cases and 4294 controls) from the global INTERHEART study found a significant interaction with a prudent diet when evaluating the risk of MI in the 9p21 chromosome. Specifically, the individuals who were homozygous for the risk allele of rs2383206 and have a low prudent diet score, had about twice the risk for MI (P = 2.11×10−9) when compared to the reference group.16 However, no significant interaction or trend was found with physical activity (P = 0.23) or smoking (P = 0.56) despite the large sample size. Although the previous study analyzed only 4 SNPs (rs10757274, rs2383206, rs10757278, and rs1333049) instead of 14 in our study, we were also not able to detect significant interactions when we evaluated each lifestyle factor separately. Additionally, differences in dietary components between studies could explain why they observed a significant interaction between the genetic markers and diet but we did not.
In general, current literature about gene–environment interactions using risk factors similar to those tested here shows heterogeneous results. A study evaluating the risk of cardiovascular disease morbidity and mortality by the SNP rs4977574 on chromosome 9p21 reported that this association was modified by smoking, but not by the other lifestyle factors analyzed (educational status and physical activity).17 However, the Atherosclerosis Risk in Communities Study did not reveal a significant interaction with this SNP and smoking, possibly due to a lack of statistical power (1653 cases versus 2309) as may be the case in our study (1534 cases).41 Another study found a significant interaction between the rs4977574 and vegetable and wine intake but not with total alcohol in 3164 cases of cardiovascular disease.18 Similarly, in our study, we could not detect a significant interaction with total alcohol intake. However, those with the highest genetic risk had higher odds of MI for any category of alcohol consumption, except for moderate intake. In addition, participants in the moderate genetic risk with moderate alcohol intake had significantly lower odds of MI compared to those with the lowest genetic risk and lowest alcohol consumption (nonalcohol consumption).
When we analyzed the joint association with each independent lifestyle component, adjusting for the other components, we found that the highest odds of MI jointly with the GRS were for smoking, followed by waist:hip ratio, diet, physical activity, and socioeconomic status. A similar gradient of population‐attributable risk of only lifestyle factors was shown in a case–control study (15 152 cases and 14 820 controls) of acute MI in 52 countries, where the strongest risk factors for MI were smoking (population‐attributable risk=36%), abdominal obesity (20.1%), daily fruit and vegetables (13.7%), physical activity (12.2%), and regular alcohol consumption (7.1%).37 In line with these results, strong evidence support that the modification of lifestyle factors could potentially decrease the risk of CHD, MI, stroke, and related clinical risk factors7, 9, 11, 36, 42, 43 even in secondary prevention.38
Despite the discrepancies among studies regarding the SNPs studied, the diet definition (factor analysis, guidelines or recommendations, or specific diet components), and outcomes that limit the comparison and replication among studies, our findings indicate that improving several lifestyle factors can serve as opportunities to reduce the likelihood of heart attacks in Costa Ricans, similar to previous studies conducted in other populations. Lifestyle modification through smoking cessation, healthy diet promotion, physical activity programs, or social assistance programs should be the cornerstone of prevention of MI and related CHD events. More importantly, a combination of these strategies may be even more protective than single behaviors, and may partly overcome the genetic predisposition that some individuals present. Although our findings highlighted that participants with the combination of poor lifestyle and genetic factors were at substantially increased risk, the results from this analysis justify and promote the need for lifestyle intervention and modification for all populations regardless of whether they carry the mutations analyzed.
Strengths of our study include the matched study design by age, sex, and area of residence; thus the associations were not likely confounded by these factors. Using randomly selected population‐based controls after matching, and conditional logistic regression for our matched sample also lend merit to our results. Additionally, we examined an overall GRS and overall LCRS comprising many lifestyle risk factors rather than individual SNPs or individual foods or nutrients. This is a more comprehensive way to evaluate the multifaceted nature of our genetic makeup and environment.
We should note some limitations of our study. Although the genetic variants used in the GRS have been associated with increased risk of CHD in European populations,6, 10, 12 they account for a modest portion of the variability in MI, and other genetic variants (for example, in genes of the inflammation response) could have a stronger effect in this population. A more comprehensive evaluation of gene–environment interactions with genetic markers in additional loci warrants future investigation. Future studies with specific designs to test this hypothesis could help generate more knowledge. Another limitation is the self‐reported dietary and other lifestyle information; thus, measurement error and misclassification were inevitable. However, we were able to validate the food frequency questionnaire with biomarkers of diet. In addition, to minimize potential recall bias among cases, dietary data collection was conducted in the participant's home as close as possible to hospital discharge. In any case, these issues may only attenuate the risk estimates.44
In conclusion, although lifestyle risk factors and genetics contribute independently and jointly to higher odds of MI, lifestyle risk factors were more strongly associated with MI among Hispanic/Latino adults living in Costa Rica. Efforts to improve lifestyle behaviors in this population, regardless of genetic susceptibility, may help prevent MI and related heart conditions.
Sources of Funding
This study was supported by grants HL49086 and HL60692 from the National Heart, Lung, and Blood Institute (NHLBI) at the National Institutes of Health. Funding was also provided by a Mentored Career Development Award to Promote Faculty Diversity in Biomedical Research from the NHLBI (Mattei, grant number K01‐HL120951). Sotos‐Prieto was supported by a research fellowship from Fundación Alfonso Martín Escudero (FAME), Spain.
Disclosures
None.
Supporting information
Table S1. Odds Ratios of Myocardial Infarction by Tertile of Lifestyle Cardiovascular Risk Score and Genetic Risk Score, for Interaction and Joint Analysis Among Hispanic/Latino Adults Living in Costa Rica
Table S2. Odds of Myocardial Infarction for the Lifestyle Cardiovascular Risk Score and the Simplified 3‐SNP Genetic Risk Score Among Hispanic/Latino Adults Living in Costa Rica
Table S3. Joint and Interaction Associations of Individual Lifestyle Cardiovascular Risk Factors With the Simplified 3‐SNP Genetic Risk Score (in Tertiles) on Myocardial Infarction Among Hispanic/Latino Adults Living in Costa Rica
Table S4. Odds Ratios of Myocardial Infarction by Tertile of Lifestyle Cardiovascular Risk Score and the Simplified 3‐SNP Genetic Risk Score, for Interaction and Joint Analysis Among Hispanic/Latino Adults Living in Costa Rica
(J Am Heart Assoc. 2016;5:e004067 doi: 10.1161/JAHA.116.004067)
References
- 1. World Health Organization . Noncommunicable diseases (NCD) country profiles. 2014. Available at: http://www.who.int/nmh/countries/cri_en.pdf. Accessed March 30, 2016.
- 2. Costa Rica, Ministerio de Salud . Encuesta Nacional de Nutricion Costa Rica, 2008–2009. Available at: http://www.paho.org/cor/index.php?option=com_docman&view=download&category_slug=alimentacion-y-nutricion&alias=67-encuesta-nacional-de-nutricion-costa-rica-2008-2009&Itemid=222&lang=en. Accessed March 30, 2016.
- 3. Institute for Health Metrics and Evaluation . 2016. Available at: http://www.healthdata.org/costa-rica. Accessed March 30, 2016.
- 4. Rodriguez T, Malvezzi M, Chatenoud L, Bosetti C, Levi F, Negri E, La Vecchia C. Trends in mortality from coronary heart and cerebrovascular diseases in the Americas: 1970–2000. Heart. 2006;92:453–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aslibekyan S, Campos H, Loucks EB, Linkletter CD, Ordovas JM, Baylin A. Development of a cardiovascular risk score for use in low‐ and middle‐income countries. J Nutr. 2011;141:1375–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Samani NJ, Erdmann J, Hall AS, Hengstenberg C, Mangino M, Mayer B, Dixon RJ, Meitinger T, Braund P, Wichmann HE, Barrett JH, Konig IR, Stevens SE, Szymczak S, Tregouet DA, Iles MM, Pahlke F, Pollard H, Lieb W, Cambien F, Fischer M, Ouwehand W, Blankenberg S, Balmforth AJ, Baessler A, Ball SG, Strom TM, Braenne I, Gieger C, Deloukas P, Tobin MD, Ziegler A, Thompson JR, Schunkert H. Genomewide association analysis of coronary artery disease. N Engl J Med. 2007;357:443–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kabagambe EK, Baylin A, Campos H. Nonfatal acute myocardial infarction in Costa Rica: modifiable risk factors, population‐attributable risks, and adherence to dietary guidelines. Circulation. 2007;115:1075–1081. [DOI] [PubMed] [Google Scholar]
- 8. Iqbal R, Anand S, Ounpuu S, Islam S, Zhang X, Rangarajan S, Chifamba J, Al‐Hinai A, Keltai M, Yusuf S. Dietary patterns and the risk of acute myocardial infarction in 52 countries: results of the INTERHEART study. Circulation. 2008;118:1929–1937. [DOI] [PubMed] [Google Scholar]
- 9. Akesson A, Weismayer C, Newby PK, Wolk A. Combined effect of low‐risk dietary and lifestyle behaviors in primary prevention of myocardial infarction in women. Arch Intern Med. 2007;167:2122–2127. [DOI] [PubMed] [Google Scholar]
- 10. Helgadottir A, Thorleifsson G, Manolescu A, Gretarsdottir S, Blondal T, Jonasdottir A, Sigurdsson A, Baker A, Palsson A, Masson G, Gudbjartsson DF, Magnusson KP, Andersen K, Levey AI, Backman VM, Matthiasdottir S, Jonsdottir T, Palsson S, Einarsdottir H, Gunnarsdottir S, Gylfason A, Vaccarino V, Hooper WC, Reilly MP, Granger CB, Austin H, Rader DJ, Shah SH, Quyyumi AA, Gulcher JR, Thorgeirsson G, Thorsteinsdottir U, Kong A, Stefansson K. A common variant on chromosome 9p21 affects the risk of myocardial infarction. Science. 2007;316:1491–1493. [DOI] [PubMed] [Google Scholar]
- 11. Sotos‐Prieto M, Bhupathiraju SN, Falcon LM, Gao X, Tucker KL, Mattei J. A Healthy Lifestyle Score is associated with cardiometabolic and neuroendocrine risk factors among Puerto Rican adults. J Nutr. 2015;145:1531–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kathiresan S, Voight BF, Purcell S, Musunuru K, Ardissino D, Mannucci PM, Anand S, Engert JC, Samani NJ, Schunkert H, Erdmann J, Reilly MP, Rader DJ, Morgan T, Spertus JA, Stoll M, Girelli D, McKeown PP, Patterson CC, Siscovick DS, O'Donnell CJ, Elosua R, Peltonen L, Salomaa V, Schwartz SM, Melander O, Altshuler D, Merlini PA, Berzuini C, Bernardinelli L, Peyvandi F, Tubaro M, Celli P, Ferrario M, Fetiveau R, Marziliano N, Casari G, Galli M, Ribichini F, Rossi M, Bernardi F, Zonzin P, Piazza A, Yee J, Friedlander Y, Marrugat J, Lucas G, Subirana I, Sala J, Ramos R, Meigs JB, Williams G, Nathan DM, MacRae CA, Havulinna AS, Berglund G, Hirschhorn JN, Asselta R, Duga S, Spreafico M, Daly MJ, Nemesh J, Korn JM, McCarroll SA, Surti A, Guiducci C, Gianniny L, Mirel D, Parkin M, Burtt N, Gabriel SB, Thompson JR, Braund PS, Wright BJ, Balmforth AJ, Ball SG, Hall A, Linsel‐Nitschke P, Lieb W, Ziegler A, Konig I, Hengstenberg C, Fischer M, Stark K, Grosshennig A, Preuss M, Wichmann HE, Schreiber S, Ouwehand W, Deloukas P, Scholz M, Cambien F, Li M, Chen Z, Wilensky R, Matthai W, Qasim A, Hakonarson HH, Devaney J, Burnett MS, Pichard AD, Kent KM, Satler L, Lindsay JM, Waksman R, Knouff CW, Waterworth DM, Walker MC, Mooser V, Epstein SE, Scheffold T, Berger K, Huge A, Martinelli N, Olivieri O, Corrocher R, McKeown P, Erdmann E, Konig IR, Holm H, Thorleifsson G, Thorsteinsdottir U, Stefansson K, Do R, Xie C, Siscovick D. Genome‐wide association of early‐onset myocardial infarction with single nucleotide polymorphisms and copy number variants. Nat Genet. 2009;41:334–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Qi L, Ma J, Qi Q, Hartiala J, Allayee H, Campos H. Genetic risk score and risk of myocardial infarction in Hispanics. Circulation. 2011;123:374–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Erdmann J, Linsel‐Nitschke P, Schunkert H. Genetic causes of myocardial infarction: new insights from genome‐wide association studies. Dtsch Arztebl Int. 2010;107:694–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Manolio TA. Cohort studies and the genetics of complex disease. Nat Genet. 2009;41:5–6. [DOI] [PubMed] [Google Scholar]
- 16. Do R, Xie C, Zhang X, Mannisto S, Harald K, Islam S, Bailey SD, Rangarajan S, McQueen MJ, Diaz R, Lisheng L, Wang X, Silander K, Peltonen L, Yusuf S, Salomaa V, Engert JC, Anand SS. The effect of chromosome 9p21 variants on cardiovascular disease may be modified by dietary intake: evidence from a case/control and a prospective study. PLoS Med. 2011;8:e1001106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hamrefors V, Hedblad B, Hindy G, Smith JG, Almgren P, Engstrom G, Sjogren M, Gransbo K, Orho‐Melander M, Melander O. Smoking modifies the associated increased risk of future cardiovascular disease by genetic variation on chromosome 9p21. PLoS One. 2014;9:e85893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hindy G, Ericson U, Hamrefors V, Drake I, Wirfalt E, Melander O, Orho‐Melander M. The chromosome 9p21 variant interacts with vegetable and wine intake to influence the risk of cardiovascular disease: a population based cohort study. BMC Med Genet. 2014;15:1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Qi L, Cornelis MC, Zhang C, van Dam RM, Hu FB. Genetic predisposition, Western dietary pattern, and the risk of type 2 diabetes in men. Am J Clin Nutr. 2009;89:1453–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sun Q, Villegas R, Delahanty R, Gao Y‐T, Long J, Williams SM, Xiang Y‐B, Cai H, Li H‐L, Hu F, Cai Q, Zheng W, Shu X‐O. Joint effect of genetic and lifestyle risk factors on type 2 diabetes risk among Chinese men and women. PLoS One. 2012;7:e49464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wassel CL, Pankow JS, Peralta CA, Choudhry S, Seldin MF, Arnett DK. Genetic ancestry is associated with subclinical cardiovascular disease in African‐Americans and Hispanics from the Multi‐Ethnic Study of Atherosclerosis. Circ Cardiovasc Genet. 2009;2:629–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. D'Agostino RB Jr, Burke G, O'Leary D, Rewers M, Selby J, Savage PJ, Saad MF, Bergman RN, Howard G, Wagenknecht L, Haffner SM. Ethnic differences in carotid wall thickness. The Insulin Resistance Atherosclerosis Study. Stroke. 1996;27:1744–1749. [DOI] [PubMed] [Google Scholar]
- 23. Tunstall‐Pedoe H, Kuulasmaa K, Amouyel P, Arveiler D, Rajakangas AM, Pajak A. Myocardial infarction and coronary deaths in the World Health Organization MONICA Project. Registration procedures, event rates, and case‐fatality rates in 38 populations from 21 countries in four continents. Circulation. 1994;90:583–612. [DOI] [PubMed] [Google Scholar]
- 24. Kabagambe EK, Baylin A, Ruiz‐Narvaez E, Rimm EB, Campos H. Alcohol intake, drinking patterns, and risk of nonfatal acute myocardial infarction in Costa Rica. Am J Clin Nutr. 2005;82:1336–1345. [DOI] [PubMed] [Google Scholar]
- 25. Kabagambe EK, Furtado J, Baylin A, Campos H. Some dietary and adipose tissue carotenoids are associated with the risk of nonfatal acute myocardial infarction in Costa Rica. J Nutr. 2005;135:1763–1769. [DOI] [PubMed] [Google Scholar]
- 26. Campos H, Mata L, Siles X, Vives M, Ordovas JM, Schaefer EJ. Prevalence of cardiovascular risk factors in rural and urban Costa Rica. Circulation. 1992;85:648–658. [DOI] [PubMed] [Google Scholar]
- 27. Campos H, Siles X. Siesta and the risk of coronary heart disease: results from a population‐based, case‐control study in Costa Rica. Int J Epidemiol. 2000;29:429–437. [PubMed] [Google Scholar]
- 28. Campos H, Bailey SM, Gussak LS, Siles X, Ordovas JM, Schaefer EJ. Relations of body habitus, fitness level, and cardiovascular risk factors including lipoproteins and apolipoproteins in a rural and urban Costa Rican population. Arterioscler Thromb. 1991;11:1077–1088. [DOI] [PubMed] [Google Scholar]
- 29. Kabagambe EK, Baylin A, Allan DA, Siles X, Spiegelman D, Campos H. Application of the method of triads to evaluate the performance of food frequency questionnaires and biomarkers as indicators of long‐term dietary intake. Am J Epidemiol. 2001;154:1126–1135. [DOI] [PubMed] [Google Scholar]
- 30. Baylin A, Kabagambe EK, Siles X, Campos H. Adipose tissue biomarkers of fatty acid intake. Am J Clin Nutr. 2002;76:750–757. [DOI] [PubMed] [Google Scholar]
- 31. World Health Organization : Report of a Joint WHO/FAO Expert Consultation. Diet, Nutrition and the Prevention of Chronic Diseases. 2003. Available at: http://apps.who.int/iris/bitstream/10665/42665/1/WHO_TRS_916.pdf. Accessed March 30, 2016.
- 32. World Health Organization : Joint FAO/WHO Expert Consultation on Human Vitamin and Mineral Requirements. Vitamin and Mineral Requirements in Human Nutrition: Folate and folic acid. p289–300. 2004. Available at: http://apps.who.int/iris/bitstream/10665/42716/1/9241546123.pdf. Accessed March 30, 2016. [Google Scholar]
- 33. Campos H, Baylin A, Willett WC. Alpha‐linolenic acid and risk of nonfatal acute myocardial infarction. Circulation. 2008;118:339–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–553. [DOI] [PubMed] [Google Scholar]
- 35. Censos. INdEy . Cifras Basicas Sobre Pobrezae Ingresos. San Jose (Costa Rica): Instituto nacional de estadistica y censos; 2009. [Google Scholar]
- 36. Sotos‐Prieto M, Bhupathiraju SN, Mattei J, Fung TT, Li Y, Pan A, Willett WC, Rimm EB, Hu FB. Changes in diet quality scores and risk of cardiovascular disease among US men and women. Circulation. 2015;132:2212–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J, Lisheng L. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case‐control study. Lancet. 2004;364:937–952. [DOI] [PubMed] [Google Scholar]
- 38. Booth JN, Levitan EB, Brown TM, Farkouh ME, Safford MM, Muntner P. Effect of sustaining lifestyle modifications (nonsmoking, weight reduction, physical activity, and Mediterranean diet) after healing of myocardial infarction, percutaneous intervention, or coronary bypass (from the REasons for Geographic and Racial Differences in Stroke Study). Am J Cardiol. 2014;113:1933–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sotos‐Prieto M, Luben R, Khaw KT, Wareham NJ, Forouhi NG. The association between Mediterranean Diet Score and glucokinase regulatory protein gene variation on the markers of cardiometabolic risk: an analysis in the European Prospective Investigation into Cancer (EPIC)‐Norfolk study. Br J Nutr. 2014;112:122–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dempfle A, Scherag A, Hein R, Beckmann L, Chang‐Claude J, Schafer H. Gene‐environment interactions for complex traits: definitions, methodological requirements and challenges. Eur J Hum Genet. 2008;16:1164–1172. [DOI] [PubMed] [Google Scholar]
- 41. Folsom AR, Nambi V, Pankow JS, Tang W, Farbakhsh K, Yamagishi K, Boerwinkle E. Effect of 9p21 genetic variation on coronary heart disease is not modified by other risk markers. The Atherosclerosis Risk in Communities (ARIC) Study. Atherosclerosis. 2012;224:435–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chomistek AK, Chiuve SE, Eliassen AH, Mukamal KJ, Willett WC, Rimm EB. Healthy lifestyle in the primordial prevention of cardiovascular disease among young women. J Am Coll Cardiol. 2015;65:43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Larsson SC, Akesson A, Wolk A. Healthy diet and lifestyle and risk of stroke in a prospective cohort of women. Neurology. 2014;83:1699–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lichtman SW, Pisarska K, Berman ER, Pestone M, Dowling H, Offenbacher E, Weisel H, Heshka S, Matthews DE, Heymsfield SB. Discrepancy between self‐reported and actual caloric intake and exercise in obese subjects. N Engl J Med. 1992;327:1893–1898. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Odds Ratios of Myocardial Infarction by Tertile of Lifestyle Cardiovascular Risk Score and Genetic Risk Score, for Interaction and Joint Analysis Among Hispanic/Latino Adults Living in Costa Rica
Table S2. Odds of Myocardial Infarction for the Lifestyle Cardiovascular Risk Score and the Simplified 3‐SNP Genetic Risk Score Among Hispanic/Latino Adults Living in Costa Rica
Table S3. Joint and Interaction Associations of Individual Lifestyle Cardiovascular Risk Factors With the Simplified 3‐SNP Genetic Risk Score (in Tertiles) on Myocardial Infarction Among Hispanic/Latino Adults Living in Costa Rica
Table S4. Odds Ratios of Myocardial Infarction by Tertile of Lifestyle Cardiovascular Risk Score and the Simplified 3‐SNP Genetic Risk Score, for Interaction and Joint Analysis Among Hispanic/Latino Adults Living in Costa Rica


